Enhancement of Fermentation Performance in the Anaerobic Co-Digestion of Chicken Manure and Corn Straw under Biogas Slurry Reflux via Air Stripping of the Digestate
Abstract
1. Introduction
2. Materials and Methods
2.1. Fermentation Materials and Inoculum
2.2. Experimental Devices
2.3. Experimental Design
2.4. Analytical Methods and Calculations
2.5. Data Processing
3. Results and Discussion
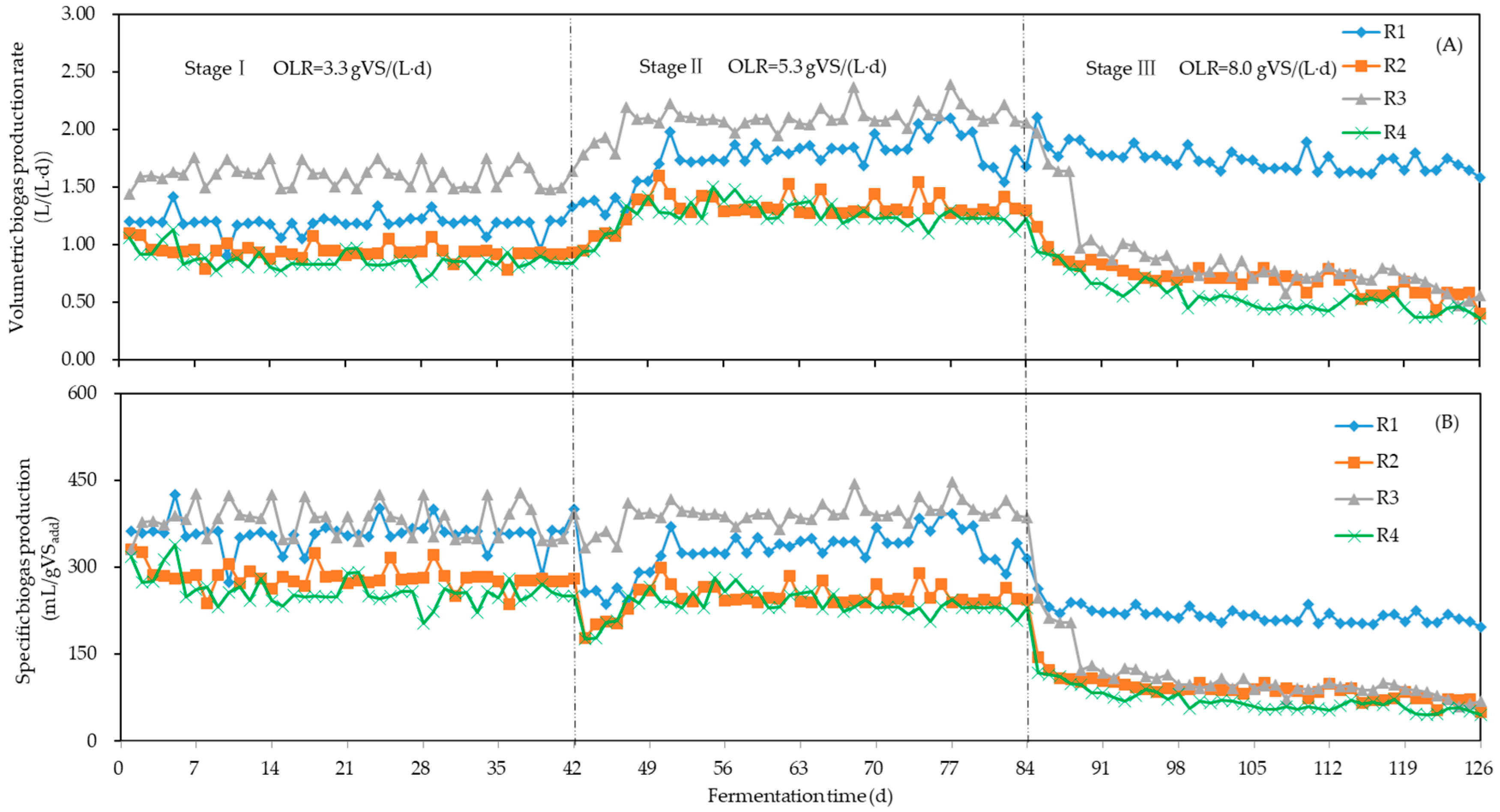
3.1. Biogas Production
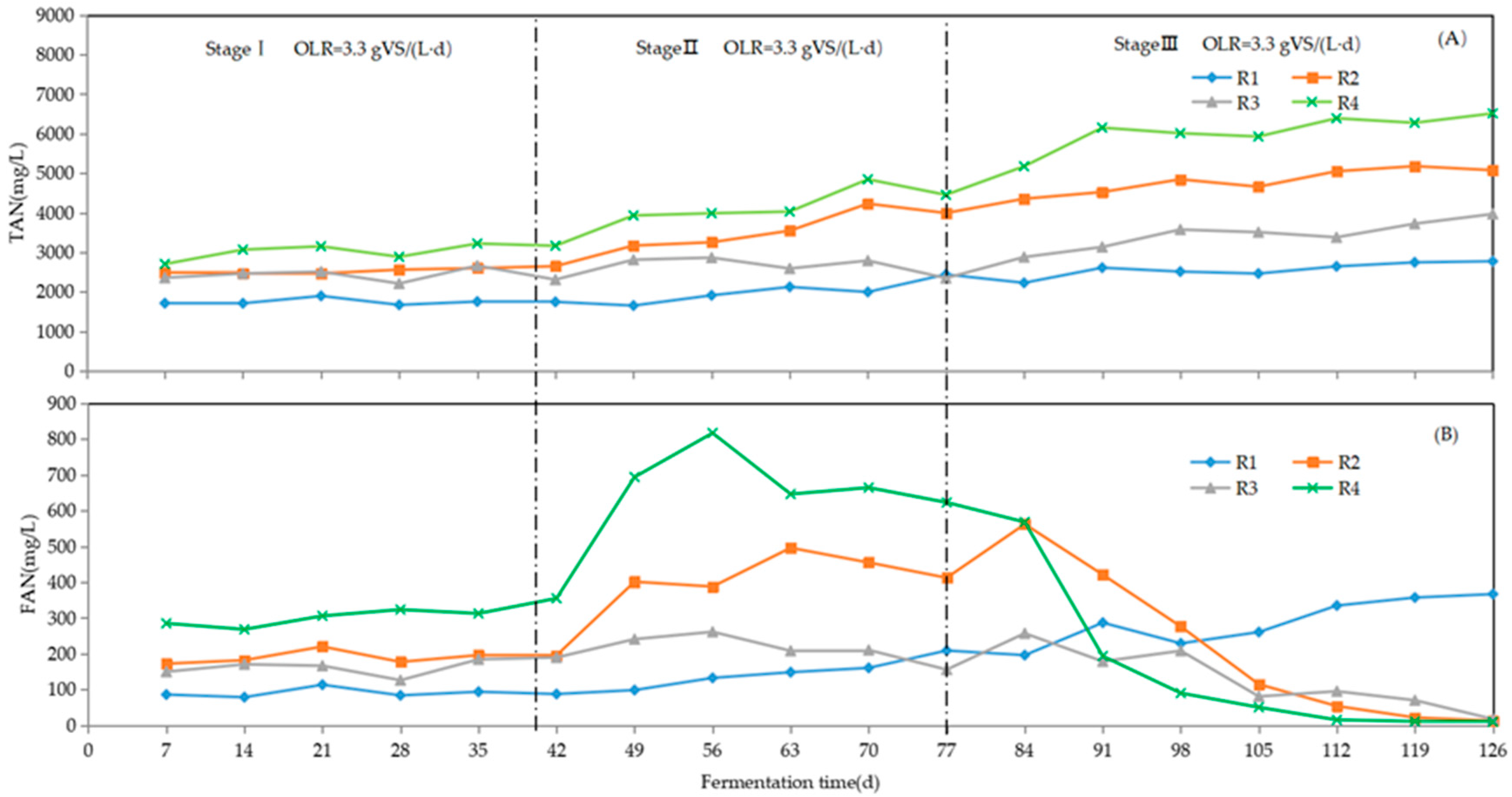
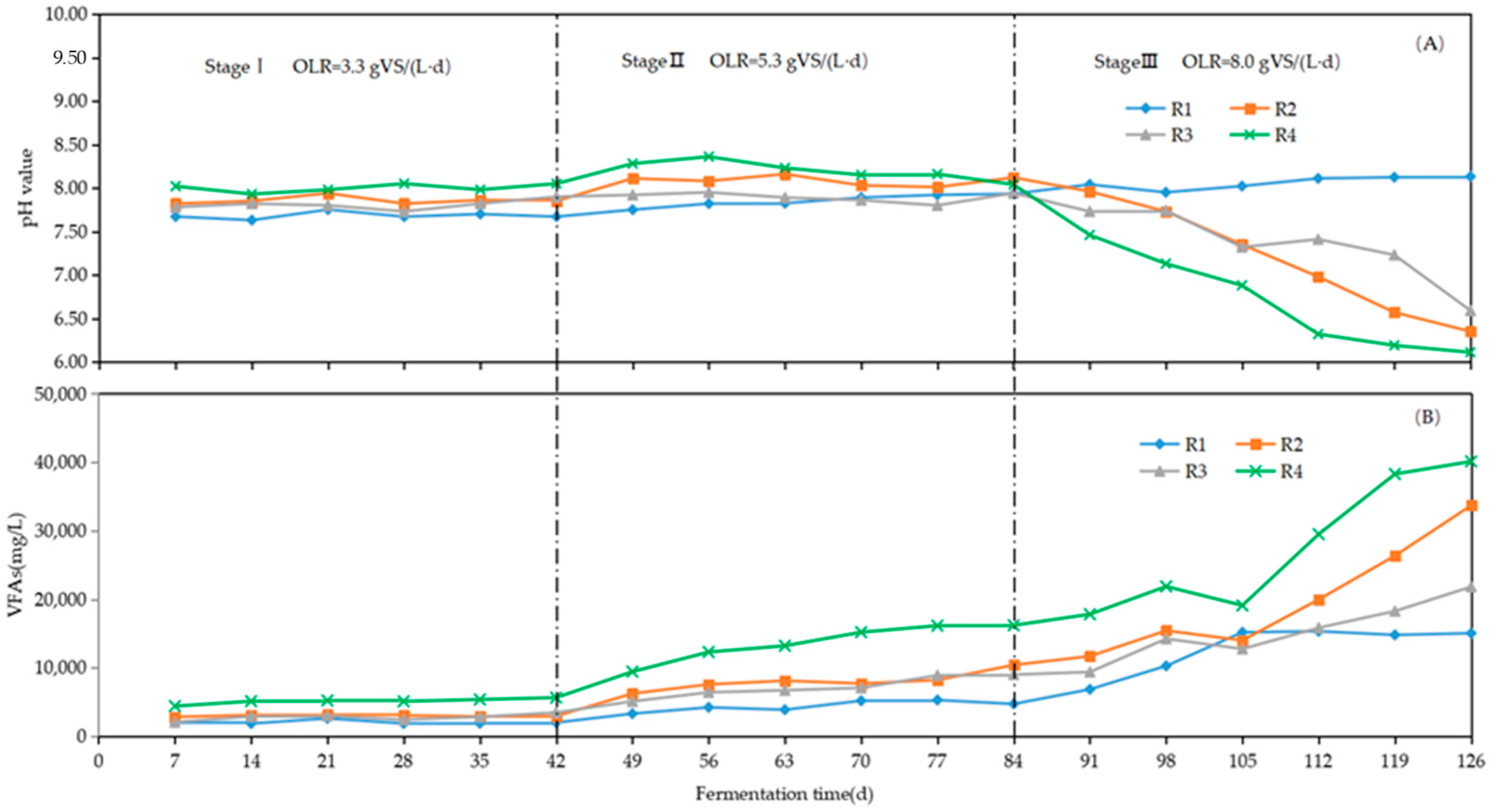
3.2. Changes in TAN, FAN, pH, and VFAs
3.2.1. TAN and FAN
3.2.2. pH and VFAs
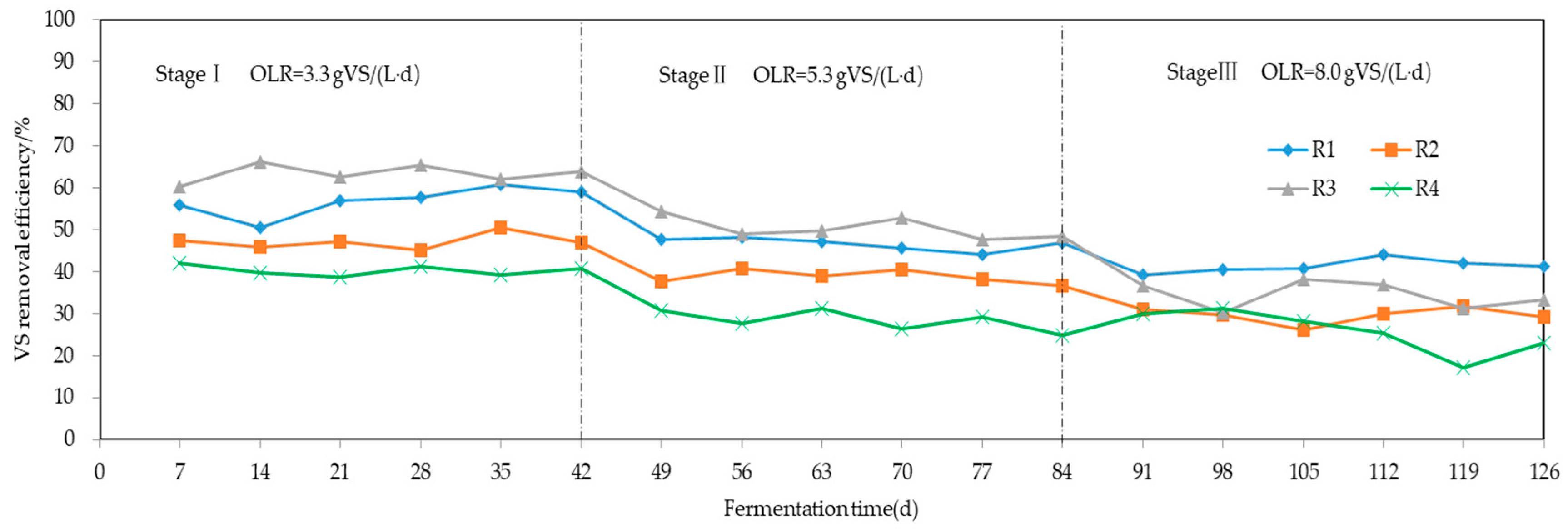
3.3. VS Removal Efficiency
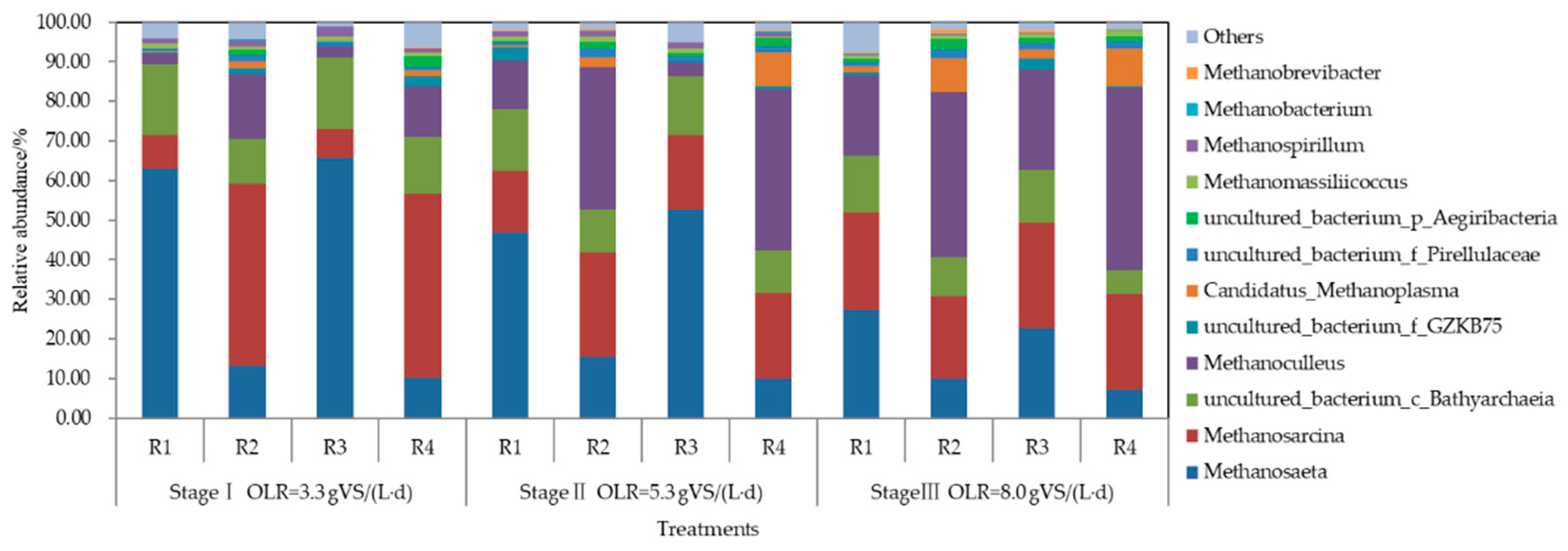
3.4. Microbial Community Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, H.; Sun, X.; Liang, B.; Chen, J.; Zhou, X. Analysis of livestock and poultry manure pollution in China and its treatment and resource utilization. J. Agro-Environ. Sci. 2020, 39, 1168–1176. [Google Scholar]
- Ministry of Agriculture and Rural Affairs of the People’s republic of China. China Agriculture Statistical Report 2007; China Agriculture Press: Beijing, China, 2008.
- Ministry of Agriculture and Rural Affairs of the People’s republic of China. China Agriculture Statistical Report 2017; China Agriculture Press: Beijing, China, 2019.
- Lu, G.; Yang, F.; Chen, H.; Du, T. Research progress on application of biogas slurry. Soils Fertil. Sci. China 2021, 339–345. [Google Scholar] [CrossRef]
- Song, Y.; Wang, G.; Li, R.; Chen, G. Research progress of biogas slurry treatment and resource utilization. Trans. Chin. Soc. Agric. Eng. 2021, 37, 237–250. [Google Scholar]
- Peng, X.; Nges, I.W.; Liu, J. Improving methane production from wheat straw by digestate liquor recirculation in continuous stirred tank processes. Renew. Energy 2016, 85, 12–18. [Google Scholar] [CrossRef]
- Chen, R.; Li, Z.; Feng, J.; Zhao, L.; Yu, J. Effects of digestate recirculation ratios on biogas production and methane yield of continuous dry anaerobic digestion. Bioresour. Technol. 2020, 316, 123963. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Liu, T.Q.; Chu, X.D.; Zheng, G.; Wang, M. Effects of biogas slurry reflux mode and reflux rate on methane production by mixed anaerobic digestion of corn straw and pig manure. J. Clean. Prod. 2023, 411, 137214. [Google Scholar] [CrossRef]
- Zheng, Z.; Cai, Y.; Zhao, Y.; Meng, X.; Zhang, Y.; Lu, C.; Hu, Y.; Cui, Z.; Wang, X. Achieve clean and efficient biomethane production by matching between digestate recirculation and straw-to-manure feeding ratios. J. Clean. Prod. 2020, 263, 121414. [Google Scholar] [CrossRef]
- Qiao, W.; Bi, S.; Xiong, L.; Ju, X.; Dong, R. Effects of ammonium on methane fermentation of chicken manure under mesophilic and thermophilic conditions. China Environ. Sci. 2019, 39, 2921–2927. [Google Scholar]
- Wu, S.; Ni, P.; Li, J.; Sun, H.; Wang, Y.; Luo, H.; Dach, J.; Dong, R. Integrated approach to sustain biogas production in anaerobic digestion of chicken manure under recycled utilization of liquid digestate: Dynamics of ammonium accumulation and mitigation control. Bioresour. Technol. 2016, 205, 75–81. [Google Scholar] [CrossRef]
- Nie, H.; Jacobi, H.F.; Strach, K.; Xu, C.; Zhou, H.; Liebetrau, J. Mono-fermentation of chicken manure: Ammonia inhibition and recirculation of the digestate. Bioresour. Technol. 2015, 178, 238–246. [Google Scholar] [CrossRef]
- Hassan, M.; Ding, W.; Shi, Z.; Zhao, S. Methane enhancement through co-digestion of chicken manure and thermo-oxidative cleaved wheat straw with waste activated sludge: A C/N optimization case. Bioresour. Technol. 2016, 211, 534–541. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, X.; Wang, Y.; Yu, D.; Wei, Y. Rsearch progress of ammonia inhibition and counter measures during anaerobic digestion of livestock wastes. Chin. J. Environ. Eng. 2018, 12, 985–998. [Google Scholar]
- Kayhanian, M. Ammonia inhibition in high-solids biogasification: An overview and practical solutions. Environ. Technol. 1999, 20, 355–365. [Google Scholar] [CrossRef]
- Pedizzi, C.; Lema, J.M.; Carballa, M. A combination of ammonia stripping and low temperature thermal pre-treatment improves anaerobic post-digestion of the supernatant from organic fraction of municipal solid waste treatment. Waste Manag. 2018, 78, 271–278. [Google Scholar] [CrossRef]
- Yellezuome, D.; Zhu, X.; Wang, Z.; Liu, R. Mitigation of ammonia inhibition in anaerobic digestion of nitrogen-rich substrates for biogas production by ammonia stripping: A review. Renew. Sustain. Energy Rev. 2022, 157, 112043. [Google Scholar] [CrossRef]
- Cuŝtin, S.; Marinŝek-Logar, R. Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process Saf. Environ. Prot. 2011, 89, 61–66. [Google Scholar]
- Li, Y.; Zhu, J.; Tang, Y.; Shi, X.; Anwar, S.; Wang, J.; Gao, L.; Zhang, J. Impact of varying mass concentrations of ammonia nitrogen on biogas production and system stability of anaerobic fermentation. Agriculture 2023, 13, 1645. [Google Scholar] [CrossRef]
- Moestedt, J.; Müller, B.; Westerholm, M.; Schnürer, A. Ammonia threshold for inhibition of anaerobic digestion of thin stillage and the importance of organic loading rate. Microb. Biotechnol. 2016, 9, 180–194. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association and Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Su, Y. Detection Technology of Biogas Fermentation; Metallurgical Industry Press: Beijing, China, 2011. [Google Scholar]
- HJ537–2009; Chinese Standard. Water Quality-Determination of Ammonia Nitrogen-Distillation-Neutralization Titration. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2009. Available online: https://www.chinesestandard.net/PDF/English.aspx/HJ537-2009 (accessed on 7 July 2024).
- Li, K.; Liu, R.; Qiong, Y.; Ma, R. Removal of nitrogen from chicken manure anaerobic digestion for enhanced biomethanization. Fuel 2018, 232, 395–404. [Google Scholar] [CrossRef]
- Han, Y.; Agyeman, F.; Green, H.; Tao, W. Stable, high-rate anaerobic digestion through vacuum stripping of digestate. Bioresour. Technol. 2022, 343, 126133. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, Y.; Yang, P.; Wang, X.; Peng, Y.; Li, L. Response of process performance and microbial community to ammonia stress in series batch experiments. Bioresour. Technol. 2020, 314, 123768. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Cai, Y.; Zhang, Y.; Zhao, Y.; Gao, Y.; Cui, Z.; Hu, Y.; Wang, X. The effects of C/N (10–25) on the relationship of substrates, metabolites, and microorganisms in “inhibited steady-state” of anaerobic digestion. Water Res. 2021, 188, 116466. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Xue, J.; Shi, C.; Yue, Z.; Peng, S. Effect of exogenous ammonia nitrogen on anaerobic digestion process of corn stover. Environ. Sci. Technol. 2013, 36, 142–147. [Google Scholar]
- Song, Y.; Hu, W.; Qiao, W.; Westerholm, M.; Wandera, S.M. Upgrading the performance of high solids feeding anaerobic digestion of chicken manure under extremely high ammonia level. Renew. Energy 2022, 194, 13–20. [Google Scholar] [CrossRef]
- Khalideh, A.; Abdullah, H. Anaerobic co-digestion mill wastewater-activated sludge effect of aerobic pretreatment on the performance of OMW anaerobic digestion. Waste Biomass Valorization 2020, 1, 4781–4788. [Google Scholar]
- Li, Y.; Zhang, S.; Chen, Z.; Huang, W. Biogas slurry reflux enhances the organic loading rate of high-solid anaerobic digestion of kitchen waste by alleviating fatty acids accumulation. Chem. Eng. J. 2024, 482, 149072. [Google Scholar] [CrossRef]
- Gonzalez-Femandez, N.; Pedizzi, C.; Lema, J.M.; Carballa, M. Air-side ammonia stripping coupled to anaerobic digestion indirectly impacts anaerobic microbiome. Microb. Biotechnol. 2019, 12, 1403–1416. [Google Scholar] [CrossRef]
- Rodriguez-Verde, I.; Regueiro, L.; Lema, J.M.; Carballa, M. Blending based optimisation and pretreatment strategies to enhance anaerobic digestion of poultry manure. Waste Manag. 2018, 71, 521–531. [Google Scholar] [CrossRef]
- Evans, P.N.; Parks, D.H.; Chadwick, G.L.; Robbins, S.J.; Orphan, V.J.; Golding, S.D.; Tyson, G.W. Methane metabolism in the archaeal phylum Bathyarchaeota revealed by genome-centric metagenomics. Science 2015, 350, 6259. [Google Scholar] [CrossRef]
- Maus, I.; Rumming, M.; Bergmann, I.; Heeg, K.; Pohl, M. Characterization of Bathyarchaeota genomes assembled from metagenomes of biofilms residing in mesophilic and thermophilic biogas reactors. Biotechnol. Biofuels 2018, 11, 167. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Achinas, S.; Zhang, Z.; Krooneman, J.; Willem, G. The biomethanation of cow manure in a continuous anaerobic digester can be boosted via a bioaugmentation culture containing Bathyarchaeota. Sci. Total Environ. 2020, 745, 141042. [Google Scholar] [CrossRef]
- Westerholm, M.; Moestedt, J.; Schnürer, A. Biogas production through syntrophic acetate oxidation and deliberate operating strategies for improved digester performance. Appl. Energy 2016, 179, 124–135. [Google Scholar] [CrossRef]

| Materials | TS/% | VS/% | C/N | pH |
|---|---|---|---|---|
| Chicken manure | 50.40 | 40.93 | 15.31 | 7.56 |
| Corn straw | 90.46 | 80.92 | 42.07 | ND |
| Inoculum | 7.73 | 5.37 | ND | 7.23 |
| Digester | HRT/d | Reflux Ratio/% | Air Stripping | OLR/gVS/(L·d) | ||
|---|---|---|---|---|---|---|
| Stage I 0~42 d | Stage II 43~84 d | Stage III 85~126 d | ||||
| R1 | 21 | 50 | Y | 3.3 | 5.3 | 8.0 |
| R2 | 21 | 50 | N | 3.3 | 5.3 | 8.0 |
| R3 | 21 | 75 | Y | 3.3 | 5.3 | 8.0 |
| R4 | 21 | 75 | N | 3.3 | 5.3 | 8.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Zhang, J.; Tang, Y.; Zhang, X.; Shi, X.; Wang, X.; Li, Y. Enhancement of Fermentation Performance in the Anaerobic Co-Digestion of Chicken Manure and Corn Straw under Biogas Slurry Reflux via Air Stripping of the Digestate. Agronomy 2024, 14, 1794. https://doi.org/10.3390/agronomy14081794
Zhu J, Zhang J, Tang Y, Zhang X, Shi X, Wang X, Li Y. Enhancement of Fermentation Performance in the Anaerobic Co-Digestion of Chicken Manure and Corn Straw under Biogas Slurry Reflux via Air Stripping of the Digestate. Agronomy. 2024; 14(8):1794. https://doi.org/10.3390/agronomy14081794
Chicago/Turabian StyleZhu, Jiaoning, Jingxuan Zhang, Yun Tang, Xiaoyuan Zhang, Xiangyuan Shi, Xiuhong Wang, and Yongping Li. 2024. "Enhancement of Fermentation Performance in the Anaerobic Co-Digestion of Chicken Manure and Corn Straw under Biogas Slurry Reflux via Air Stripping of the Digestate" Agronomy 14, no. 8: 1794. https://doi.org/10.3390/agronomy14081794
APA StyleZhu, J., Zhang, J., Tang, Y., Zhang, X., Shi, X., Wang, X., & Li, Y. (2024). Enhancement of Fermentation Performance in the Anaerobic Co-Digestion of Chicken Manure and Corn Straw under Biogas Slurry Reflux via Air Stripping of the Digestate. Agronomy, 14(8), 1794. https://doi.org/10.3390/agronomy14081794





