Stress Response of Citrus Leaves under Mechanical Damage and Huanglongbing Disease Infection Using Plasmonic TiO2 Nanotube Substrate-Based Imprinting Mass Spectrometry Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of the Pesticide Standards and AuNPs
2.3. Preparation of the AuNP-hPDA-TDNT Substrate
2.4. Preparation of Imprinted Citrus Leaves That Were Infected by Huanglongbing Disease, Mechanically Damaged, and Fed Larvae for MSI Analysis
2.5. Metabolite Extraction of Citrus Leaves for Ultra-Performance Liquid Chromatography Cou-Pled with Mass Spectrometry (UPLC–MS) Experiments
2.6. UPLC-MS Conditions for Metabolite Identification in Citrus Leaves
2.7. SALDI-MS and Imprinting MSI Experiments
2.8. Data Analysis
3. Results and Discussion
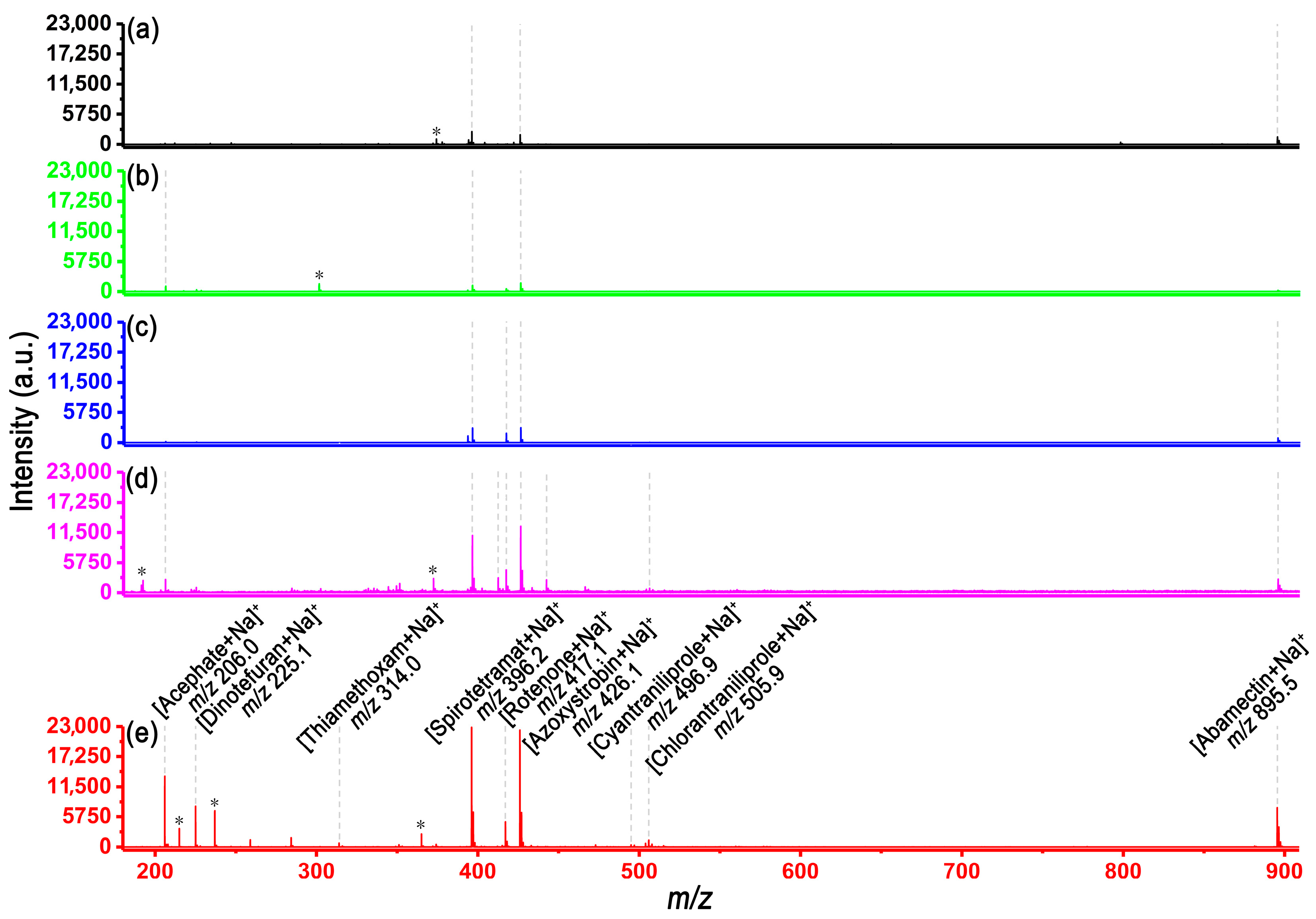
3.1. SALDI-MS Analysis of Various Pesticide Molecules
3.2. Imprinting SALDI-MSI of Citrus Leaves
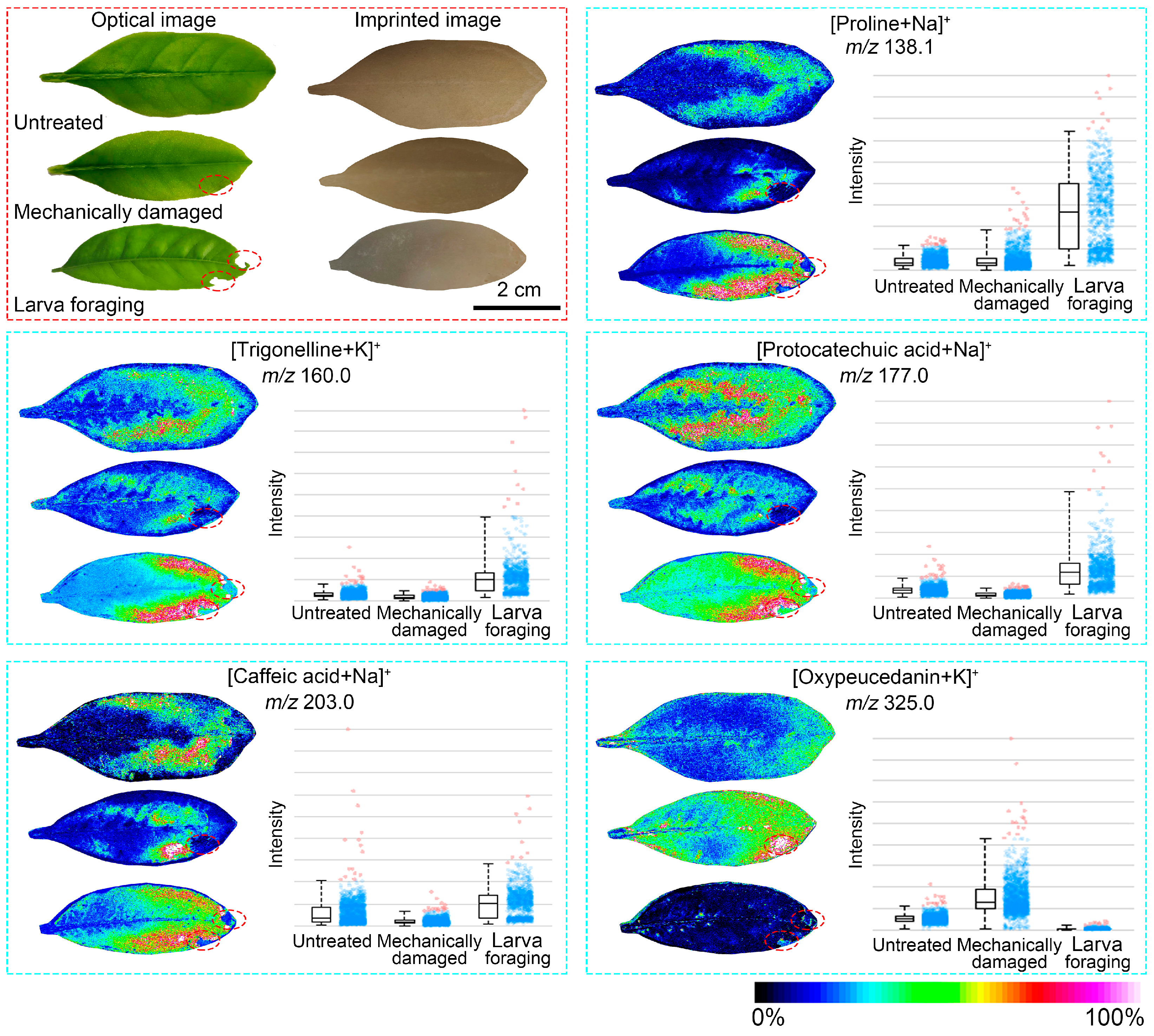
3.3. Stress Response of Citrus Leaves to Insect Feeding and Mechanical Damage
3.4. Stress Response of Citrus after Mechanical Damage for Different Repair Durations
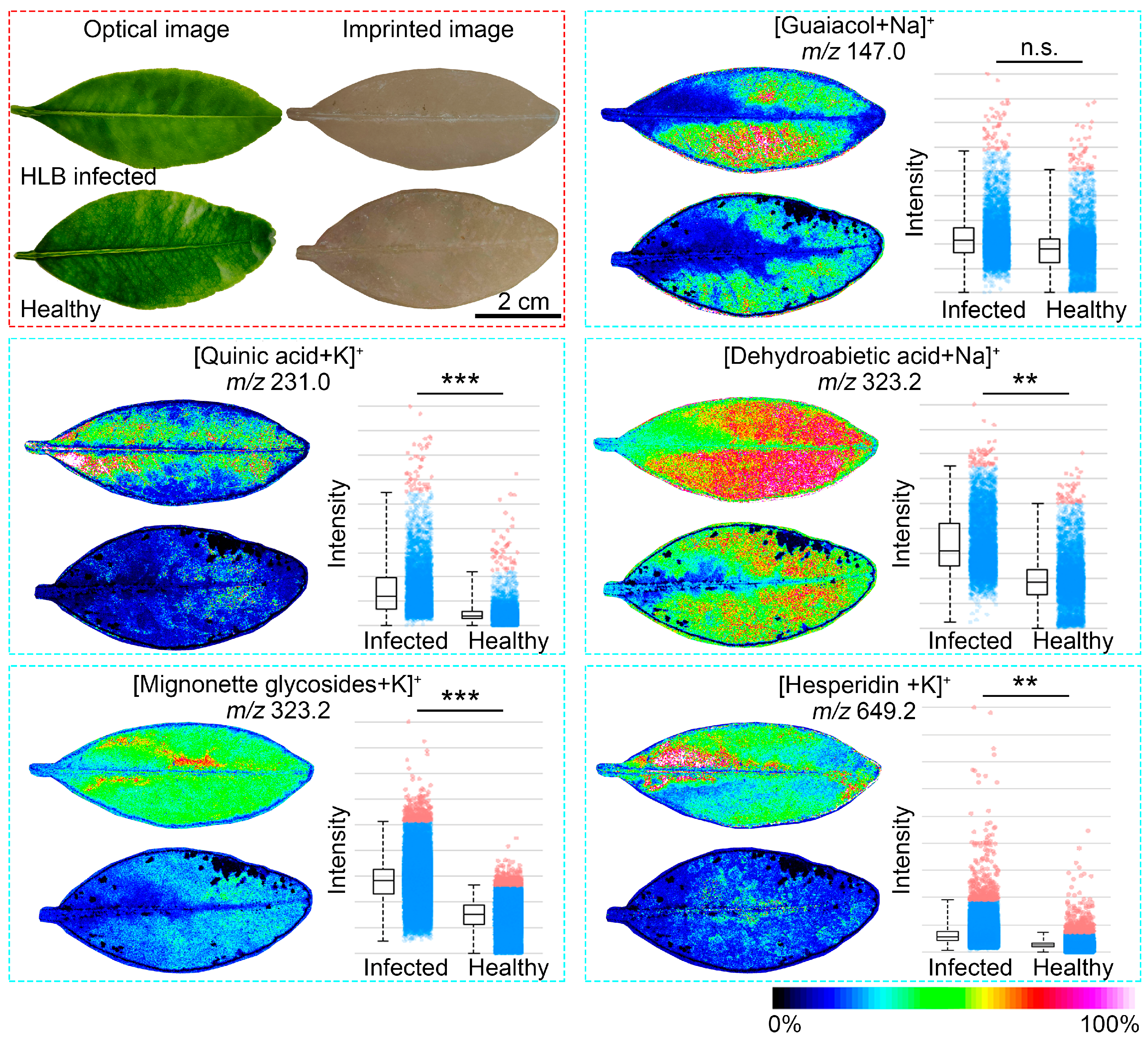
3.5. Stress Response of Citrus Leaves during Infection with HLB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gottwald, T.R. Current epidemiological understanding of citrus Huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Pang, Z.; Huang, X.; Xu, J.; Pandey, S.S.; Li, J.; Achor, D.S.; Vasconcelos, F.N.C.; Hendrich, C.; Huang, Y.; et al. Citrus Huanglongbing is a pathogen-triggered immune disease that can be mitigated with antioxidants and gibberellin. Nat. Commun. 2022, 13, 529. [Google Scholar] [CrossRef]
- Li, X.; Ruan, H.; Zhou, C.; Meng, X.; Chen, W. Controlling citrus Huanglongbing: Green sustainable development route is the future. Front. Plant Sci. 2021, 12, 760481. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, A.; Wang, S.; Hao, Y.; Cui, M.; Liu, L.; Luo, L. Metabolic response of Citrus limon to Asian citrus psyllid infestation revealed by EESI-MS and HPLC. Anal. Biochem. 2020, 609, 113973. [Google Scholar] [CrossRef]
- Lee, J.A.; Halbert, S.E.; Dawson, W.O.; Robertson, C.J.; Keesling, J.E.; Singer, B.H. Asymptomatic spread of Huanglongbing and implications for disease control. Proc. Natl. Acad. Sci. USA 2015, 112, 7605–7610. [Google Scholar] [CrossRef]
- Wang, K.; Liao, Y.; Meng, Y.; Jiao, X.; Huang, W.; Liu, T.C.-y. The early, rapid, and non-destructive detection of citrus Huanglongbing (HLB) based on microscopic confocal Raman. Food Anal. Methods 2019, 12, 2500–2508. [Google Scholar] [CrossRef]
- Xue, A.; Liu, Y.; Li, H.; Cui, M.; Huang, X.; Wang, W.; Wu, D.; Guo, X.; Hao, Y.; Luo, L. Early detection of Huanglongbing with EESI-MS indicates a role of phenylpropanoid pathway in citrus. Anal. Biochem. 2022, 639, 114511. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.; Vergara, F.; Muck, A.; Svatoš, A.; Gershenzon, J. Nonuniform distribution of glucosinolates in Arabidopsis thaliana leaves has important consequences for plant defense. Proc. Natl. Acad. Sci. USA 2008, 105, 6196–6201. [Google Scholar] [CrossRef]
- Yan, S.; Bhawal, R.; Yin, Z.; Thannhauser, T.W.; Zhang, S. Recent advances in proteomics and metabolomics in plants. Mol. Hortic. 2022, 2, 17. [Google Scholar] [CrossRef]
- Aharoni, A.; Goodacre, R.; Fernie, A.R. Plant and microbial sciences as key drivers in the development of metabolomics research. Proc. Natl. Acad. Sci. USA 2023, 120, e2217383120. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.A.; Fernie, A.R. Evolutionary history of plant metabolism. Annu. Rev. Plant Biol. 2021, 72, 185–216. [Google Scholar] [CrossRef]
- Perez de Souza, L.; Alseekh, S.; Scossa, F.; Fernie, A.R. Ultra-high-performance liquid chromatography high-resolution mass spectrometry variants for metabolomics research. Nat. Methods 2021, 18, 733–746. [Google Scholar] [CrossRef]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass spectrometry-based metabolomics: A guide for annotation, quantification and best reporting practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Horn, P.J.; Chapman, K.D. Imaging plant metabolism in situ. J. Exp. Bot. 2023, 75, 1654–1670. [Google Scholar] [CrossRef]
- Yin, Z.; Huang, W.; Fernie, A.R.; Yan, S. Mass spectrometry imaging techniques: A versatile toolbox for plant metabolomics. Trends Plant Sci. 2023, 28, 250–251. [Google Scholar] [CrossRef]
- Yin, Z.; Dong, T.; Huang, W.; Du, M.; Chen, D.; Fernie, A.R.; Yi, G.; Yan, S. Spatially resolved metabolomics reveals variety-specific metabolic changes in banana pulp during postharvest senescence. Food Chem. X 2022, 15, 100371. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Li, P.; Du, M.; Ma, L.; Huang, Y.; Yin, Z.; Zhang, Y.; Chen, D.; Xu, H.; Wu, X. Spatiotemporal visualization of insecticides and fungicides within fruits and vegetables using gold nanoparticle-immersed paper imprinting mass spectrometry imaging. Nanomaterials 2021, 11, 1327. [Google Scholar] [CrossRef]
- Yin, Z.; Huang, W.; Li, K.; Fernie, A.R.; Yan, S. Advances in mass spectrometry imaging for plant metabolomics—Expanding the analytical toolbox. Plant J. 2024; early view. [Google Scholar] [CrossRef]
- Wang, T.; Cheng, X.; Xu, H.; Meng, Y.; Yin, Z.; Li, X.; Hang, W. Perspective on advances in laser-based high-resolution mass spectrometry imaging. Anal. Chem. 2020, 92, 543–553. [Google Scholar] [CrossRef]
- Ma, S.; Leng, Y.; Li, X.; Meng, Y.; Yin, Z.; Hang, W. High spatial resolution mass spectrometry imaging for spatial metabolomics: Advances, challenges, and future perspectives. TrAC Trends Anal. Chem. 2023, 159, 116902. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Zeng, H.-S.; Jin, H.L.; Wang, H.B. Applications of mass spectrometry imaging in botanical research. Adv. Biotechnol. 2024, 2, 6. [Google Scholar] [CrossRef]
- Dong, Y.; Aharoni, A. Image to insight: Exploring natural products through mass spectrometry imaging. Nat. Prod. Rep. 2022, 39, 1510–1530. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.H.; Verdin, A.; De Pauw, E.; Malherbe, C.; Eppe, G. Surface-assisted laser desorption/ionization mass spectrometry imaging: A review. Mass Spectrom. Rev. 2022, 41, 373–420. [Google Scholar] [CrossRef]
- Lu, M.; Yang, X.; Yang, Y.; Qin, P.; Wu, X.; Cai, Z. Nanomaterials as assisted matrix of laser desorption/ionization time-of-flight mass spectrometry for the analysis of small molecules. Nanomaterials 2017, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Pomastowski, P.; Buszewski, B. Complementarity of matrix- and nanostructure-assisted laser desorption/ionization approaches. Nanomaterials 2019, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, B.; Malitsky, S.; Rogachev, I.; Aharoni, A.; Kaftan, F.; Svatoš, A.; Franceschi, P. Sample preparation for mass spectrometry imaging of plant tissues: A review. Front. Plant Sci. 2016, 7, 60. [Google Scholar] [CrossRef]
- Wu, X.; Qin, R.; Wu, H.; Yao, G.; Zhang, Y.; Li, P.; Xu, Y.; Zhang, Z.; Yin, Z.; Xu, H. Nanoparticle-immersed paper imprinting mass spectrometry imaging reveals uptake and translocation mechanism of pesticides in plants. Nano Res. 2020, 13, 611–620. [Google Scholar] [CrossRef]
- Xia, J.; Lou, G.; Zhang, L.; Huang, Y.; Yang, J.; Guo, J.; Qi, Z.; Li, Z.; Zhang, G.; Xu, S.; et al. Unveiling the spatial distribution and molecular mechanisms of terpenoid biosynthesis in Salvia miltiorrhiza and S. grandifolia using multi-omics and DESI–MSI. Hortic. Res. 2023, 10, uhad109. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, R.G.; Pradeep, T. Understanding the molecular signatures in leaves and flowers by desorption electrospray ionization mass spectrometry (DESI MS) imaging. J. Agric. Food Chem. 2013, 61, 7477–7487. [Google Scholar] [CrossRef]
- Hemalatha, R.G.; Ganayee, M.A.; Pradeep, T. Electrospun nanofiber mats as “smart surfaces” for desorption electrospray ionization mass spectrometry (DESI MS)-based analysis and imprint imaging. Anal. Chem. 2016, 88, 5710–5717. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Buriak, J.M.; Siuzdak, G. Desorption-ionization mass spectrometry on porous silicon. Nature 1999, 399, 243–246. [Google Scholar] [CrossRef]
- Samarah, L.Z.; Tran, T.H.; Stacey, G.; Vertes, A. Mass spectrometry imaging of bio-oligomer polydispersity in plant tissues by laser desorption ionization from silicon nanopost arrays. Angew. Chem. Int. Ed. 2021, 60, 9071–9077. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Z.; Wang, T.; Jiang, X.; Qu, X.; Duan, W.; Xi, F.; He, Z.; Wu, J. Tissue imprinting on 2D nanoflakes-capped silicon nanowires for lipidomic mass spectrometry imaging and cancer diagnosis. ACS Nano 2022, 16, 6916–6928. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Du, M.; Huang, Y.; Xu, Y.; Chen, Y.; Ma, L.; Xie, Q.; Zhu, X.; Chen, Z.; Xu, H.; et al. Plasmonic polydopamine-modified TiO2 nanotube substrates for surface-assisted laser desorption/ionization mass spectrometry imaging. Nano Res. 2023, 16, 3028–3039. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, W.; Zhang, H.; Zhong, M.; Cao, W.; Li, Z.; Huang, X.; Nie, Z.; Liu, J.; Li, P.; et al. Polydopamine-modified substrates for high-sensitivity laser desorption ionization mass spectrometry imaging. ACS Appl. Mater. Interfaces 2019, 11, 46140–46148. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, H.; Jia, J.; Zhang, X.; Ma, X.; Zhong, M.; Ouyang, Z. Antireflection surfaces for biological analysis using laser desorption ionization mass spectrometry. Research 2018, 2018, 5439729. [Google Scholar] [CrossRef] [PubMed]
- Sakai, R.; Kondo, H.; Ishikawa, K.; Ohta, T.; Hiramatsu, M.; Tanaka, H.; Hori, M. Effects of high-quality carbon nanowalls ionization-assisting substrates on surface-assisted laser desorption/ionization mass spectrometry performance. Nanomaterials 2023, 13, 63. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Na, H.-K.; Kwack, S.-J.; Ryoo, S.-R.; Lee, Y.; Hong, S.; Hong, S.; Jeong, Y.; Min, D.-H. Synergistic effect of graphene oxide/MWCNT films in laser desorption/ionization mass spectrometry of small molecules and tissue imaging. ACS Nano 2011, 5, 4550–4561. [Google Scholar] [CrossRef]
- Iakab, S.A.; Ràfols, P.; Tajes, M.; Correig-Blanchar, X.; García-Altares, M. Gold nanoparticle-assisted black silicon substrates for mass spectrometry imaging applications. ACS Nano 2020, 14, 6785–6794. [Google Scholar] [CrossRef]
- Sun, S.; Liu, W.; Yang, J.; Wang, H.; Qian, K. Nanoparticle-assisted cation adduction and fragmentation of small metabolites. Angew. Chem. Int. Ed. 2021, 60, 11310–11317. [Google Scholar] [CrossRef]
- Manjavacas, A.; Liu, J.G.; Kulkarni, V.; Nordlander, P. Plasmon-induced hot carriers in metallic nanoparticles. ACS Nano 2014, 8, 7630–7638. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, E.P.; Su, C.-H.; Lee, H.-J.; Hsu, C.-C.; Yang, Y.-L. Visualizing vinca alkaloids in the petal of Catharanthus roseus using functionalized titanium oxide nanowire substrate for surface-assisted laser desorption/ionization imaging mass spectrometry. Plant J. 2021, 105, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-N.; Li, B. Monolithic gold nanoparticles/thiol-β-cyclodextrin-functionalized TiO2 nanowires for enhanced SALDI MS detection and imaging of natural products. Anal. Chem. 2022, 94, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xu, Y.; Huang, Y.; Chen, Y.; Zhao, Y.; Yan, B.; Zhu, X.; Chen, Z.; Xu, H.; Yin, Z.; et al. Mapping molecular signatures in plant leaves, flowers, and fruits by a TiO2 nanotube-based plasmonic chip for imprint mass spectrometry imaging. ACS Agric. Sci. Technol. 2023, 3, 119–130. [Google Scholar] [CrossRef]
- Wu, Q.; Chu, J.L.; Rubakhin, S.S.; Gillette, M.U.; Sweedler, J.V. Dopamine-modified TiO2 monolith-assisted LDI MS imaging for simultaneous localization of small metabolites and lipids in mouse brain tissue with enhanced detection selectivity and sensitivity. Chem. Sci. 2017, 8, 3926–3938. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Huang, L.; Zhang, R.; Xu, W.; Huang, J.; Gurav, D.D.; Vedarethinam, V.; Chen, R.; Lou, J.; Wang, Q.; et al. Metabolic fingerprinting on a plasmonic gold chip for mass spectrometry based in vitro diagnostics. ACS Cent. Sci. 2018, 4, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Mohammadin, K.; Shafique, Z.; Amjad, S.T.; Asad, M.H.H.b. Citrus flavonoids as potential therapeutic agents: A review. Phytother. Res. 2022, 36, 1417–1441. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Liu, X.; Srivastava, A.K.; Tan, Q.; Low, W.; Yan, X.; Wu, S.; Sun, X.; Hu, C. Foliar nutrition alleviate citrus plants from Asian citrus psyllid feeding by affecting leaf structure and secondary metabolism. Sci. Hortic. 2023, 309, 111667. [Google Scholar] [CrossRef]
- Galili, G.; Amir, R.; Fernie, A.R. The regulation of essential amino acid synthesis and accumulation in plants. Annu. Rev. Plant Biol. 2016, 67, 153–178. [Google Scholar] [CrossRef] [PubMed]
- Galili, G.; Amir, R. Fortifying plants with the essential amino acids lysine and methionine to improve nutritional quality. Plant Biotechnol. J. 2013, 11, 211–222. [Google Scholar] [CrossRef]
- Gonda, I.; Lev, S.; Bar, E.; Sikron, N.; Portnoy, V.; Davidovich-Rikanati, R.; Burger, J.; Schaffer, A.A.; Tadmor, Y.; Giovannonni, J.J.; et al. Catabolism of L–methionine in the formation of sulfur and other volatiles in melon (Ucumis melo L.) fruit. Plant J. 2013, 74, 458–472. [Google Scholar] [CrossRef]
- Roje, S. S-Adenosyl-L-methionine: Beyond the universal methyl group donor. Phytochemistry 2006, 67, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.Y.; Sugiyama, K.; Sawada, Y.; Tohge, T.; Obayashi, T.; Suzuki, A.; Araki, R.; Sakurai, N.; Suzuki, H.; Aoki, K.; et al. Omics-based identification of Arabidopsis Myb transcription factors regulating aliphatic glucosinolate biosynthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 6478–6483. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Matsukawa, T.; Kajiyama, S. Metabolomic analysis of primary metabolites in citrus leaf during defense responses. J. Biosci. Bioeng. 2017, 123, 376–381. [Google Scholar] [CrossRef] [PubMed]
- de Moraes Pontes, J.G.; Vendramini, P.H.; Fernandes, L.S.; de Souza, F.H.; Pilau, E.J.; Eberlin, M.N.; Magnani, R.F.; Wulff, N.A.; Fill, T.P. Mass spectrometry imaging as a potential technique for diagnostic of Huanglongbing disease using fast and simple sample preparation. Sci. Rep. 2020, 10, 13457. [Google Scholar] [CrossRef]
- Hijaz, F.M.; Manthey, J.A.; Folimonova, S.Y.; Davis, C.L.; Jones, S.E.; Reyes-De-Corcuera, J.I. An HPLC-MS characterization of the changes in sweet orange leaf metabolite profile following infection by the bacterial pathogen Candidatus Liberibacter asiaticus. PLoS ONE 2013, 8, e79485. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Hijaz, F.; Davis, C.; Folimonova, S.; Manthey, J.; Reyes-De-Corcuera, J. GC-MS analysis of secondary metabolites in leaves from orange trees infected with HLB: A 9-month study. Proc. Fla. State Hortic. Soc. 2012, 125, 75–83. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Chen, D.; Chen, X.; Wu, X. Stress Response of Citrus Leaves under Mechanical Damage and Huanglongbing Disease Infection Using Plasmonic TiO2 Nanotube Substrate-Based Imprinting Mass Spectrometry Imaging. Agronomy 2024, 14, 1797. https://doi.org/10.3390/agronomy14081797
Sun Y, Chen D, Chen X, Wu X. Stress Response of Citrus Leaves under Mechanical Damage and Huanglongbing Disease Infection Using Plasmonic TiO2 Nanotube Substrate-Based Imprinting Mass Spectrometry Imaging. Agronomy. 2024; 14(8):1797. https://doi.org/10.3390/agronomy14081797
Chicago/Turabian StyleSun, Yaming, Dong Chen, Xiran Chen, and Xinzhou Wu. 2024. "Stress Response of Citrus Leaves under Mechanical Damage and Huanglongbing Disease Infection Using Plasmonic TiO2 Nanotube Substrate-Based Imprinting Mass Spectrometry Imaging" Agronomy 14, no. 8: 1797. https://doi.org/10.3390/agronomy14081797
APA StyleSun, Y., Chen, D., Chen, X., & Wu, X. (2024). Stress Response of Citrus Leaves under Mechanical Damage and Huanglongbing Disease Infection Using Plasmonic TiO2 Nanotube Substrate-Based Imprinting Mass Spectrometry Imaging. Agronomy, 14(8), 1797. https://doi.org/10.3390/agronomy14081797





