Abstract
Sake, which is produced mainly from japonica rice (Oryza sativa subsp. japonica), is one of the most important alcohol products in Japan. In this study, we aimed to investigate a hypothesis that the early root endophytic bacterial communities in Japanese sake rice cultivars would be distinct from those in table rice cultivars, comparing four sake rice cultivars and two table rice cultivars. Rice roots in the vegetative stage were collected 0, 3, and 6 weeks after transplanting, and 16S rRNA gene amplicon sequencing revealed significant differences in bacterial community composition diversity between the sake and table rice cultivars. The root endophytic bacterial communities at the transplanting differed significantly between the rice cultivars, indicating differences in each seed-derived endophytic community. After an overall dominance of Pantoea and Methylobacterium-Methylorubrum at the transplanting, the endophytic community was gradually replaced by soil-derived bacteria that varied by the rice cultivars. Notably, PERMANOVA results showed that the rice endophytic bacterial community composition differed significantly between the sake and table rice cultivars (p < 0.001). These results highlight the distinct root endophytic bacterial composition in the sake rice cultivars compared to those in the table rice cultivars, supporting our hypothesis.
1. Introduction
Rice serves as the primary staple food in over 100 countries worldwide, with its production playing a pivotal role in addressing global food security concerns [1]. This significance is particularly pronounced in regions such as Asia, Sub-Saharan Africa, and South America, where rice consumption forms a substantial portion of dietary intake for millions of people. Given its widespread cultivation and consumption, any advancements in rice production methods hold significant promise for alleviating hunger and improving food security on a global scale. China holds the distinction of being the world’s largest producer of rice, with evidence of the earliest domesticated rice dating back as far as 9000 years ago in the Yangtze Valley [2]. China extensively cultivates two primary subspecies of Asian rice, indica and japonica. In addition, many hybrid cultivars, showing substantial hybrid vigor, have been effectively bred and extensively cultivated nationwide [3]. These cultivars often exhibit enhanced disease resistance, increased yield potential, and improved adaptability compared to their parental lines. In comparison to the traditional indica and japonica rice cultivars, indica–japonica hybrid rice demonstrates increased plant height and yield potential, as well as enhanced resistance to environmental stresses, as reported by Wei et al. [3], Bhadraray et al. [4], and Zhu et al. [5]. In previous studies, Edwards et al. [6] examined the composition and assembly of the root-associated bacterial community in six rice cultivars affiliated with japonica and indica. The study revealed a small but significant variation in the microbiota attributable to the host genotypes.
Japan has a rich tradition of sake rice cultivation which is essential for sake production. Sake, primarily made from japonica rice, holds significant cultural and economic importance as one of the Japanese national alcohol products. Currently, Japan is characterized by a rich tapestry of sake-brewing rice cultivars, each boasting a unique genetic heritage deeply intertwined with the nation’s cultural and agricultural landscape. The sake rice cultivars, meticulously bred and cultivated over generations, form the cornerstone of Japan’s esteemed sake tradition, contributing to its diverse flavor profiles and regional nuances. Sake brewing cultivars are characterized by generally lower protein contents, larger grain sizes, and the presence of a floury white structure in the center of the grain, commonly referred to as “shinpaku” in contrast to normal table rice cultivars.
Hashimoto et al. [7] underscored the significance of the diversity of sake rice cultivars, highlighting the myriad genetic backgrounds that underpin Japan’s vibrant sake industry. While Japan cultivates approximately 100 distinct types of sake rice cultivars, a significant portion of sake production is derived from Yamadanishiki (Yam), Gohyakumangoku (Goh), and Miyamanishiki rice cultivars. Regarding the protein characteristics of various rice cultivars, Yam is the most esteemed cultivar employed in sake production, which exhibits lower levels of protein and glutelin compared to Goh, as reported by Okuda et al. [8]. Goh is the second most widely cultivated cultivar. Kamenoo (Kam) and Omachi (Oma) are relatively older cultivars suitable for sake brewing. Oma represents the ancient sake rice cultivar, serving as the progenitor to both Yam and Goh cultivars. Oma is one of the few remaining pure cultivars of sake rice, having not been crossbred with other ones. On the contrary, Koshihikari (Kos) is one of the most popular table rice cultivars in Japan. It has been utilized as a progenitor in the development of other Japanese table rice cultivars, such as Akitakomachi, Hitomebore, and Hinohikari. Another cultivar of table rice, Nipponbare (Nip) is utilized as the reference genome in many studies focusing on rice genomics. In the International Rice Genome Sequencing Project (IRGSP), completed in 2005, the genomic sequence of Nip was meticulously deciphered to a high standard of quality [9].
Given its crucial role in Japanese society, substantial research has focused on understanding the characteristics of each sake rice cultivar. Many studies have assessed the suitability for brewing, alongside comprehensive genome analyses [10]. However, despite the significance, there have been few studies on the relationship between sake rice and symbiotic microorganisms. No study has yet examined the distinct community composition of bacterial endophytes in sake rice cultivars compared to those in table rice ones, as far as we know. Xu et al. [11] and Edwards et al. [6] highlighted variations in endophyte community compositions between different rice cultivars. From their results, it is expected that the endophytic bacterial communities in sake rice cultivars exhibit significant differences from those in table rice cultivars.
The aim of this study is to characterize the root endophytic bacterial communities of Japanese sake rice cultivars in the vegetative stage and to compare them with those of other rice cultivars. We hypothesized that there would be distinct endophytic communities among the sake and table rice cultivars and investigated this hypothesis by conducting a cultivation study with four Japanese sake rice cultivars and two table rice cultivars.
2. Materials and Methods
2.1. Soil Collection and Processing
For this research, a soil sample was collected in July 2021 from a drained paddy field located at the Shindori Station, Field Center for Sustainable Agriculture and Forestry, Niigata University, Niigata, Japan (coordinates: N37.86, E138.96). The sample was dried in the open air for seven days, then crushed and sieved through a 2 mm mesh. Subsequently, the processed soil was stored in cold storage at 4 °C. This soil was identified as alluvial gley lowland soil. It was chosen because rice fields with this soil type are widely distributed in Niigata Prefecture, one of the most famous rice-producing regions in Japan [12]. The soil physicochemical properties were described in our previous study [13].
2.2. Pre-Incubation of Soil and Experimental Set-Up
For the preparation of microbial source, 40 g of soil sample sieved to a 2 mm size was carefully weighed into individual polystyrene woven bags. Subsequently, these bags were immersed in a bath filled with sterile water. The pre-incubation of soil was carried out in sterile water under anaerobic and dark conditions for 21 days at room temperature. This period allowed for the adaptation, proliferation, and stabilization of microbial communities within each soil sample.
To characterize the endophytic bacterial communities, four Japanese sake rice cultivars Goh, Kam, Oma, and Yam, and two table rice cultivars Nip and Kos were used (Table 1). The seeds of each rice cultivar were stored separately in a plastic bag at 4 °C cold storage until germination.

Table 1.
Rice types and cultivars used in this study.
The rice seeds underwent de-husking and surface sterilization procedures outlined by Asiloglu et al. [14]. Subsequently, they were germinated under sterile conditions and allowed to develop for 14 days.
The experimental pots used in this study were prepared as previously described by Samuel et al. [13]. Each pot, 13.0 cm in height and 8.5 cm in inner diameter, was meticulously prepared with specific attributes as described by Samuel et al. [13]: a black polystyrene lid featuring a central opening of 2.5 cm in diameter to facilitate the emergence of a rice plant, and a 50 mL column (11.0 cm in height and 2.5 cm in inner diameter) equipped with four perforations (each 2 mm in diameter), internally lined with a piece of polypropylene mesh sheet. This column was vertically affixed to the inner base of the pot and lined with mesh sheets to enable the permeation of water and bacteria into and out of the inner column while restricting the extension of rice roots beyond the column. One soil bag per experimental pot, pre-incubated for 21 days, was delicately transferred using sterile forceps. Subsequently, 14-day-old sterile rice seedlings were transplanted into each pot. Five hundred mL of sterile water, referred to as the hydroponic solution, was added and maintained during rice cultivation. To minimize the known soil factors affecting endophytic bacterial communities, no nutrient supplementation was incorporated into the experimental setup, as nutrient levels in soil have been reported to impact such communities [15,16]. The rice plants were cultivated for 6 weeks in a growth chamber maintained at 25/30 °C (day/night), with 16 h of daylight (photoperiod) (250 µmol m−2 s−1) and a relative humidity of 70% as described in a previous study [13]. All the rice plants were placed in the same growth chamber, with their positions being randomized twice weekly to ensure uniformity in the lighting and relative humidity conditions until the final sampling.
2.3. Sample Collection
The samplings were conducted at three different time points, 0, 3, and 6 weeks after transplanting as in our previous study [13], with six different cultivars collected at each time point. Each sample was taken in triplicate, resulting in a total of 54 samples (3 time points × 6 samples per time point × 3 replicates). The first sampling was to illustrate seed-derived initial endophytic bacterial community. The samplings at the 3rd and 6th week were to understand the re-placement of them with soil-derived endophytic bacteria. Destructive sampling procedures were implemented, following the protocol outlined by Samuel et al. [13].
Rice roots were isolated from the shoots utilizing sterile scissors and subsequently washed extensively under running tap water. Surface sterilization of the roots was then carried out by immersing them in sterile water for 5 min, followed by a 2 min immersion in 70% ethanol, a 5 min immersion in 2.5% sodium hypochlorite, a 1 min immersion in 70% ethanol, and a final 5 min immersion in sterile water, repeated twice. Finally, the entire root system of each plant was homogenized in liquid nitrogen and mixed thoroughly. All samples were then stored under −20 °C until DNA extraction.
2.4. DNA Extraction and Sequencing
Genomic DNA extraction from the homogenized root samples was carried out utilizing an ISOPLANT kit (Nippon Gene, Tokyo, Japan), following the established protocol outlined by Samuel et al. [13], employing the methodology described in the study previously mentioned. We used the ISOPLANT kit because it has been well used in Japan and it allowed us to extract sufficiently high-quality DNA from rice roots in our previous studies [13].
The DNA extracts from all samples were utilized as templates for a polymerase chain reaction (PCR) employing the universal primer pair 515F and 806R [17], which included overhang adapter sequences for the Nextera XT index primers (Illumina, San Diego, CA, USA). The PCR for amplification of the V4 region of the 16S rRNA gene was conducted with a final reaction volume of 25 µL. In brief, the PCR reaction mixture comprised 1 µL of template DNA, 0.1 µL each of forward and reverse primers (50 µM, 10P), 11.5 µL H2O, and 12.5 µL Ex Premier DNA Polymerase (Takara Bio, Kusatsu, Japan). The PCR conditions for amplifying endophytic bacterial genes were established as follows: initial denaturation at 94 °C for 2 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 57 °C for 30 s, and elongation at 72 °C for 20 s, with a final extension at 72 °C for 5 min. Amplicon purification was conducted using Agencourt AMPure XP (Beckman Coulter, Brea, CA, USA) following the manufacturer’s instructions. An index PCR was performed under the following thermocycling conditions: initial denaturation at 95 °C for 3 min; 12 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and elongation at 72 °C for 30 s; and a final elongation step at 72 °C for 5 min. This was accompanied by a second purification step of the amplicons as previously described. Amplicon quantification was carried out using the QuantiFluor device (Promega, Madison, WI, USA), followed by pooling equimolar concentrations of the purified amplicons. Finally, the pooled amplicons were subjected to paired-end sequencing on the Illumina MiSeq platform, generating reads of 2 × 300 bp, utilizing the MiSeq reagent kit v3.
2.5. Bioinformatics and Statistical Analysis
The initial processing of the raw FASTQ files excluded sequences categorized as Archaea, Eukaryota, chloroplast, and mitochondria. After this, we could obtain 21,720–129,002 raw paired reads and then performed rarefaction by randomly selecting 10,000 sequences per sample with a quality score above 30, in line with Samuel et al. [13]. This was executed using the QIIME II platform (version 2023.2; http://qiime2.org/ (accessed on 7 February 2024)) [18]. To create amplicon sequence variants (ASVs), paired-end sequences underwent denoising via the DADA2 plugin within QIIME2 [19]. Forward and reverse reads were shortened at 240 bp and 150 bp, respectively, and sequences occurring only once or twice were discarded after merging the paired-end reads. Taxonomic classification was carried out with the q2-feature-classifier plugin, referencing the Silva database (SILVA SSU Ref NR 138 sequences) [20]. Finally, the 16S rRNA gene amplicon sequences produced in this research were submitted to the DNA Data Bank of Japan (DDBJ) under the accession number PRJDB18248. The bacterial community diversity in the experimental samples was assessed using the Shannon index. A heatmap of the top 20 abundant genera was created based on their relative abundances. The data were normalized and standardized across samples, and clustering was performed using the Ward D2 method. To depict the common and unique bacterial taxa at the genus level within the endosphere at different time points (0, 3, and 6 weeks after transplanting), a Venn diagram was generated. Bacterial genera with a relative abundance of 0.1% or higher, averaged across replicates, were included in the Venn diagram, created with InteractiVenn [21]. It showcased the shared and distinct bacterial taxa among different cultivars at the specified time intervals. Beta diversity among bacterial communities in rice roots was evaluated using principal coordinate analysis (PCoA) based on Bray–Curtis dissimilarity, performed with the R vegan package in the R software (version 4.3.2; https://www.r-project.org/ (accessed on 27 February 2024) [22]. Furthermore, permutational multivariate analysis of variance (PERMANOVA; 9999 permutations) was applied using the adonis function to statistically assess differences (p < 0.05) in endophytic bacterial community composition across the six rice cultivars. To identify differentially abundant bacterial taxa among samples, Linear Discriminant Analysis Effect Size (LEfSe) [23] was applied.
3. Results and Discussion
3.1. Bacterial Community Compositions
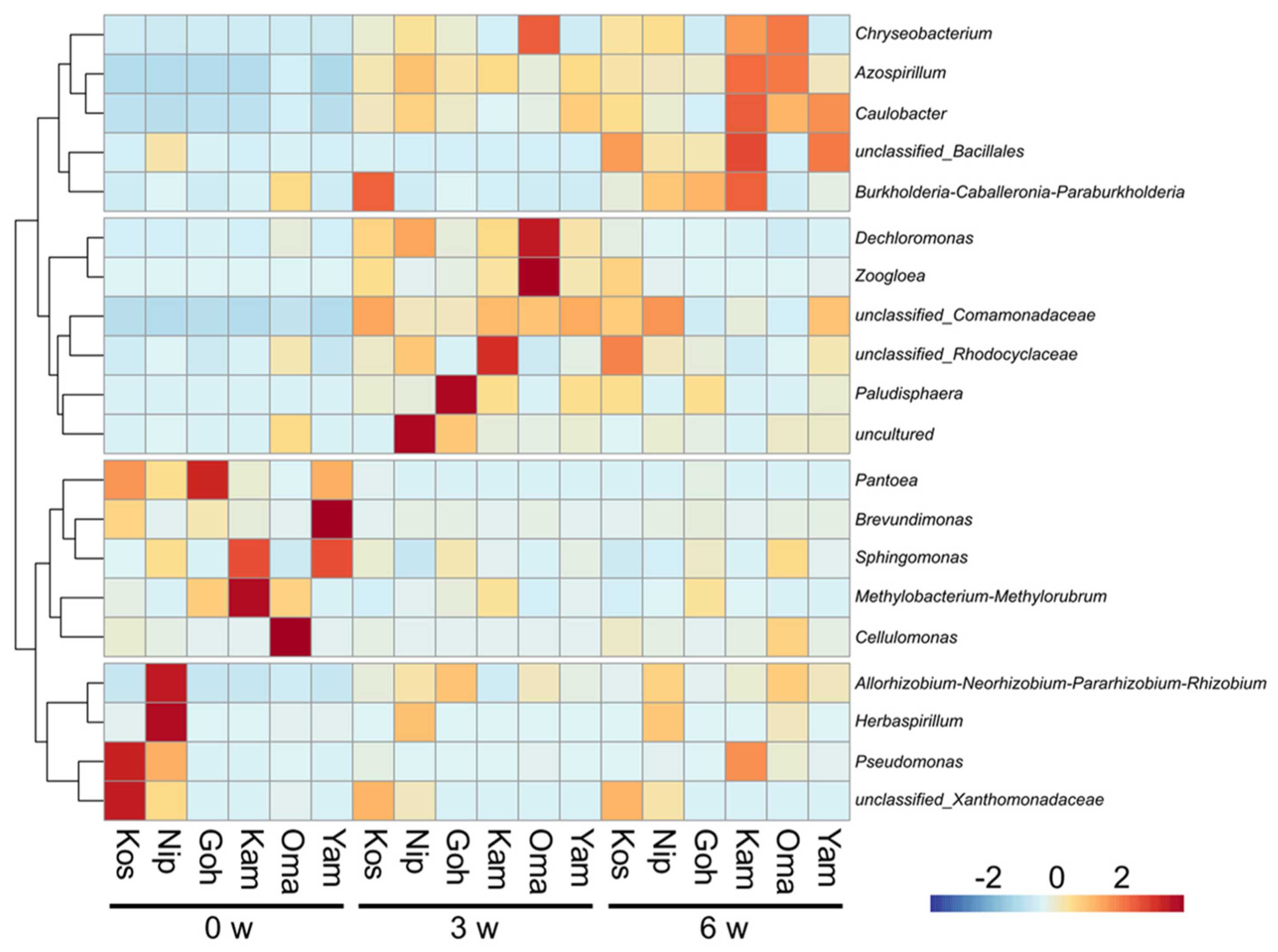
Figure 1 displays a heatmap depicted based on the relative abundances of top 20 abundant genera found as the root endophytic bacteria in the six rice cultivars at 0, 3, and 6 weeks after transplanting. In all rice cultivars, the bacterial communities varied temporally up to the 6th week.

Figure 1.
A cluster heatmap depicted based on the relative abundances of top 20 abundant genera in the six rice cultivars (the abbreviations are shown in Table 1) at 0, 3, and 6 weeks (W) after transplanting. The color intensity indicates differences in the relative abundances after normalization and standardization across the samples.
Cluster analysis of the dynamics of the predominant endophytic bacteria in rice roots at the genus level revealed that they can be broadly classified into four groups (Figure 1).
The first group includes Pantoea, a seed-derived bacterial genus. It exhibited dominance across all rice cultivars initially, e.g., at 66.3% in Goh, at 38.1% in Kos, and at 31.2% in Yam. The second dominant bacterial genus was Methylobacterium-Methylorubrum in Kam at 36.0% followed by Goh and Oma at 11.4 and 11.0%, respectively. In the sake rice cultivars, their prevalences tended to be more pronounced compared to those in the table rice cultivars. It may indicate a common feature of the root endophytic bacterial community within the sake rice cultivars.
The second group is likely to be specific to the table rice cultivars, Kos and Nip. It includes Rhizobium-related genus, Herbaspirillum, Pseudomonas, and Xanthomonadaceae-related genus.
The third showed dominance 3 weeks after transplanting, of which the dominance of Comamonadaceae replaced Pantoea and Methylobacterium-Methylorubrum at 19.6% in Kos, 17.5% in Kam, and 15.9% in Oma. Paludisphaera was most dominant at 37.3% in Goh, but less at 8.6% in Kam and Yam. The last group slowly builds a predominance in the rice roots. Azospirillum which replaced Comamonadaceae in Oma, accounting for 10.0% of the total.
The relative abundances of initial endophytic bacteria tended to be similar but were diversified by the 6th week in Kos, Nip, Goh, Kam, Oma, and Yam. It is reasonable to assume that the changes in the diversity of root endophytic bacteria with rice growth observed in this study were caused by the soil acting as a source of rhizobacteria [24]. Importantly, the root endophytic bacterial community composition differed among the rice cultivars, as well as among sake and table rice cultivars.
3.2. Bacterial Diversity
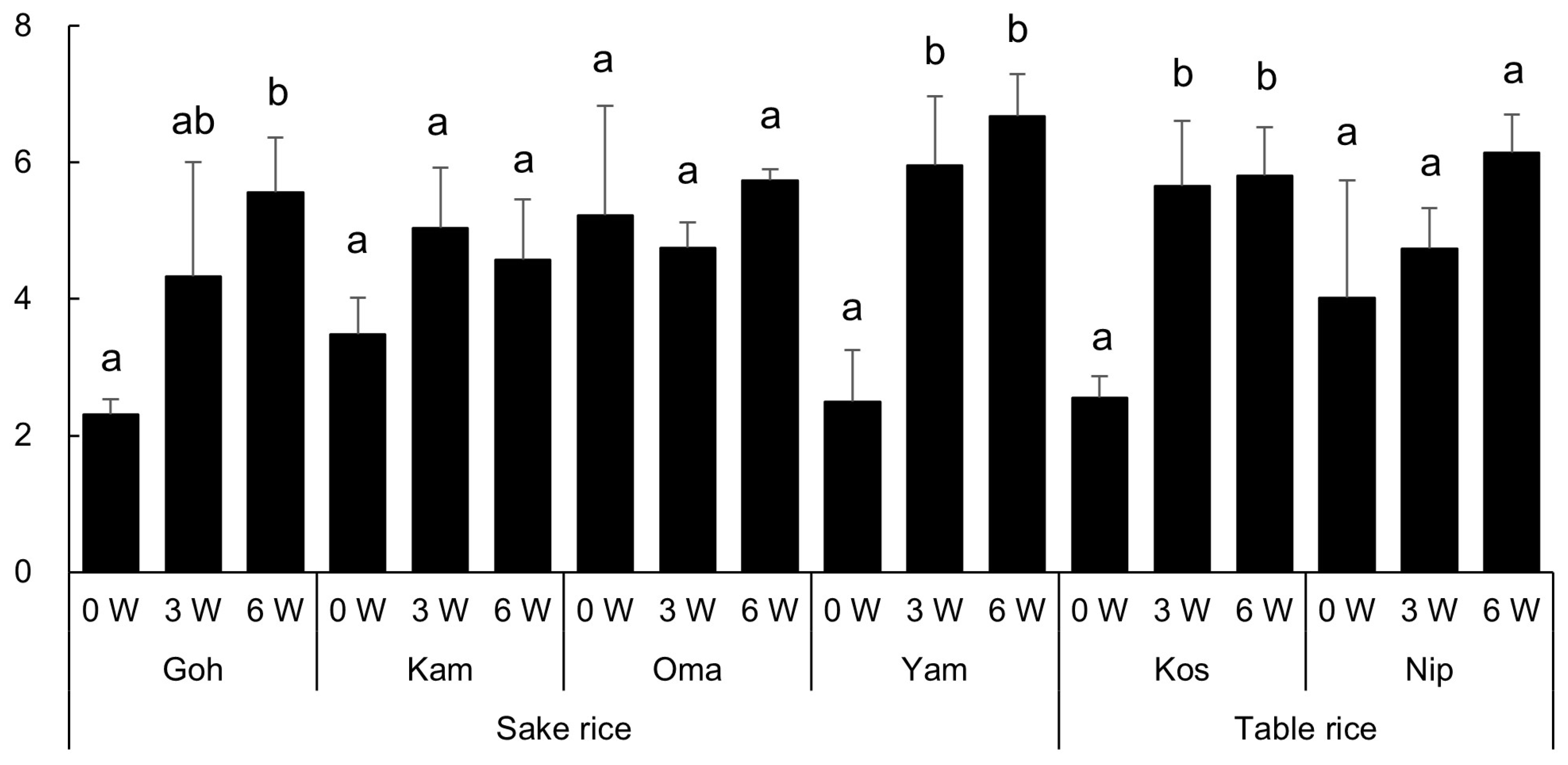
The alpha diversity metrics for the endophytic bacterial communities are summarized in Table S1. The diversity of the root endophytic bacterial community was estimated by the Shannon index for each sample and the results of the multiple comparisons (Tukey test, p < 0.05) were displayed in Figure 2.

Figure 2.
The Shannon indices of the root endophytic bacterial community of the six rice cultivars (the abbreviations are shown in Table 1) 0, 3, and 6 weeks (W) after transplanting. Different letters indicate significant differences within each rice cultivar (Tukey test, p < 0.05).
As shown in Table 2, the ANOVA results evaluating microbial diversity in the root endophytes revealed that the growth period (week) had a highly significant effect on microbial diversity (F = 32.91, p < 0.001). This indicates that time plays a critical role in shaping the community structure of root endophytes. In contrast, the effect of rice cultivar alone on microbial diversity was not significant. However, the interaction between week and cultivar was found to have a significant impact on microbial diversity (F = 2.79, p < 0.05). This finding suggests that different rice cultivars may influence root bacterial communities differently at specific growth stages.

Table 2.
Summary of ANOVA for Shannon index calculated for the root endophytic bacterial community.
Table 3 shows the summary of PERMANOVA results evaluating beta diversity based on the Bray–Curtis dissimilarity, indicating that the bacterial community composition in the rice roots differed significantly within the six rice cultivars and even between the sake rice cultivars and the table rice cultivars (p < 0.05).

Table 3.
Summary of nested PERMANOVA results evaluating beta diversity based on the Bray–Curtis dissimilarity for the bacterial community composition in the rice roots.
The result indicates that the rice type and cultivar were significant factors of variation in both the initial and subsequent stages. The influence of rice cultivars was initially large and remained an important factor of variation until the 6th week. The large residual variance suggests that other factors not included in this model are also influential.
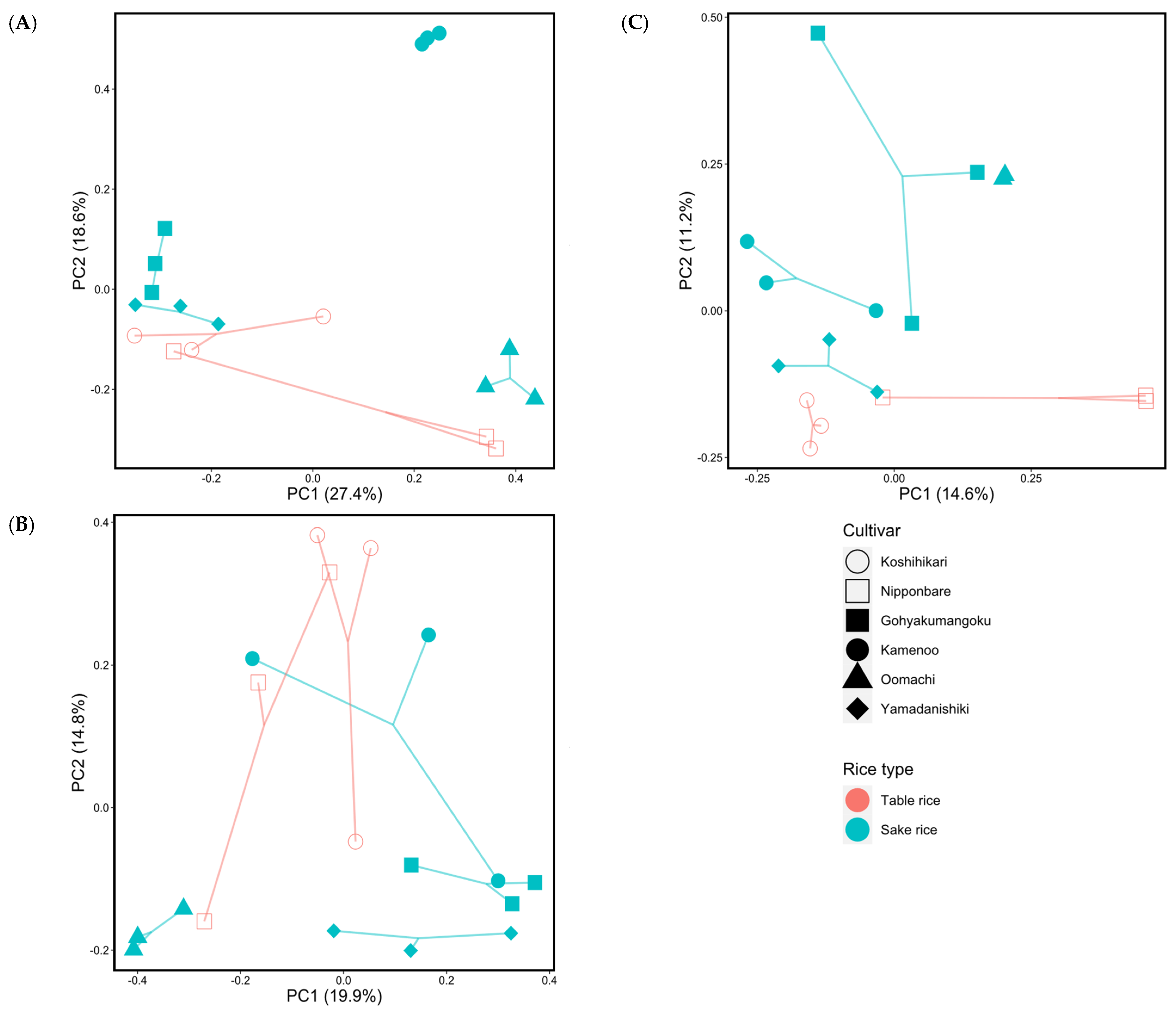
PCoA analysis performed based on the Bray–Curtis dissimilarity provided a visual representation of the bacterial beta diversity in the six cultivars, and the resultant effect on assemblages of the root endophytic bacterial communities 0, 3, and 6 weeks after transplanting (Figure 3). The changes in the plot patterns here apparently reflected differences in the rice cultivars over time.

Figure 3.
Results of principal coordinate analysis (PCoA) of the endophytic bacterial community composition in roots, based on Bray–Curtis dissimilarity illustrating the impact of rice cultivars on them 0 (A), 3 (B), and 6 (C) weeks after transplanting. Different colors represent the distinct table and sake rice cultivars, while shapes indicate the various rice cultivars.
Approximately 46% of the microbial community dissimilarities observed between the root endophytes were explained by the first principal component (PC1) axis, accounting for 27.4%, and the second principal component (PC2) axis, accounting for 18.6% at transplanting (Figure 3A). Approximately 34.7% of the microbial community dissimilarities observed between the root endophytes were explained by the first principal component (PC1) axis, accounting for 19.9%, and the second principal component (PC2) axis, accounting for 14.8%, 3 weeks after transplanting (Figure 3B). Approximately 25.8% of the microbial community dissimilarities observed between the root endophytes 6 weeks after transplanting were explained by the first principal component (PC1) axis, accounting for 14.6%, and the second principal component (PC2) axis, accounting for 11.2%, as shown in Figure 3C.
LEfSe analysis revealed the prominence of various taxa within the phylum Proteobacteria (Table S4). At the transplanting, the table rice cultivars were characterized by high LDA scores for class Gammaproteobacteria, while the sake rice cultivars showed significant presences of class Alphaproteobacteria. The table rice cultivars then continued to exhibit higher relative abundances of class Gammaproteobacteria, specifically the orders Xanthomonadales and Burkholderiales, and, in addition, the phylum Verrucomicrobiota also became prominent 6 weeks after transplanting. However, the sake rice cultivars showed a marked increase in the phylum Bacteroidota 3 weeks after transplanting, and then the relative abundances of the order Rhizobiales in the class Alphaproteobacteria emerged.
These results indicate that the endophytic bacterial communities formed in both types of rice roots change differently, resulting in significantly higher relative abundances of certain taxa, respectively. Namely, the table rice cultivars tend to be associated with species in the class Gammaproteobacteria, while the sake rice cultivars show relatively strong associations with those in the class Alphaproteobacteria. It may be due to differences in the internal nutrient conditions of each rice type, and/or the interaction between root exudates and endophytic bacteria [25], although further research is needed to confirm this point.
3.3. Shared and Unique Endophytic Bacterial Taxa
A Venn diagram showing the shared and unique root endophytic bacterial taxa for the sake and table rice types collected at the transplanting is illustrated in Figure S1A. Among the bacterial genera found in the sake rice cultivars, five were identified as shared taxa, namely Paenibacillus, Sphingomonas, Brevundimonas, Methylobacterium-Methylorubrum, and Pantoea. Among the table rice cultivars, 25 shared taxa were identified, such as Pseudomonas, Methylonatrum, Azospirillum, Paenibacillus. While there were 53 shared taxa, 46 and 74 taxa were identified to be specific to the sake and table rice types, respectively.
The number of bacterial taxa detected at the 3rd week was 177 (Figure S1B), almost the same as the 173 detected at the transplanting. The number of bacterial taxa specific to the sake rice type increased to 76, while that specific to the table rice type decreased to 55.
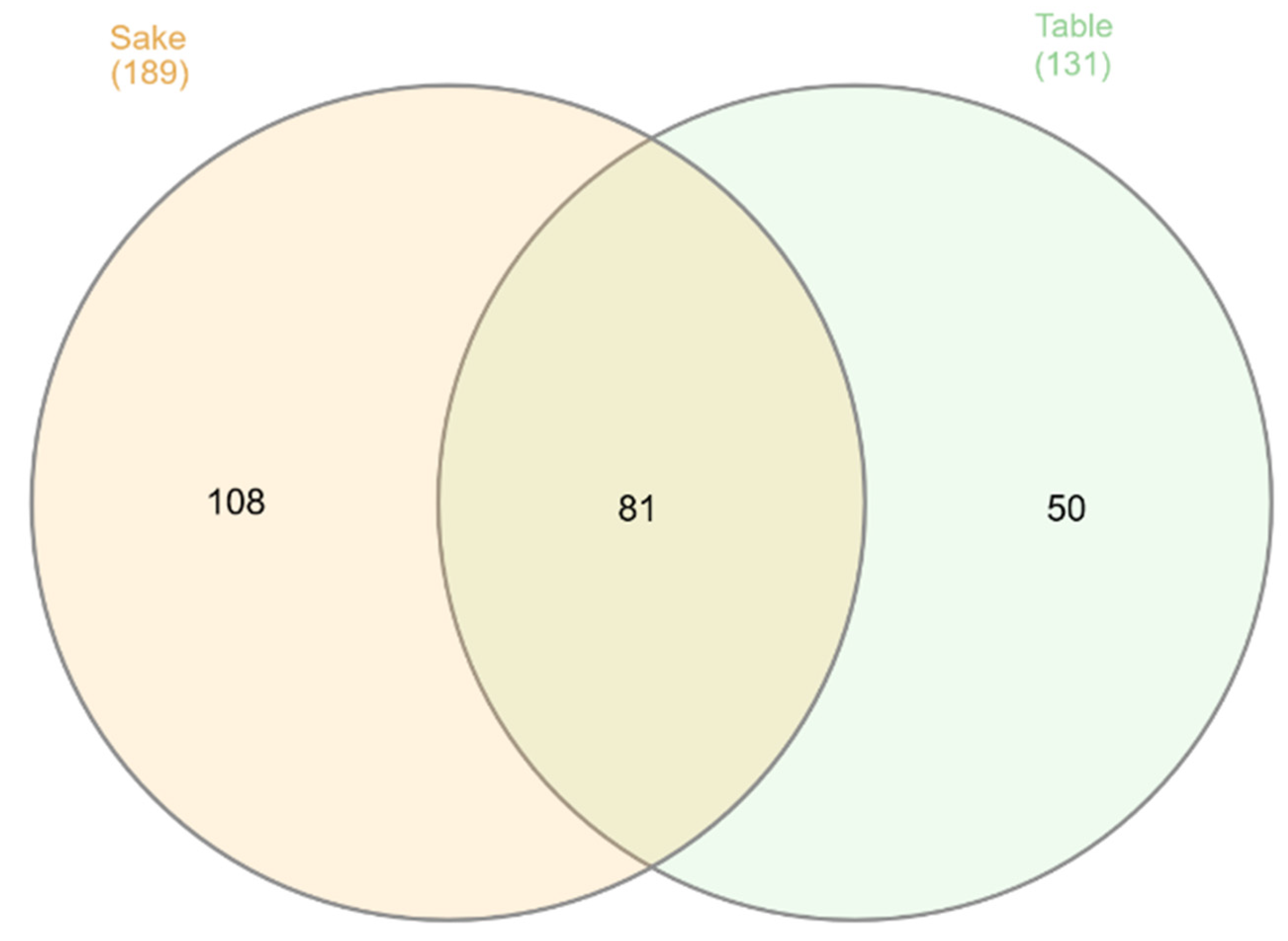
At the 6th week, the number of the root endophytic bacterial genera increased to 239 (Figure 4). Sake rice specific taxa increased to 108, although table rice specific taxa decreased to 50. It is characteristic of the table rice type that the number of specific endophytic taxa decreases.

Figure 4.
A Venn diagram depicting the distribution of unique and shared root endophytic bacterial genera between the sake rice type and the table rice type 6 weeks after transplanting. The values within the overlapping regions represent numbers of the genera common to both compartments, while those outside the overlaps indicate ones specific to each compartment. The numbers in parentheses indicate the number of genera detected in the sake rice type and table rice type.
After further classification, it was found that Goh showed the most unique endophytic bacterial taxa, while Kam and Kos had relatively few (Table S2). In addition, a total of 19 endophytic bacterial taxa were shared by roots of the 6 rice cultivars at the 6th week (Table S3). Other bacterial groups including Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium, Azospirillum, Pseudomonas, Sporomusaceae, Comamonadaceae, Caulobacter, Pleomorphomonas, Bradyrhizobium, and Sphingomonas detected 6 weeks after transplanting made differences in the endophytic bacterial community compositions between the rice cultivars more apparent.
3.4. Effects of Rice Types, Cultivars, and Time
This study was conducted to confirm the hypothesis that sake rice cultivars exhibit different root endophyte communities from table rice cultivars. Four sake rice cultivars and two table rice cultivars were grown in a soil bag–hydroponic pot culture method and their root endophytic bacterial communities were compared for 6 weeks after transplanting. Next-generation sequencing investigations have uncovered a substantial diversity of endophytic bacteria within rice roots [6,26,27,28]. In our study, a total of 19 endophytic bacterial taxa were found to be shared by the 6 rice cultivars with a relative abundance of 1% or more. Proteobacteria, Firmicutes, Actinobacteriota, Bacteroidota, Desulfobacterota, and Planctomycetota were identified as the predominant phyla. This fact is consistent with Ding et al. [27], Moronta-Barrios et al. [29], and Reinhold-Hurek and Hurek [30], who recognized them as the primary bacterial endophytes present in rice roots. Different cultivars exhibited varying levels of bacterial diversity and richness (Figure 2). In our previous study, we observed that the diversity and richness of endophytic bacteria community in rice roots varied significantly depending on the soil added as a microbial source, which also affected the rice growth [24].
It is generally known that endophytic microbiota has positive impacts on plant growth through improving nutrient absorption and/or increasing resistance to both biotic and abiotic stressors, as mentioned by Castrillo et al. [31] and Kim and Lee [32]. In this study, Proteobacteria were shown to constitute the most significant portion of the endophytic bacterial communities in the rice roots 6 weeks after transplanting.
Based on the findings presented in Table 3, we can understand that the cultivation period and rice cultivars cause significant variation in the endophytic bacterial community compositions in the rice roots. The rice roots collected at transplanting (0 weeks) exhibited distinct bacterial communities between the rice cultivars, indicating variation of seed-derived endophytic bacteria. This is consistent with Nannipieri et al. [33], who emphasized the importance of sampling time. Santoyo et al. [34] also demonstrated that the endophytic microbiota varies with the growth and developmental stages of the host plants including rice. In this study, the alpha diversity indices of the endophytic bacterial communities in the rice roots at 6 weeks after transplanting were significantly higher than those at 3 weeks after transplanting. Endophytic bacterial diversity and richness initially varied across root bacteria. By the 6th week, endophytic bacterial diversity differed among cultivars, following fluctuations observed from the 3rd week onwards (Figure 4). Previous studies [35,36] showed that the endophytic microbial composition in rice roots fluctuates significantly in the early growth stages but stabilizes markedly in the later stages. Our results were consistent with their findings. Moreover, Zhang et al. [37] reported that indica and japonica rice cultivars exhibited unique root microbiota compositions. Bacterial taxa enriched in indica cultivars demonstrated greater diversity than those enriched in japonica cultivars and were particularly rich in genera associated with nitrogen metabolisms. Xun et al. [38] found that bacterial assemblages characterized by high phylogenetic diversity exhibit greater resilience and stability to environmental disturbances. Bacterial dominance and suppression in the endosphere including roots may be attributed to competitive interactions over resources and habitats [39] and selective recruitment of certain bacterial species over others by the host plant [40]. These complex mechanisms governing the formation and function of microbial communities in the plant rhizosphere may represent the dynamic nature of plant–microbe interactions.
PERMANOVA results revealed that rice type and cultivar significantly affected the endophytic bacterial community composition of rice roots 0, 3, and 6 weeks after transplanting (Table 3). Notably, the endophytic bacterial community at the transplanting differed between the rice cultivars, indicating the existence of a seed-derived endophytic community specific to each cultivar. PCoA based on Bray–Curtis dissimilarity was conducted on the rice root endophytic bacterial communities identified 0, 3, and 6 weeks after transplanting to explore their changes as well as the influence of rice type and cultivar. As shown in Figure 3, the root endophytic bacterial communities formed distinct clusters for each cultivar during the growth period, with clear differences between the sake and table rice cultivars.
3.5. Predominant Endophytic Bacterial Taxa
The genus-level analysis indicated that certain bacterial endophytes that may promote plant growth were predominant in the rice roots (Figure 1). Venn diagram analysis showed that 19 bacterial genera were shared between the six rice cultivars and, notably, Burkholderia-Caballeronia-Paraburkholderia emerged as the predominant genus within the endophytic bacterial community after 6-week cultivation, regardless of the rice cultivars (Figure 4). Earlier research has demonstrated that Pseudomonas species effectively colonize the rhizosphere [41]. In previous research, plant-associated bacterial clusters such as Xanthomonas, Pantoea, and Pseudomonas [42,43] and Burkholderia and Herbaspirillum [44] were identified in rice roots, which were also detected as the major components of the rice root endophytic bacterial communities in this study (Figure 1).
Focusing on endophytes derived from rice seeds, the presence of Pantoea, Pseudomonas, and Sphingomonas was described [43,45,46]. Among them, Pantoea can cause vertical transmission, as suggested by Zhang et al. [43]. In this study, Pantoea was also detected as one of the dominant endophytic genera (Figure 1) that could be seed-derived. This is because Pantoea is known to possess endophytic capabilities, often colonizing plant tissues internally without causing negative effects and establishing a symbiotic relationship with the host plant [47]. This finding aligns with the observations of Hardoim et al. [46].
In addition, Pseudomonads are known as predominant microorganisms in aerobic environments [48], which can establish an endophytic association with the rice roots [42,43]. However, the soil bag–hydroponic system used in this study is known to negatively affect the ability of Pseudomonas to establish as rice root endophytes [13], which may be due to the existence of nonmotile Pseudomonas endophytes as Turnbull et al. [49]. Therefore, the relative abundance of Pseudomonads identified in this study is likely to be underestimated. Further research is needed to fully elucidate the formation mechanisms of endophytic bacterial communities in rice roots.
4. Conclusions
Focusing on sake rice cultivars, this study aims to trace changes in the root endophytic bacterial communities during the vegetative stage to understand how endophyte community formation differs from that of table rice cultivars. To confirm the hypothesis that sake rice cultivars exhibit different root endophyte communities compared to table rice cultivars, four sake rice cultivars and two table rice cultivars were grown for 6 weeks after transplanting using the soil bag–hydroponic method, and root endophytic bacterial communities were compared over time.
The predominant endophytic bacteria in rice roots could be broadly classified into four groups, as shown in Figure 1. The first group including Pantoea tended to be shared by the six rice cultivars. The second group was the table rice specific initial bacteria. Both are likely to be seed-derived bacteria. The third, e.g., Comamonadaceae and Paludisphaera, showed dominance 3 weeks after transplanting. The last group, including Azospirillum, slowly built a predominance in the rice roots at the later stage.
Root endophytic bacterial diversity tended to differ throughout the entire growth period following transplanting (Table 3). Although the soil bacterial community provided as the inoculum should be similar, a clear difference in the root endophytic bacterial community was found between the two rice types, sake rice cultivars and table rice cultivars.
Therefore, we conclude that the experimental findings in this study fully support our hypothesis that sake rice cultivars can exhibit distinct endophytic communities compared to table rice cultivars in the vegetative stage. Differences between the rice cultivars could be influenced by variations in their respective root exudates, known to play an important role in the colonization of endophytic bacteria. This point needs to be investigated in more detail in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14081769/s1, Figure S1: Venn diagrams depicting the distribution of unique and shared root endophytic bacterial genera between the sake rice type and the table rice type 0 (A) and 3 (B) weeks after transplanting. The values within the overlapping regions represent numbers of the genera common to both compartments, while those outside the overlaps indicate ones specific to each compartment. The numbers in parentheses indicate the number of genera detected in the sake rice type and table rice type; Figure S2: Venn diagrams depicting the distribution of unique and shared root endophytic bacterial genera between the rice cultivars 0 (A), 3 (B), and 6 (C) weeks after transplanting. The values within the overlapping regions represent numbers of the genera common to multiple compartments, while those outside the overlaps indicate ones specific to each compartment. Goh, Kam, Kos, Nip, Oma, and Yam mean rice cultivars as shown in Table 1. The number in parentheses indicate the number of genera detected in each rice cultivar; Table S1: Summary of alpha diversities of the rice root endophytic bacterial community 0, 3, and 6 weeks after transplanting; Table S2: Summary of the unique bacterial taxa identified at the genus level and their relative abundances 6 weeks after transplanting; Table S3: A list of 19 shared bacterial taxa identified at the genus level 6 weeks after transplanting; Table S4: Biomarkers of the root endophytic bacteria for the sake and table rice cultivars satisfying both LDA score > 2.0 and p < 0.05 identified by Linear Discriminant Analysis Effect Size (LEfSe) analysis 0, 3, and 6 weeks (w) after transplanting.
Author Contributions
Conceptualization, K.S. and N.H.; methodology, S.S., S.O.S., K.S. and N.H.; validation, K.S. and N.H.; formal analysis, S.S. and K.S.; investigation, S.S., S.O.S., K.S. and N.H.; resources, K.S. and N.H.; writing—original draft preparation, S.S.; writing—review and editing, S.S., S.O.S., K.S. and N.H.; visualization, S.S. and K.S.; supervision, K.S. and N.H.; funding acquisition, K.S. and N.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI grant number 24K01651 and Foundation of Sasaki Environment technology (2022).
Data Availability Statement
The sequence data presented in this study are openly available in the DDBJ Sequenced Read Archive under the accession number PRJDB18248. The others are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Food and Agriculture Organization (FAO); International Fund for Agricultural Development (IFAD); The United Nations Children’s Fund (UNICEF); World Food Programme (WFP); World Health Organization (WHO). The State of Food Security and Nutrition in the World. 2022. Repurposing Food and Agricultural Policies to Make Healthy Diets More Affordable; Food and Agriculture Organization (FAO): Rome, Italy, 2022.
- Molina, J.; Sikora, M.; Garud, N.; Flowers, J.M.; Rubinstein, S.; Reynolds, A.; Huang, P.; Jackson, S.; Schaal, B.A.; Bustamante, C.D. Molecular evidence for a single evolutionary origin of domesticated rice. Proc. Natl. Acad. Sci. USA 2011, 108, 8351–8356. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Hu, L.; Zhu, Y.; Xu, D.; Zheng, L.; Chen, Z.; Hu, Y.; Cui, P.; Guo, B.; Dai, Q.; et al. Different characteristics of nutrient absorption and utilization between inbred japonica super rice and inter-sub-specific hybrid super rice. Field Crops Res. 2018, 218, 88–96. [Google Scholar] [CrossRef]
- Bhadraray, S.; Purakayastha, T.; Chhonkar, P. Phosphorus mobilization in hybrid rice rhizosphere compared to high yielding varieties under integrated nutrient management. Biol. Fertil. Soils 2002, 35, 73–78. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, H.; Guo, B.; Xu, K.; Huo, Z. Physicochemical properties of indica-japonica hybrid rice starch from Chinese varieties. Food Hydrocoll. 2016, 63, 356–363. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-Medellin, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Z.; Mori, N.; Kawamura, M. Genetic diversity and phylogeny of Japanese sake-brewing rice as revealed by AFLP and nuclear and chloroplast SSR markers. Theor. Appl. Genet. 2004, 109, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Joyo, M.; Tamamoto, Y. Analysis of protein composition in rice cultivar used for sake brewing, and their effects on nitrogen compounds in sake. Cereal Chem. 2018, 95, 320–329. [Google Scholar] [CrossRef]
- Matsumoto, T.; Wu, J.; Itoh, T. The Nipponbare genome and the next generation of rice genomics research in Japan. Rice 2016, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Okada, R.; Nemoto, Y.; Endo-Higashi, N. Synthetic control of flowering in rice independent of the cultivation environment. Nat. Plants 2017, 3, 17039. [Google Scholar] [CrossRef]
- Xu, Y.; Ge, Y.; Song, J. Assembly of root-associated microbial community of typical rice cultivars in different soil types. Biol. Fertil. Soils 2020, 56, 249–260. [Google Scholar] [CrossRef]
- Honma, T.; Ohba, H.; Ohyama, T. Effects of soil management on changes of soil carbon content in alluvial paddy soil in Niigata. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010. [Google Scholar]
- Samuel, S.O.; Suzuki, K.; Asiloglu, R.; Harada, N. Soil-root interface influences the assembly of the endophytic bacterial community in rice plants. Biol. Fertil. Soils 2021, 58, 35–48. [Google Scholar] [CrossRef]
- Asiloglu, R.; Shiroishi, K.; Suzuki, K.; Turgay, O.C.; Murase, J.; Harada, N. Protist-enhanced survival of a plant growth promoting rhizobacteria, Azospirillum sp. B510, and the growth of rice (Oryza sativa L.) plants. Appl. Soil Ecol. 2020, 154, 103599. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, Z.; Gai, X.; Du, X.; Bian, F.; Yang, C.; Gao, G.; Wen, X. Changes of root endophytic bacterial community along a chronosequence of intensively managed lei bamboo (Phyllostachys praecox) forests in subtropical China. Microorganisms 2019, 7, 616. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Ali, Q.; Sohail, M.A.; Ashraf, M.F.; Saleem, M.H.; Hussain, S.; Zhou, L. Diversity and Taxonomic Distribution of Endophytic Bacterial Community in the Rice Plant and Its Prospective. Int. J. Mol. Sci. 2021, 22, 10165. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Core R Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.r-project.org/ (accessed on 27 February 2024).
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Samuel, S.O.; Suzuki, K.; Asiloglu, R.; Harada, N. Rice endophytic communities are strongly dependent on microbial communities specific to each soil. Biol. Fertil. Soils 2023, 59, 733–746. [Google Scholar] [CrossRef]
- Bacilio-Jiménez, M.; Aguilar-Flores, S.; Ventura-Zapata, E. Chemical characterization of root exudates from rice (Oryza sativa) and their effects on the chemotactic response of endophytic bacteria. Plant Soil 2003, 249, 271–277. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, F.; Zhang, X.; Dai, X.; Dong, X.; Song, W. Endophytic bacterial diversity in rice (Oryza sativa L.) roots estimated by 16S rDNA sequence analysis. Microb. Ecol. 2008, 55, 415–424. [Google Scholar] [CrossRef]
- Ding, L.; Cui, H.; Nie, S.; Long, X.; Duan, G.; Zhu, Y. Microbiomes inhabiting rice roots and rhizosphere. FEMS Microbiol. Ecol. 2019, 95, fiz040. [Google Scholar] [CrossRef] [PubMed]
- Walitang, D.; Samaddar, S.; Roy Choudhury, A.; Chatterjee, P.; Ahmed, S.; Sa, T. Diversity and Plant Growth-Promoting Potential of Bacterial Endophytes in Rice. In Plant Growth Promoting Rhizobacteria (PGPR): Prospects for Sustainable Agriculture; Sayyed, R., Reddy, M., Antonius, S., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Moronta-Barrios, F.; Gionechetti, F.; Pallavicini, A.; Marys, E.; Venturi, V. Bacterial microbiota of rice roots: 16S-based taxonomic profiling of endophytic and rhizospheric diversity, endophytes isolation and simplified endophytic community. Microorganisms 2018, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Reinhold-Hurek, B.; Hurek, T. Living inside plants: Bacterial endophytes. Curr. Opin. Plant Biol. 2011, 14, 435–443. [Google Scholar] [CrossRef]
- Castrillo, G.; Teixeira, P.; Paredes, S.H.; Law, T.F.; Lorenzo, L.D.; Feltcher, M.E. Root microbiota drive direct integration of phosphate stress and immunity. Nature 2017, 543, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, Y. The rice microbiome: A model platform for crop holobiome. Phytobiomes J. 2020, 4, 5–18. [Google Scholar] [CrossRef]
- Nannipieri, P.; Penton, C.R.; Purahong, W.; Schloter, M.; van Elsas, J.D. Recommendations for soil microbiome analyses. Biol. Fertil. Soils 2019, 55, 765–766. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Edwards, J.A.; Santos-Medellín, C.M.; Liechty, Z.S.; Nguyen, B.; Lurie, E.; Eason, S. Compositional shifts in root-associated bacterial and archaeal microbiota track the plant life cycle in field-grown rice. PLoS Biol. 2018, 16, e2003862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, N.; Liu, Y.; Zhang, X.; Hu, B.; Qin, Y. Root microbiota shift in rice correlates with resident time in the field and developmental stage. Sci. China Life Sci. 2018, 61, 613–621. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.X.; Zhang, N. NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 2019, 37, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Xun, W.; Liu, Y.; Li, W.; Ren, Y.; Xiong, W.; Xu, Z. Specialized metabolic functions of keystone taxa sustain soil microbiome stability. Microbiome 2021, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Kandel, S.; Joubert, P.; Doty, S. Bacterial endophyte colonization and distribution within plants. Microorganisms 2017, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Afzal, I.; Shinwari, Z.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.; Défago, G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Sahu, K.P.; Kumar, A.; Sakthivel, K.; Reddy, B.; Kumar, M.; Patel, A.; Sheoran, N.; Gopalakrishnan, S.; Prakash, G.; Rathour, R.; et al. Deciphering core phyllomicrobiome assemblage on rice genotypes grown in contrasting agroclimatic zones: Implications for phyllomicrobiome engineering against blast disease. Environ. Microbiol. 2022, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, Y.N.; Wang, X.; Liao, K.; He, S.; Zhao, X.; Guo, H.; Zhao, D.; Wei, H.L. Dynamics of rice microbiomes reveal core vertically transmitted seed endophytes. Microbiome 2022, 10, 216. [Google Scholar] [CrossRef]
- Mano, H.; Morisaki, H. Endophytic bacteria in the rice plant. Microbes Environ. 2008, 23, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Kaga, H.; Mano, H.; Tanaka, F.; Watanabe, A.; Kaneko, S.; Morisaki, H. Rice seeds as sources of endophytic bacteria. Microbes Environ. 2009, 24, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; Hardoim, C.C.P.; van Overbeek, L.S.; van Elsas, J.D. Dynamics of seed-borne rice endophytes on early plant growth stages. PLoS ONE 2012, 7, e30438. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, V.; Shantharaj, D.; Li, G.; Seyfferth, A.L.; Janine Sherrier, D.; Bais, H.P. A natural rice rhizospheric bacterium abates arsenic accumulation in rice (Oryza sativa L.). Planta 2015, 242, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Anzai, Y.; Kim, H.; Park, J.; Wakabayashi, H.; Oyaizu, H. Phylogenetic affiliation of the Pseudomonads based on 16S rRNA sequence. Int. J. Syst. Evol. Microbiol. 2000, 50, 1563–1589. [Google Scholar] [CrossRef]
- Turnbull, G.A.; Morgan, J.A.W.; Whipps, J.M.; Saunders, J.R. The role of motility in the in vitro attachment of Pseudomonas putida PaW8 to wheat roots. FEMS Microbiol. Ecol. 2001, 35, 57–65. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).