Inducing Aquilaria sinensis (Lour.) Spreng “Qinan” Agarwood Formation with Chemical Reagents
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Determination of Non-Structural and Structural Carbohydrates
2.3. Essential Oil Extraction and Content Determination
2.4. Essential Oil Composition Determination
2.5. Determinations of Net Photosynthetic Rates, Flavonoids, Phenols and Total Antioxidant Capacity
2.6. Statistical Analysis
3. Results
3.1. Visual Appearance of Agarwood and Essential Oils
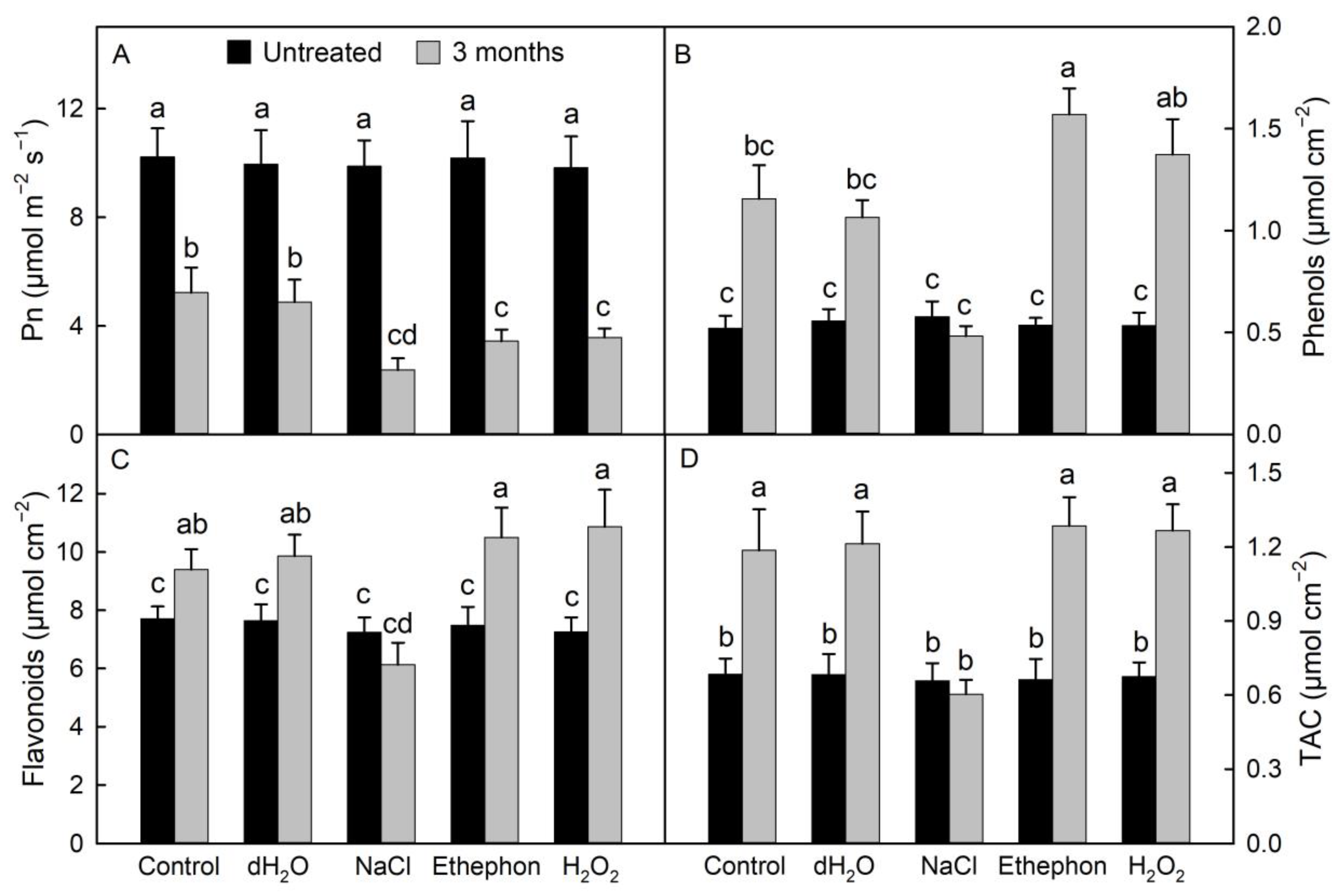
3.2. Changes in the Net Photosynthetic Rates and Antioxidant Capacity
3.3. Changes in the Structural and Non-Structural Carbohydrates
3.4. Agarwood Essential Oil Content and Components
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ding, X.P.; Mei, W.L.; Lin, Q.; Wang, H.; Wang, J.; Peng, S.Q.; Li, H.L.; Zhu, J.H.; Li, W.; Wang, P.; et al. Genome sequence of the agarwood tree Aquilaria sinensis (Lour.) Spreng: The first chromosome-level draft genome in the Thymelaeceae family. Gigascience 2020, 9, giaa013. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.W.; Xu, Y.H.; Yu, C.C.; Lv, F.F.; Tang, X.L.; Gao, Z.H.; Zhang, Z.; Wang, H.; Liu, Y.; Wei, J.H. WRKY44 represses expression of the wound-induced sesquiterpene biosynthetic gene ASS1 in Aquilaria sinensis. J. Exp. Bot. 2020, 71, 1128–1138. [Google Scholar] [PubMed]
- Naziz, P.S.; Das, R.; Sen, S. The scent of stress: Evidence from the unique fragrance of agarwood. Front. Plant Sci. 2019, 10, 840. [Google Scholar] [CrossRef] [PubMed]
- CITES. Checklist of CITES Species. 2005. Available online: https://checklist.cites.org (accessed on 23 February 2023).
- López-Sampson, A.; Cernusak, L.A.; Page, T. Relationship between leaf functional traits and productivity in Aquilaria crassna (Thymelaeaceae) plantations: A tool to aid in the early selection of high-yielding trees. Tree Physiol. 2017, 37, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Shivanand, P.; Arbie, N.F.; Krishnamoorthy, S.; Ahmad, N. Agarwood-the fragrant molecules of a wounded tree. Molecules 2022, 27, 3386. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Q.; Liao, Y.C.; Chen, H.J.; Zhang, Z. Chemical solution is an efficient method to induce the formation of 2-(2-phenylethyl) chromone derivatives in Aquilaria sinensis. Phytochem. Lett. 2017, 19, 64–70. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Y.Y.; Feng, J.; Chen, D.L.; Yang, Y.; Liu, P.W.; Yu, Z.X.; Wei, J.H. Remarkable phytochemical characteristics of Chi-Nan agarwood induced from new-found Chi-Nan germplasm of Aquilaria sinensis compared with ordinary agarwood. Int. J. Anal. Chem. 2021, 2021, 5593730. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Liu, P.W.; Lv, F.F.; Zhang, Y.X.; Yang, Y.; Wei, J.H. Genetic relationship and source species identification of 58 Qi-Nan germplasms of species in China that easily form agarwood. PLoS ONE 2022, 17, e0270167. [Google Scholar] [CrossRef]
- Chhipa, H.; Chowdhary, K.; Kaushik, N. Artificial production of agarwood oil in Aquilaria sp. by fungi: A review. Phytochem. Rev. 2017, 16, 835–860. [Google Scholar] [CrossRef]
- Liao, G.; Dong, W.H.; Yang, J.L.; Li, W.; Wang, J.; Mei, W.L.; Dai, H.F. Monitoring the chemical profile in agarwood formation within one year and speculating on the biosynthesis of 2-(2-phenylethyl) chromones. Molecules 2018, 23, 1261. [Google Scholar] [CrossRef]
- Tan, C.S.; Isa, N.M.; Ismail, I.; Zainal, Z. Agarwood induction: Current developments and future perspectives. Front. Plant Sci. 2019, 10, 122. [Google Scholar] [CrossRef]
- Fang, H.X.; Zhou, Q.; Cheng, S.C.; Zhou, X.; Wei, B.D.; Zhao, Y.B.; Ji, S.J. 24-epibrassinolide alleviates postharvest yellowing of broccoli via improving its antioxidant capacity. Food Chem. 2021, 365, 130529. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, A.; Planas-Marquès, M.; Capellades, M.; Valls, M.; Coll, N.S. Blocking intruders: Inducible physico-chemical barriers against plant vascular wilt pathogens. J. Exp. Bot. 2021, 72, 184–198. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.F.; Cui, Z.Y.; Xu, D.P. Morphological, physiological, biochemical and molecular analyses reveal wounding-induced agarwood formation mechanism in two types of Aquilaria sinensis (Lour.) Spreng. Ind. Crops Prod. 2022, 178, 114603. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.Q.; Wang, H.; Mei, W.L.; Dai, H.F. Natural products in agarwood and plants: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 528–565. [Google Scholar] [CrossRef]
- Qi, S.Y.; He, M.L.; Lin, L.D.; Zhang, C.H.; Hu, L.J.; Zhang, H.Z. Production of 2-(2-phenylethyl) chromones in cell suspension cultures of Aquilaria sinensis. Plant Cell Tiss. Organ. Cult. 2005, 83, 217–221. [Google Scholar] [CrossRef]
- Naef, R. The volatile and semi-volatile constituents of agarwood, the infected heartwood of Aquilaria species: A review. Flavour Frag. J. 2011, 26, 73–89. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A. Natural occurring 2-(2-phenylethyl) chromones, structure elucidation and biological activities. Nat. Prod. Res. 2015, 29, 1489–1520. [Google Scholar] [CrossRef]
- Azren, P.D.; Lee, S.Y.; Emang, D.; Mohamed, R. History and perspectives of induction technology for agarwood production from cultivated Aquilaria in Asia: A review. J. For. Res. 2019, 30, 1–11. [Google Scholar] [CrossRef]
- Ito, M.; Okimoto, K.; Yagura, T.; Honda, G.; Kiuchi, F.; Shimada, Y. Induction of sesquiterpenoid production by methyl jasmonate in Aquilaria sinensis cell suspension culture. J. Essent. Oil Res. 2005, 17, 175–180. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, H.Q.; Yang, Y.; Zhang, Z.; Wei, J.H.; Meng, H.; Chen, W.P.; Feng, J.D.; Gan, B.C.; Chen, X.Y.; et al. Whole-tree agarwood-inducing technique: An efficient novel technique for producing high-quality agarwood in cultivated Aquilaria sinensis trees. Molecules 2013, 18, 3086–3106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.N.; Xue, S.Y.; Song, J.; Zhou, X.R.; Zhou, D.H.; Liu, X.J.; Hong, Z.; Xu, D.P. Effects of various artificial agarwood-induction techniques on the metabolome of Aquilaria sinensis. BMC Plant Biol. 2021, 21, 591. [Google Scholar] [CrossRef]
- Carrodus, B.B. Carbon dioxide and formation of heartwood. New Phytol. 1971, 70, 939–943. [Google Scholar] [CrossRef]
- Johannes, S.; Von Weikersthal-Drachenberg, K.F.; Zielen, S. Comparison of outcomes following ultra short course specific immunotherapy in juvenile and adult patients with asthma. Allergy 2008, 63, 526–527. [Google Scholar]
- Nilsson, M.; Wikman, S.; Eklund, L. Induction of discolored wood in scots pine (Pinus sylvestris). Tree Physiol. 2002, 22, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.Y.; Li, X.F.; Xu, D.P.; Yang, Z.J.; Zhang, N.N.; Liu, X.J.; Hong, Z. Physiological changes during heartwood formation induced by plant growth regulators in Dalbergia odorifera (Leguminosae). IAWA J. 2021, 42, 217–234. [Google Scholar] [CrossRef]
- Lin, L.S.; Guo, D.W.; Huang, J.; Zhang, X.D.; Zhang, L.; Wei, C.X. Molecular structure and enzymatic hydrolysis properties of starches from high-amylose maize inbred lines and their hybrids. Food Hydrocoll. 2016, 58, 246–254. [Google Scholar] [CrossRef]
- Liu, F.L.; Jensen, C.R.; Andersen, M.N. Drought stress effect on carbohydrate concentration in soybean leaves and pods during early reproductive development: Its implication in altering pod set. Field Crops Res. 2004, 86, 1–13. [Google Scholar] [CrossRef]
- Han, Y.Y.; Xu, T.Y.; Chen, H.; Tang, M. Sugar metabolism and 14-3-3 protein genes expression induced by arbuscular mycorrhizal fungi and phosphorus addition to response drought stress in Populus cathayana. J. Plant Physiol. 2023, 288, 154075. [Google Scholar] [CrossRef]
- Nazli, R.I.; Gulnaz, O.; Kafkas, E.; Tansi, V. Comparison of different chemical pretreatments for their effects on fermentable sugar production from miscanthus biomass. Biomass Convers. Bior. 2023, 13, 6471–6479. [Google Scholar] [CrossRef]
- Li, Y.H.; Zou, X.B.; Shen, T.T.; Shi, J.Y.; Zhao, J.W.; Holmes, M. Determination of geographical origin and anthocyanin content of black goji berry (Lycium ruthenicum Murr.) using near-infrared spectroscopy and chemometrics. Food Anal. Methods 2017, 10, 1034–1044. [Google Scholar]
- Nguyen, T.T.N.; Trinh, N.Y.; Le, P.K. Recovery yield and bioactivities evaluation on essential oil and ethanolic extract of star anise (Illicium verum Hook. f.). Chem. Eng. J. 2021, 83, 205–210. [Google Scholar]
- Lv, F.F.; Yang, Y.; Sun, P.W.; Zhang, Y.; Liu, P.W.; Fan, X.H.; Xu, Y.H.; Wei, J.H. Comparative transcriptome analysis reveals different defence responses during the early stage of wounding stress in germplasm and ordinary Aquilaria sinensis. BMC Plant Biol. 2022, 22, 464. [Google Scholar] [CrossRef]
- Howes, M.J.R.; Simmonds, M.S.J.; Kite, G.C. Evaluation of the quality of sandalwood essential oils by gas chromatography-mass spectrometry. J. Chromatogr. A 2004, 1028, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G.; Ghisalberti, E.L.; Plummer, J.A.; Barbour, E.L. Quantitative co-occurrence of sesquiterpenes; a tool for elucidating their biosynthesis in Indian sandalwood, Santalum album. Phytochemistry 2006, 67, 2463–2468. [Google Scholar] [CrossRef] [PubMed]
- Samadi, M.; Abidin, Z.Z.; Yoshida, H.; Yunus, R.; Biak, D.R.A. Towards higher oil yield and quality of essential oil extracted from Aquilaria malaccensis wood via the subcritical technique. Molecules 2020, 25, 3872. [Google Scholar] [CrossRef] [PubMed]
- Sciarrone, D.; Costa, R.; Ragonese, C.; Tranchida, P.Q.; Tedone, L.; Santi, L.; Dugo, P.; Dugo, G.; Mondello, L. Application of a multidemensional gas chromatography system with simultaneous mass spectrometric and flame ionization detection to the analysis of sandalwood oil. J. Chromatogr. A 2011, 1218, 137–142. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Liu, X.J.; Xu, D.P.; Hong, Z.; Zhang, N.N.; Cui, Z. Effects of drought and host on the growth of Santalum album seedlings in pot culture. Int. J. Mol. Sci. 2022, 23, 11241. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Uscola, M.; Jacobs, D.F. The role of stored carbohydrates and nitrogen in the growth and stress tolerance of planted forest trees. New For. 2015, 46, 813–839. [Google Scholar] [CrossRef]
- Hishamuddin, M.S.; Lee, S.Y.; Isa, N.M.; Lamasudin, D.U.; Zainal Abidin, S.A.; Mohamed, R. Time-based LC-MS/MS analysis provides insights into early responses to mechanical wounding, a major trigger to agarwood formation in Aquilaria malaccensis Lam. Rsc. Adv. 2019, 9, 18383–18393. [Google Scholar] [CrossRef]
- Richardson, A.D.; Carbone, M.S.; Keenan, T.F.; Czimczik, C.I.; Hollinger, D.Y.; Murakami, P.; Schaberg, P.G.; Xu, X.M. Seasonal dynamics and age of stem wood nonstructural carbohydrates in temperate forest trees. New Phytol. 2013, 197, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees-from what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Liang, J.; Tang, G.M.; Wang, X.F.; Liu, F.C.; Zhao, D.C. Drought stress affects on growth, water use efficiency, gas exchange and chlorophyll fluorescence of Juglans rootstocks. Sci. Hortic. 2019, 250, 230–235. [Google Scholar] [CrossRef]
- Liu, P.W.; Zhang, X.L.; Yang, Y.; Sui, C.; Xu, Y.H.; Wei, J.H. Interxylary phloem and xylem rays are the structural foundation of agarwood resin formation in the stems of Aquilaria sinensis. Trees-Struct. Funct. 2019, 33, 533–542. [Google Scholar] [CrossRef]
- Seydel, C.; Kitashova, A.; Fürtauer, L.; Nägele, T. Temperature-induced dynamics of plant carbohydrate metabolism. Physiol. Plant. 2021, 174, e13602. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Macías, J.P.; García, Y.C.; Núñez, M.; Díaz, K.; Olea, A.F.; Espinoza, L. Plant growth-defense trade-offs: Molecular processes leading to physiological changes. Int. J. Mol. Sci. 2021, 22, 693. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.F.; Zhong, S.L.; Grierson, D. Recent advances in ethylene research. J. Exp. Bot. 2009, 60, 3311–3336. [Google Scholar] [CrossRef] [PubMed]
- Vanderauwera, S.; Zimmermann, P.; Rombauts, S.; Vandenabeele, S.; Langebartels, C.; Gruissem, W.; Inzé, D.; Van Breusegem, F. Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol. 2005, 139, 806–821. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.H.; Cheng, Q.W.; da Silva, J.A.T.; Fang, L.; Ma, G.H. Elicitors modulate young sandalwood (Santalum album L.) growth, heartwood formation, and concrete oil synthesis. Plants 2021, 10, 339. [Google Scholar] [CrossRef]
- Lv, F.F.; Li, S.S.; Feng, J.; Liu, P.W.; Gao, Z.H.; Yang, Y.; Xu, Y.H.; Wei, J.H. Hydrogen peroxide burst triggers accumulation of jasmonates and salicylic acid inducing sesquiterpene biosynthesis in wounded Aquilaria sinesis. J. Plant Physiol. 2019, 234, 167–175. [Google Scholar] [CrossRef]
- Hieno, A.; Naznin, H.A.; Inaba-Hasegawa, K.; Yokogawa, T.; Hayami, N.; Nomoto, M.; Tada, Y.; Yokogawa, T.; Higuchi-Takeuchi, M.; Hanada, K.; et al. Transcriptome analysis and identification of a transcriptional regulatory network in the response to H2O2. Plant Physiol. 2019, 180, 1629–1646. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Liu, X.; Li, X.; Fang, X.; Xiong, Y.; Xu, D. Inducing Aquilaria sinensis (Lour.) Spreng “Qinan” Agarwood Formation with Chemical Reagents. Agronomy 2024, 14, 1727. https://doi.org/10.3390/agronomy14081727
Zhang Q, Liu X, Li X, Fang X, Xiong Y, Xu D. Inducing Aquilaria sinensis (Lour.) Spreng “Qinan” Agarwood Formation with Chemical Reagents. Agronomy. 2024; 14(8):1727. https://doi.org/10.3390/agronomy14081727
Chicago/Turabian StyleZhang, Qilei, Xiaojin Liu, Xiaofei Li, Xiaoying Fang, Yongmei Xiong, and Daping Xu. 2024. "Inducing Aquilaria sinensis (Lour.) Spreng “Qinan” Agarwood Formation with Chemical Reagents" Agronomy 14, no. 8: 1727. https://doi.org/10.3390/agronomy14081727
APA StyleZhang, Q., Liu, X., Li, X., Fang, X., Xiong, Y., & Xu, D. (2024). Inducing Aquilaria sinensis (Lour.) Spreng “Qinan” Agarwood Formation with Chemical Reagents. Agronomy, 14(8), 1727. https://doi.org/10.3390/agronomy14081727



