Abstract
Selenium (Se) is one of the human essential elements and the input of Se for its biofortification is common in rice production to meet the demand for Se in the population. Biological nano-selenium (nano-Se) is a new type of nanoscale microbial synthetic material. However, the effects of biological nano-Se on aromatic rice performance metrics, such as yield formation, grain quality parameters, and the biosynthesis of 2-acetyl-1-pyrroline (2-AP, the key component of aromatic rice aroma), have rarely been reported. Therefore, this study conducted a field experiment with two cropping seasons and two aromatic rice genotypes to explore the effects of the foliar application of biological nano-Se on aromatic rice performance metrics. The results showed that the foliar application of biological nano-Se at 3–4 days before panicle differentiation or the heading stage increased the grain yield of aromatic rice. Dry matter accumulation and the leaf area index increased under Nano-Se application. Furthermore, the foliar application of Nano-Se at 3–4 days before panicle differentiation significantly enhanced the activity of peroxidase, superoxide dismutase, catalase and reduced malondialdehyde content. The foliar application of Nano-Se at the grain-filling stage also increased 2-AP content. In addition, nano-Se application substantially increased the grain Se content in aromatic rice.
1. Introduction
Rice (Oryza sativa L.) is one of the main food crops in China and occupies an important position in China’s agricultural production [1]. However, with the improvement of people’s material living standards, the quality requirements of staple crops are becoming higher, and the focus of rice production is gradually changing from high yield to high quality [2]. Aromatic rice is a special kind of rice that can emit fragrance in stems, leaves and the panicles of plant organs, except roots [3]. Among the complex volatile compounds detected in aromatic rice, 2-acetyl-1-pyrroline (2-AP) is considered to be the most significant compound affecting the aromatic traits of aromatic rice [4]. Because of the consumer’s ideal pursuit of the good smell, good taste, good looks, and high nutrition of rice, the amount of aromatic rice produced is far from meeting the market demand. Therefore, it is the urgent desire of scientists and producers related to aromatic rice to increase the yield and aroma of aromatic rice.
Selenium (Se) is a trace element that has recently received a lot of attention. It is a metal-like element that is both toxic and nutritious to organisms [5]. As an indispensable nutrient in the human diet and one of the essential structural elements, a moderate intake of selenium can improve human immunity, prevent many diseases such as Keshan disease, cataracts, heart disease, etc. [6], and even effectively counteract the toxic effects of heavy metals on the kidneys, reproductive glands, and central nervous system [7]. Selenium is not only beneficial to the human body, but also an essential element for plant growth. The biological activity and physiological function of selenium in plants depend not only on the total concentration of exogenous selenium, but also on the chemical form of selenium [8]. In the process of studying the conversion of selenium (Se) by biological macromolecules (such as proteins), it is often found that there is a phenomenon of red material formation [9]. These substances have been identified as red particles of the element selenium with a diameter of about 5–200 nm. They are called biological nano-Se because of their apparent biological effects [10]. The emergence of biological nano-Se has solved the problem of the high-cost and toxic chemicals used in the synthesis of nano-Se by chemical methods. Because of its unique high safety, easy absorption, and high biocompatibility, it has attracted wide attention [11,12]. More and more scholars have studied the physiological and biochemical functions of bio-nano-selenium on plants, and have achieved phased research results. Studies have shown that the application of bio-nano-selenium in agriculture mainly plays a positive role in improving crop growth [13,14], enhancing crop resistance [15], and inhibiting the accumulation of heavy metals in crops [16,17].
At present, most of the studies on the effect of nano-Se on the growth of rice are carried out by chemically synthesized nano-Se, while the effect of biological nano-Se on the formation and growth of aromatic rice fragrance has not been explored. Therefore, in this study, five modes of biological nano-Se application were adopted, and two kinds of high-quality conventional aromatic rice were used as experimental materials to explore the effects of biological nano-Se and its spraying period on the yield, quality, aroma, and selenium content of aromatic rice. Our findings will provide the latest guidance information for the production and application of biological nano-Se on aromatic rice.
2. Materials and Methods
2.1. Materials and Test Site Overview
Field trials were conducted at the Experimental Station of South China Agricultural University in Zengcheng District, Guangzhou City, Guangdong Province, China (23°14′ N, 113°38′ E) in the early and late cropping seasons of 2023. The climatic type of this trial site was a subtropical monsoon climate and the meteorological data are shown in Table 1. The experimental soil was sandy loam with 21.87 g kg−1 of organic matter, 1.54 g kg−1 of total nitrogen, 1.11 g kg−1 of total phosphorus, 20.85 g kg−1 of total potassium, and a 5.71 pH. Two commercial aromatic rice cultivars, 19Xiang and Qingxiangyou19Xiang, provided by the Laboratory of Rice Cultivation, College of Agriculture, South China Agricultural University, were used as the plant materials. More information on these cultivars could be found in the China Rice Data Center (https://www.ricedata.cn/, accessed on 1 March 2023). In the early cropping season, the sowing was completed on 10 March. The transplanting was completed on 28 March. The harvest was completed on 10 July. In the late cropping season, the sowing was completed on 14 July. The transplanting was completed on 29 July. The harvest was completed on 23 October. A commercial fertilizer (N:P2O5:K2O = 15:4:6) was applied with 600 kg ha−1 as the basal before transplanting and 300 kg ha−1 as topdressing at the tillering stage in both the early and late seasons. After transplanting, standing water of a 3–4 cm layer was maintained until the end of the tillering stage. Then, the water was drained for about a week to control the production of infertile tillers. At the following periods, a shallow water layer of 3–4 cm was maintained to the grain-filling stage. Diseases, insects, and weeds were strictly controlled using pesticides and herbicides following the routine management measures adopted by the local farmers.

Table 1.
Meteorological data during the experiment.
2.2. Experimental Design
Six treatments were adopted in the present experiment: no foliar application (CK), foliar application one time at 3–4 days before panicle differentiation (T1), foliar application one time at the heading stage (T2), foliar application one time at 10 days after the heading stage (T3), foliar application two times at 3–4 days before panicle differentiation and the heading stage, respectively (T4), and foliar application two times at 3–4 days before panicle differentiation and 10 days after the heading stage (T5). The experiment was arranged in a split-zone design, with varieties as the main zone and treatments as sub-zones and each plot was 40 m2. The applied Se concentration is 6.67 mg L−1. The nano-Se was produced and provided by Green Huinong Biotechnology (Shenzhen) Co., Ltd., Shenzhen, China, which was synthesized through microbial fermentation with selenite as the material. The average particle size of nano-Se was 13.767 nm.
2.3. Determination Items and Methods
2.3.1. Determination of Yield and Yield-Related Traits of Aromatic Rice
The random sampling method was used at harvest, according to the yield of a 1 m2 harvest as a repetition, each plot was repeated three times, and the actual yield was obtained after natural drying. Effective panicles were randomly selected and collected from five plants in each plot to measure the number of grains per panicle, the seed-setting rate, and the 1000-grain weight using a fully automated seed analyzer, OPTO-Agri (Riom, France). The seed-setting rate is the percentage of filled grains to the total number of grains per panicle. The 1000-grain weight was measured by counting and weighting a thousand filled grains in three repetitions.
2.3.2. Determination of Grain Quality of Aromatic Rice
After the rice was naturally dried, 500 g of each plot was placed in a cool and ventilated place for 2 months to determine the quality traits. The brown rice rate was estimated using a rice huller (Hiroshima, Japan). The milled rice and head rice recovery rates were calculated by using a Jingmi testing rice grader (Taizhou, China) to polish a minute. Then, the chalky grain rate and chalkiness degree of rice were determined by the Wanshen SC-E rice appearance quality analysis system, with 4 repetitions.
2.3.3. Determination of Leaf Photosynthetic Rate, Leaf Area Index (LAI), and Dry Matter Weight
The net photosynthetic rate of the main stem leaves was measured using a LI-6800 portable photosynthesis meter (LI-COR, Lincoln, NE, USA) on a sunny day without cloud cover. The determination method of the LAI and above-ground dry matter quality is selecting 4 representative rice plants in each plot, and then randomly selecting 1 rice plant and selecting 15 complete leaves as standard leaves to measure their length and width. The leaf area of the standard leaves was measured according to the length–width coefficient method of 0.75 × length × width. After drying at 80 °C for 48 h, the total weight of the above-ground part was weighed. At the same time, the leaf area of the whole rice plant was calculated according to the specific gravity method after the standard leaves were dried and weighed, and the LAI was calculated according to the planting density.
2.3.4. Determination of Parameters Related to Antioxidant Reactions
Methods for the determination of Superoxide dismutase (SOD), peroxidase (POD), Catalase (CAT) activity and Malondialdehyde (MDA) content referred to those of Chen et al. [18]. Weigh the ground fresh sample of 0.4 g in a 5 mL centrifuge tube, add 4 mL of pH 7.8 PBS to shock and mix, ice bath ultrasound for 20 min, and place it in a centrifuge at 8000 rpm for 15 min at 4 °C. The supernatant was taken as the test solution in a 2.2 mL 96-well plate and stored at 4 °C.
2.3.5. Determination of 2-AP Content in Grains
After harvest, 40 g of grain was taken from each plot, and stored at −80 °C. During the determination, 2 g of the ground sample was weighed and placed in a glass bottle, 10 mL of chromatographic pure dichloromethane was added, the sealing ring was covered, the cap was tightened and shaken slightly, and the rubber band was tied together with a rubber band and placed in a water-filled ultrasonic cleaner, at 40 KHZ, 40 °C, that was ultrasonic for 240 min. The sample bottle was taken out and cooled to room temperature. After adding an appropriate amount of anhydrous sodium sulfite, the supernatant was immediately absorbed with a 2 mL bacterial syringe and injected into the headspace sample bottle through an organic needle filter membrane (pore size 0.22 μm, 13 mm). Then, the gas chromatography analyzer (Shimadzu (Kyoto, Japan), GC-MS QP 2010 Plus) was used for determination [19].
2.3.6. Determination of Pyrroline Content in Aromatic Rice Grain
A total of 0.15 g of the ground fresh sample was weighed in a 5 mL centrifuge tube, added to 4 mL of a 50 mM Tris-HCl buffer (pH 7.6), and shaken well. After ultrasonication in an ice bath for 30 min, it was placed in a centrifuge at 8000 rpm and 4 °C for 20 min. The supernatant was placed into 96-well plates as the test solution to determine the pyrroline content [20].
2.3.7. Determination of Selenium Content
The milled rice was obtained after the rice was screened by the rice huller and the rice miller, and the milled rice was ground and determined according to the standard ‘GB 5009.268-2016 Determination of multi-elements in food’ [21].
2.3.8. Data Processing and Analysis
The experimental data were analyzed using Microsoft Office Excel 2021 (Microsoft, Washington, DC, USA) and SPSS 21.0 (International Business Machines Corporation (IBM), New York, NY, USA). The least significant difference (LSD) test was used for multiple comparisons (p < 0.05).
3. Results
3.1. Effect of Biological Nano-Se on Yield and Yield-Related Traits
Foliar application of biological nano-Se significantly influenced the yield formation of aromatic rice (Table 2). Analysis of variance showed that biological nano-Se had different degrees of increase in the seed-setting rate, 1000-grain weight, and yield of aromatic rice. Compared to CK, T1, T4, and T5 treatments had the best yield-increasing effect. Compared to CK, the yield of T1, T4, and T5 increased by 11.34%, 8.55%, and 10.22%, respectively, in the early season of 19Xiang. Compared to CK, the yield of T1, T4, and T5 treatments increased by 7.18%, 5.51%, and 7.18%, respectively, for Qingxiangyou19Xiang in the early season. In the late season of 19Xiang, T1, T4, and T5 treatments increased the yield by 12.41%, 8.35%, and 11.9%, respectively, compared to CK. In the late season of Qingxiangyou19Xiang, the yield of T1, T4, and T5 treatments increased by 5.29%, 4.71%, and 6.67% respectively, compared to CK. In addition, there was no significant difference between the T1, T4 and T5 treatments. Moreover, the seed-setting rate of the T1, T2, T4, and T5 treatments was higher than that of CK in the two planting seasons and for two varieties. Compared to CK, biological nano-Se treatment increased the 1000-grain weight of two seasons and for two varieties, but the difference did not reach a significant level.

Table 2.
Effects of biological nano-Se application on yield and yield-related traits.
3.2. Effect of Biological Nano-Se on Grain Quality Attributes
Foliar application of biological nano-Se significantly affected the grain quality of aromatic rice (Table 3). Variance analysis showed that biological nano-Se significantly impacted the brown rice rate, milled rice rate, chalky rice rate, and chalkiness. In terms of milling quality, T3 and T5 treatments had the best improvement effect. In the early season of 19Xiang, T3 and T5 treatments increased the milled rice rate by 2.14% and 1.05%, respectively, compared to CK. In the early season of Qingxiangyou19Xiang, T3 and T5 treatments increased the milled rice rate by 2.86% and 2.92%, respectively, compared to CK. In the late season of 19Xiang, T3 and T5 treatments increased the milled rice rate by 3.20% and 1.33%, respectively, compared to CK. In the late season of Qingxiangyou19Xiang, T3 and T5 treatments increased the milled rice rate by 3.03% and 3.08%, respectively, compared to CK. In terms of appearance quality, compared to CK, the decrease in chalkiness in the early season of 19Xiang could reach 15.03% under the biological nano-Se application. In the early season of Qingxiangyou19Xiang, the decrease in chalkiness could reach 17.58% under the biological nano-Se application. In the late season, the decrease in chalkiness in the late season could reach 27.08% and 14.60% for 19Xiang and Qingxiangyou19Xiang, respectively, under the biological nano-Se application.

Table 3.
Effects of biological nano-Se application on grain quality attributes.
3.3. Effects of Biological Nano-Se on the Net Photosynthetic Rate
Foliar application of biological nano-Se impacted the net photosynthetic rate (Figure 1). The variation trend in the net photosynthetic rate of aromatic rice leaves in early and late seasons varied with different varieties. Compared to CK, T1, T4, and T5 treatments had an obvious increase in 19Xiang, while in Qingxiangyou19Xiang, there was an obvious increase in all places. For 19Xiang, the maximum increase in biological nano-Se treatment in early and late seasons reached 28.89% and 24.81%, respectively. For Qingxiangyou19Xiang, the maximum increase in biological nano-Se treatment in early and late seasons can reach 28.71% and 19.32%.
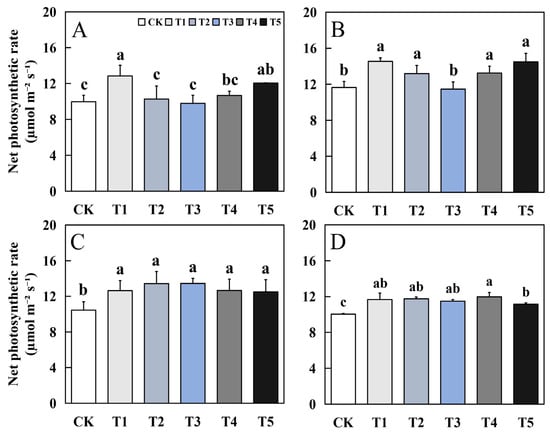
Figure 1.
Effects of biological nano-Se application on the net photosynthetic rate of aromatic rice (A) for 19Xiang in the early season; (B) for 19Xiang in the late season; (C) for Qingxiangyou19Xiang in the early season; (D) for Qingxiangyou19Xiang in the late season. No foliar application (CK), foliar application one time at 3–4 days before panicle differentiation (T1), foliar application one time at the heading stage (T2), foliar application one time at 10 days after the heading stage (T3), foliar application two times at 3–4 days before panicle differentiation and the heading stage, respectively (T4), foliar application two times at 3–4 days before panicle differentiation and 10 days after the heading stage (T5). Bars sharing a common letter do not differ significantly at (p ≤ 0.05) in each cropping season according to the LSD test. The same as below.
3.4. Effect of Biological Nano-Se on the Leaf Area Index (LAI)
Foliar application of biological nano-Se significantly impacted the LAI of fragrant rice (Figure 2). The LAI is the ratio of the total area of plant leaves per unit area to the planted area. The LAI of the two aromatic rice varieties at the mature stage was basically the same in the early and late seasons. Compared to CK, each treatment had different degrees of increase, among which T1, T4, and T5 treatments had the best effect. For the early season of 19Xiang, the increase of T1, T4, and T5 treatments was 14.94%, 10.11%, and 11.26%, respectively. In the late season of 19Xiang, the increase of T1, T4, and T5 treatments was 12.97%, 10.33%, and 22.20%. For the first season of Qingxiangyou19Xiang, the increase of T1, T4, and T5 treatments was 21.38%, 18.16%, and 22.76%, respectively. In the late season of Qingxiangyou19Xiang, the increase of T1, T4, and T5 treatments was 37.31%, 37.82%, and 20.47%.
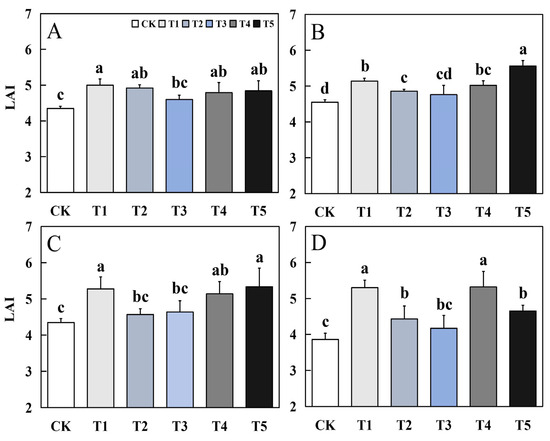
Figure 2.
Effects of biological nano-Se application on the LAI of aromatic rice (A) for 19Xiang in the early season; (B) for 19Xiang in the late season; (C) for Qingxiangyou19Xiang in the early season; (D) for Qingxiangyou19Xiang in the late season. No foliar application (CK), foliar application one time at 3–4 days before panicle differentiation (T1), foliar application one time at the heading stage (T2), foliar application one time at 10 days after the heading stage (T3), foliar application two times at 3–4 days before panicle differentiation and the heading stage, respectively (T4), foliar application two times at 3–4 days before panicle differentiation and 10 days after the heading stage (T5). The LAI (leaf area index) is the ratio of the total area of plant leaves per unit area to the planted area. Bars sharing a common letter do not differ significantly at (p ≤ 0.05) in each cropping season according to the LSD test.
3.5. Effect of Biological Nano-Se on Aboveground Dry Matter Weight
Foliar application of biological nano-Se significantly impacted the aboveground dry matter weight of fragrant rice (Figure 3). Except for T3 treatment, the other treatments had different degrees of increase in the dry matter quality of the aboveground part at the mature stage compared to CK. Among them, T1, T4, and T5 treatments had the best effect, and there was no significant difference between the three treatments. Compared to CK, the maximum increase of biological nano-Se treatment in the early and late seasons of 19Xiang can reach 10.42% and 12.94%; in Qingxiangyou19Xiang, the maximum increase in early and late seasons was 6.05% and 9.13%.
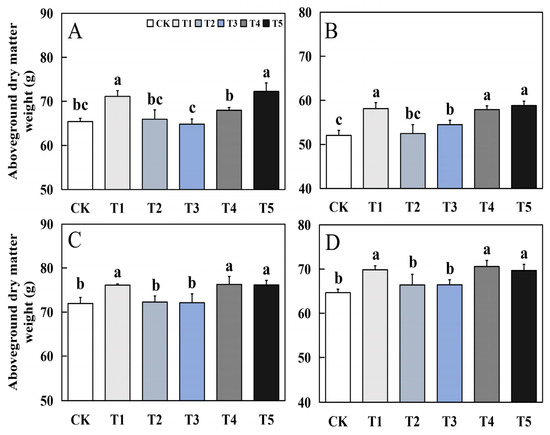
Figure 3.
Effect of biological nano-Se application on the aboveground dry matter of aromatic rice (A) for 19Xiang in the early season; (B) for 19Xiang in the late season; (C) for Qingxiangyou19Xiang in the early season; (D) for Qingxiangyou19Xiang in the late season. No foliar application (CK), foliar application one time at 3–4 days before panicle differentiation (T1), foliar application one time at the heading stage (T2), foliar application one time at 10 days after the heading stage (T3), foliar application two times at 3–4 days before panicle differentiation and the heading stage, respectively (T4), foliar application two times at 3–4 days before panicle differentiation and 10 days after the heading stage (T5). Bars sharing a common letter do not differ significantly at (p ≤ 0.05) in each cropping season according to the LSD test.
3.6. Effects of Biological Nano-Se on the Antioxidant System
Foliar application of biological nano-Se significantly impacted the antioxidant system in terms of the Superoxide dismutase (SOD), peroxidase (POD), Catalase (CAT) and Malondialdehyde (MDA) of fragrant rice (Figure 4, Figure 5, Figure 6 and Figure 7). The biological nano-Se treatment showed different trends in the performance of SOD activity in different varieties of aromatic rice leaves at the mature stage. In 19Xiang, except for T3 treatment, the other treatments had different degrees of increase compared to CK, and the maximum increase in early and late seasons could reach 16.78% and 29.08%. In the first season of Qingxiangyou19Xiang, T1 and T4 treatments had a significant increase compared to CK, with an increase of 9.89% and 10.9%; in the late season, each treatment had different degrees of increase compared to CK, and the maximum increase could reach 24.63%.
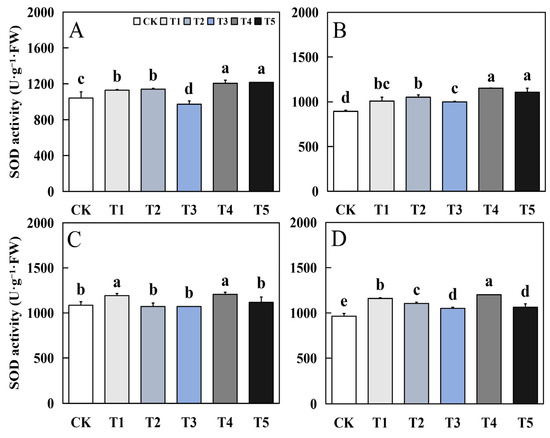
Figure 4.
Effects of biological nano-Se application on the SOD activity of aromatic rice (A) for 19Xiang in the early season; (B) for 19Xiang in the late season; (C) for Qingxiangyou19Xiang in the early season; (D) for Qingxiangyou19Xiang in the late season. No foliar application (CK), foliar application one time at 3–4 days before panicle differentiation (T1), foliar application one time at the heading stage (T2), foliar application one time at 10 days after the heading stage (T3), foliar application two times at 3–4 days before panicle differentiation and the heading stage, respectively (T4), foliar application two times at 3–4 days before panicle differentiation and 10 days after the heading stage (T5). FW, fresh weight. Bars sharing a common letter do not differ significantly at (p ≤ 0.05) in each cropping season according to the LSD test.
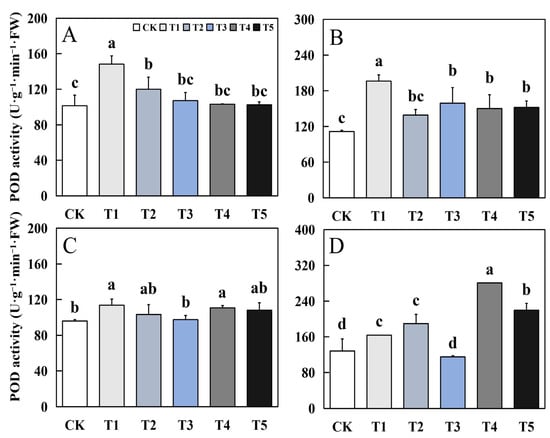
Figure 5.
Effects of biological nano-Se application on the POD activity of aromatic rice (A) for 19Xiang in the early season; (B) for 19Xiang in the late season; (C) for Qingxiangyou19Xiang in the early season; (D) for Qingxiangyou19Xiang in the late season. No foliar application (CK), foliar application one time at 3–4 days before panicle differentiation (T1), foliar application one time at the heading stage (T2), foliar application one time at 10 days after the heading stage (T3), foliar application two times at 3–4 days before panicle differentiation and the heading stage, respectively (T4), foliar application two times at 3–4 days before panicle differentiation and 10 days after the heading stage (T5). FW, fresh weight. Bars sharing a common letter do not differ significantly at (p ≤ 0.05) in each cropping season according to the LSD test.
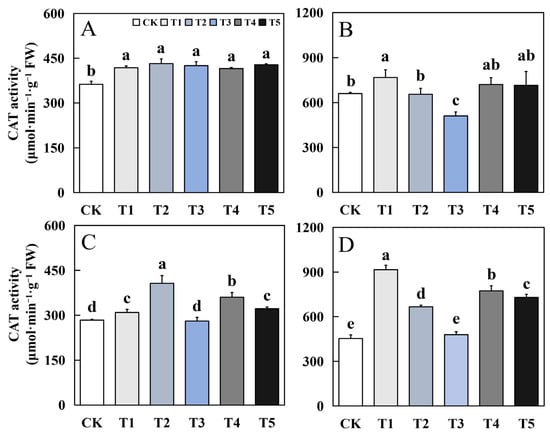
Figure 6.
Effects of biological nano-Se application on the CAT activity of aromatic rice (A) for 19Xiang in the early season; (B) for 19Xiang in the late season; (C) for Qingxiangyou19Xiang in the early season; (D) for Qingxiangyou19Xiang in the late season. No foliar application (CK), foliar application one time at 3–4 days before panicle differentiation (T1), foliar application one time at the heading stage (T2), foliar application one time at 10 days after the heading stage (T3), foliar application two times at 3–4 days before panicle differentiation and the heading stage, respectively (T4), foliar application two times at 3–4 days before panicle differentiation and 10 days after the heading stage (T5). FW, fresh weight. Bars sharing a common letter do not differ significantly at (p ≤ 0.05) in each cropping season according to the LSD test.
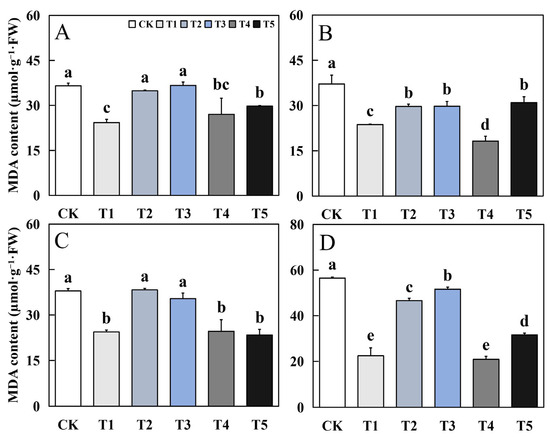
Figure 7.
Effects of biological nano-Se application on the MDA content of aromatic rice (A) for 19Xiang in the early season; (B) for 19Xiang in the late season; (C) for Qingxiangyou19Xiang in the early season; (D) for Qingxiangyou19Xiang in the late season. No foliar application (CK), foliar application one time at 3–4 days before panicle differentiation (T1), foliar application one time at the heading stage (T2), foliar application one time at 10 days after the heading stage (T3), foliar application two times at 3–4 days before panicle differentiation and the heading stage, respectively (T4), foliar application two times at 3–4 days before panicle differentiation and 10 days after the heading stage (T5). FW, fresh weight. Bars sharing a common letter do not differ significantly at (p ≤ 0.05) in each cropping season according to the LSD test.
The effect of biological nano-Se treatment on POD activity of mature aromatic rice leaves showed different trends in different varieties. In 19Xiang, the biological nano-Se treatment had different degrees of increase compared to CK, among which T1 treatment had the best effect, with an increase of 46.13% and 75.95% in early and late seasons. In Qingxiangyou19Xiang, except for T3 treatment, the other treatments had different degrees of increase compared to CK, and the maximum increase in early and late seasons could reach 18.44% and 118.59%.
The effect of biological nano-Se treatment on CAT activity in mature fragrant rice leaves was generally consistent in different varieties. Except for T3 treatment, the other biological nano-Se treatments were enhanced to varying degrees compared to CK. Compared to CK, the highest increase in the early and late seasons of 19Xiang was 19.27% and 16.22%. In Qingxiangyou19Xiang, the maximum increase in early and late seasons was 43.29% and 101.92%.
The effect of biological nano-Se treatment on the MDA content in leaves of mature aromatic rice was generally consistent in different varieties. Compared to CK, T1, T4 and T5 treatments had different degrees of decline, and there was no significant difference between the three treatments. Compared to CK, the decrease of T1, T4, and T5 treatments in the early season of 19Xiang was 33.53%, 25.98%, and 18.53%, respectively. In the late season of 19Xiang, the decrease of T1, T4, and T5 treatments was 36.24%, 51.01%, and 16.65%, respectively. In the first season of Qingxiangyou19Xiang, the decrease of T1, T4 and T5 treatments was 35.63%, 35.07%, and 38.34%. In the late season of Qingxiangyou19Xiang, the decrease of T1, T4, and T5 treatments was 60.33%, 63.07% and 44.06%.
3.7. Effects of Bio-Nano-Se on 2-AP Content
Foliar application of biological nano-Se significantly impacted the 2-AP content of aromatic rice (Figure 8). The effect of biological nano-Se treatment on the 2-AP content of aromatic rice grains at the maturity stage was generally consistent in different varieties. Compared to CK, except for the early season of Qingxiangyou19Xiang, T3 and T5 treatments had different degrees of aroma enhancement effects. In the early season of Qingxiangyou19Xiang, compared to CK, the decrease of T3 and T5 was 0.56% and 4.5%, and in the late season of Qingxiangyou19Xiang there were growth increases of 17.19% and 15.59%. The maximum increase in the early and late seasons of 19Xiang was 15.45% and 5.47%.
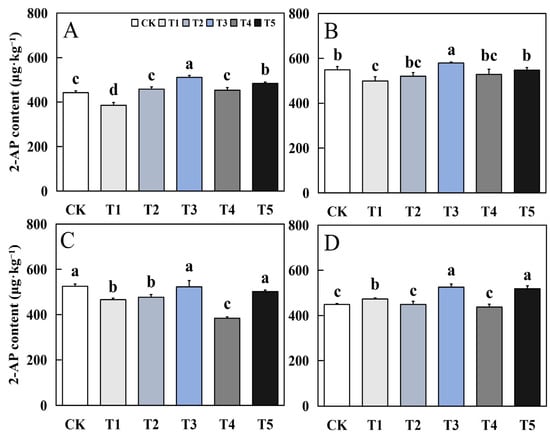
Figure 8.
Effects of biological nano-Se application on grain 2-AP content of aromatic rice (A) for 19Xiang in the early season; (B) for 19Xiang in the late season; (C) for Qingxiangyou19Xiang in the early season; (D) for Qingxiangyou19Xiang in the late season. No foliar application (CK), foliar application one time at 3–4 days before panicle differentiation (T1), foliar application one time at the heading stage (T2), foliar application one time at 10 days after the heading stage (T3), foliar application two times at 3–4 days before panicle differentiation and the heading stage, respectively (T4), foliar application two times at 3–4 days before panicle differentiation and 10 days after the heading stage (T5). 2-AP, 2-acetyl-1-pyrroline. Bars sharing a common letter do not differ significantly at (p ≤ 0.05) in each cropping season according to the LSD test.
3.8. Effects of Biological Nano-Se on Grain Pyrroline Content of Aromatic Rice
Foliar application of biological nano-Se significantly impacted the pyrroline content of aromatic rice (Figure 9). The effect of biological nano-Se treatment on the pyrroline content of aromatic rice grains at the mature stage was generally consistent in different varieties. Compared to CK, T3 and T5 treatments had different degrees of increase. In the early season of 19Xiang, the increase of T3 and T5 was 28.4% and 10.06%. In the late season of 19Xiang, the increase of T3 and T5 was 6.02% and 1.81%. In the early season of Qingxiangyou19Xiang, the increase of T3 and T5 was 10.9% and 5.21%; in the late season of Qingxiangyou19Xiang, the increase of T3 and T5 was 9.85% and 13.85%.
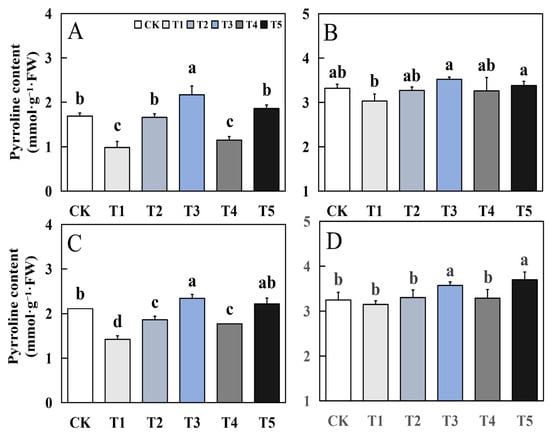
Figure 9.
Effects of biological nano-Se application on grain pyrroline content of aromatic rice (A) for 19Xiang in the early season; (B) for 19Xiang in the late season; (C) for Qingxiangyou19Xiang in the early season; (D) for Qingxiangyou19Xiang in the late season. No foliar application (CK), foliar application one time at 3–4 days before panicle differentiation (T1), foliar application one time at the heading stage (T2), foliar application one time at 10 days after the heading stage (T3), foliar application two times at 3–4 days before panicle differentiation and the heading stage, respectively (T4), foliar application two times at 3–4 days before panicle differentiation and 10 days after the heading stage (T5). FW, fresh weight. Bars sharing a common letter do not differ significantly at (p ≤ 0.05) in each cropping season according to the LSD test.
3.9. Effects of Bio-Nano-Se on Selenium Content in Aromatic Rice
Foliar application of biological nano-Se significantly increased the grain Se content of aromatic rice (Figure 10). The effect of biological nano-Se treatment on the selenium content of fragrant rice was generally consistent in different varieties. Compared to CK, each treatment of biological nano-Se increased to varying degrees, among which T3 and T5 treatments had the best selenium enrichment effect. In the early and late seasons of 19Xiang, the maximum increase was 3.87 times and 3.53 times. The maximum increase in the early and late seasons of Qingxiangyou19Xiang was 1.82 times and 2.5 times.
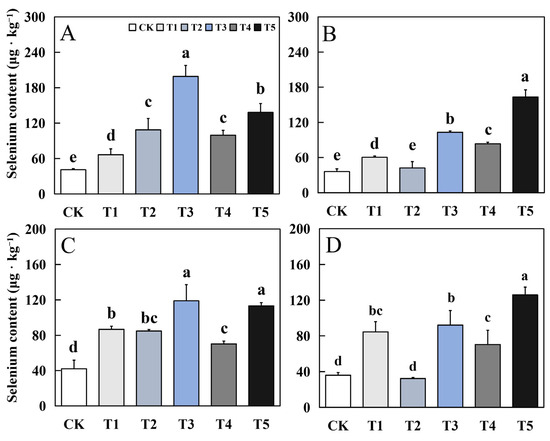
Figure 10.
Effects of biological nano-Se application on grain Se content of aromatic rice (A) for 19Xiang in the early season; (B) for 19Xiang in the late season; (C) for Qingxiangyou19Xiang in the early season; (D) for Qingxiangyou19Xiang in the late season. No foliar application (CK), foliar application one time at 3–4 days before panicle differentiation (T1), foliar application one time at the heading stage (T2), foliar application one time at 10 days after the heading stage (T3), foliar application two times at 3–4 days before panicle differentiation and the heading stage, respectively (T4), foliar application two times at 3–4 days before panicle differentiation and 10 days after the heading stage (T5). Bars sharing a common letter do not differ significantly at (p ≤ 0.05) in each cropping season according to the LSD test.
4. Discussion
The present study revealed the effects of biological nano-Se application on aromatic rice performance metrics including growth, yield formation, grain quality attributes, Se biofortification, and 2-AP biosynthesis. Firstly, our results showed that the application of bio-nano-selenium greatly increased the yield of aromatic rice, which is consistent with the findings of Kadhim et al. [22] and Badawy et al. [23]. The study by Kadhim et al. [22] showed that the additional application of bio-nano-selenium treatment increased yield by 7.83% over CK at the same level of NPK. The study by Badawy et al. [23] showed that the application of NPs-Se protected rice plants from salinity, which significantly improved the yield of rice and was more efficient in improving the yield components of NPs-Se as compared to the same treatment of NPs-Si. The increase in yield formation may be due to improved dry matter accumulation which resulted from enhanced photosynthesis [24]. Our results showed that the foliar spraying of biological nano-Se not only increased the net photosynthetic rate, but also increased the LAI, which led to an increase in dry matter accumulation. The study by Smith et al. [25] revealed that delaying leaf senescence and a longer green leaf duration could improve the photosynthetic capacity. This argument was verified by Du et al. [26] who reported that enhancing the photosynthetic capacity of grain-filling leaves under water immersion conditions could increase wheat yield. Since rice yield depends largely on the number of assimilates produced, spraying a biological nano-Se treatment can obtain longer green leaf duration and more green leaf exposure area to improve the photosynthetic capacity of rice, thereby increasing the aboveground dry matter weight of aromatic rice, and finally, maximizing yield [27]. In addition to photosynthesis, another explanation for the delay of leaf senescence is the improvement of antioxidant-related substances [28]. In addition to T3 treatment, under the other biological nano-Se foliar spraying treatments, the leaves of aromatic rice at the mature stage showed higher antioxidant enzyme activities (SOD, POD, CAT) and lower MDA contents, which alleviated the damage of reactive oxygen species (ROS) or other peroxide free radicals to the cell membrane system and delayed the decline of leaf physiological function [29]. This is highly consistent with the findings of Yu et al. [30], who demonstrated that SOD, POD, and CAT activities were significantly increased in redwood fruits after nano-Se treatment.
In the processing of rice, the removal of husks and bran layers from grains to produce storage-resistant milled rice is crucial [31]. This study found that foliar application of bio-nano-Se at 10 days after the heading stage significantly increased the brown rice rate and milled rice rate of aromatic rice, which was consistent with the study by Gao et al. [32]. Our findings indicated that rice plants treated with bio-nano-Se can harvest more milled rice, thereby increasing the income of aromatic rice production. The appearance quality of rice is also of great significance to rice production and application [33] because it significantly affects the rice price and consumers’ purchase intention. This study found that biological nano-Se treatment at the heading stage of aromatic rice significantly reduced the chalky grain rate and chalkiness of aromatic rice. Chalkiness is the opaque white part of rice, which is caused by the poor filling of amyloplasts and proteasomes in the endosperm and the formation of air between them [34]. Our result is highly similar to that of the study of Luo et al. [35]. The study by Barman et al. [36] showed that the chalkiness characteristics of grains were mainly affected by the increase in α-amylase activity during the grain-filling stage. Under Cadmium (Cd) stress, α-amylase activity was significantly up-regulated. We believed that the improvement in the appearance quality of aromatic rice may be due to the reduction in a-amylase activity in rice grains under the treatment of biological nano-Se foliar spraying at the heading, which in turn reduces the chalkiness characteristics of aromatic rice. Therefore, more studies should be carried out at the physiological and molecular levels to reveal the mechanism behind the regulation of chalkiness by nano-Se treatment. The quality characteristics of rice are mostly related to the accumulation of protein and starch in grains [37,38]. We believed that the improvement of the quality of aromatic rice by bio-nano-Se may be due to its promotion of photosynthesis, thereby increasing the dry matter weight of the aboveground part, and then coordinating the filling of starch and protein in the grain to improve the quality [39].
Previous studies have shown that 2-AP content is a characteristic component that distinguishes aromatic and non-aromatic rice. It is a highly heritable trait that is genetically controlled and also influenced by various external factors [40]. In addition to improving the growth and development of aromatic rice, the foliar application of biological nano-Se also changed the synthesis of aromatic rice aroma 2-AP to varying degrees. The study by Poonlaphdecha et al. [41] showed that 1-pyrroline is a limiting factor for the formation of 2AP in aromatic and non-aromatic rice varieties. In our study, the foliar application of bio-nano-Se at 10 days after the heading increased the content of pyrroline, the direct precursor of 2-AP aroma synthesis in plants, thereby changing the content of 2-AP. In addition, we observed that the contents of 2-AP and 1-pyrroline were highly similar in different treatments under the treatment of biological nano-Se, which was consistent with the results of the study by Luo et al. [42], who demonstrated that the application of Ethylenediaminetetraacetic acid-chelated selenium (EDTA-Se) enhanced the biosynthesis of 2-AP in fragrant rice by adjusting the content of direct precursor 1-pyrroline.
Biological nano-Se can produce a better spatial distribution of active ingredients on the surface of leaves [43]. The small size, low toxicity, and high compatibility of biological nano-Se make the application effect of biological nano-Se better than other forms of selenium fertilizer [44]. In addition, rice can quickly convert biological nano-Se into organic Se and store it in plant tissues, and its conversion efficiency is better than that of selenite and selenate treatments [45]. In this study, foliar application of biological nano-Se significantly increased the selenium content of aromatic rice, which was consistent with previous research results [46,47]. According to the national standard ‘Q/ZLMY 0009 S-2021 selenium-enriched rice’ [48], the selenium content of rice is between 150 and 400 mg·kg−1. Although the fragrant rice treated with biological nano-Se failed to meet the standard of selenium-enriched rice, which may be caused by the insufficient concentration of biological nano-Se in this experiment, compared to CK, the treatment of biological nano-Se increased exponentially. In the future, more studies should be conducted at the physiological and molecular level to reveal the regulation mechanism on the yield formation, grain quality, and 2-AP biosynthesis of aromatic rice after Nano-Se application.
5. Conclusions
This study showed the effects of different application modes of biological nano-Se on aromatic rice performance metrics in terms of growth, yield formation, grain quality, 2-AP biosynthesis, and Se biofortification. The treatment applied biological nano-Se twice at 3–4 days before panicle differentiation and 10 days after the heading stage exhibited the best improvement in the growth, grain yield, grain Se content, and grain 2-AP content. However, it is important to recognize the subtle relationship between yield and 2-AP content, which may be influenced by multiple factors. The simple application of biological nano-Se at 3–4 days before panicle differentiation resulted in a decrease in 2-AP content along with an increase in grain yield, and interestingly, the supplementary application of biological nano-Se at 10 days after the heading stage along with at 3–4 days before panicle differentiation ensured that there was no significant change in yield without a significant decrease in 2-AP content. Our findings highlight the complex balance between optimizing yield and reducing aroma loss in aromatic rice cultivation. In conclusion, this study underscores the significant positive impact of the foliar application of biological nano-Se on reducing aroma loss while increasing yield in aromatic rice varieties. This promising strategy provides a theoretical basis for the sustainable, efficient, high-yielding, and high-quality development of aromatic rice production in southern China.
Author Contributions
Conceptualization: X.T. and J.Q.; methodology: X.T., X.Y. and C.Z.; investigation: Q.G., L.L., Q.Z., W.Y. and Z.L.; data curation: Q.G., Q.Z., W.Y. and Z.L.; formal analysis: Q.G., L.L. and H.L.; validation: Q.G., Q.Z., W.Y. and Z.L.; writing—original draft preparation: Q.G., L.L. and H.L.; writing—review and editing: Q.G. and H.L.; funding acquisition: X.T., X.Y., C.Z. and J.Q.; All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (31971843), the Special Rural Revitalization Funds of Guangdong Province (2021KJ382), Guangzhou Science and Technology Project (202103000075), and the Guangdong Basic and Applied Basic Research Foundation (2023A1515010738 and 2024A1515012709).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Authors Xianghai Yu and Changjian Zuo were employed by the company Green Huinong Biotechnology (Shenzhen) Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zheng, H.; Zhang, J.; Zhuang, H.; Zeng, X.Q.; Tang, J.; Wang, H.L.; Chen, H.; Li, Y.; Ling, Y.H.; He, G.H.; et al. Gene mapping and candidate gene analysis of multi—floret spikelet 3 (mfs3) in rice (Oryza sativa L.). J. Integr. Agric. 2019, 18, 2673–2681. [Google Scholar] [CrossRef]
- Kaewmungkun, K.; Tongmark, K.; Chakhonkaen, S.; Sangarwut, N.; Wasinanon, T.; Panyawut, N.; Ditthab, K.; Sikaewtung, K.; Qi, Y.; Dapha, S.; et al. Development of new aromatic rice lines with high eating and cooking qualities. J. Integr. Agric. 2023, 22, 679–690. [Google Scholar] [CrossRef]
- Behera, P.K.; Panda, D. Germplasm Resources, Genes and Perspective for Aromatic Rice. Rice Sci. 2023, 30, 294–305. [Google Scholar] [CrossRef]
- Mo, Z.; Huang, J.; Xiao, D.; Ashraf, U.; Duan, M.; Pan, S.; Tian, H.; Xiao, L.; Zhong, K.; Tang, X. Supplementation of 2-Ap, Zn and La Improves 2-Acetyl-1-Pyrroline Concentrations in Detached Aromatic Rice Panicles in Vitro. PLoS ONE 2016, 11, e0149523. [Google Scholar] [CrossRef]
- Jablonska, E.; Vinceti, M. Selenium and Human Health: Witnessing a Copernican Revolution? J. Env. Sci. Health C Env. Carcinog. Ecotoxicol. Rev. 2015, 33, 328–368. [Google Scholar] [CrossRef]
- Feng, M.; Wang, X.; Xiong, H.; Qiu, T.; Zhang, H.; Guo, F.; Jiang, L.; Sun, Y. Anti-inflammatory effects of three selenium-enriched brown rice protein hydrolysates in LPS-induced RAW264. 7 macrophages via NF-κB/MAPKs signaling pathways. J. Funct. Foods 2021, 76, 104320. [Google Scholar] [CrossRef]
- Han, M.; Liu, K.; Rashid, M.T.; Zhu, G.; Wei, Y. Status and distribution of selenium in selenium-enriched peanut sprouts. Grain Oil Sci. Technol. 2024. in print. [Google Scholar] [CrossRef]
- Dalia, A.M.; Loh, T.C.; Sazili, A.Q.; Samsudin, A.A. Influence of bacterial organic selenium on blood parameters, immune response, selenium retention and intestinal morphology of broiler chickens. BMC Vet. Res. 2020, 16, 365. [Google Scholar] [CrossRef]
- Srivastava, P.; Kowshik, M. Anti-neoplastic selenium nanoparticles from Idiomarina sp. PR58-8. Enzyme. Microb. Technol. 2016, 95, 192–200. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, K.S. Role of nano-selenium in health and environment. J. Biotechnol. 2021, 325, 152–163. [Google Scholar] [CrossRef]
- Zambonino, M.C.; Quizhpe, E.M.; Mouheb, L.; Rahman, A.; Agathos, S.N.; Dahoumane, S.A. Biogenic Selenium Nanoparticles in Biomedical Sciences: Properties, Current Trends, Novel Opportunities and Emerging Challenges in Theranostic Nanomedicine. Nanomaterials 2023, 13, 424. [Google Scholar] [CrossRef]
- Malyugina, S.; Skalickova, S.; Skladanka, J.; Slama, P.; Horky, P. Biogenic Selenium Nanoparticles in Animal Nutrition: A Review. Agriculture 2021, 11, 1244. [Google Scholar] [CrossRef]
- Sonali, J.M.I.; Gayathri, K.V.; Rangasamy, G.; Kumar, P.S.; Rajagopal, R. Efficacy of Biogenic Selenium Nanoparticles from Pseudomonas Libanesis Towards Growth Enhancement of Okra. Waste Biomass Valorization 2024, 15, 1793–1806. [Google Scholar] [CrossRef]
- Li, L.; Xiong, Y.; Wang, Y.; Wu, S.; Xiao, C.; Wang, S.; Cheng, S.; Cheng, H. Effect of Nano-Selenium on Nutritional Quality of Cowpea and Response of ABCC Transporter Family. Molecules 2023, 28, 1398. [Google Scholar] [CrossRef]
- Mellinas, C.; Jimenez, A.; Garrigos, M.D.C. Microwave-Assisted Green Synthesis and Antioxidant Activity of Selenium Nanoparticles Using Theobroma cacao L. Bean Shell Extract. Molecules 2019, 24, 4048. [Google Scholar] [CrossRef]
- Wang, X.; Pan, X.; Gadd, G.M. Soil dissolved organic matter affects mercury immobilization by biogenic selenium nanoparticles. Sci. Total Environ. 2019, 658, 8–15. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, Y.; Zhu, N.; Jin, H. Foliar application of biosynthetic nano-selenium alleviates the toxicity of Cd, Pb, and Hg in Brassica chinensis by inhibiting heavy metal adsorption and improving antioxidant system in plant. Ecotoxicol. Environ. Saf. 2022, 240, 113681. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, L.; Cheng, S.; Ren, Y.; Deng, H.; Wang, X.; Li, Y.; Tang, X.; Wang, Z.; Mo, Z. Regulation of 2-acetyl-1-pyrroline and grain quality in early-season indica fragrant rice by nitrogen and silicon fertilization under different plantation methods. J. Integr. Agric. 2024, 23, 511–535. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, Q.; Lai, R.; Zhang, S.; Yi, W.; Tang, X. Regulation of 2-Acetyl-1-pyrroline Content in Fragrant Rice under Different Temperatures at the Grain-Filling Stage. J. Agric. Food. Chem. 2024, 72, 10521–10530. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, Y.; Liang, F.; Li, Q.; Zhao, Q.; He, Y.; Lin, X.; Qin, X.; Cheng, S. Effect of foliar copper application on grain yield, 2-acetyl-1-Pyrroline and copper content in fragrant rice. Plant Physiol. Biochem. 2022, 182, 154–166. [Google Scholar] [CrossRef]
- GB 5009.268-2016; National Standard for Food Safety Determination of Multi-Elements in Food. National Health and Family Planning Commission; State Food and Drug Administration: Beijing, China, 2016. (In Chinese)
- Kadhim Al-Karaawi, M.D.; Attia Al-Juthery, H.W. Effect of NPK, NPK Organic Fertilizers and Spraying Nano-Vanadium and Nano-Selenium on the Growth and Yield of Rice. IOP Conf. Ser. Earth Environ. Sci. 2022, 1060, 12035. [Google Scholar] [CrossRef]
- Badawy, S.A.; Zayed, B.A.; Bassiouni, S.M.A.; Mahdi, A.H.A.; Majrashi, A.; Ali, E.F.; Seleiman, M.F. Influence of Nano Silicon and Nano Selenium on Root Characters, Growth, Ion Selectivity, Yield, and Yield Components of Rice (Oryza sativa L.) Under Salinity Conditions. Plants 2021, 10, 1657. [Google Scholar] [CrossRef]
- Xiong, D. Perspectives of improving rice photosynthesis for higher grain yield. Crop Environ. 2024, 3, 123–137. [Google Scholar] [CrossRef]
- Smith, M.R.; Rao, I.M.; Merchant, A. Source-Sink Relationships in Crop Plants and their Influence on Yield Development and Nutritional Quality. Front. Plant Sci. 2018, 9, 1889. [Google Scholar] [CrossRef]
- Du, X.; Xi, M.; Wei, Z.; Chen, X.; Wu, W.; Kong, L. Raised bed planting promotes grain number per spike in wheat grown after rice by improving spike differentiation and enhancing photosynthetic capacity. J. Integr. Agric. 2023, 22, 1631–1644. [Google Scholar] [CrossRef]
- Murchie, E.H.; Burgess, A.J. Casting light on the architecture of crop yield. Crop Environ. 2022, 1, 74–85. [Google Scholar] [CrossRef]
- Rizwan, M.; Nawaz, A.; Irshad, S.; Manoharadas, S. Exogenously applied melatonin enhanced chromium tolerance in pepper by up-regulating the photosynthetic apparatus and antioxidant machinery. Sci. Hortic. 2024, 323, 112468. [Google Scholar] [CrossRef]
- Cheng, S.; Fang, Z.; Cheng, X.; Wu, Y.; Mo, L.; Yan, C.; Zhou, L.; Ren, Y. Modulation of Antioxidant Attributes and Grain Yield in Fragrant Rice by Exogenous Cu Application. J. Plant Growth Regul. 2023, 42, 1937–1952. [Google Scholar] [CrossRef]
- Yu, H.; Miao, P.; Li, D.; Wu, Y.; Zhou, C.; Pan, C. Improving red pitaya fruit quality by nano-selenium biofortification to enhance phenylpropanoid and betalain biosynthesis. Ecotoxicol. Environ. Saf. 2023, 267, 115653. [Google Scholar] [CrossRef]
- Siaw, M.O.; Mcclung, A.M.; Mauromoustakos, A.; Wang, Y. Influence of bran layer on rice milling quality. Cereal Chem. 2023, 100, 1234–1249. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, M.; Xu, J.; Xu, F.; Zhang, W. The application of organic selenium (SeMet) improve the photosynthetic characteristics, yield and quality of hybrid rice. Plant Physiol. Biochem. 2024, 208, 108457. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, H.; Chen, Y.; Zou, D.; Luo, Y.; Tang, Q. Progress and challenges of rice ratooning technology in Hunan Province, China. Crop Environ. 2023, 2, 101–110. [Google Scholar] [CrossRef]
- Mitsui, T.; Yamakawa, H.; Kobata, T. Molecular physiological aspects of chalking mechanism in rice grains under high-temperature stress. Plant Prod. Sci. 2016, 19, 22–29. [Google Scholar] [CrossRef]
- Luo, H.; He, L.; Du, B.; Pan, S.; Mo, Z.; Duan, M.; Tian, H.; Tang, X. Biofortification with chelating selenium in fragrant rice: Effects on photosynthetic rates, aroma, grain quality and yield formation. Field Crops Res. 2020, 255, 107909. [Google Scholar] [CrossRef]
- Barman, F.; Kundu, R. Foliar application of selenium affecting pollen viability, grain chalkiness, and transporter genes in cadmium accumulating rice cultivar: A pot study. Chemosphere 2023, 313, 137538. [Google Scholar] [CrossRef]
- Du, B.; Wu, Q.; Jiang, S.; Zhang, D.; Qiao, Y.; Xie, Y.; Xu, J.; Zhu, J. Effects of exogenous α-ketoglutaric acid on 2-acetyl-1-pyrroline, yield formation and grain quality characters of aromatic rice. Phyton-Int. J. Exp. Bot. 2021, 90, 437–447. [Google Scholar] [CrossRef]
- Reis, H.; de Queiroz, B.J.; Silva, V.M.; Santos, E.F.; Tavanti, R.; Putti, F.F.; Young, S.D.; Broadley, M.R.; White, P.J.; Dos, R.A. Agronomic biofortification with selenium impacts storage proteins in grains of upland rice. J. Sci. Food. Agric. 2020, 100, 1990–1997. [Google Scholar] [CrossRef]
- Paul, N.C.; Tasmim, M.T.; Imran, S.; Mahamud, M.A.; Chakrobortty, J.; Rabbi, R.H.M.; Sarkar, S.K.; Paul, S.K. Nutrient Management in Fragrant Rice: A Review. Agric. Sci. 2021, 12, 1538–1554. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, Y.; Yang, D.; Liu, H.; Zhang, Y.; Wang, X.; Bai, F.; Cheng, S. Foliar methyl jasmonate (MeJA) application increased 2-acetyl-1-Pyrroline (2-AP) content and modulated antioxidant attributes and yield formation in fragrant rice. J. Plant Physiol. 2023, 282, 153946. [Google Scholar] [CrossRef]
- Poonlaphdecha, J.; Gantet, P.; Maraval, I.; Sauvage, F.; Menut, C.; Morere, A.; Boulanger, R.; Wuest, M.; Gunata, Z. Biosynthesis of 2-acetyl-1-pyrroline in rice calli cultures: Demonstration of 1-pyrroline as a limiting substrate. Food Chem. 2016, 197, 965–971. [Google Scholar] [CrossRef]
- Luo, H.W.; He, L.X.; Du, B.; Wang, Z.M.; Zheng, A.X.; Lai, R.F.; Tang, X.R. Foliar application of selenium (Se) at heading stage induces regulation of photosynthesis, yield formation, and quality characteristics in fragrant rice. Photosynthetica 2019, 57, 1007–1014. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, C.; Zhao, G.; Stoll, S.; Ren, F.; Leng, X. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J. Nanobiotechnology 2017, 15, 4. [Google Scholar] [CrossRef]
- Huang, S.; Qin, H.; Jiang, D.; Lu, J.; Zhu, Z.; Huang, X. Bio-nano selenium fertilizer improves the yield, quality, and organic selenium content in rice. J. Food Compos. Anal. 2024, 132, 106348. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Li, K.; Wan, Y.; Wang, Q.; Zhuang, Z.; Guo, Y.; Li, H. Uptake, translocation and biotransformation of selenium nanoparticles in rice seedlings (Oryza sativa L.). J. Nanobiotechnol. 2020, 18, 103. [Google Scholar] [CrossRef]
- Huang, W.; Liu, Z.; Zhang, Y.; Zhu, Y.; Wang, D. Effects of Soil Nano-Conditioning Agent on Selenium Content, Yield and Quality of Pepper. Integr. Ferroelectr. 2021, 216, 94–107. [Google Scholar] [CrossRef]
- Huang, S.; Yu, K.; Xiao, Q.; Song, B.; Yuan, W.; Long, X.; Cai, D.; Xiong, X.; Zheng, W. Effect of bio-nano-selenium on yield, nutritional quality and selenium content of radish. J. Food Compos. Anal. 2023, 115, 104927. [Google Scholar] [CrossRef]
- Q/ZLMY 0009 S-2021; Selenium-Enriched Rice. COFCO Rice (Ningxia) Co., Ltd.: Pingluo, China, 2021. (In Chinese)
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).