Effects of Scallop Shells and Starfish (Asterias amurensis) on Stabilization of Metalloid (As) and Heavy Metal (Pb and Zn)-Contaminated Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Contaminated Soil Collection and Characteristics
2.2. Stabilizer Processing
2.3. Stabilization Treatment and Elution
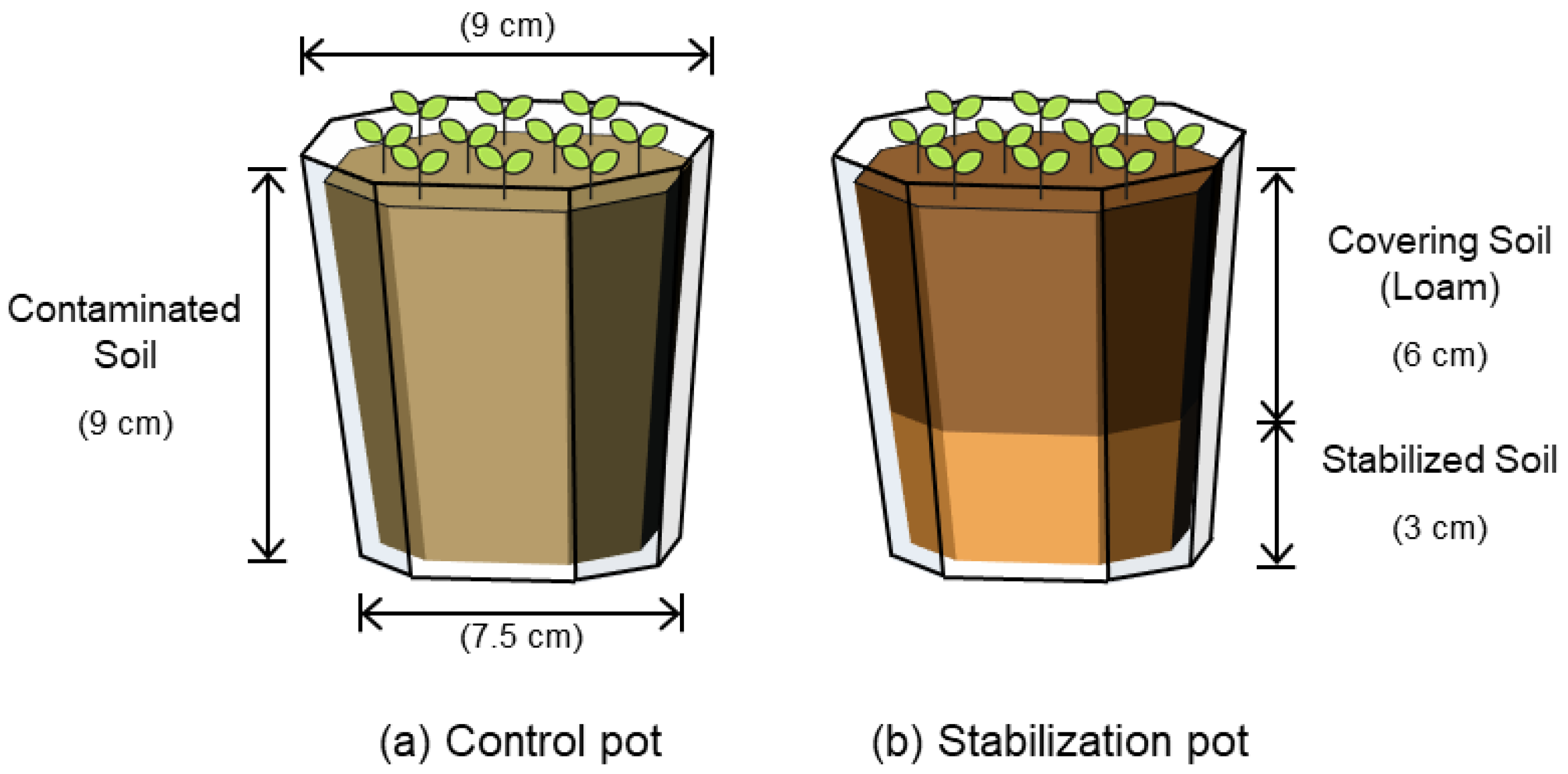
2.4. Evaluation of Heavy Metal Transition to Crops
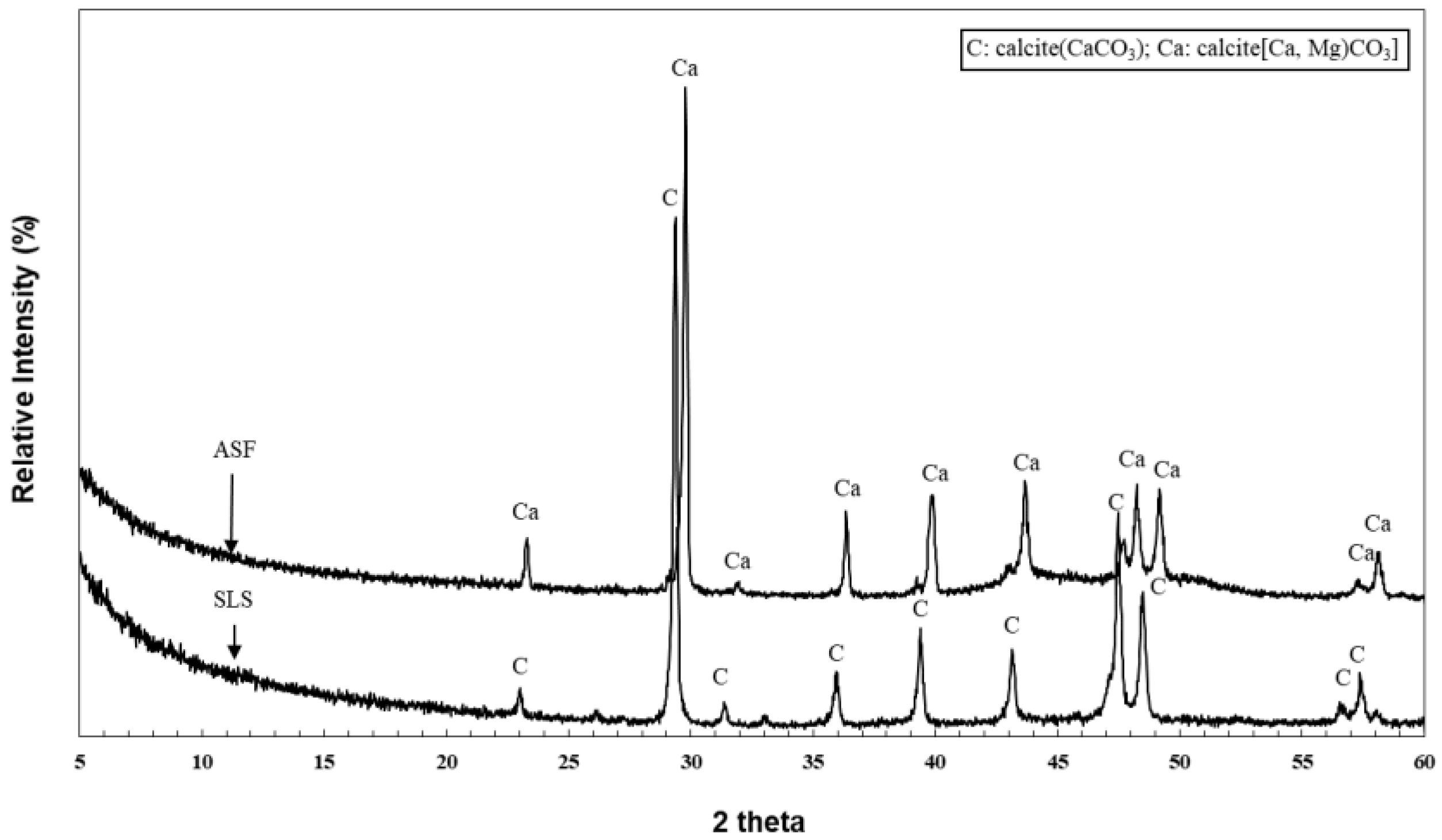
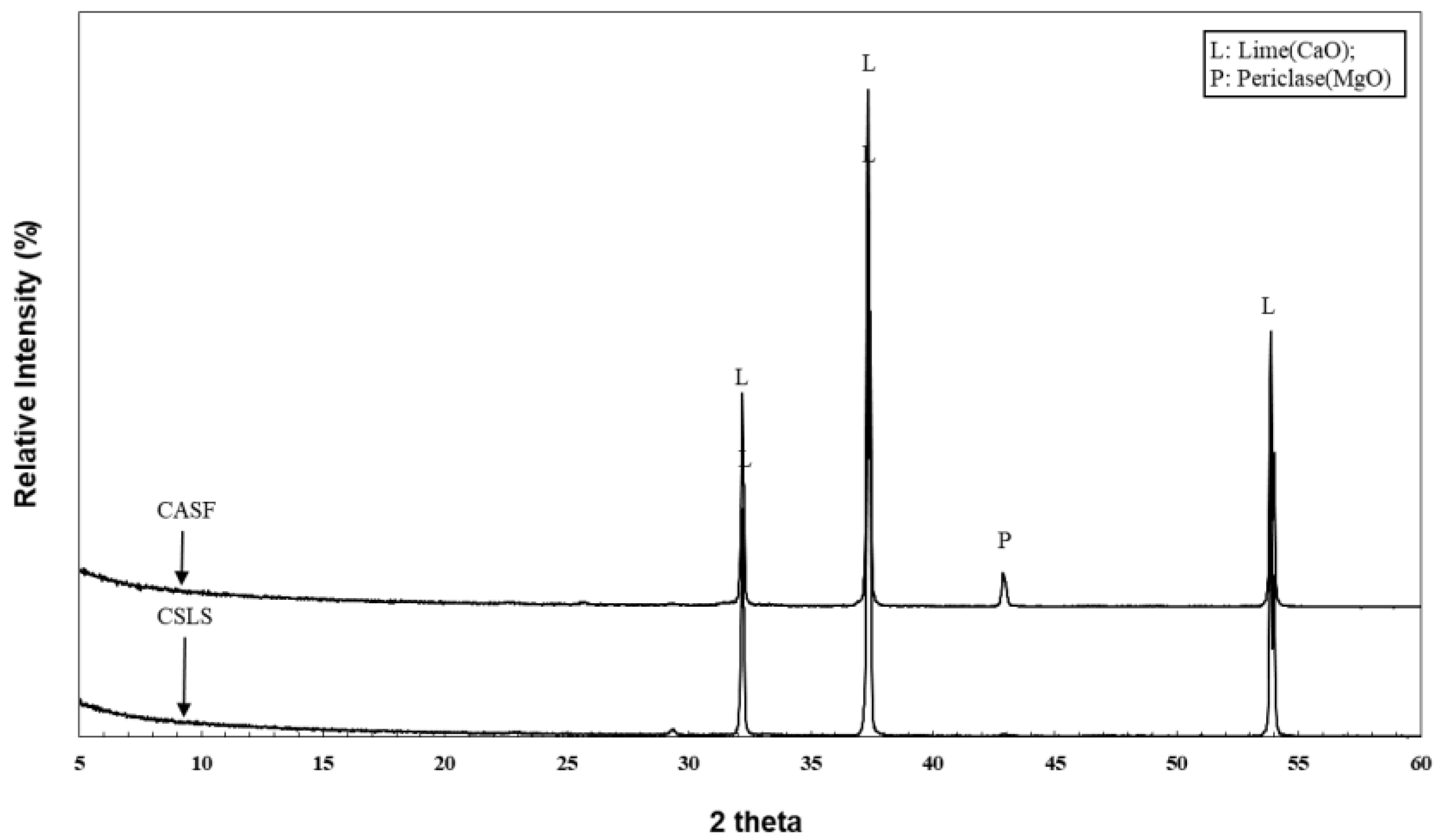
2.5. XRD Analysis
2.6. SEM-EDX Analysis
3. Results and Discussion
3.1. XRD Analysis Results
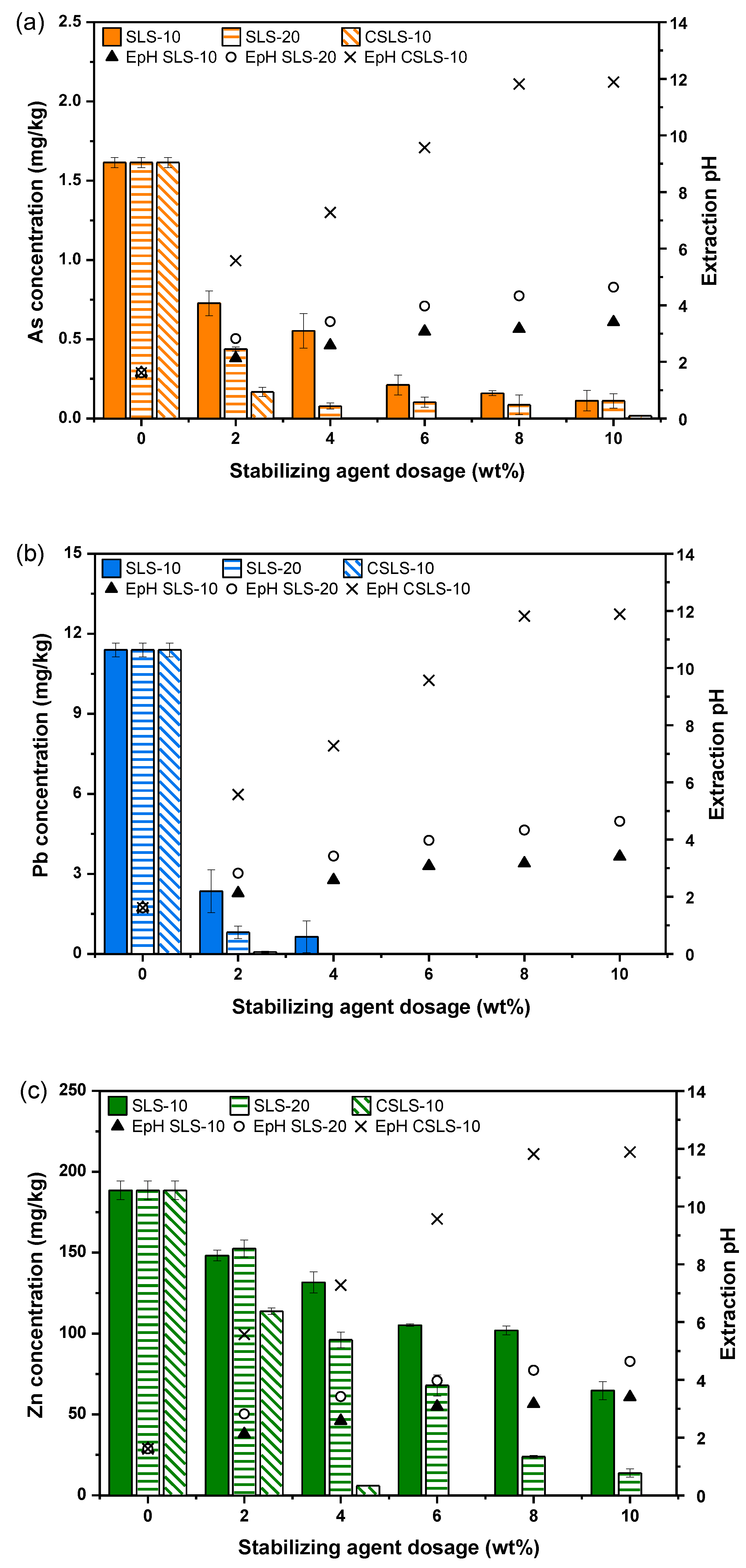
3.2. Stabilization Efficiency Assessment
3.3. Crop Cultivation and Heavy Metal Transition Evaluation
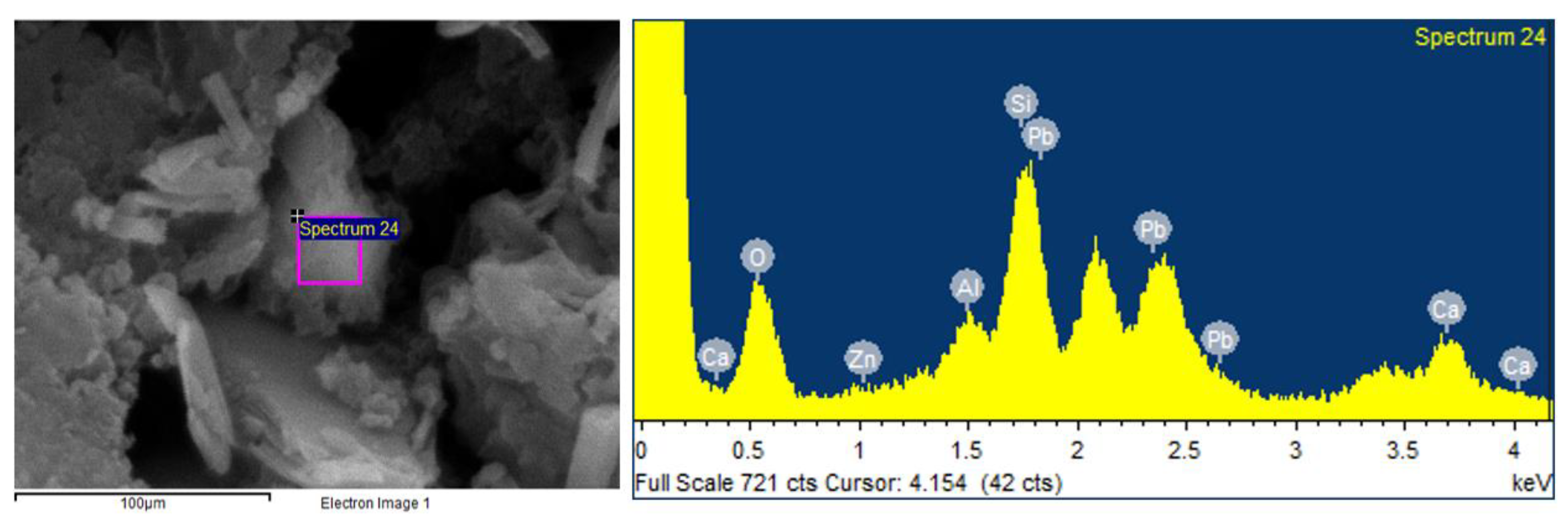
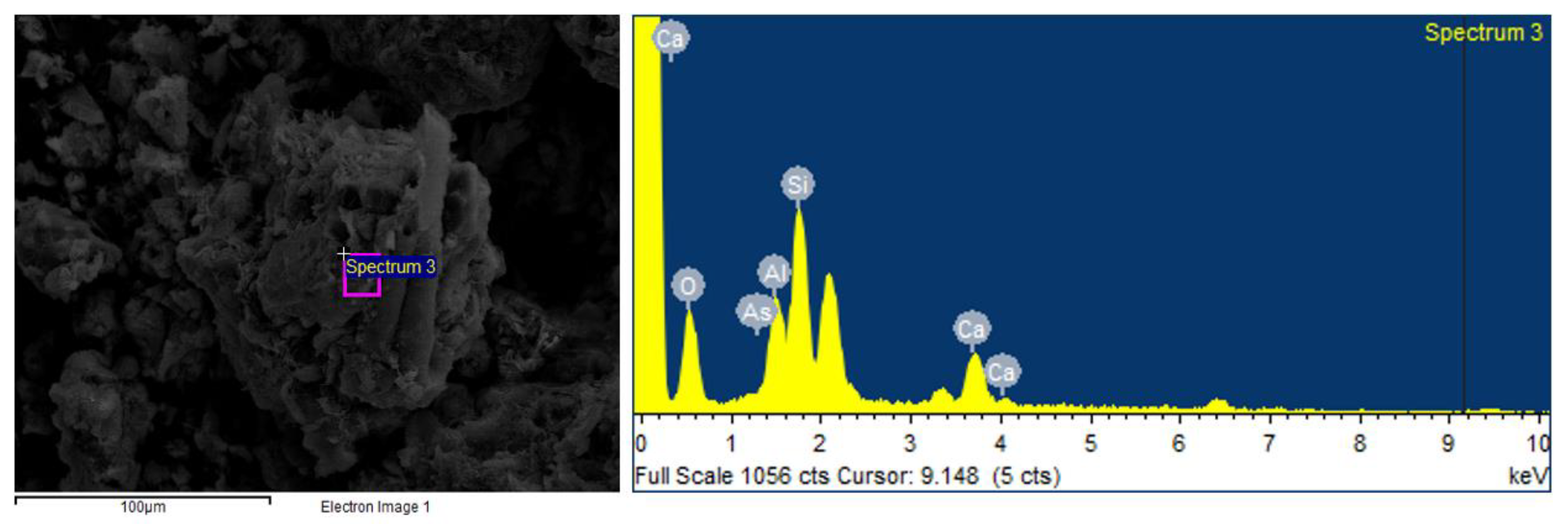
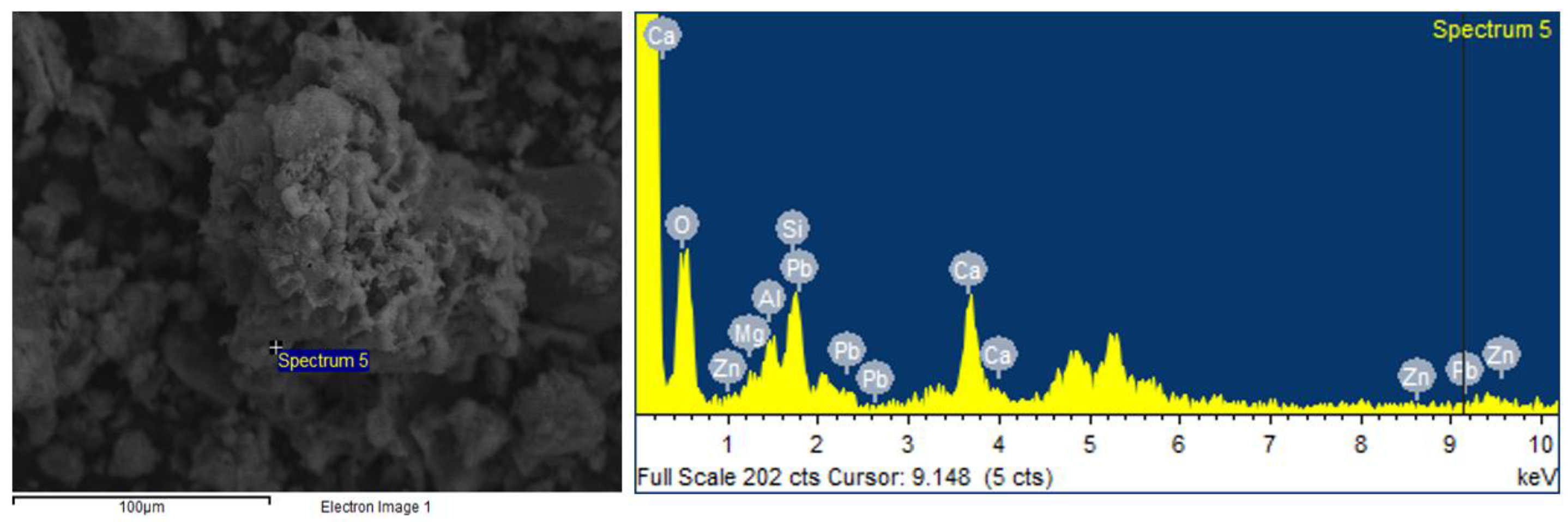
3.4. SEM-EDX Analysis Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Song, S.; Wang, R.; Liu, Z.; Meng, J.; Sweetman, A.J.; Jenkins, A.; Ferrier, R.C.; Li, H.; Luo, W. Impacts of soil and water pollution on food safety and health risks in China. Environ. Int. 2015, 77, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Kang, M.W.; Choi, G.H.; Oh, S.J.; Kim, D.J.; Lee, S.S. Assessment of soil pollutant distribution characteristics and heavy metal pollution in Korea. Korean J. Environ. Agric. 2022, 41, 9–15. [Google Scholar] [CrossRef]
- Ra, K.; Kim, J.K.; Lee, J.M.; Lee, S.Y.; Kim, E.S.; Kim, K.T. Characteristics and risk assessment of heavy metals in the stormwater runoffs from industrial region discharged into Shihwa Lake. J. Korean Soc. Mar. Environ. Energy 2014, 17, 283–296. [Google Scholar] [CrossRef]
- Deng, L.; Zeng, G.; Fan, C.; Lu, L.; Chen, X.; Chen, M.; Wu, H.; He, X.; He, Y. Response of rhizosphere microbial community structure and diversity to heavy metal co-pollution in arable soil. Appl. Microbiol. Biotechnol. 2015, 99, 8259–8269. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Mirza, M.A.; Choudhary, M.A.; Kim, K.H.; Raza, W.; Raza, N.; Lee, S.S.; Zhang, M.; Lee, J.H.; Sarfraz, M. Spatial distribution of heavy metals in crops in a wastewater irrigated zone and health risk assessment. Environ. Res. 2019, 168, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Gil, C.; Boluda, R.; Ramos, J. Determination and evaluation of cadmium, lead and nickel in greenhouse soils of Almería (Spain). Chemosphere 2004, 55, 1027–1034. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Food and Rural Affairs (MAFRA). A Study on the Necessity of Establishment and Operation of Farmland Environmental Management System; Ministry of Agriculture, Food and Rural Affairs (MAFRA): Sejong, Republic of Korea, 2015; p. 197.
- Korea Mine Rehabilitation and Mineral Resources Corporation (KOMIR). Statistics of National Mine (by Ore Type); Korea Mine Rehabilitation and Mineral Resources Corporation (KOMIR): Wonju, Republic of Korea, 2021. [Google Scholar]
- Hwang, S.; Myung, S.; Han, Y. A Study of Policy Alternatives for Managing Heavy-Metal Contaminated Agricultural Soils by Natural Causes; Korea Environment Institute (KEI): Sejong, Republic of Korea, 2017; p. 79. [Google Scholar]
- Venkateswarlu, K.; Nirola, R.; Kuppusamy, S.; Thavamani, P.; Naidu, R.; Megharaj, M. Abandoned metalliferous mines: Ecological impacts and potential approaches for reclamation. Rev. Environ. Sci. Biotechnol. 2016, 15, 327–354. [Google Scholar] [CrossRef]
- Kim, M.G.; Kim, K.J.; Jeong, G.C. Assessment of the cause and pathway of contamination and sustainability in an abandoned mine. J. Eng. Geol. 2018, 28, 411–429. [Google Scholar]
- Ministry of Environment (MOE). Investigation of Soil Contamination in Abandoned Mine; Ministry of Environment (MOE): Sejong, Republic of Korea, 2015; p. 19.
- Ministry of Food and Drug Safety (MFDS). Lead Risk Assessment Report; Ministry of Food and Drug Safety (MFDS): Cheongju, Republic of Korea, 2016; p. 80.
- Sanchez, T.R.; Perzanowski, M.; Graziano, J.H. Inorganic arsenic and respiratory health, from early life exposure to sex-specific effects: A systematic review. Environ. Res. 2016, 147, 537–555. [Google Scholar] [CrossRef]
- Park, J.J.; Shin, E.C. Trends in contaminated soil remediation rechnologies. J. Kor. Geoenviron. Soc. 2008, 9, 14–29. [Google Scholar]
- Cho, H.R.; Kim, D.J.; Lee, J.H. Applicable remediation technologies for different types of soils contaminated by heavy metals: A review. J. Agric. Life Sci. 2016, 47, 32–38. [Google Scholar]
- Shanableh, A.; Kharabsheh, A. Stabilization of Cd, Ni and Pb in soil using natural zeolite. J. Hazard. Mater. 1996, 45, 207–217. [Google Scholar] [CrossRef]
- Wang, S.; Vipulanandan, C. Leachability of lead from solidified cement-fly ash binders. Cem. Concr. Res. 1996, 26, 895–905. [Google Scholar] [CrossRef]
- Li, X.; Poon, C.S.; Sun, H.; Lo, I.; Kirk, D.W. Heavy metal speciation and leaching behaviors in cement based solidified/stabilized waste materials. J. Hazard. Mater. 2001, 82, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Moirou, A.; Xenidis, A.; Paspaliaris, I. Stabilization Pb, Zn, and Cd-contaminated soil by means of natural zeolite. Soil Sediment. Contam. 2001, 10, 251–267. [Google Scholar] [CrossRef]
- Gray, C.; Dunham, S.; Dennis, P.; Zhao, F.; McGrath, S. Field evaluation of in situ remediation of a heavy metal contaminated soil using lime and red-mud. Environ. Pollut. 2006, 142, 530–539. [Google Scholar] [CrossRef]
- Yin, C.-Y.; Mahmud, H.B.; Shaaban, M.G. Stabilization/solidification of lead-contaminated soil using cement and rice husk ash. J. Hazard. Mater. 2006, 137, 1758–1764. [Google Scholar] [CrossRef]
- Mahabadi, A.A.; Hajabbasi, M.; Khademi, H.; Kazemian, H. Soil cadmium stabilization using an Iranian natural zeolite. Geoderma 2007, 137, 388–393. [Google Scholar] [CrossRef]
- Chen, Q.; Tyrer, M.; Hills, C.D.; Yang, X.; Carey, P. Immobilisation of heavy metal in cement-based solidification/stabilisation: A review. Waste Manag. 2009, 29, 390–403. [Google Scholar] [CrossRef]
- Shi, W.-y.; Shao, H.-b.; Li, H.; Shao, M.-a.; Du, S. Progress in the remediation of hazardous heavy metal-polluted soils by natural zeolite. J. Hazard. Mater. 2009, 170, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Taki, G.; Nguyen, X.P.; Jo, Y.T.; Kim, J.; Park, J.H. Heavy metal stabilization in contaminated soil by treatment with calcined cockle shell. Environ. Sci. Pollut. Res. 2017, 24, 7177–7183. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.C.; Lee, J.Y.; Yoon, Y.J.; Lee, B.y.; Choi, S.i. Improvement of heavy metal stabilizers using agricultural and industrial by-products. J. Korea Soc. Waste Manag. 2021, 38, 70–77. [Google Scholar] [CrossRef]
- Moon, D.H.; Koutsospyros, A. Stabilization of lead-contaminated mine soil using natural waste materials. Agriculture 2022, 12, 367. [Google Scholar] [CrossRef]
- Koh, I.H.; Hwan, L.S.; Lee, W.S.; Chang, Y.Y. Assessment on the transition of arsenic and heavy metal from soil to plant according to stabilization process using limestone and steelmaking slag. J. Soil Groundwater Environ. 2013, 18, 63–72. [Google Scholar] [CrossRef]
- Koh, I.H.; Kim, J.; Kim, G.S.; Park, M.S.; Kang, D.M.; Ji, W.H. Stabilization of agricultural soil contaminated by arsenic and heavy metals using biochar derived from Buffalo weed. J. Soil Groundwater Environ. 2016, 21, 87–100. [Google Scholar] [CrossRef]
- Ministry of Oceans and Fisheries (MOF). Fisheries Production Statistics; Ministry of Oceans and Fisheries (MOF): Sejong, Republic of Korea, 2024.
- Quantum. Hotamet (Shellmet). Available online: https://quantum.ne.jp/en/projects/hotamet/ (accessed on 21 June 2024).
- Ministry of Oceans and Fisheries (MOF). Designated Harmful Marine Organisms. Available online: https://www.meis.go.kr/mes/marineLife/harmful/species.do (accessed on 30 June 2024).
- Park, S.H.; An, J.; Koutsospyros, A.; Moon, D.H. Assessment of the stabilization of Cu-, Pb-, and Zn-contaminated fine soil using cockle shells, scallop shells, and starfish. Agriculture 2023, 13, 1414. [Google Scholar] [CrossRef]
- Ok, Y.S.; Oh, S.E.; Ahmad, M.; Hyun, S.; Kim, K.R.; Moon, D.H.; Lee, S.S.; Lim, K.J.; Jeon, W.T.; Yang, J.E. Effects of natural and calcined oyster shells on Cd and Pb immobilization in contaminated soils. Environ. Earth Sci. 2010, 61, 1301–1308. [Google Scholar] [CrossRef]
- Moon, D.H.; Cheong, K.H.; Kim, T.-S.; Khim, J.; Choi, S.B.; Moon, O.R.; Ok, Y.S. Stabilization of As in soil contaminated with chromated copper arsenate (CCA) Using calcinated oyster shells. Korean J. Environ. Agric. 2009, 28, 378–385. [Google Scholar] [CrossRef]
- Ahmad, M.; Hashimoto, Y.; Moon, D.H.; Lee, S.S.; Ok, Y.S. Immobilization of lead in a Korean military shooting range soil using eggshell waste: An integrated mechanistic approach. J. Hazard. Mater. 2012, 209, 392–401. [Google Scholar] [CrossRef]
- Lim, J.E.; Sung, J.K.; Sarkar, B.; Wang, H.; Hashimoto, Y.; Tsang, D.C.; Ok, Y.S. Impact of natural and calcined starfish (Asterina pectinifera) on the stabilization of Pb, Zn and As in contaminated agricultural soil. Environ. Geochem. Health 2017, 39, 431–441. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Environmental Research (NIER). The Korean Standard Test (KST) Methods; Ministry of Environment (MOE): Sejong, Republic of Korea, 2008.
- Lee, S.; Kim, J.; Jeong, S.W. Analysis of the organic matter content for soil samples taken at the new points of Korea soil quality monitoring network. J. Korean Soc. Environ. Eng. 2016, 38, 641–646. [Google Scholar] [CrossRef]
- Jang, B.C.; Gang, S.S.; Kim, R.Y.; Kim, M.S.; Kim, Y.H.; Song, G.C.; Yoon, H.B.; Lee, Y.J. Methods of Soil Chemical Analysis; National Academy of Agricultural Science (NAAS): Wanju, Republic of Korea, 2010; p. 313. [Google Scholar]
- Hyun, B.G.; Hur, S.O.; Ok, J.H.; Hwang, S.A.; Oh, B.Y.; Son, J.U.; Lee, S.I. Agricultural Soil Physical Property Investigation Methods and Analysis Methods; National Academy of Agricultural Science (NAAS): Wanju, Republic of Korea, 2022; p. 105. [Google Scholar]
- Gilchrist, J.D. Extraction Metallurgy; Pergamon Press: London, UK, 1986; p. 145. [Google Scholar]
- Korea Mine Rehabilitation and Mineral Resources Corporation (KOMIR). Guidebook: Mine Rehabilitation Technology in Korea; Korea Mine Rehabilitation and Mineral Resources Corporation (KOMIR): Wonju, Republic of Korea, 2022. [Google Scholar]
- Mistry of Food and Drug Safety (MFDS). Korean Food Code; Mistry of Food and Drug Safety (MFDS): Seoul, Republic of Korea, 2022.
- MDI. Jade Version 7.1; Material’s Data Inc.: Livermore, CA, USA, 2005. [Google Scholar]
- ICDD. Powder Diffraction File.PDF-2 Database Release; International Centre for Diffraction Data: Newtown Square, PA, USA, 2002. [Google Scholar]
- Moon, D.H.; Jung, S.P.; Koutsospyros, A. Assessment of the stabilization of mercury contaminated soil using starfish. Agriculture 2022, 12, 542. [Google Scholar] [CrossRef]
- Panpho, P.; Vittayakorn, N.; Sumang, R. Synthesis, scrutiny, and applications of bio-adsorbents from cockle shell waste for the adsorption of Pb and Cd in aqueous solution. Crystals 2023, 13, 552. [Google Scholar] [CrossRef]
- Suwannasingha, N.; Kantavong, A.; Tunkijjanukij, S.; Aenglong, C.; Liu, H.-B.; Klaypradit, W. Effect of calcination temperature on structure and characteristics of calcium oxide powder derived from marine shell waste. J. Saudi Chem. Soc. 2022, 26, 101441. [Google Scholar] [CrossRef]
- Lim, J.H.; Cui, M.C.; Moon, D.H.; Khim, J.H. Stabilization of heavy metal contaminated soil amended with waste cow bone. J. Environ. Sci. Int. 2010, 19, 255–260. [Google Scholar] [CrossRef]
- Lei, J.; Peng, B.; Liang, Y.-J.; Min, X.-B.; Chai, L.-Y.; Ke, Y.; You, Y. Effects of anions on calcium arsenate crystalline structure and arsenic stability. Hydrometallurgy 2018, 177, 123–131. [Google Scholar] [CrossRef]
- Zama, E.F.; Li, G.; Tang, Y.-T.; Reid, B.J.; Ngwabie, N.M.; Sun, G.-X. The removal of arsenic from solution through biochar-enhanced precipitation of calcium-arsenic derivatives. Environ. Pollut. 2022, 292, 118241. [Google Scholar] [CrossRef]
- Koo, N.; Jo, H.; Lee, S.; Kim, J. Using response surface methodology to assess the effects of iron and spent mushroom substrate on arsenic phytotoxicity in lettuce (Lactuca sativa L.). J. Hazard. Mater. 2011, 192, 381–387. [Google Scholar] [CrossRef]
- Kim, M.S.; Min, H.; Kim, J.G.; Koo, N.; Park, J.S.; Bak, G.I. Effects of various amendments on heavy metal stabilization in acid and alkali soils. Korean J. Environ. Agric. 2014, 33, 1–8. [Google Scholar] [CrossRef]
- Yoo, J.H.; Lee, J.W.; Yoon, J.H.; Kim, M.H.; Kim, S.I.; Kim, S.C. Effect of organic matter of various C:N ratios on the solubility of arsenic, manganese, and iron in paddy soil and on arsenic availability to plants. J. Environ. Anal. Health Toxicol. 2020, 23, 211–221. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, H.; Chen, Z.; Zhu, Y.G. Arsenic uptake by rice is influenced by microbe-mediated arsenic redox changes in the rhizosphere. Environ. Sci. Technol. 2014, 48, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, Y.S.; Park, S.H.; Moon, D.H. Stabilization of heavy metal contaminated soil around an abandoned mine using starfish (Asterina pectinifera) and cockle shell. J. Korean Soc. Environ. Eng. 2024, 46, 33–47. [Google Scholar] [CrossRef]
- Moon, D.H.; Cheong, K.H.; Koutsospyros, A.; Chang, Y.Y.; Hyun, S.; Ok, Y.S.; Park, J.H. Assessment of waste oyster shells and coal mine drainage sludge for the stabilization of As-, Pb-, and Cu-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 2362–2370. [Google Scholar] [CrossRef] [PubMed]
- Bothe, J.V.; Brown, P.W. Arsenic immobilization by calcium arsenate formation. Environ. Sci. Technol. 1999, 33, 3806–3811. [Google Scholar] [CrossRef]
- Lu, Q.; Xiao, H.; Du, Y.; Du, D. Using CaO to stabilize arsenic sulfide slag by moderate temperature calcination. Environ. Earth Sci. 2017, 76, 1–6. [Google Scholar] [CrossRef]
- Sparks, D.L. Environmental Soil Chemistry; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.R.; Pullammanappallil, P.; Cao, X. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Power, L.M.; Cheng, T.; Rastghalam, Z.S. Cu and Zn adsorption to a heterogeneous natural sediment: Influence of leached cations and natural organic matter. Chemosphere 2016, 144, 1973–1979. [Google Scholar] [CrossRef]
- Zhao, X.L.; Masaihiko, S. Amelioration of cadmium polluted paddy soils by porous hydrated calcium silicate. Water Air Soil Pollut. 2007, 183, 309–315. [Google Scholar] [CrossRef]

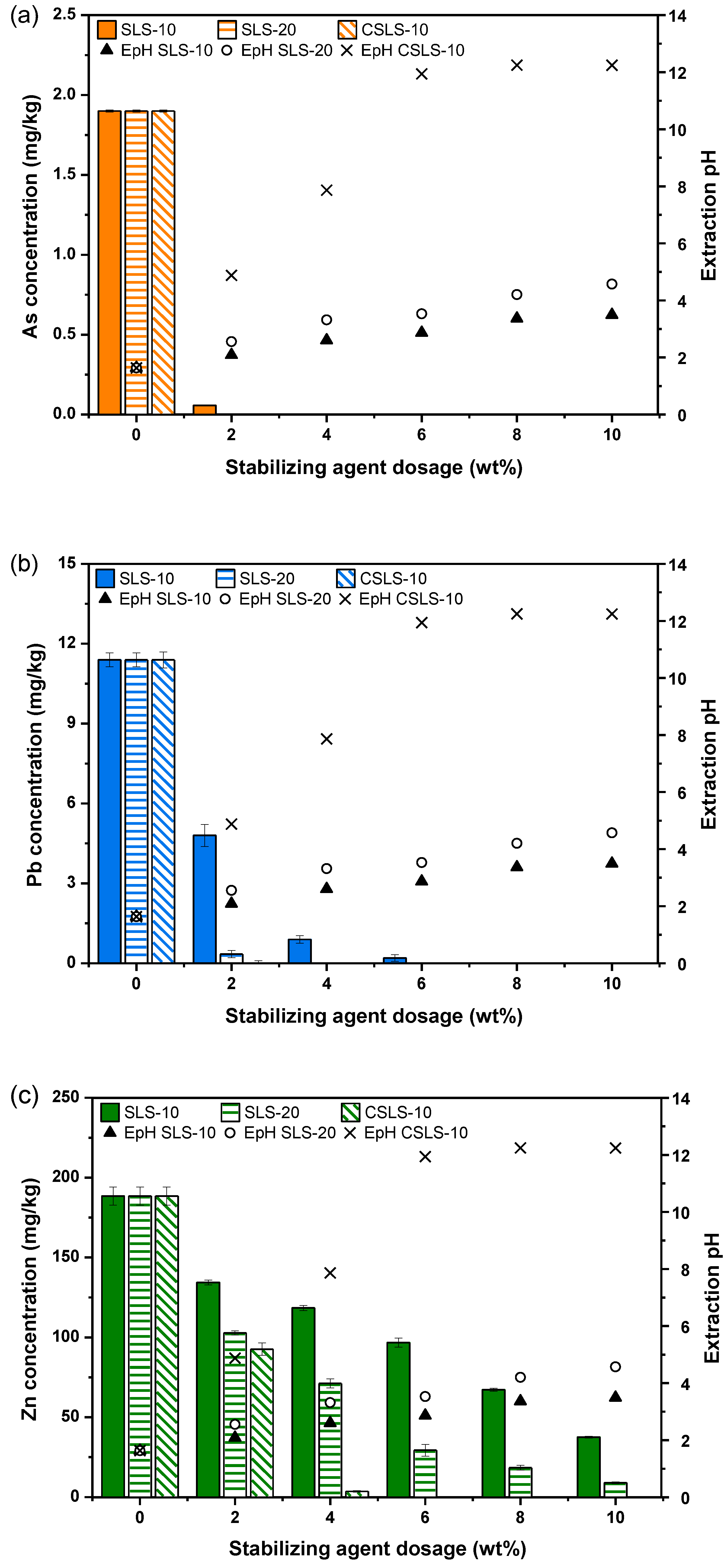
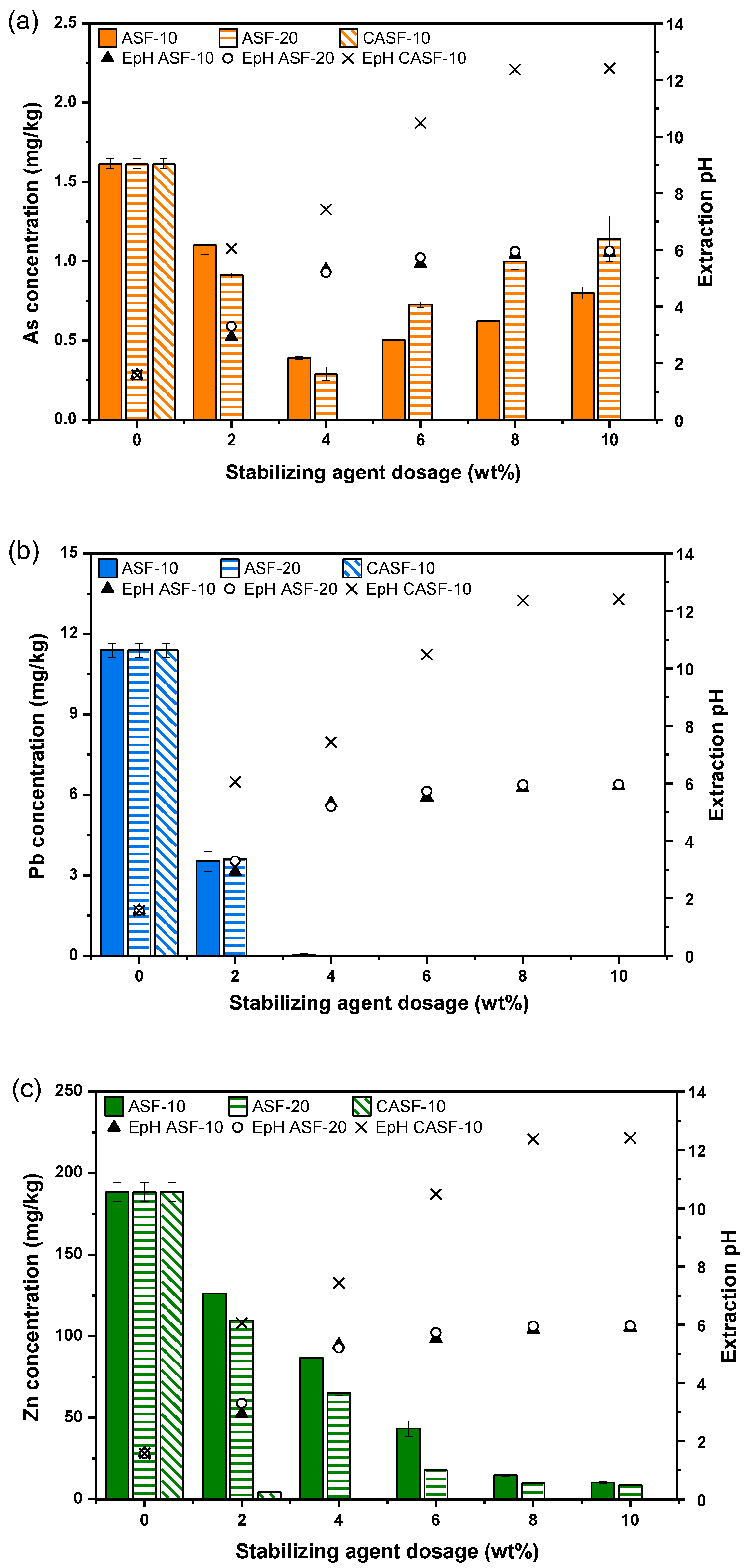
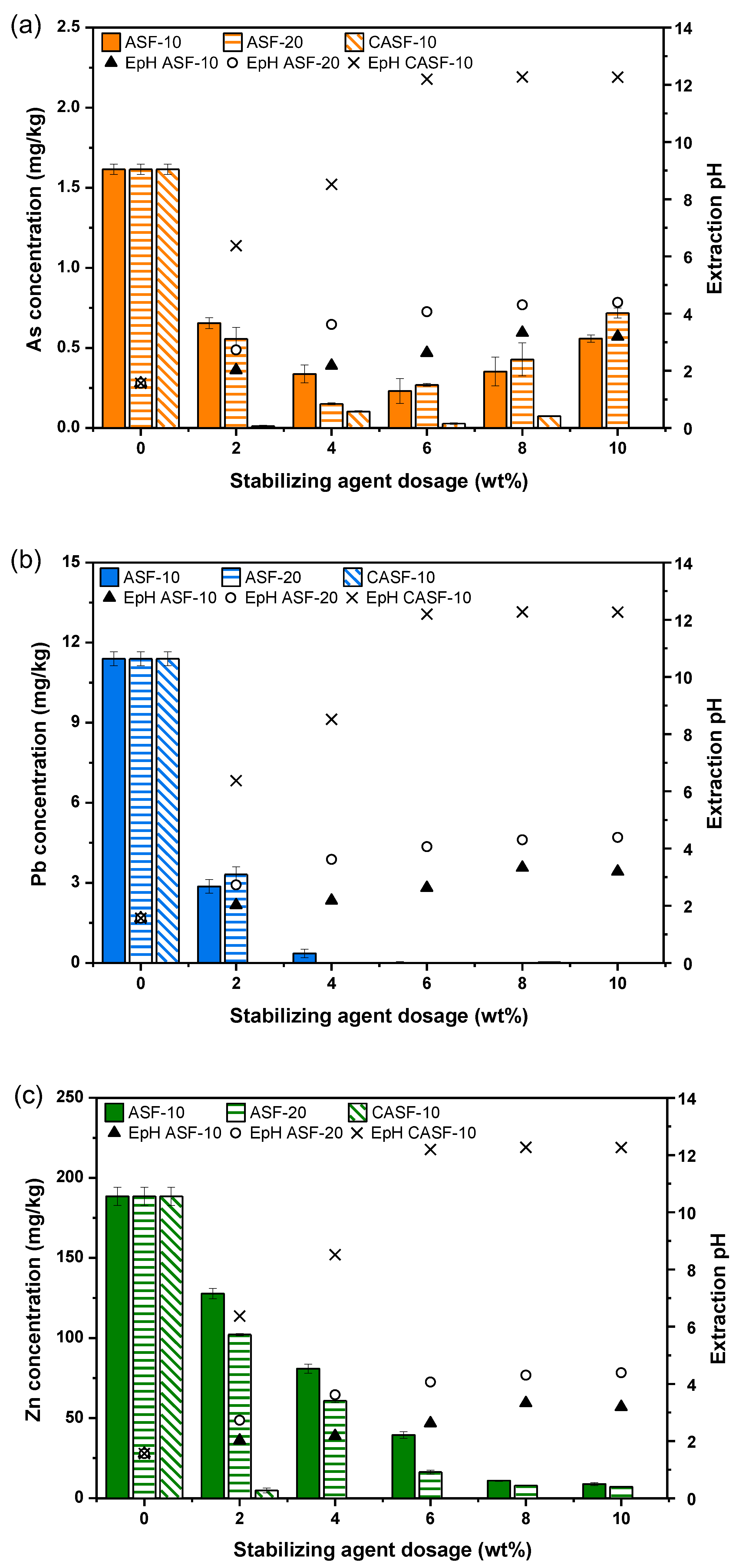

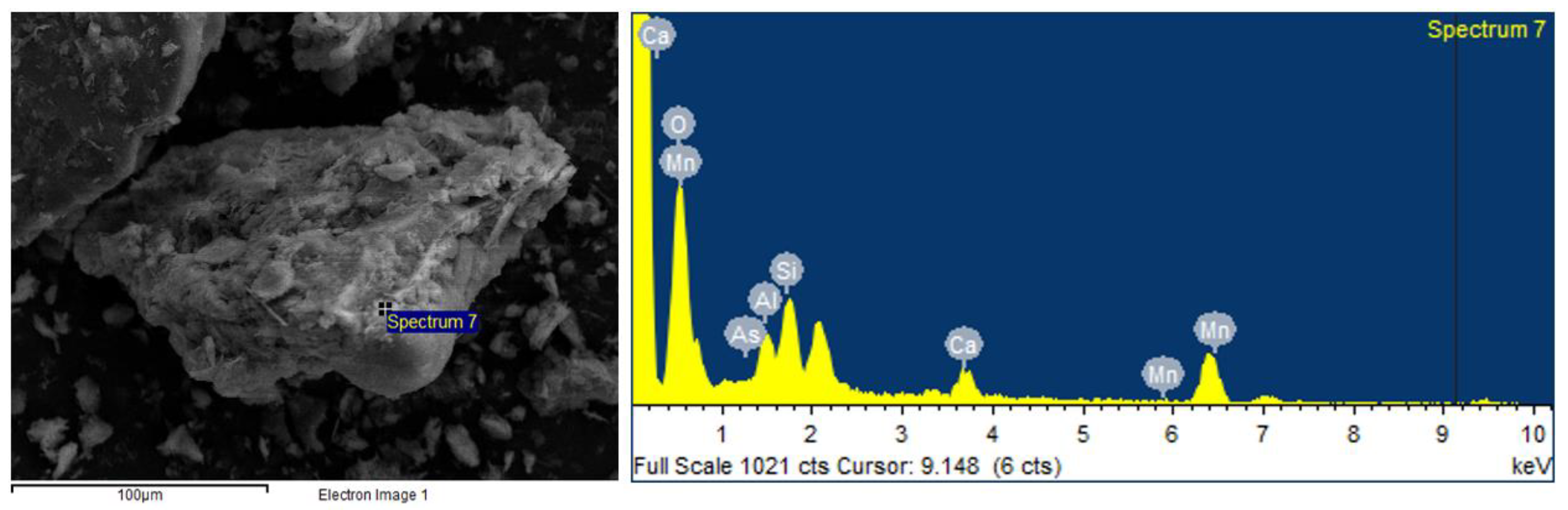
| Soil Properties | Contaminated Soil | Regulatory Limit (Korean Warning Standard) 1 | |
|---|---|---|---|
| Contaminants (mg/kg) | As | 57.0 | 25 |
| Pb | 466.8 | 200 | |
| Zn | 700.7 | 300 | |
| Organic matter content 2 (%) | 5.4 | ||
| pH (1:5) | 6.8 | ||
| EC (dS/m) | 0.36 | ||
| CEC (cmol+/kg) | 23.8 | ||
| Composition 3 (%) | Sand | 67.7 | |
| Silt | 17.2 | ||
| Clay | 15.1 | ||
| Texture 4 | Sandy Loam | ||
| Major Chemical Composition (%) | Contaminated Soil | SLS | CSLS | ASF | CASF |
|---|---|---|---|---|---|
| SiO2 | 62.12 | 0.073 | 0.113 | 0.135 | 0.126 |
| Al2O3 | 17.97 | 0.205 | 0.154 | 0.053 | 0.130 |
| K2O | 4.459 | 0.008 | 0.012 | 0.355 | 0.158 |
| Fe2O3 | 4.317 | 0.065 | 0.051 | 0.069 | 0.053 |
| Na2O | 1.546 | 0.824 | 0.974 | 2.161 | 2.510 |
| SO3 | 1.243 | 0.597 | 0.580 | 5.483 | 2.450 |
| MgO | 0.987 | 0.603 | 0.749 | 5.754 | 13.74 |
| CaO | 0.844 | 94.59 | 96.18 | 67.60 | 78.78 |
| MnO | 0.528 | 0.007 | 0.007 | 0.008 | 0.004 |
| P2O5 | 0.428 | 0.122 | 0.129 | 1.203 | 0.633 |
| LOI 1 | 5.400 | 2.050 | - | 17.64 | - |
| pH | 6.50 | 9.42 | 12.51 | 7.37 | 12.29 |
| Sample ID | Contaminated Soil (g) | Stabilizing Agent (g) | L:S Ratio |
|---|---|---|---|
| Control | 50 | 0 | 30:1 |
| 2 wt.% SLS-10/SLS-20/CSLS-10 ASF-10/ASF-20/CASF-10 | 50 | 1 | 30:1 |
| 4 wt.% SLS-10/SLS-20/CSLS-10 ASF-10/ASF-20/CASF-10 | 50 | 2 | 30:1 |
| 6 wt.% SLS-10/SLS-20/CSLS-10 ASF-10/ASF-20/CASF-10 | 50 | 3 | 30:1 |
| 8 wt.% SLS-10/SLS-20/CSLS-10 ASF-10/ASF-20/CASF-10 | 50 | 4 | 30:1 |
| 10 wt.% SLS-10/SLS-20/CSLS-10 ASF-10/ASF-20/CASF-10 | 50 | 5 | 30:1 |
| Pot | Leaf Length 1 (cm) | Weight 2 (g) | Contaminant Transition Concentration (mg/kg) | ||
|---|---|---|---|---|---|
| As | Pb | Zn | |||
| Regulatory Limit (Korean Standard) 3 | - | - | - | 0.300 | - |
| Control | 9.4 (±0.48) | 7.3 (±0.31) | ND 4 | 0.353 (±0.01) | 25.58 (±3.49) |
| SLS-20 | 20.3 (±0.37) | 19.5 (±0.31) | ND | ND | 5.371 (±0.31) |
| CSLS-10 | 21.0 (±0.43) | 21.1 (±0.37) | ND | ND | 4.709 (±0.08) |
| ASF-20 | 21.4 (±1.23) | 19.0 (±0.80) | ND | ND | 7.779 (±0.25) |
| CASF-10 | 20.5 (±1.57) | 20.7 (±0.83) | ND | ND | 3.734 (±0.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.H.; Park, S.H.; Koutsospyros, A.; Moon, D.H. Effects of Scallop Shells and Starfish (Asterias amurensis) on Stabilization of Metalloid (As) and Heavy Metal (Pb and Zn)-Contaminated Soil. Agronomy 2024, 14, 1781. https://doi.org/10.3390/agronomy14081781
Park SH, Park SH, Koutsospyros A, Moon DH. Effects of Scallop Shells and Starfish (Asterias amurensis) on Stabilization of Metalloid (As) and Heavy Metal (Pb and Zn)-Contaminated Soil. Agronomy. 2024; 14(8):1781. https://doi.org/10.3390/agronomy14081781
Chicago/Turabian StylePark, Se Hyun, Sang Hyeop Park, Agamemnon Koutsospyros, and Deok Hyun Moon. 2024. "Effects of Scallop Shells and Starfish (Asterias amurensis) on Stabilization of Metalloid (As) and Heavy Metal (Pb and Zn)-Contaminated Soil" Agronomy 14, no. 8: 1781. https://doi.org/10.3390/agronomy14081781






