Potential for Grain Sorghum as a Trap and Nursery Crop for Helicoverpa zea and Its Natural Enemies and Dissemination of HearNPV into Cotton
Abstract
:1. Introduction
2. Materials and Methods
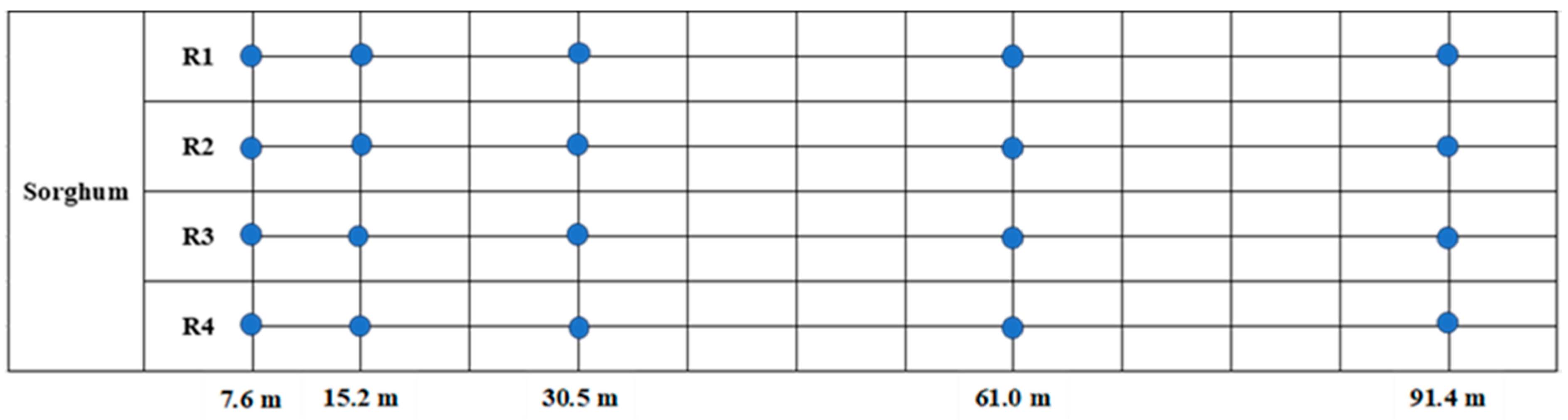
2.1. Locations, Experimental Design, and Treatments
2.2. Proof-of-Concept Experiment
2.3. Validation Experiment
2.4. HearNPV Infection Analysis
2.5. Statistical Analyses
3. Results
3.1. Proof-of-Concept Experiment
3.2. Validation Experiment
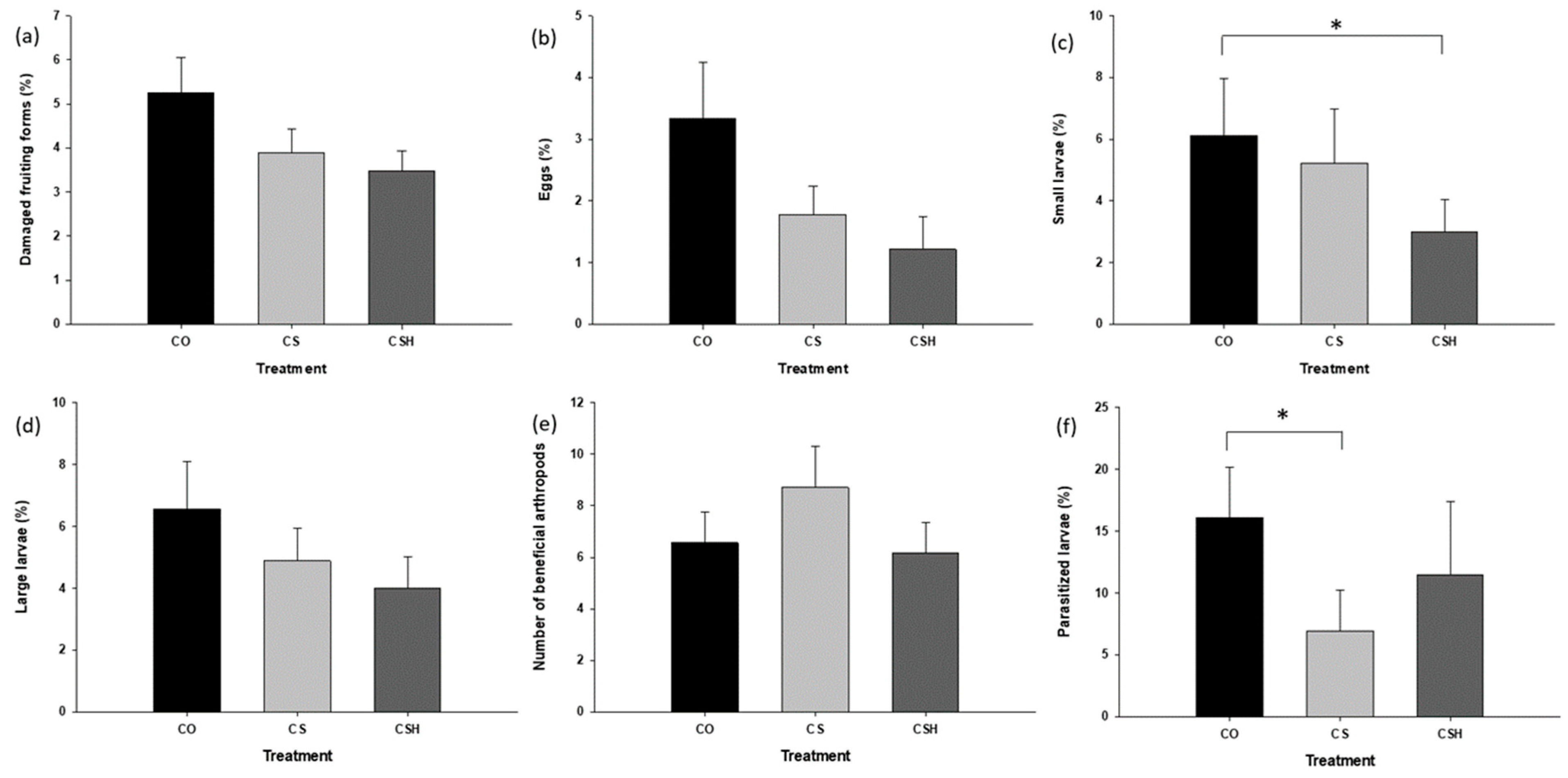
3.2.1. Comparing Damaged Fruit Incidence, H. zea Larvae, and Beneficial Arthropod Density between Cotton-Only, Non-Treated Cotton–Sorghum, and HearNPV-Treated Cotton–Sorghum
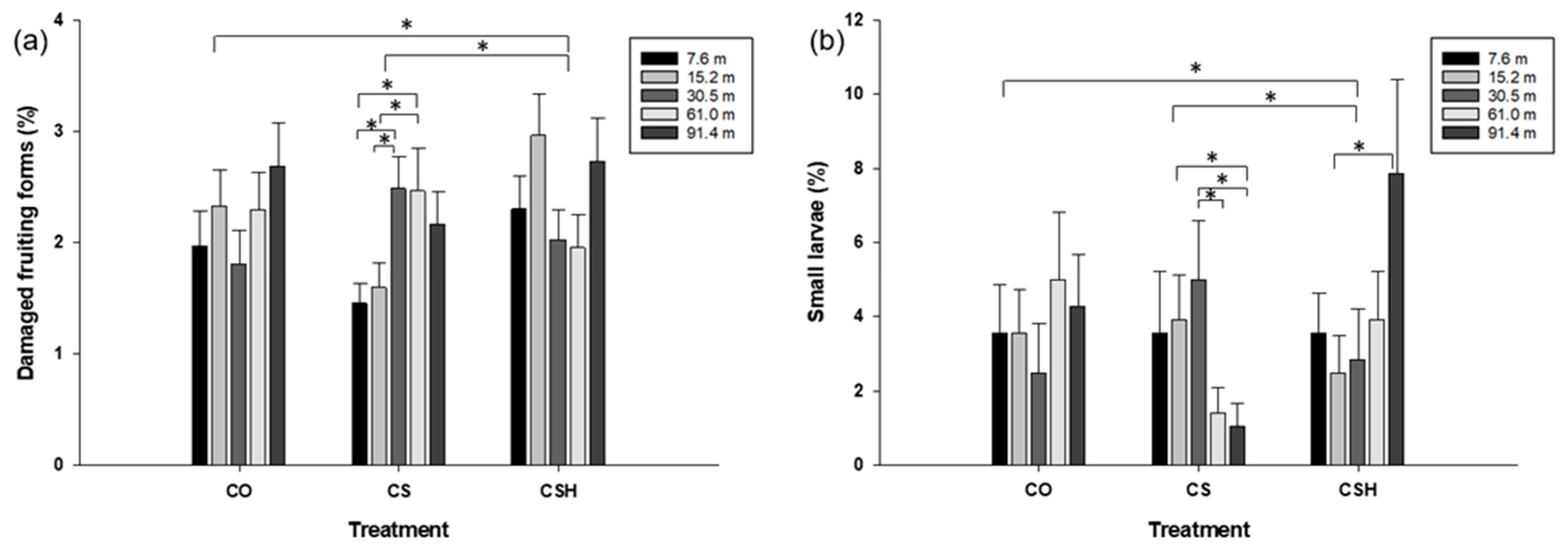
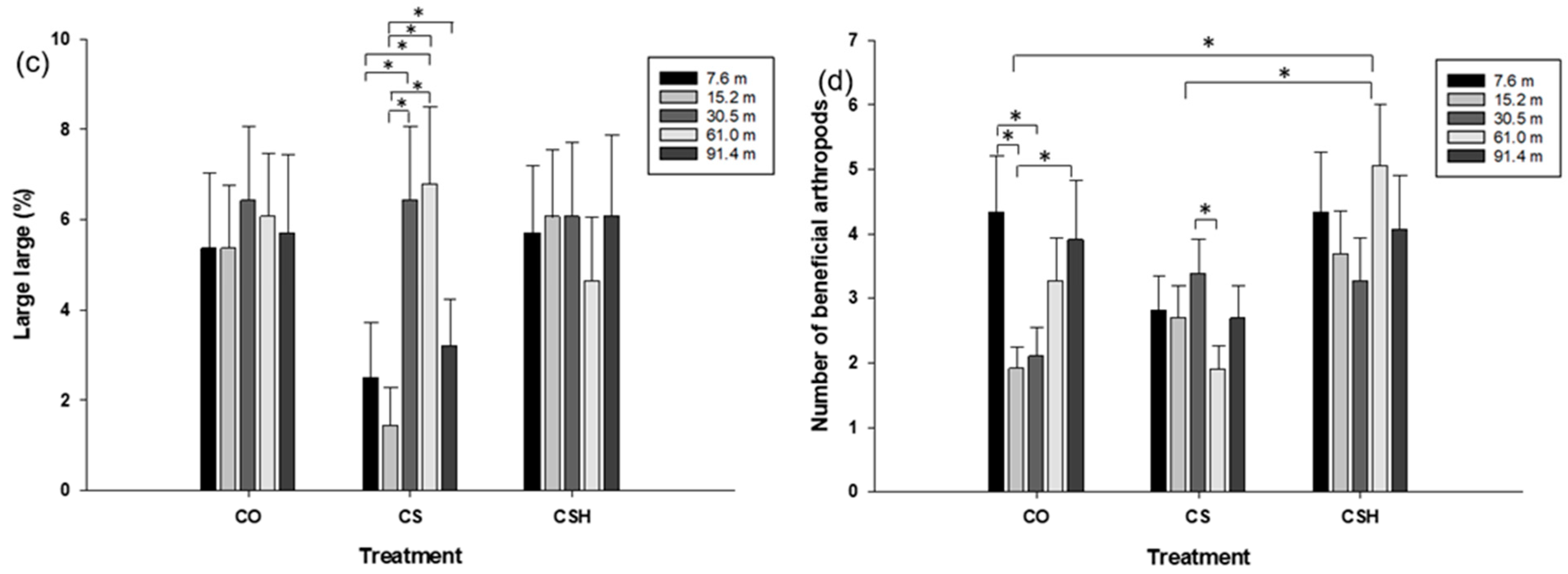
3.2.2. Comparing Damaged Fruit Incidence, H. zea Larvae, and Beneficial Arthropod Density between Transect Locations within Cotton-Only, Non-Treated Cotton–Sorghum, and HearNPV-Treated Cotton–Sorghum
3.3. Beneficial Arthropods Observed
3.4. HearNPV Infection Analysis
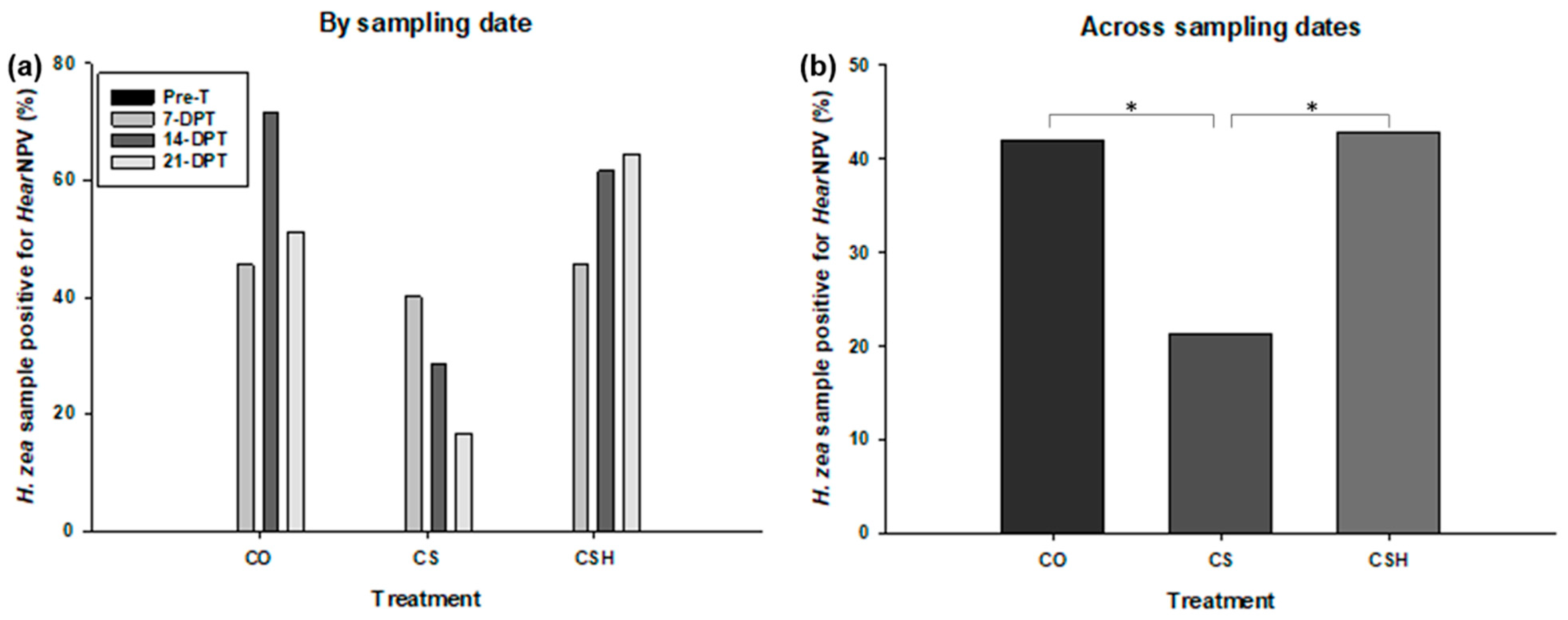
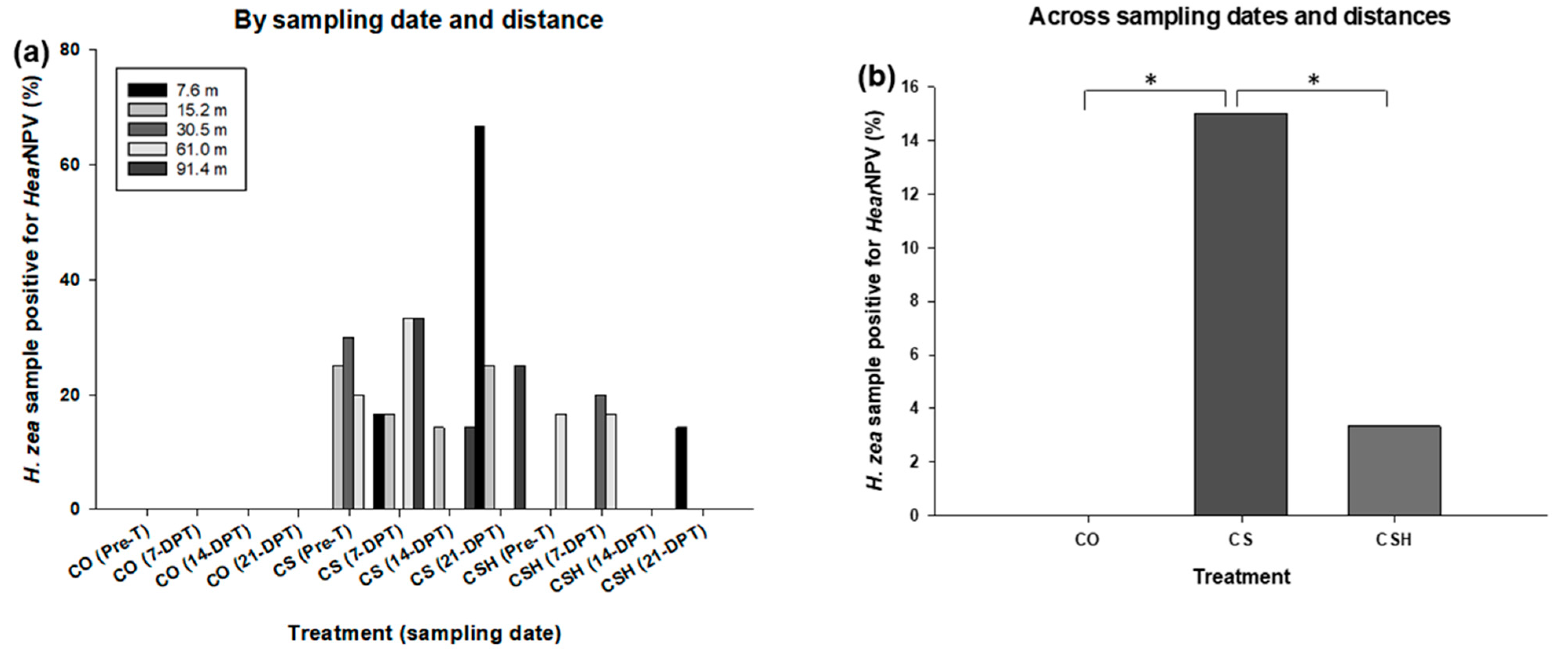
3.4.1. Helicoverpa zea Samples
3.4.2. Beneficial Arthropod Samples
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carpenter, J.E. Peer-reviewed surveys indicate positive impact of commercialized GM crops. Nat. Biotechnol. 2010, 28, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Li, Y.H.; Wu, K.M. Risk assessment and ecological effects of transgenic Bacillus thuringiensis crops on non-target organisms. J. Invert. Plant Biol. 2011, 53, 520–538. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Carrière, Y. Successes and failures of transgenic Bt crops: Global patterns of field-evolved resistance. In Bt Resistance: Characterization and Strategies for GM Crops Producing Bacillus thuringiensis Toxins; Soberón, M., Gao, Y., Bravo, A., Eds.; CABI Press: Wallingford, UK, 2015; pp. 1–14. [Google Scholar]
- Dively, G.P.; Venugopal, P.D.; Finkenbinder, C. Field-evolved resistance in corn earworm to Cry proteins expressed by transgenic sweet corn. PLoS ONE 2016, 11, e0169115. [Google Scholar] [CrossRef] [PubMed]
- Kerns, D.L.; Yang, F.; Lorenz, G.M.; Gore, J.; Catchot, A.L.; Stewart, S.D.; Brown, S.A.; Cook, D.R.; Seiter, N. Value of Bt technology for bollworm management. In Proceedings of the Beltwide Cotton Conference, Memphis, TN, USA, 3–5 January 2018. [Google Scholar]
- Reisig, D.D.; Huseth, A.S.; Bachelor, J.S.; Aghaee, M.; Braswell, L.; Burrack, H.J.; Flanders, K.; Greene, J.K.; Herbert, D.A.; Jacobson, A.; et al. Long-term empirical and observational evidence of practical Helicoverpa zea resistance to cotton with pyramided Bt toxins. J. Econ. Entomol. 2018, 111, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Reisig, D.; Kerns, D.; Gore, J.; Musser, F. Managing pyrethroid- and Bt-resistant bollworm in southern U.S. cotton. Crops Soils 2019, 52, 30–35. [Google Scholar] [CrossRef]
- Little, N.S.; Elkins, B.H.; Mullen, R.M.; Perera, O.P.; Parys, K.A.; Allen, K.C.; Boykin, D.L. Differences between two populations of bollworm, Helicoverpa zea (Lepidoptera: Noctuidae), with variable measurements of laboratory susceptibilities to Bt toxins exposed to non-Bt and Bt cottons in large field cages. PLoS ONE 2019, 14, e0212567. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; González, J.C.S.; Williams, J.; Cook, D.C.; Gilreath, R.T.; Kerns, D.L. Occurrence and ear damage of Helicoverpa zea on transgenic Bacillus thuringiensis maize in the field in Texas, US and its susceptibility to Vip3A protein. Toxins 2019, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Guoa, J.; Brown, S.; Head, G.P.; Priced, P.A.; Paula-Moraese, S.; Nif, X.; Dimase, M.; Huanga, F. Field-evolved resistance of Helicoverpa zea (Boddie) to transgenic maize expressing pyramided Cry1A.105/Cry2Ab2 proteins in northeast Louisiana, the United States. J. Invertebr. Pathol. 2019, 163, 11–20. [Google Scholar] [CrossRef]
- Musser, F.R.; Greene, J.K.; Kerns, D.; Stewart, S.D.; Parajulee, M.N.; Lorenz, G.M.; Jones, M.; Herbert, D.A.; Taylor, S.; Phillips, P.M.; et al. Monitoring bollworms for pyrethroid resistance. In Proceedings of the Beltwide Cotton Conference, Memphis, TN, USA, 20 January 2017. [Google Scholar]
- Vyavhare, S.S.; Kerns, D.L. Managing Cotton Insects in Texas; Texas A&M AgriLife Extension Publication ENTO-PU-158: College Station, TX, USA, 2022; Available online: https://lubbock.tamu.edu/files/2022/07/managing-cotton-insects-in-texas.pdf (accessed on 1 December 2022).
- Roditakis, E.; Vasakis, E.; Grispou, M.; Stavrakaki, M.; Nauen, R.; Gravouil, M.; Bassi, A. First report of Tuta absoluta resistance to diamide insecticides. J. Pest Sci. 2015, 88, 9–16. [Google Scholar] [CrossRef]
- Adams, A.; Gore, J.; Catchot, A.; Musser, F.; Cook, D.; Krishnan, N.; Irby, T. Susceptibility of Helicoverpa zea (Lepidoptera: Noctuidae) neonates to diamide insecticides in the Midsouthern and Southeastern United States. J. Econ. Entomol. 2016, 109, 2205–2209. [Google Scholar] [CrossRef]
- Calvin, W.; Yang, F.; Brown, S.A.; Catchot, A.L.; Crow, W.D.; Cook, D.R.; Gore, J.; Kurtz, R.; Lorenz, G.M.; Seiter, N.J.; et al. Development of economic thresholds toward bollworm (Lepidoptera: Noctuidae), management in Bt cotton, and assessment of the benefits from treating Bt cotton with insecticide. J. Econ. Entomol. 2021, 114, 2493–2504. [Google Scholar] [CrossRef] [PubMed]
- Musser, F.R.; Catchot, B.D. Insecticide resistance monitoring for bollworm and soybean looper. In Proceedings of the Beltwide Cotton Conference, Memphis, TN, USA, 4–6 January 2022. [Google Scholar]
- Allen, K.C.; Little, N.S.; Perera, O.P. Susceptibilities of Helicoverpa zea (Lepidoptera: Noctuidae) populations from the Mississippi Delta to a Diamide insecticide. J. Econ. Entomol. 2023, 116, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Stomph, T.; Dordas, C.; Baranger, A.; Rijk, J.; Dong, B.; Evers, J.; Gu, C.; Li, L.; Simon, J.; Jensen, E.S.; et al. Designing intercrops for high yield, yield stability and efficient use of resources: Are there principles? Adv. Agron. 2020, 160, 1–50. [Google Scholar] [CrossRef]
- Smith, H.A.; McSorley, R. Intercropping and pest management: A review of major concepts. Am. Entomol. 2000, 46, 154–161. [Google Scholar] [CrossRef]
- VanTine, M.; Verlinden, S. Managing Insects and Disease Damage under an Organic System. Extension Service; West Virginia University: Morgantown, WV, USA, 2003; Available online: https://organic.wvu.edu/files/d/f47962a9-d1e3-4bc8-a4d3-b7728548c30a/insects.pdf (accessed on 3 April 2023).
- Jones, G.A.; Gillett, J.L. Intercropping with sunflowers to attract beneficial insects in organic agriculture. Fla. Entomol. 2005, 88, 91–96. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C. Can we make crops more attractive to the natural enemies of herbivores? Entomol. Ornithol. Herpetol. 2012, 1, e102. [Google Scholar] [CrossRef]
- King, E.G.; Coleman, R.J. Potential for biological control of Heliothis species. Ann. Rev. Entomol. 1989, 34, 53–75. [Google Scholar] [CrossRef]
- Tillman, P.G.; Mullinix, B.G. Grain sorghum as a trap crop for corn earworm (Lepidoptera: Noctuidae) in cotton. Environ. Entomol. 2004, 33, 1371–1380. [Google Scholar] [CrossRef]
- Knutson, A.; Ruberson, J. Field Guide to Predators, Parasites and Pathogens Attacking Insect and Mite Pests of Cotton; Texas A&M University Extension Publication E-357: College Station, TX, USA, 2005; Available online: https://www.researchgate.net/publication/26904773_Field_Guide_to_Predators_Parasites_and_Pathogens_Attacking_Insect_and_Mite_Pests_of_Cotton_Recognizing_the_Good_Bugs_in_Cotton (accessed on 22 February 2021).
- Safarzoda, S.; Bahlai, C.A.; Fox, A.F.; Landis, D.A. The role of natural enemy foraging guilds in controlling cereal aphids in Michigan wheat. PLoS ONE 2014, 9, e114230. [Google Scholar] [CrossRef]
- Ratnadass, A.; Togola, M.; Cissé, B.; Vassal, J.M. Potential of sorghum and physic nut (Jatropha curcas) for management of plant bugs (Hemiptera: Miridae) and cotton bollworm (Helicoverpa armigera) on cotton in an assisted trap-cropping strategy. J. SAT Agric. Res. 2009, 7, 1–7. [Google Scholar]
- Piñero, J.C.; Manandhar, R. Effects of increased crop diversity using trap crops, flowering plants, and living mulches on vegetable insect pests. Trends Entomol. 2015, 11, 91–109. [Google Scholar]
- Sarkar, S.C.; Wang, E.; Wu, S.; Lei, Z. Application of trap cropping as companion plants for the management of agricultural pests: A review. Insects 2018, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Duraimurugan, P.; Regupathy, A. Push-pull strategy with trap crops, neem and nuclear polyhedrosis virus for insecticide resistance management in Helicoverpa armigera (Hubner) in cotton. Amer. J. Appl. Sci. 2005, 2, 1042–1048. [Google Scholar]
- Roome, R.E. Field trials with a nuclear polyhedrosis virus and Bacillus thuringiensis against larvae of Heliothis armigera (Hb.) (Lepidoptera, Noctuidae) on sorghum and cotton in Botswana. Bull. Entomol. Res. 1975, 65, 507–514. [Google Scholar] [CrossRef]
- Roome, R.E.; Daoust, R.A. Survival of the nuclear polyhedrosis virus of Heliothis armigera on crops and in soil in Botswana. J. Invert. Path. 1975, 27, 7–12. [Google Scholar] [CrossRef]
- Gettig, R.R.; McCarthy, W.J. Genotypic variation among wild isolates of Heliothis spp nuclear polyhedrosis viruses from different geographical regions. Virology 1982, 117, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Black, J.L.; Lorenz, G.M.; Cato, A.J.; Bateman, N.R.; Seiter, N.J. Efficacy of Helicoverpa armigera Nucleopolyhedrovirus on Soybean for Control of Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) in Arkansas Agriculture. Insects 2022, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Black, J.L.; Lorenz, G.M.; Cato, A.J.; Faske, T.R.; Popham, H.J.R.; Paddock, K.J.; Bateman, N.R.; Seiter, N.J. Field Studies on the horizontal transmission potential by voluntary and involuntary carriers of Helicoverpa armigera nucleopolyhedrovirus (Baculoviridae). J. Econ. Entomol. 2019, 112, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Yearian, W.C.; Young, S.Y. Persistence of Heliothis nuclear polyhedrosis virus on cotton plant parts. Environ. Entomol. 1974, 3, 1035–1036. [Google Scholar] [CrossRef]
- Young, S.Y.; Yearian, W.C.; Kim, K.S. Effect of dew from cotton and soybean foliage on activity of Heliothis nuclear polyhedrosis virus. J. Invert. Path. 1977, 29, 105–111. [Google Scholar] [CrossRef]
- McLeod, P.J.; Yearian, W.C.; Young III, S.Y. Inactivation of Baculovirus heliothis by ultraviolet irradiation, dew, and temperature. J. Invertebr. Pathol. 1977, 30, 237–241. [Google Scholar] [CrossRef]
- Merchant, M.E.; Teetes, G.L. Evaluation of selected sampling methods for panicle infesting insect pests of sorghum. J. Econ. Entomol. 1992, 85, 2418–2424. [Google Scholar] [CrossRef]
- Knutson, A.E.; Muegge, M.A.; Wilson, L.T.; Naranjo, S.E. Evaluation of sampling methods and development of sample plans for estimating predator densities in cotton. J. Econom. Entomol. 2008, 101, 1501–1509. [Google Scholar] [CrossRef]
- SAS Institute. User’s Manual, Version 13.1; SAS Institute: Cary, NC, USA, 2013.
- Calvin, W. Investigating Action Thresholds and Alternative Management Approaches to Control Cotton Bollworm [Helicoverpa zea (Boddie)] Infestations; Texas A&M University ProQuest Dissertations & Theses: College Station, TX, USA, 2023; p. 30873167. [Google Scholar]
| Sorghum Hybrid | Minimum Days to 50% Bloom | Maximum Days to 50% Bloom |
|---|---|---|
| SP 78M30 | 72 | 76 |
| SP 74M21 | 69 | 74 |
| SP 68M57 | 66 | 71 |
| SP 31A15 | 54 | 58 |
| SP 43M80 | 58 | 62 |
| 251 | 50 | 54 |
| Order | Family | Common Name | Benefit |
|---|---|---|---|
| Araneae | Thomisidae | Crab spider | Predator |
| Salticidae | Jumping spider | Predator | |
| Araneidae | Orb-weaver spider | Predator | |
| Oxyopidae | Lynx spider | Predator | |
| Coleoptera | Coccinellidae | Lady beetle | Predator |
| Diptera | Syrphidae | Hoverfly | Predator |
| Tachinidae | Tachinid fly | Parasitoid | |
| Hemiptera | Pentatomidae | Spined soldier bug | Predator |
| Reduviidae | Assassin bug | Predator | |
| Geocoridae | Big-eyed bug | Predator | |
| Anthocoridae | Minute pirate bug | Predator | |
| Miridae | Cotton fleahopper | Predator | |
| Nabidae | Damsel bug species | Predator | |
| Hymenoptera | Formicidae | Fire ant | Predator |
| Braconidae | Braconid wasp | Parasitoid | |
| Neuroptera | Chrysopidae | Green lacewing | Predator |
| Hemerobiidae | Brown lacewing | Predator |
| Year | Arthropod Groups | n a | No. Positive Sample | % Positive Sample | n a | No. Positive Sample | % Positive Sample | n a | No. Positive Sample | % Positive Sample |
|---|---|---|---|---|---|---|---|---|---|---|
| Cotton-Only | Non-Treated Cotton–Sorghum | Treated Cotton–Sorghum | ||||||||
| 2020 | Chrysopidae | 5 | 0 | 0 | 15 | 0 | 0 | 31 | 4 | 12.9 |
| Coccinellidae | 10 | 0 | 0 | 28 | 0 | 0 | 52 | 1 | 1.9 | |
| Pentatomidae | 0 | 0 | 0 | 1 | 0 | 0 | 4 | 1 | 25 | |
| Reduviidae | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 50 | |
| Combined | 15 | 0 | 0 | 45 | 0 | 0 | 89 | 7 | 7.9 | |
| 2021 | Coccinellidae | 24 | 0 | 0 | 50 | 3 | 6 | 43 | 2 | 4.7 |
| Pentatomidae | 0 | 0 | 0 | 3 | 2 | 66.7 | 2 | 0 | 0 | |
| Reduviidae | 1 | 0 | 0 | 4 | 1 | 25 | 1 | 1 | 100 | |
| Formicidae | 19 | 0 | 0 | 57 | 2 | 3.5 | 72 | 3 | 4.2 | |
| Anthocoridae | 4 | 0 | 0 | 31 | 0 | 0 | 40 | 1 | 2.5 | |
| Spiders * | 11 | 0 | 0 | 58 | 6 | 10.3 | 60 | 1 | 1.7 | |
| Combined | 59 | 0 | 0 | 203 | 14 | 6.9 | 218 | 8 | 3.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvin, W.; Gore, J.; Greene, J.; Perkin, L.; Kerns, D.L. Potential for Grain Sorghum as a Trap and Nursery Crop for Helicoverpa zea and Its Natural Enemies and Dissemination of HearNPV into Cotton. Agronomy 2024, 14, 1779. https://doi.org/10.3390/agronomy14081779
Calvin W, Gore J, Greene J, Perkin L, Kerns DL. Potential for Grain Sorghum as a Trap and Nursery Crop for Helicoverpa zea and Its Natural Enemies and Dissemination of HearNPV into Cotton. Agronomy. 2024; 14(8):1779. https://doi.org/10.3390/agronomy14081779
Chicago/Turabian StyleCalvin, Wilfrid, Jeffrey Gore, Jeremy Greene, Lindsey Perkin, and David L. Kerns. 2024. "Potential for Grain Sorghum as a Trap and Nursery Crop for Helicoverpa zea and Its Natural Enemies and Dissemination of HearNPV into Cotton" Agronomy 14, no. 8: 1779. https://doi.org/10.3390/agronomy14081779






