Photosynthetic Limitations and Growth Traits of Four Arabica Coffee (Coffea arabica L.) Genotypes under Water Deficit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Site Conditions
2.2. Plant Material
2.3. Experimental Design
2.4. Water Deficit Experiment
2.5. Water Status
2.6. Gas Exchange
2.7. A/Ci Curves
2.8. Growth
2.9. Statistical Analysis
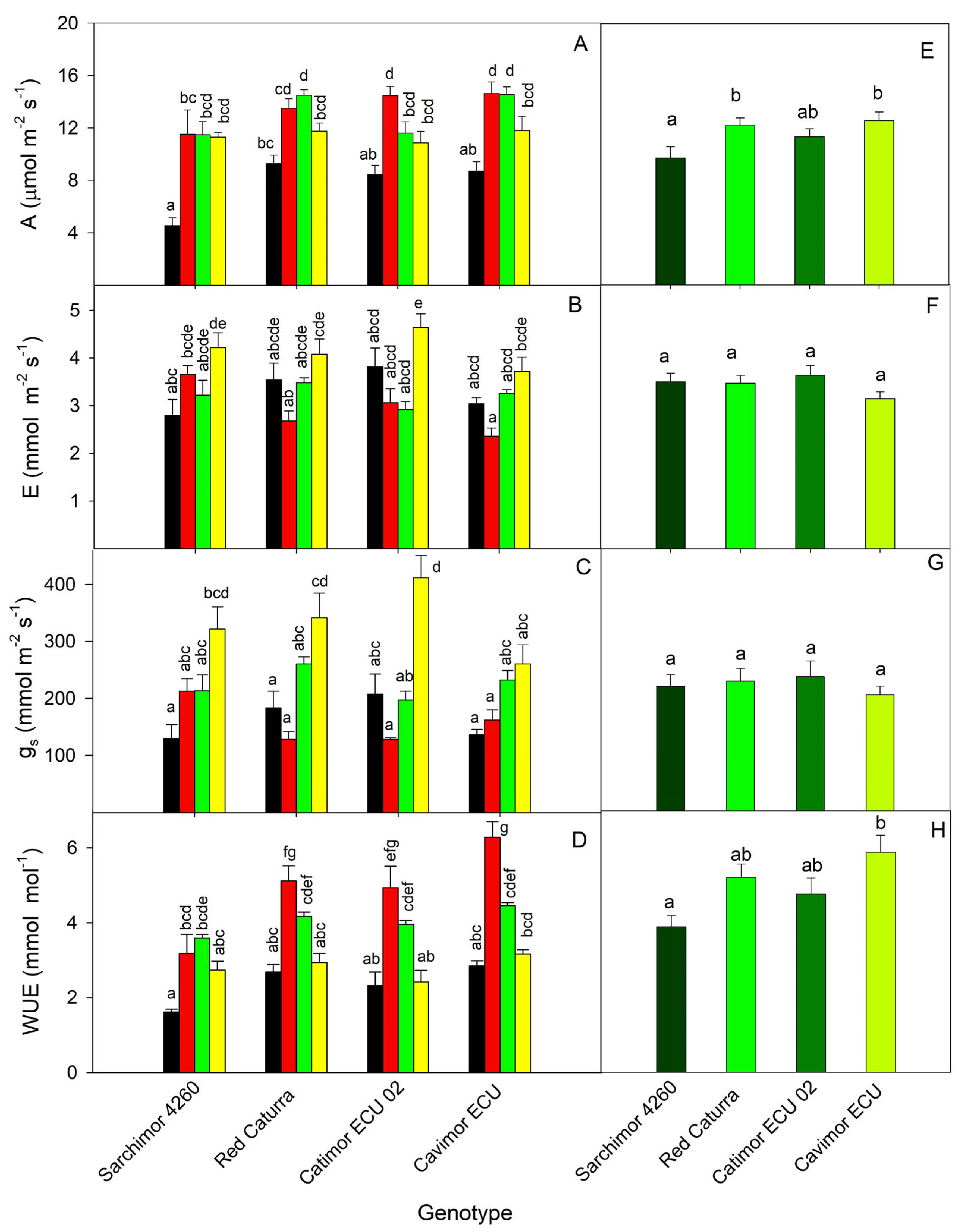
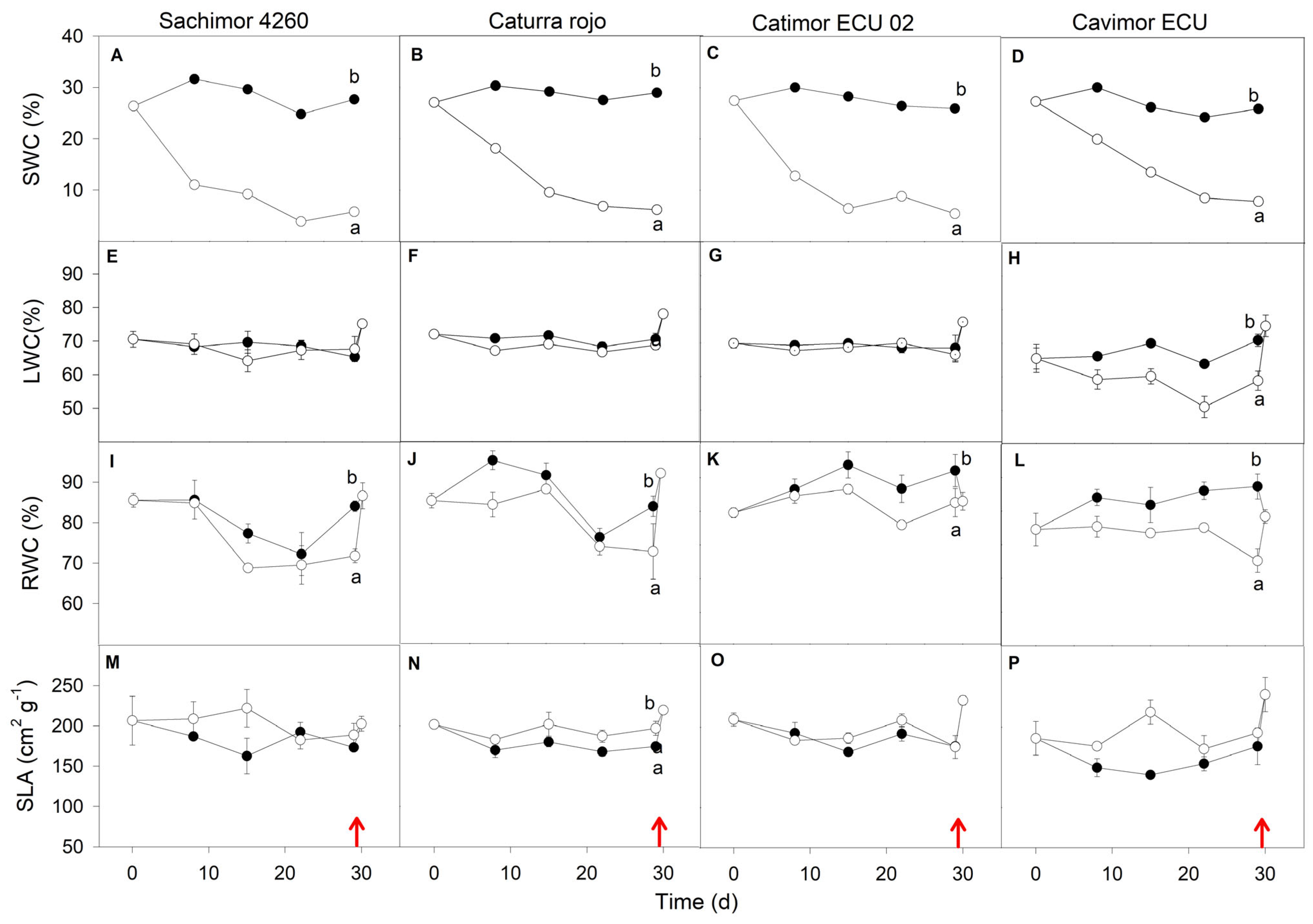
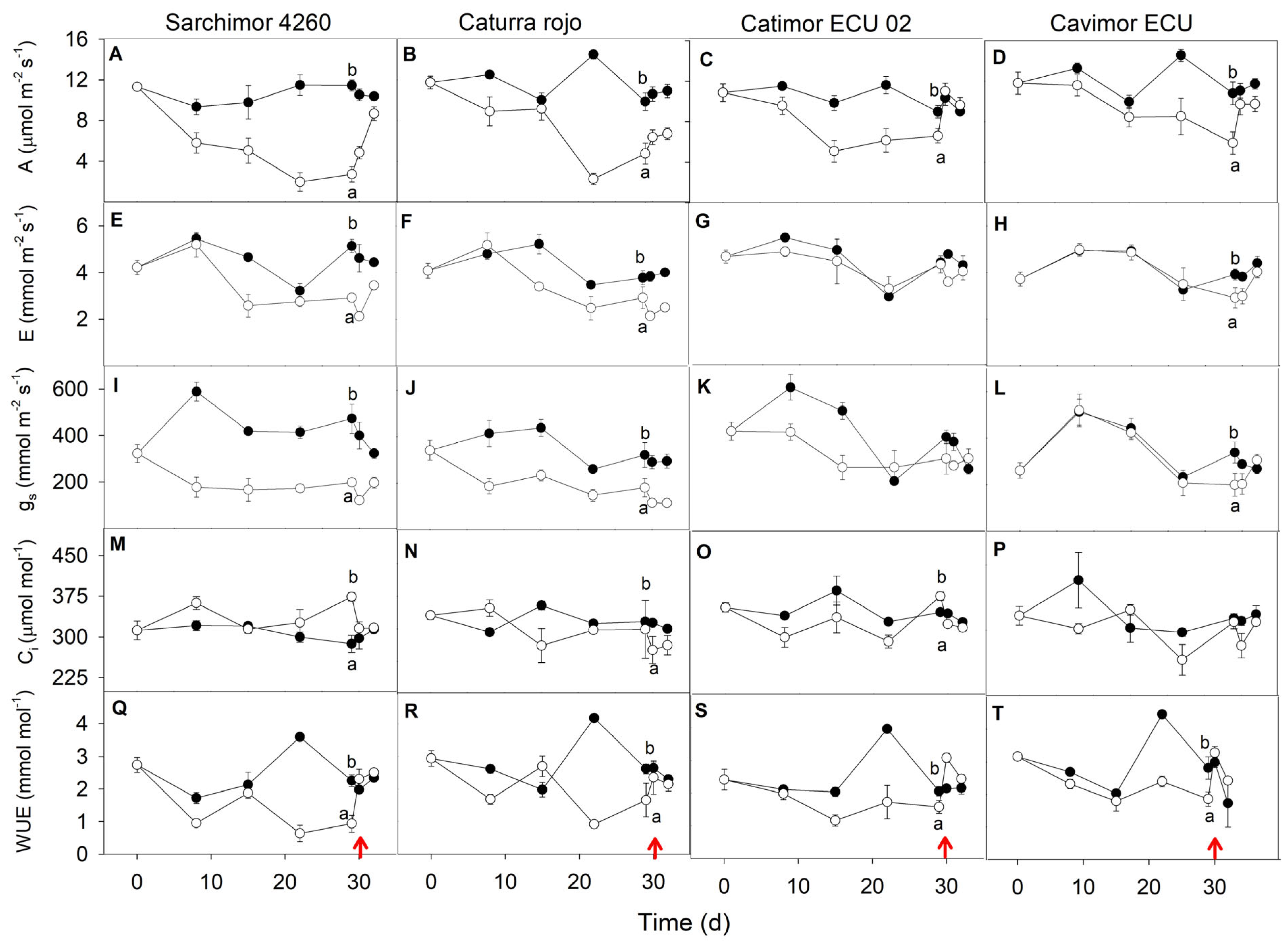
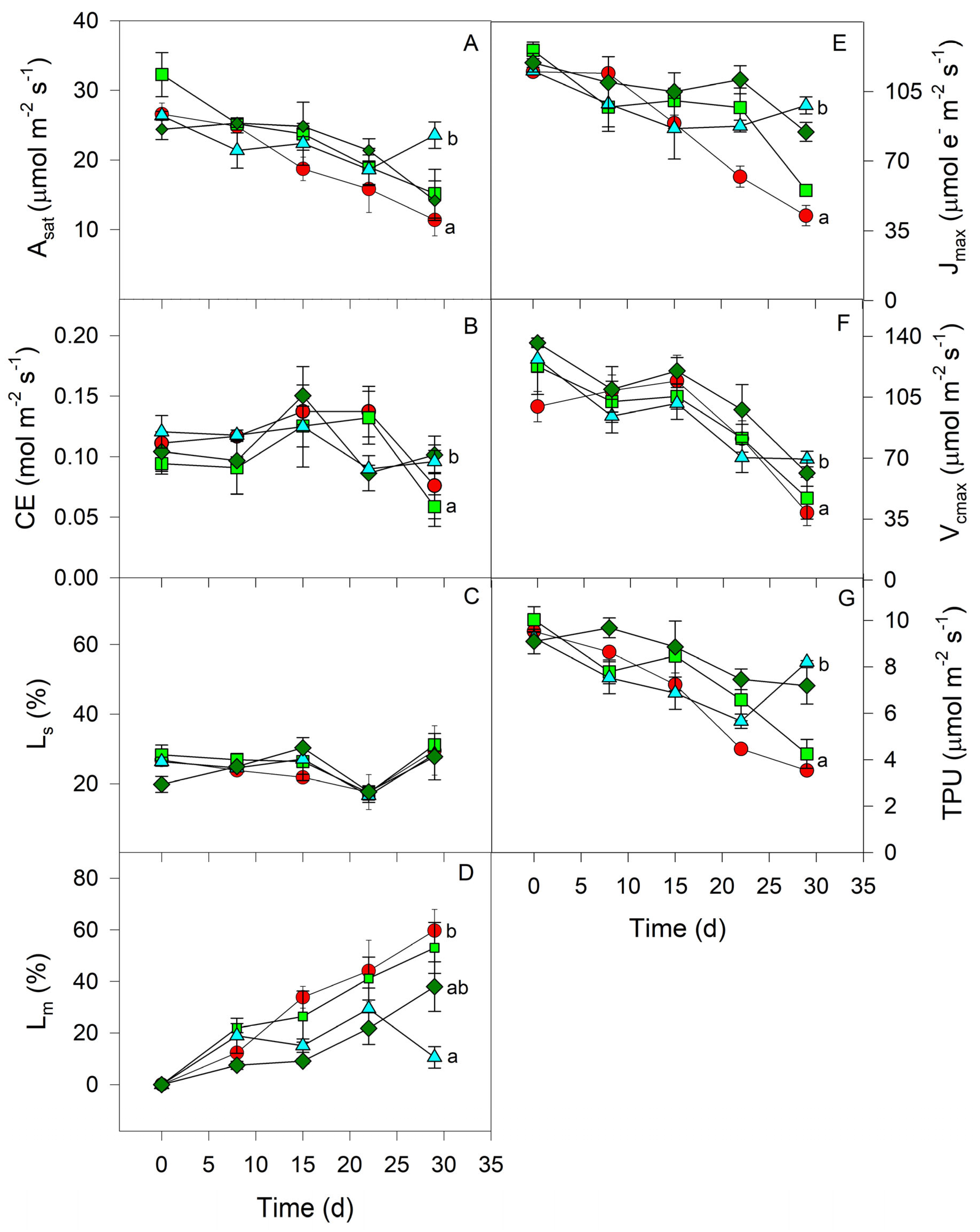
3. Results
3.1. Genotype Physiological Characterization
3.2. Water Deficit Experiment
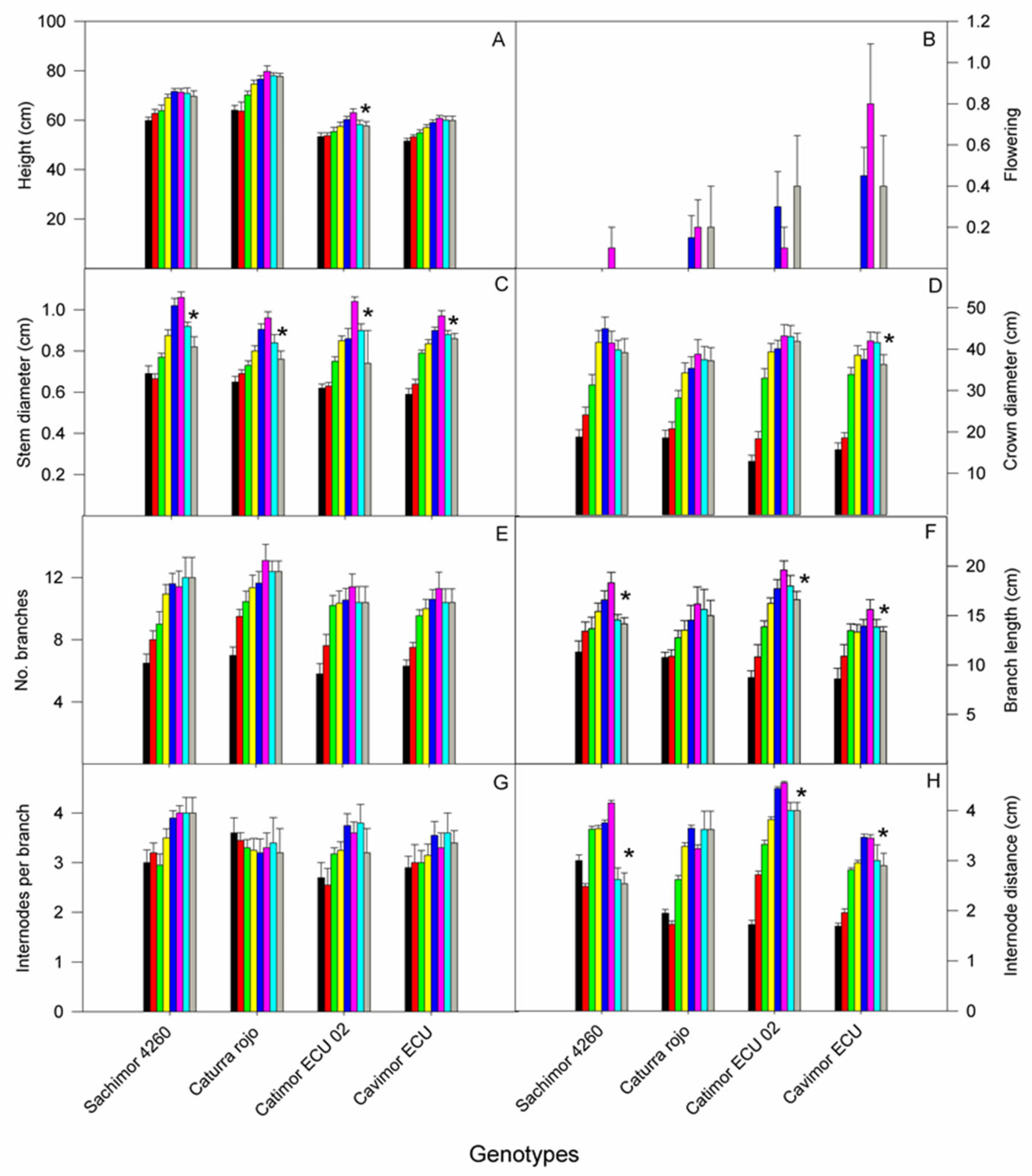
3.3. Growth
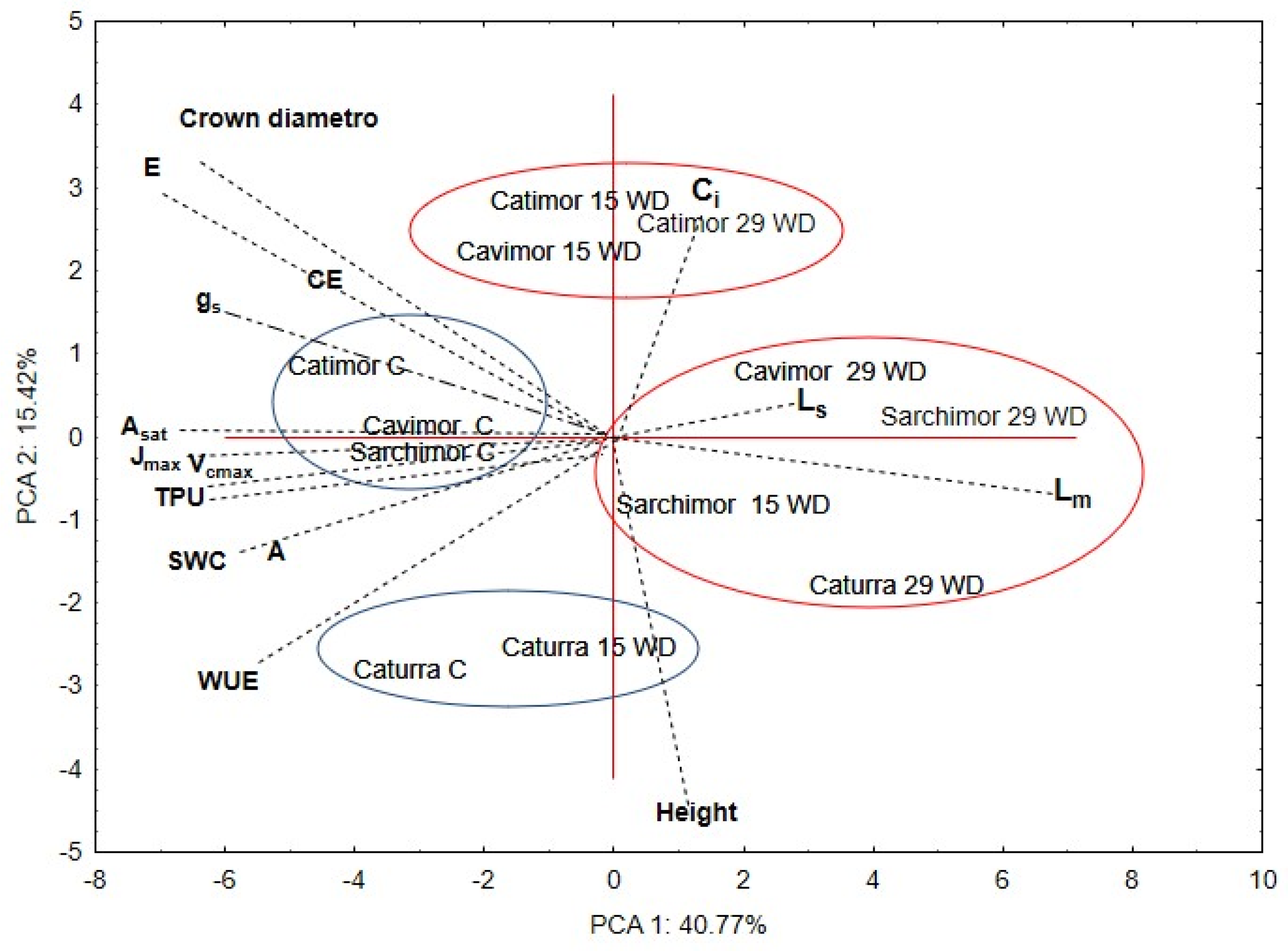
3.4. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caporaso, N.; Whitworth, M.B.; Grebby, S.; Fisk, I.D. Non-destructive analysis of sucrose, caffeine and trigonelline on single green coffee beans by hyperspectral imaging. Food Res. Int. 2018, 106, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Ma, G.; Bai, X.; Li, J.; Zhao, M.; Su, L.; Zhou, H. The influence of leaf anatomical traits on photosynthesis in Catimor type Arabica coffee. Bever. Plant Res. 2024, 4, e002. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Rahn, E.; Läderach, P.; Ghini, R.; Ramalho, J.C. Why could the coffee crop endure climate change and global warming to a greater extent than previously estimated? Clim. Chang. 2019, 152, 167–178. [Google Scholar] [CrossRef]
- Semedo, J.N.; Rodrigues, A.P.; Lidon, F.C.; Pais, I.P.; Marques, I.; Gouveia, D.; Armengaud, J.; Silva, M.J.; Martins, S.; Semedo, M.C.; et al. Intrinsic non-stomatal resilience to drought of the photosynthetic apparatus in Coffea spp. is strengthened by elevated air [CO2]. Tree Physiol. 2021, 41, 708–727. [Google Scholar] [CrossRef] [PubMed]
- ICO (International Coffee Organization). 2021. Available online: https://icocoffee.org/ (accessed on 12 December 2021).
- ICO (International Coffee Organization). 2024. Available online: https://icocoffee.org/ (accessed on 1 March 2024).
- Duicela, L. Café robusta: Producción y poscosecha. In Humus ESPAM MFL; Calceta-Manabí-Ecuador: Calceta, Ecuador, 2017; p. 292. ISBN 9789942859587. (In Spanish) [Google Scholar]
- Cavatte, P.C.; Rodríguez-López, N.F.; Martins, S.C.V.; Mattos, M.S.; Sanglard, L.M.; DaMatta, F.M. Functional analysis of the relative growth rate, chemical composition, construction and maintenance costs, and the payback time of Coffea arabica L. leaves in response to light and water availability. J. Exp. Bot. 2012, 63, 3071–3082. [Google Scholar] [CrossRef] [PubMed]
- DaMatta, F.M. Exploring drought tolerance in coffee: A physiological approach with some insights for plant breeding. Braz. J. Plant Physiol. 2004, 16, 1–6. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Ramalho, J.D. Impacts of drought and temperature stress on coffee physiology and production: A review. Braz. J. Plant Physiol. 2006, 18, 55–81. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Godoy, A.G.; Menezes-Silva, P.E.; Martins, S.C.V.; Sanglard, L.M.V.; Morais, L.E.; Torre-Neto, A.; Ghini, R. Sustained enhancement of photosynthesis in coffee trees grown under free-air CO2 enrichment conditions: Disentangling the contributions of stomatal, mesophyll, and biochemical limitations. J. Exp. Bot. 2016, 167, 341–352. [Google Scholar] [CrossRef]
- Martins, M.Q.; Rodrigues, W.P.; Fortunato, A.S.; Leitão, A.E.; Rodrigues, A.P.; Pais, I.P.; Martins, L.D.; Silva, M.J.; Rebodero, F.M.; Partelli, F.L.; et al. Protective response mechanisms to heat stress in interaction with high [CO2] Conditions in Coffea spp. Front. Plant Sci. 2016, 7, 947. [Google Scholar] [CrossRef]
- Rodríguez-López, N.F.; Martins, S.C.V.; Cavatte, P.C.; Silva, P.E.M.; Morais, L.E.; Pereira, L.F.; Reis, J.V.; Ávila, R.T.; Godoy, A.G.; Lavinski, A.O.; et al. Morphological and physiological acclimations of coffee seedlings to growth over a range of fixed or changing light supplies. Environ. Exp. Bot. 2014, 102, 1–10. [Google Scholar] [CrossRef]
- Tezara, W.; Duicela, G.L.A.; Reynel Chila, V.H.; Nazareno Ortiz, R.; Bolaños Ortega, M.J. Seasonal changes in gas exchange and yield of 21 genotypes of Coffea arabica. Bot. Sci. 2022, 100, 1000–1013. [Google Scholar] [CrossRef]
- Duicela, L. Productividad y Estabilidad Ambiental de Clones de Café Robusta en Distintas Localidades Cafetaleras del Ecuador. Ph.D. Thesis, Universidad del Zulia, Maracaibo, Venezuela, 2021. (In Spanish) [Google Scholar] [CrossRef]
- Gornall, J.; Betts, R.; Burke, E.; Clark, R.; Camp, J.; Willet, K.; Wiltshire, A. Implications of climate change for agricultural productivity in the early twenty–first century. Philos. Trans. R. Soc. 2010, 365, 2973–2989. [Google Scholar] [CrossRef] [PubMed]
- DaMatta, F.M.; Grandis, A.; Arenque, B.C.; Buckeridge, M.S. Impacts of climate changes on crop physiology and food quality. Food Res. Int. 2010, 43, 1814–1823. [Google Scholar] [CrossRef]
- Semedo, J.N.; Rodrigues, W.P.; Dubberstein, D.; Martins, M.Q.; Martins, L.D.; Pais, I.P.; Rodrigues, A.P.; Leitão, A.E.; Partelli, F.L.; Campostrini, E.; et al. Coffee responses to drought, warming and high [CO2] in a context of future climate change scenarios. In Theory and Practice of Climate Adaptation. Climate Change Management; Alves, F., Leal Filho, W., Azeiteiro, U., Eds.; Climate Change Management; Springer: Cham, Switzerland, 2018; pp. 465–477. [Google Scholar] [CrossRef]
- Rodrigues, A.P.; Pais, I.P.; Leitão, A.E.; Dubberstein, D.; Lidon, F.C.; Marques, I.; Semedo, J.N.; Rakocevic, M.; Scotti-Campos, P.; Campostrini, E.; et al. Uncovering the wide protective responses in Coffea spp. leaves to single and superimposed exposure of warming and severe water deficit. Front. Plant Sci. 2024, 14, 1320552. [Google Scholar] [CrossRef] [PubMed]
- Merga, D.; Beksisa, L. Mechanisms of drought tolerance in coffee (Coffea arabica L.): Implication for genetic improvement program: Review. Am. J. BioSci. 2023, 11, 63–70. [Google Scholar] [CrossRef]
- Läderach, P.; Ramirez-Villegas, J.; Navarro-Racines, C.; Zelaya, C.; Martinez-Valle, A.; Jarvis, A. Climate change adaptation of coffee production in space and time. Clim. Chang. 2017, 141, 47–62. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation and integration of mechanisms and processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef] [PubMed]
- DaMatta, F.M.; Chaves, A.R.M.; Pinheiro, H.A.; Ducatti, C.; Loureiro, M.E. Drought tolerance of two field-grown clones of Coffea canephora. Plant Sci. 2003, 164, 111–117. [Google Scholar] [CrossRef]
- Pinheiro, H.A. Physiological and Morphological Adaptations as associated with Drought Tolerance in Robusta Coffee (Coffea canephora Pierre var. kouillou). Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2004. [Google Scholar]
- Chekol, H.; Warkineh, B.; Shimber, T.; Mierek-Adamska, A.; Dabrowska, G.B.; Degu, A. Drought stress responses in arabica coffee genotypes: Physiological and metabolic insights. Plants 2024, 13, 828. [Google Scholar] [CrossRef]
- INAMHI. Red de Estaciones Automáticas Hidrometeorológicas. 2016. Available online: https://www.inamhi.gob.ec/informacion-en-linea/ (accessed on 1 May 2021).
- Jones, H.G. Plants and Microclimate. A Quantitative Approach to Environmental Plant Physiology; Cambridge University Press: Cambridge, UK, 1992; p. 465. [Google Scholar]
- Tezara, W.; Torres-Domínguez, T.S.; Loyaga, D.W.; Nazareno-Ortiz, R.; Reynel-Chila, V.H.; Bolaños Ortega, M.J. Photosynthetic activity of oil palm (Elaeis guineensis) and interspecific hybrid genotypes (Elaeis oleifera × Elaeis guineensis), and response of hybrids to water déficit. Sci. Hortic. 2021, 287, 110263. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Jacob, J.; Lawlor, D.W. Stomatal and mesophyll limitations of photosynthesis in phosphate deficient sunflower, maize and wheat plants. J. Exp. Bot. 1991, 42, 1003–1011. [Google Scholar] [CrossRef]
- Sharkey, T.D. What gas exchange data can tell us about photosynthesis? Plant Cell Environ. 2015, 39, 1161–1163. [Google Scholar] [CrossRef] [PubMed]
- Tezara Fernández, W.; Mendoza Cortez, P.J.; Loyaga Guerrero, W.D.; Reynel Chila, V.H.; Bolaños Ortega, M.J. Capacidad fotosintética de 15 clones de café robusta (Coffea canephora) en Esmeraldas, Ecuador. Rev. Espamciencia 2020, 11, 19–27. [Google Scholar] [CrossRef]
- Reyes-Herrera, D.F.; Sánchez-Reinoso, A.D.; Lombardini, L.; Restrepo-Díaz, H. Physiological responses of coffee (Coffea arabica L.) plants to biochar application under water deficit conditions. Not. Bot. Horti Agrobot. 2023, 51, 12873. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Ronchi, C.P.; Maestri, M.; Barros, R.S. Ecophysiology of coffee growth and production. Braz. J. Plant Physiol. 2007, 19, 485–510. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Maestri, M.; Barros, R.S.; Regazzi, A.J. Water relations of coffee leaves (Coffea arabica and C. canephora) in response to drought. J. Horticult. Sci. 1993, 68, 741–746. [Google Scholar] [CrossRef]
- Campostrini, E.; Maestri, M. Photosynthetic potential of five genotypes of Coffea canephora Pierre. Rev. Bras. Fisiol. Veg. 1998, 10, 13–18. [Google Scholar]
- DaMatta, F.M.; Loos, R.A.; Rodrigues, R.; Barros, R.S. Actual and potential photosynthetic rates of tropical crop species. Braz. J. Plant. Physiol. 2001, 13, 24–32. [Google Scholar] [CrossRef]
- Martins, S.C.V.; Galmés, J.G.; Molins, A.; DaMatta, F.M. Improving the estimation of mesophyll conductance: On the role of electron transport rate correction and respiration. J. Exp. Bot. 2013, 64, 3285–3298. [Google Scholar] [CrossRef]
- Martins, S.C.V.; Galmés, J.; Cavatte, P.C.; Pereira, L.F.; Ventrella, M.C.; DaMatta, F.M. Understanding the low photosynthetic rates of sun and shade coffee leaves: Bridging the gap on the relative roles of hydraulic, diffusive and biochemical constraints to photosynthesis. PLoS ONE 2014, 9, e95571. [Google Scholar] [CrossRef] [PubMed]

| Hour of Day |
Ca (µmol mol−1) |
PPFD (µmol m−2 s−1) |
Ta (°C) |
RH (%) | ∆w (kPa) |
|---|---|---|---|---|---|
| 08:00 | 510 ± 2 | 30 ± 3 | 24.8 ± 0.4 | 82.8 ± 0.4 | 0.5 ± 0.02 |
| 13:00 | 414 ± 7 | 260 ± 70 | 33.1 ± 0.7 | 53.7 ± 0.7 | 2.3 ± 0.08 |
| 17:00 | 418 ± 5 | 50 ± 6 | 28.4 ± 0.6 | 66.2 ± 0.6 | 1.3 ± 0.05 |
| Name | Traits and Genetic Origin |
|---|---|
| Sarchimor 4260 | Sarchimor from CIFC based on the cross of Hybrid Villa Sarchi × Timor Hybrid. Various genetic lines of Sarchimor are being evaluated in Ecuador, and to date have excellent agronomic, productive and resistance to coffee rust. |
| Red Caturra | Caturra are mutants of the coffee variety Bourbon, native to Brazil. This variety is considered to have a wide range of adaptability, high production, good agronomic and organoleptic characteristics, but susceptible to coffee rust. |
| Cavimor ECU | Developed at the Coffee Rust Research Center (CIFC, Oeiras, Portugal) based on the crossing of Hybrid Catuaí × Catimor. Several Cavimor genetic lines currently being evaluated showed resistant to coffee rust. |
| Catimor ECU 02 | The CIFC has developed the hybrid Catimor, the result of the cross between Caturra and Timor. This hybrid shows great genetic variability and resistance to coffee rust. |
| Parameters | Abbreviations | Units |
|---|---|---|
| Net photosynthetic rate | A | μmol m−2 s−1 |
| A at saturating intercellular [CO2] | Asat | μmol m−2 s−1 |
| Carboxylation efficiency | CE | mol m−2 s−1 |
| Intercellular [CO2] | Ci | μmol mol−1 |
| Transpiration rate | E | mmol m−2 s−1 |
| Electron transport rate | J | μmol e− m−2 s−1 |
| Maximum electron transport rate | Jmax | μmol e− m−2 s−1 |
| Stomatal conductance | gs | mmol m−2 s−1 |
| CO2 compensation point | Γ | μmol mol−1 |
| Mesophyll limitation | Lm | % |
| Relative stomatal limitation | Ls | % |
| Leaf water content | LWC | No units |
| Photosynthetic photon flux density | PPFD | μmol photons m−2 s−1 |
| Relative humidity | RH | % |
| Relative water content | RWC | No units |
| Soil water content | SWC | % |
| Triose phosphate utilization rate | TPU | μmol m−2 s−1 |
| Air water vapor pressure deficit | ∆W | KPa |
| Maximum rate of carboxylation of Rubisco | Vcmax | μmol m−2 s−1 |
| Water deficit | WD | |
| Water use efficiency | WUE | mmol CO2 mol H2O−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tezara, W.; Loyaga, D.W.; Reynel Chila, V.H.; Herrera, A. Photosynthetic Limitations and Growth Traits of Four Arabica Coffee (Coffea arabica L.) Genotypes under Water Deficit. Agronomy 2024, 14, 1713. https://doi.org/10.3390/agronomy14081713
Tezara W, Loyaga DW, Reynel Chila VH, Herrera A. Photosynthetic Limitations and Growth Traits of Four Arabica Coffee (Coffea arabica L.) Genotypes under Water Deficit. Agronomy. 2024; 14(8):1713. https://doi.org/10.3390/agronomy14081713
Chicago/Turabian StyleTezara, Wilmer, Daniel W. Loyaga, Víctor H. Reynel Chila, and Ana Herrera. 2024. "Photosynthetic Limitations and Growth Traits of Four Arabica Coffee (Coffea arabica L.) Genotypes under Water Deficit" Agronomy 14, no. 8: 1713. https://doi.org/10.3390/agronomy14081713
APA StyleTezara, W., Loyaga, D. W., Reynel Chila, V. H., & Herrera, A. (2024). Photosynthetic Limitations and Growth Traits of Four Arabica Coffee (Coffea arabica L.) Genotypes under Water Deficit. Agronomy, 14(8), 1713. https://doi.org/10.3390/agronomy14081713







