The Promotion of Dark Septate Endophytes on the Performance and Active Ingredients Accumulation of Astragalus mongholicus under Cadmium Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Materials and Culture Substrate
2.2. Inoculation Assay
2.3. DSE Colonization of Plant Roots
2.4. Plant Growth Parameters and Cd Contents
2.5. IAA and Active Ingredient Content
2.6. Soil Physicochemical Properties
2.7. Statistical Analyses
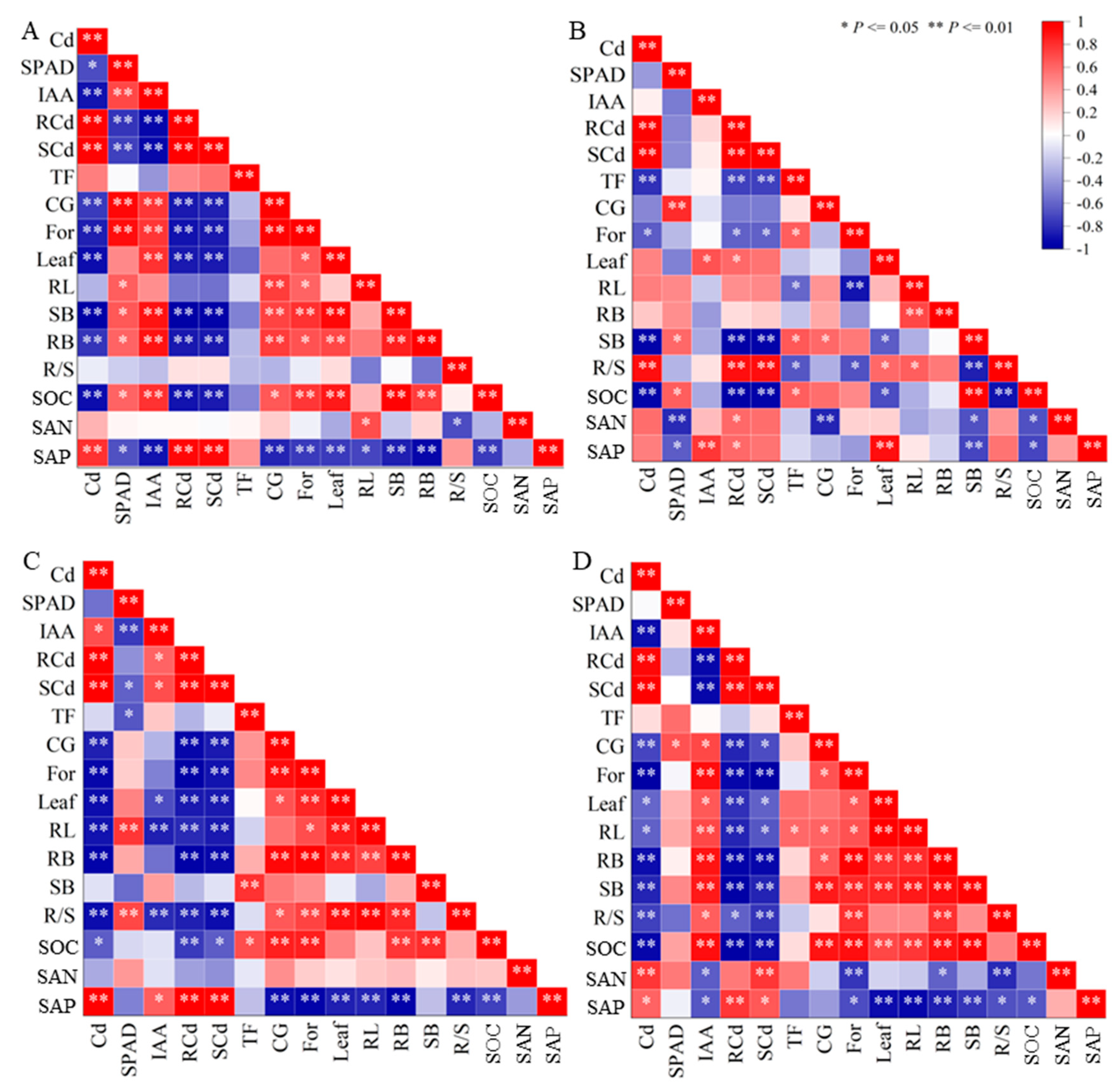
3. Results
3.1. Quantification of DSE Colonization
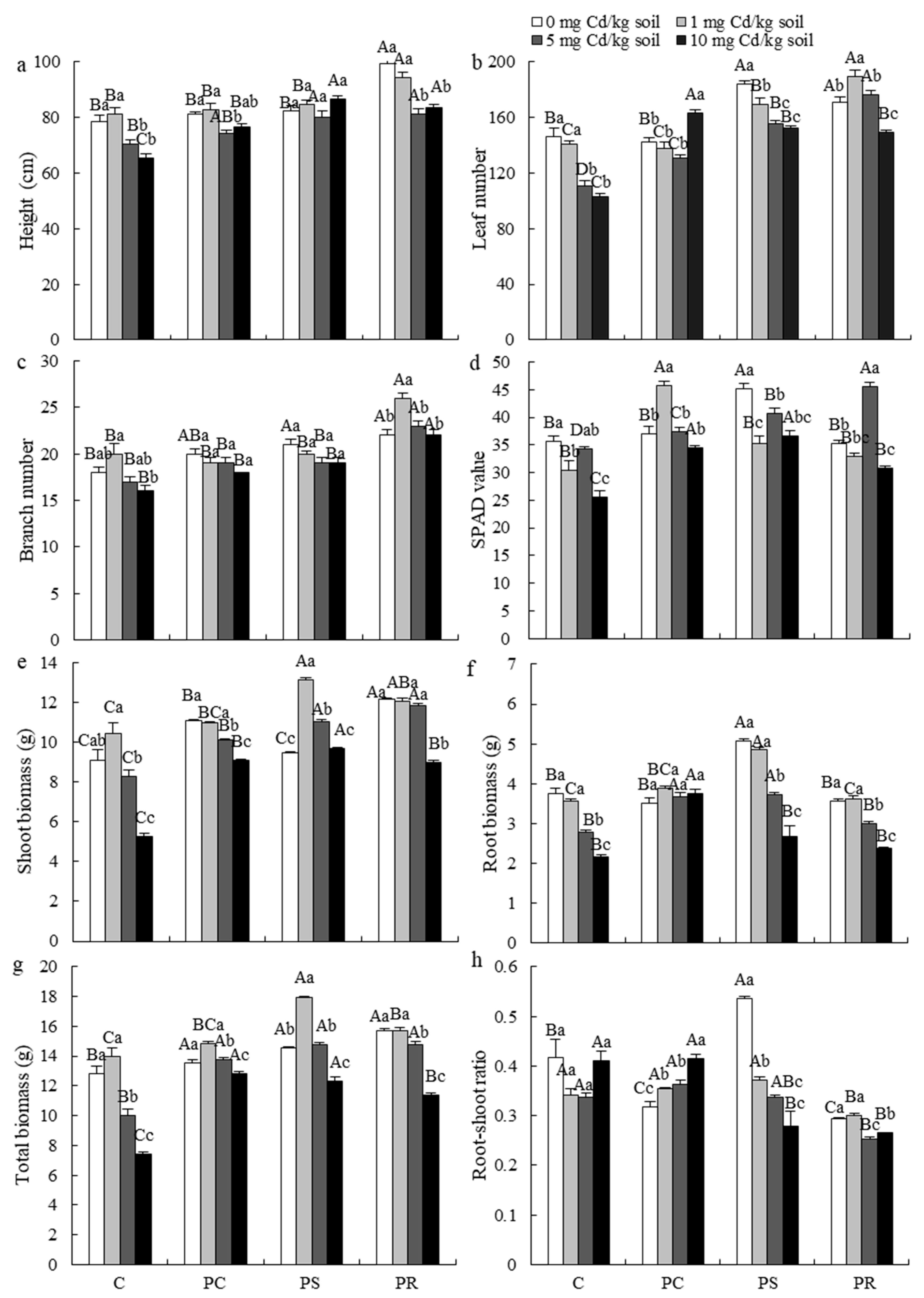
3.2. Plant Growth Parameters
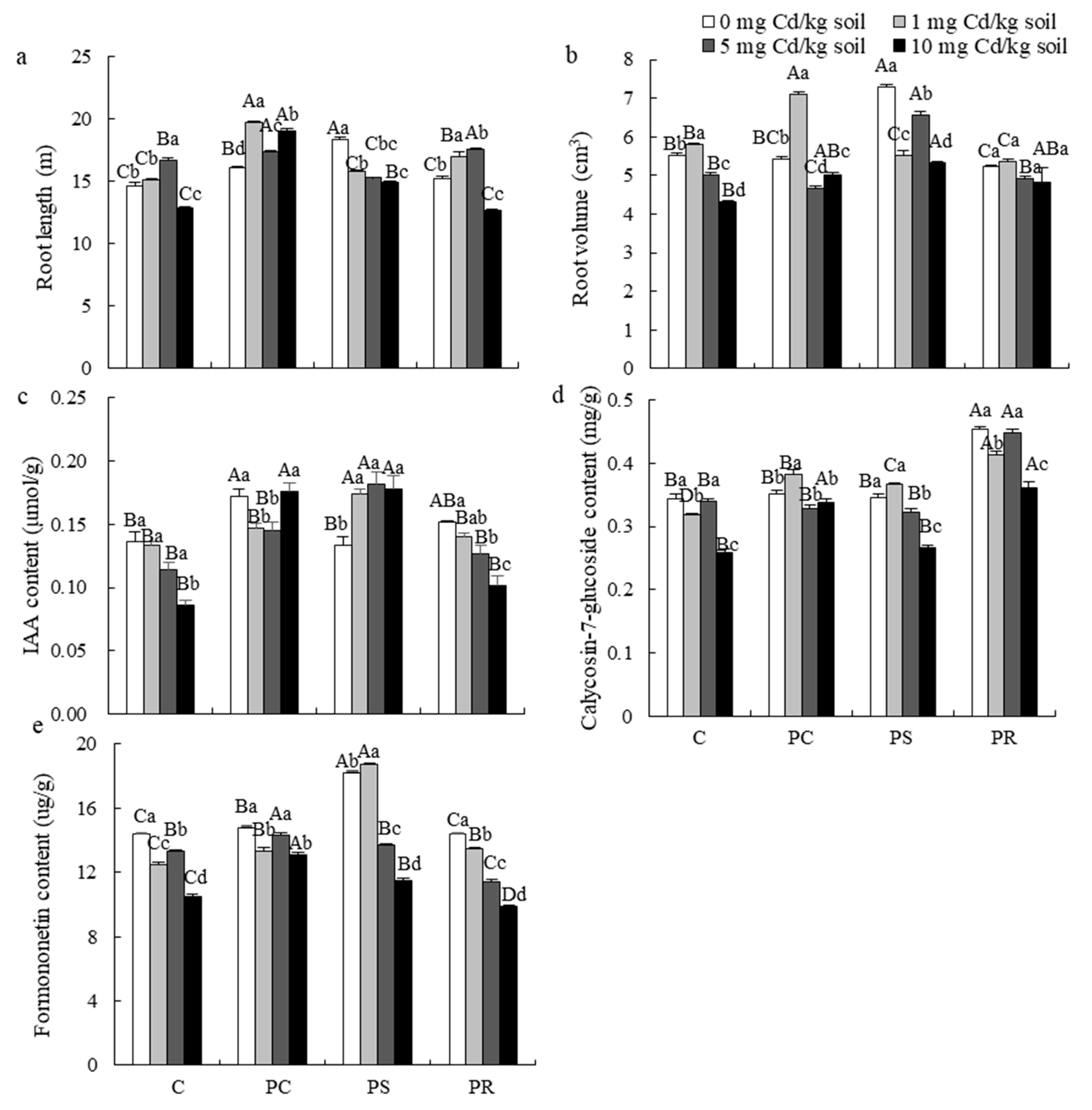
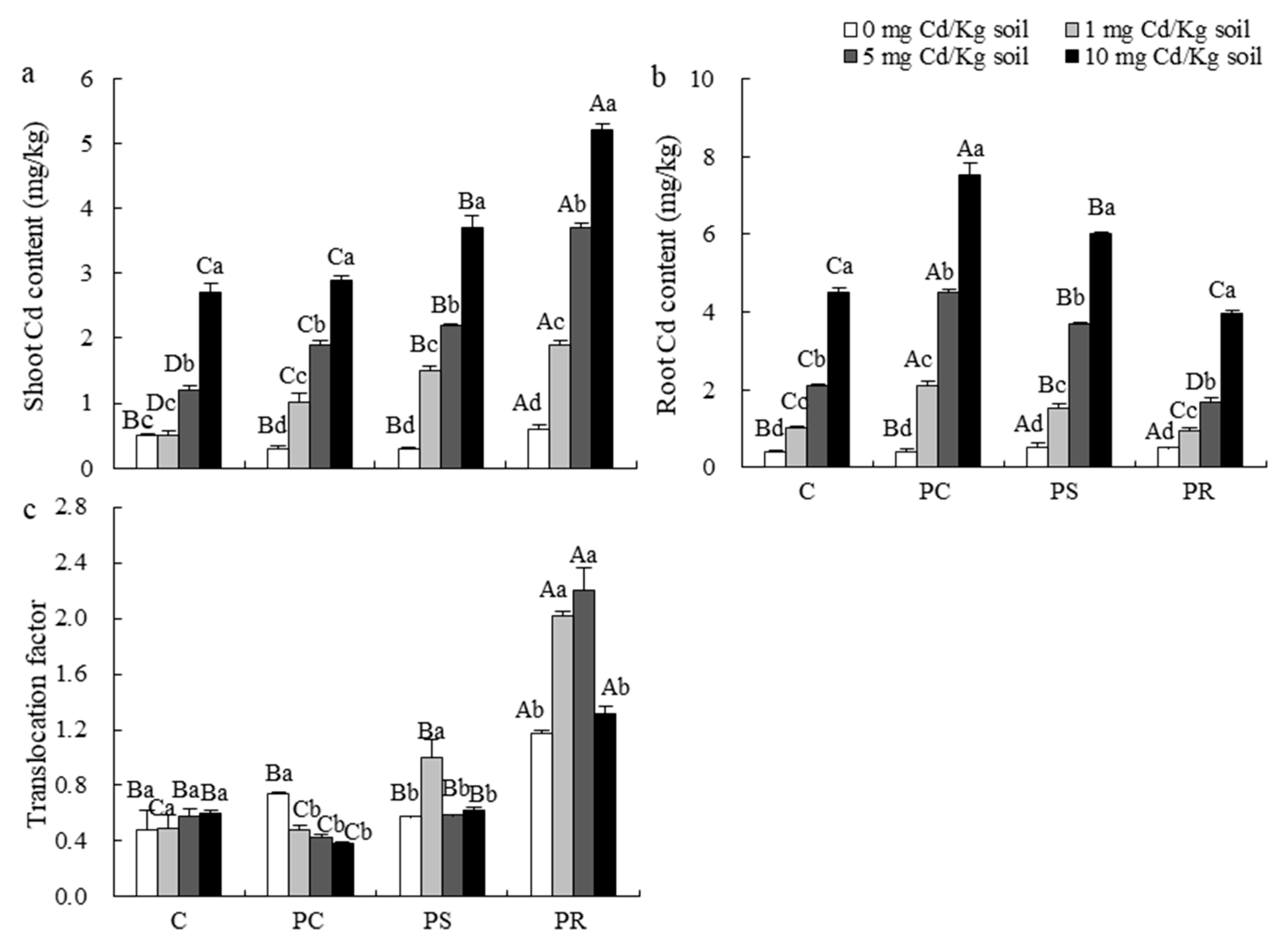
3.3. IAA, Active Ingredients and Cd Content
3.4. Soil Physicochemical Properties
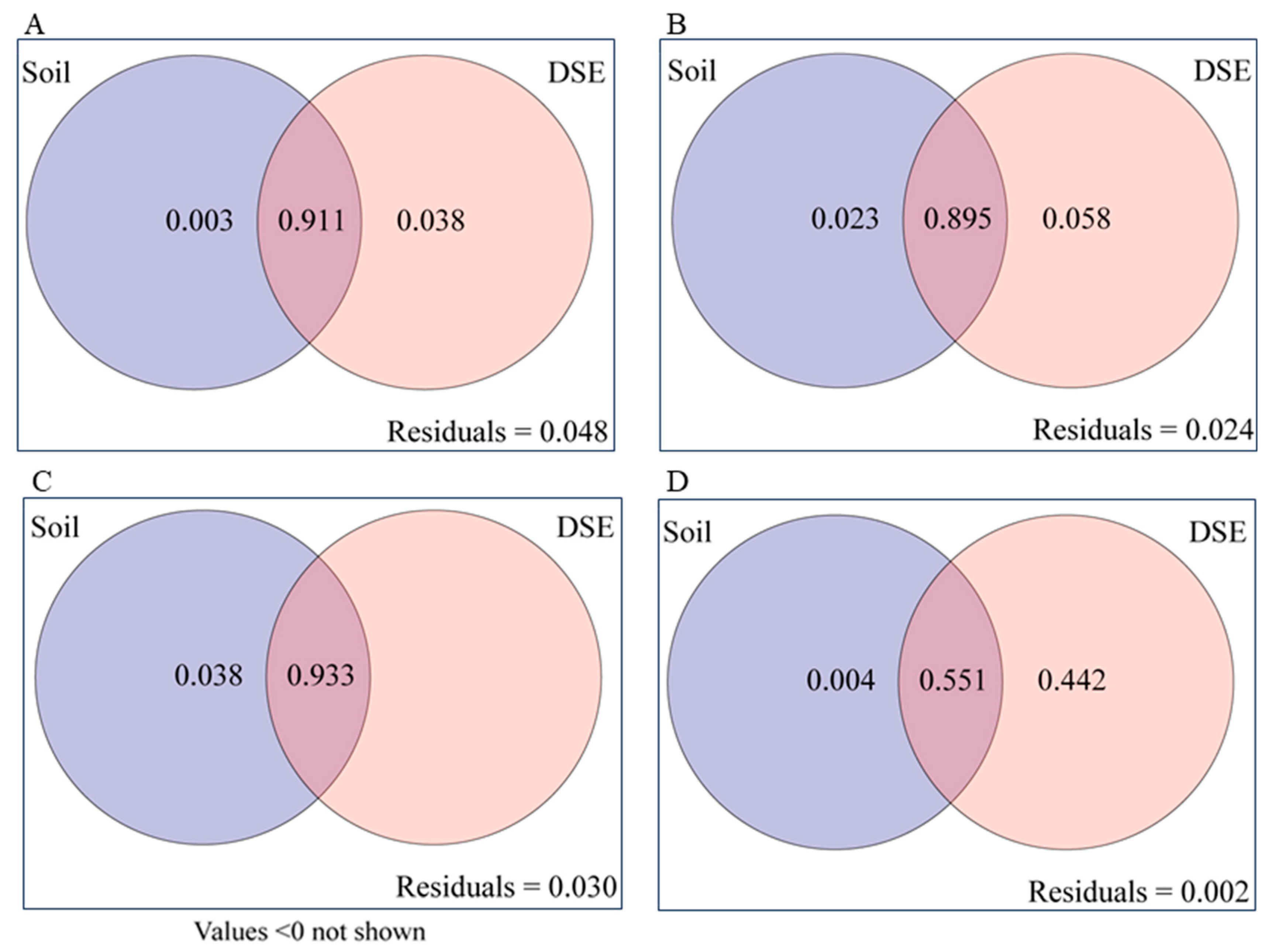
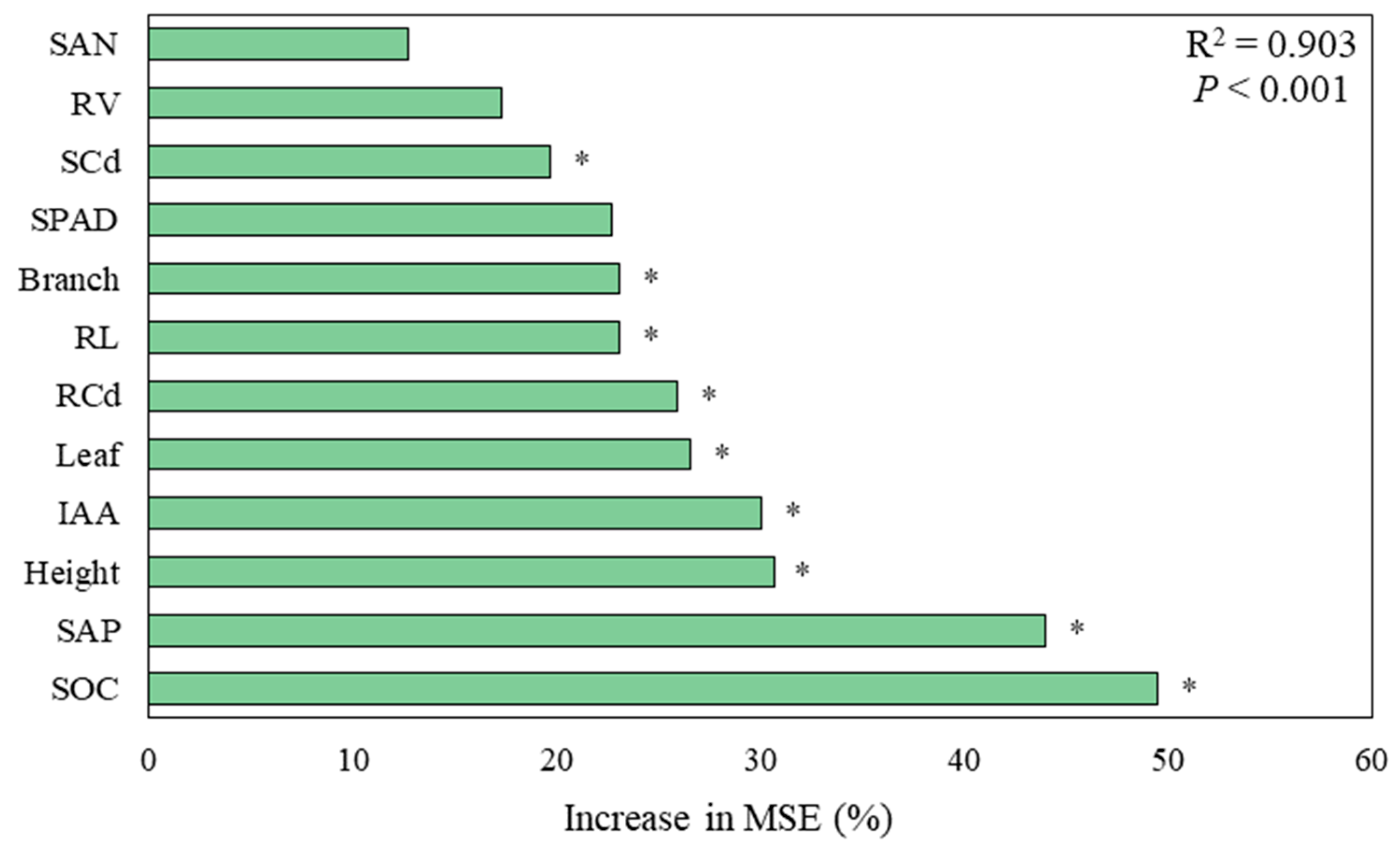
3.5. Factors Affecting the Growth of the Host Plant
4. Discussion
4.1. Effect of DSE Strains on the Growth of the Host Plant
4.2. Effect of DSE Inoculation on Active Ingredients
4.3. Effect of DSE Strains on Cd Absorption in Medicinal Plants
4.4. Effect of DSE Strains on Soil Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, C.L.; Chen, Y.F.; Fang, S.Z.; Wang, Y.C.; Zheng, D.X.; Chen, C.X.; Jin, S.F. Cadmium contamination of Chinese agricultural soils. Fresen. Environ. Bull. 2022, 31, 2466–2473. [Google Scholar]
- Deram, A.; Languereau, F.; Haluwyn, C.V. Mycorrhizal and endophytic fungal colonization in Arrhenatherum elatius L. roots according to the soil contamination in heavy metals. Soil Sediment Contam. China Int. J. 2011, 20, 114–127. [Google Scholar] [CrossRef]
- Zhao, L.H.; Yang, Y.H.; Hu, Y.C.; Yang, S.H.; Jin, H.Y.; Wei, J.H.; Yang, M.H. Analysis and countermeasures of heavy metal pollution in traditional Chinese medicine. Chin. Tradit. Herbal Drug. 2020, 45, 1190–1206. [Google Scholar]
- Zhang, Y.; Li, T.; Zhao, Z.W. Colonization characteristics and composition of dark septate endophytes (DSE) in a lead and zinc slag heap in Southwest China. Soil Sediment Contam. 2013, 22, 532–545. [Google Scholar] [CrossRef]
- Hou, L.F.; Yu, J.; Zhao, L.L.; He, X.L. Dark septate endophytes improve the growth and the tolerance of Medicago sativa and Ammopiptanthus mongolicus under cadmium stress. Front. Microbiol. 2020, 10, 3061. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, T.; Wang, C.; Zhang, X.; Xu, L.; Xu, R.; Zhao, Z. The effects of dark septate endophyte (DSE) inoculation on tomato seedlings under Zn and Cd stress. Environ. Sci. Pollut. Res. 2018, 25, 35232–35241. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Jumpponen, A.; Trappe, J.M. Dark septate endophytes: A review of facultative biotrophic root-colonizing fungi. New Phytol. 1998, 140, 295–310. [Google Scholar] [CrossRef]
- Gonzalez Mateu, M.; Baldwin, A.H.; Maul, J.E.; Yarwood, S.A. Dark septate endophyte improves salt tolerance of native and invasive lineages of Phragmites australis. ISME J. 2020, 14, 1943–1954. [Google Scholar] [CrossRef]
- Akhtar, N.; Wani, A.K.; Dhanjal, D.S.; Mukherjee, S. Insights into the beneficial roles of dark septate endophytes in plants under challenging environment: Resilience to biotic and abiotic stresses. World J. Microbiol. Biotechnol. 2022, 38, 79. [Google Scholar] [CrossRef]
- Uma, E.; Sathiyadash, K.; Loganathan, J.; Muthukumar, T. Tree species as hosts for arbuscular mycorrhizal and dark septate endophyte fungi. J. Forestry Res. 2012, 23, 641–649. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Shahzad, R.; Imran, M.; Jan, R.; Kim, K.M.; Lee, I.J. Synergistic association of endophytic fungi enhances Glycine max L. resilience to combined abiotic stresses: Heavy metals, high temperature and drought stress. Ind. Crops Prod. 2020, 143, 111931. [Google Scholar] [CrossRef]
- Zhang, H.H.; Tang, M.; Chen, H.; Cui, H. Effects of a dark-septate endophytic isolate LBF-2 on the medicinal plant Lycium barbarum L. J. Microbiol. 2012, 50, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Hamayun, M.; Kang, S.M.; Kim, Y.H.; Jung, H.Y.; Lee, J.H.; Lee, I.J. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: An example of Paecilomyces Formosus LHL10. BMC Microbiol. 2012, 12, 3. [Google Scholar] [CrossRef]
- Baum, C.; Hrynkiewicz, K.; Szymańska, S.; Vitow, N.; Hoeber, S.; Fransson, P.M.A.; Weih, M. Mixture of Salix genotypes promotes root colonization with dark septate endophytes and changes P cycling in the mycorrhizosphere. Front. Microbiol. 2018, 9, 1012. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Q.; Lv, Y.L.; Meng, Z.X.; Chen, J.; Guo, S.X. The promoting role of an isolate of dark-septate fungus on its host plant Saussurea involucrata Kar. et Kir. Mycorrhiza 2010, 20, 127–135. [Google Scholar] [CrossRef]
- Zhu, Z.B.; Fan, J.Y.; Guo, Q.S.; Liu, Z.Y.; Zhu, G.S. The growth and medicinal quality of Epimedium wushanense are improved by an isolate of dark septate fungus. Pharm. Biol. 2015, 53, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.Y.; Yue, Z.C.; Li, S.T.; Pan, Y.Y.; Chu, Z.Y.; Ban, Y.H.; Xu, Z.Y. Alleviation of salt stress and changes in glycyrrhizic acid accumulation by dark septate endophytes in Glycyrrhiza glabra grown under salt stress. J. Agric. Food Chem. 2024, 72, 14557–14569. [Google Scholar] [CrossRef]
- Pan, X.M.; Zhang, S.R.; Zhong, Q.M.; Gong, G.S.; Wang, G.Y.; Guo, X.; Xu, X.X. Effects of soil chemical properties and fractions of Pb, Cd, and Zn on bacterial and fungal communities. Sci. Total Environ. 2020, 715, 136904. [Google Scholar] [CrossRef]
- Campbell, T.P.; Ulrich, D.E.M.; Toyoda, J.; Thompson, J.; Munsky, B.; Albright, M.B.N.; Bailey, V.L.; Tfaily, M.M.; Dunbar, J. Microbial communities influence soil dissolved organic carbon concentration by altering metabolite composition. Front. Microbiol. 2021, 12, 799014. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, A.; Dennis, P.G.; Forstner, C.; Raghavendra, A.K.H.; Mathesius, U.; Richardson, A.E.; Delhaize, E.; Gilliham, M.; Watt, M.; Ryan, P.R. Manipulating exudate composition from root apices shapes the microbiome throughout the root system. Plant Physiol. 2021, 187, 2279–2295. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Y.; Guo, Y.X.; Wang, Y.Y.; Yan, X.; Guo, X.; Lei, Z.H.; Niu, J.P.; Liang, J.P.; Li, Z.Y. Nitrogen-fixing bacteria promote growth and bioactive components accumulation of Astragalus mongholicus by regulating plant metabolism and rhizosphere microbiota. BMC Microbiol. 2024, 24, 261. [Google Scholar]
- Salducci, M.D.; Folzer, H.; Issartel, J.; Rabier, J.; Masotti, V.; Prudent, P.; Affre, L.; Hardion, L.; Tatoni, T.; Laffont-Schwob, I. How can a rare protected plant cope with the metal and metalloid soil pollution resulting from past industrial activities? Phytometabolites, antioxidant activities and root symbiosis involved in the metal tolerance of Astragalus tragacantha. Chemosphere 2019, 217, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Li, B.Z.; Jia, X.; Sun, S.Y.; Su, Y.L.; Chen, G.L. Differential expression of calycosin-7-O-β-D-glucoside biosynthesis genes and accumulation of related metabolites in different organs of Astragalus membranaceus Bge. var. mongholicus (Bge.) Hsiao under drought stress. Appl. Biochem. Biotechnol. 2022, 194, 3182–3195. [Google Scholar] [PubMed]
- Ding, M.; Bao, Y.; Liang, H.; Zhang, X.; Li, B.; Yang, R.; Zeng, N. Potential mechanisms of formononetin against inflammation and oxidative stress: A review. Front. Pharmacol. 2024, 15, 1368765. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Liu, S.; Wang, Y.; Li, X.; Zheng, L.; Zhao, M. Neuroprotective effects of formononetin against NMDA-induced apoptosis in cortical neurons. Phytother. Res. 2013, 27, 1770–1775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, S.; Qian, D.; Shang, E.X.; Duan, J.A. Analysis of interaction property of calycosin-7-O-β-D-glucoside with human gut microbiota. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 963, 16–23. [Google Scholar] [CrossRef]
- Ruan, J.Q.; Li, S.; Li, Y.P.; Wu, W.J.; Lee, S.M.; Yan, R. The presystemic interplay between gut microbiota and orally administered calycosin-7-O-β-D-Glucoside. Drug Metabol. Dispos. Biol. Fate Chem. 2015, 43, 1601–1611. [Google Scholar] [CrossRef]
- Han, L.; Zuo, Y.; He, X.; Hou, Y.; Li, M.; Li, B. Plant identity and soil variables shift the colonisation and species composition of dark septate endophytes associated with medicinal plants in a northern farmland in China. Appl. Soil Ecol. 2021, 167, 104042. [Google Scholar] [CrossRef]
- GB15618-2018; Soil Environmental Quality—Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2018. (In Chinese)
- Wu, Y.F.; Li, X.; Yu, L.; Wang, T.Q.; Wang, J.N.; Liu, T.T. Review of soil heavy metal pollution in China: Spatial distribution, primary sources, and remediation alternatives. Resour. Conserv. Recycl. 2022, 181, 106261. [Google Scholar] [CrossRef]
- Wen, X.C.H.; He, X.L.; Ren, Y.; Li, M.; Zhao, L.L. Characteristics of microbial community structure in rhizosphere soil of Fengfeng mining area. J. Hebei Agric. Univ. 2018, 41, 44–51. (In Chinese) [Google Scholar]
- Han, J.; Zhao, J.L.; He, X.L. Characteristics of heavy metals enrichment in plant species growing in Baiyangdian. J. Hebei Agric. Univ. 2016, 3, 31–36. (In Chinese) [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–163. [Google Scholar] [CrossRef]
- Biermann, B.; Linderman, R.G. Quantifying vesicular-arbuscular mycorrhizae: A proposed method towards standardization. New Phytol. 1981, 87, 63–67. [Google Scholar] [CrossRef]
- Torelli, A.; Trotta, A.; Acerbi, A.; Arcidiaconol, G.; Berta, G.; Branca, C. IAA and ZR content in leek (Allium porrum L.), as influenced by P nutrition and arbuscular mycorrhizae, in relation to plant development. Plant Soil 2000, 226, 29–35. [Google Scholar] [CrossRef]
- National Pharmacopoeia Commission. Chinese Pharmacopoeia; China Medical Science and Technology Press: Beijing, China, 2015. [Google Scholar]
- Rawls, W.J.; Pachepsky, Y.A.; Ritchie, J.C.; Sobecki, T.M.; Bloodworth, H. Effect of soil organic carbon on soil water retention. Geoderma 2003, 116, 61–76. [Google Scholar] [CrossRef]
- Bao, S.D. Agrochemical Analysis of Soil; Chinese Agricultural Press: Beijing, China, 2000; pp. 44–49. [Google Scholar]
- Tarafdar, J.C.; Marschner, H. Phosphatase activity in the rhizosphere and hyphosphere of VA mycorrhizal wheat supplied with inorganic and organic phosphorus. Soil Biol. Biochem. 1994, 26, 387–395. [Google Scholar] [CrossRef]
- Knapp, D.G.; Pintye, A.; Kovács, G.M. The dark side is not fastidious-dark septate endophytic fungi of native and invasive plants of semiarid sandy areas. PLoS ONE 2012, 7, e32570. [Google Scholar] [CrossRef]
- Knapp, D.G.; Kovács, G.M.; Zajta, E. Dark septate endophytic Pleosporalean genera from semiarid areas. Persoonia 2015, 35, 87–100. [Google Scholar] [CrossRef]
- Spagnoletti, F.N.; Tobar, N.E.; Fernández Di Pardo, A.; Chiocchio, V.M.; Lavado, R.S. Dark septate endophytes present different potential to solubilize calcium, iron and aluminum phosphates. Appl. Soil Ecol. 2017, 111, 25–32. [Google Scholar] [CrossRef]
- Liu, N.; Jacquemyn, H.; Liu, Q.; Shao, S.C.; Ding, G.; Xing, X. Effects of a dark septate fungal endophyte on the growth and physiological response of seedlings to drought in an Epiphytic Orchid. Front. Microbiol. 2022, 13, 961172. [Google Scholar] [CrossRef] [PubMed]
- Mikheev, V.S.; Struchkova, I.V.; Ageyeva, M.N.; Brilkina, A.A.; Berezina, E.V. The role of Phialocephala fortinii in improving plants’ phosphorus nutrition: New puzzle pieces. J. Fungi 2022, 8, 1225. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Khalid, M.; Kayani, S.I.; Tang, K.X. The ameliorative effects of exogenous inoculation of Piriformospora indica on molecular, biochemical and physiological parameters of Artemisia annua L. under arsenic stress condition. Ecotox. Environ. Saf. 2020, 206, 111202. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Z.; Dai, M.D.; Zhu, J.N.; Liu, X.H.; Lin, F.C. Dark septate endophyte Falciphora oryzae-assisted alleviation of cadmium in rice. J. Hazard. Mater. 2021, 419, 126435. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Dai, M.X.; Zhang, G.Q.; Yang, Z.X.; He, Y.M.; Zhan, F.D. Effects of the dark septate endophyte (DSE) Exophiala pisciphila on the growth of root cell wall polysaccharides and the cadmium content of Zea mays L. under cadmium stress. J. Fungi. 2021, 7, 1035. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.H.; Xu, Z.Y.; Yang, Y.R.; Zhang, H.; Chen, H.; Ming, T. Effect of dark septate endophytic fungus Gaeumannomyces cylindrosporus on plant growth, photosynthesis and Pb tolerance of maize (Zea mays L.). Pedosphere 2017, 27, 283–292. [Google Scholar] [CrossRef]
- Shi, G.R.; Xia, S.L.; Liu, C.F.; Zhang, Z. Cadmium accumulation and growth response to cadmium stress of eighteen plant species. Environ. Sci. Pollut. R. 2016, 23, 23071–23080. [Google Scholar] [CrossRef]
- Berthelot, C.; Leyval, C.; Foulon, J.; Chalot, M.; Blaudez, D. Plant growth promotion, metabolite production and metal tolerance of dark septate endophytes isolated from metal-polluted poplar phytomanagement sites. FEMS Microbiol. Ecol. 2016, 92, fiw144. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.H.; Tang, M.; Chen, H.; Xu, Z.Y.; Zhang, H.H.; Yang, Y.R. The response of dark septate endophytes (DSE) to heavy metals in pure culture. PLoS ONE 2012, 7, e47968. [Google Scholar] [CrossRef]
- Huot, C.; Zhou, Y.; Philp, J.N.M.; Denton, M.D. Root depth development in tropical perennial forage grasses is related to root angle, root diameter and leaf area. Plant Soil 2020, 456, 145–158. [Google Scholar] [CrossRef]
- Mayerhofer, M.S.; Kernaghan, G.; Harper, K.A. The effects of fungal root endophytes on plant growth: A meta-analysis. Mycorrhiza 2013, 23, 119–128. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Hussain, S.; Bano, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.A.; Khan, F.; Chen, Y.; Wu, C. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: Consequences for changing environment. Environ. Sci. Pollut. Res. 2015, 22, 4907–4921. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Irshad, M.; Hussain, A.; Qadir, M.; Murad, W.; Khan, A.; Awais, M.; Alrefaei, A.F.; Ali, S. EDTA and IAA ameliorates phytoextraction potential and growth of sunflower by mitigating Cu-induced morphological and biochemical injuries. Life 2023, 13, 759. [Google Scholar] [CrossRef]
- Yuan, Z.L.; Dai, C.C.; Chen, L.Q. Regulation and accumulation of secondary metabolites in plant-fungus symbiotic system. Afr. J. Biotechnol. 2007, 6, 1266–1271. [Google Scholar]
- Wu, F.L.; Li, Y.; Tian, W.; Sun, Y.; Chen, F.; Zhang, Y.; Zhai, Y.; Zhang, J.; Su, H.; Wang, L. A novel dark septate fungal endophyte positively affected blueberry growth and changed the expression of plant genes involved in phytohormone and flavonoid biosynthesis. Tree Physiol. 2020, 40, 1080–1094. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Jia, M.; Chen, L.; Zheng, C.J.; Rahman, K.; Han, T.; Qin, L.P. The regulatory mechanism of fungal elicitor-induced secondary metabolite biosynthesis in medical plants. Crit. Rev. Microbiol. 2016, 43, 238–261. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, Y.; Ding, G. Morphogenesis and metabolomics reveal the compatible relationship among Suillus bovinus, Phialocephala fortinii, and their co-host, Pinus massoniana. Microbiol. Spectr. 2023, 11, e01453-23. [Google Scholar] [CrossRef]
- Yakti, W.; Kovács, G.M.; Vági, P.; Franken, P. Impact of dark septate endophytes on tomato growth and nutrient uptake. Plant Ecol. Divers. 2018, 11, 637–648. [Google Scholar] [CrossRef]
- He, Y.; Yang, Z.; Li, M.; Jiang, M.; Zhan, F.; Zu, Y. Effects of a dark septate endophyte (DSE) on growth, cadmium content and physiology in maize under cadmium stress. Environ. Sci. Pollut. Res. Int. 2017, 24, 18494–18504. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Du, L.; Tsona, N.T.; Wang, W. The interaction of trace heavy metal with lipid monolayer in the sea surface microlayer. Chemosphere 2018, 196, 323–330. [Google Scholar] [CrossRef]
- Parrotta, L.; Guerriero, G.; Sergeant, K.; Cai, G.; Hausman, J.F. Target or barrier? The cell wall of early- and later-diverging plants vs cadmium toxicity: Differences in the response mechanisms. Front. Plant Sci. 2015, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Priyadarshini, S.S.; Cousins, B.G.; Pradhan, N. Metal-Fungus interaction: Review on cellular processes underlying heavy metal detoxification and synthesis of metal nanoparticles. Chemosphere 2021, 274, 129976. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Li, T.; Liu, G.Y.; Smith, J.M.; Zhao, Z.W. Unraveling the role of dark septate endophyte (DSE) colonizing maize (Zea mays) under cadmium stress: Physiological, cytological and genic aspects. Sci. Rep. 2016, 6, 22028. [Google Scholar] [CrossRef]
- Zhan, F.D.; He, Y.M.; Zu, Y.Q.; Li, T.; Zhao, Z.W. Characterization of melanin isolated from a dark septate endophyte (DSE), Exophiala pisciphila. World J. Microbiol. Biotechnol. 2011, 27, 2483–2489. [Google Scholar] [CrossRef]
- Qiu, Q.; Wang, Y.; Yang, Z.; Yuan, J. Effects of phosphorus supplied in soil on subcellular distribution and chemical forms of cadmium in two Chinese flowering cabbage (Brassica parachinensis L.) cultivars differing in cadmium accumulation. Food Chem. Toxicol. 2011, 49, 2260–2267. [Google Scholar] [CrossRef]
- Zhan, F.; He, Y.; Li, Y.; Li, T.; Yang, Y.Y.; Toor, G.S.; Zhao, Z.W. Subcellular distribution and chemical forms of cadmium in a dark septate endophyte (DSE), Exophiala pisciphila. Environ. Sci. Pollut. Res. 2015, 22, 17897–17905. [Google Scholar] [CrossRef]
- Li, Y.; Wei, M. Evaluation on adsorption capacity of low organic matter soil for hydrophobic organic pollutant. J. Environ. Chem. Eng. 2022, 10, 107561. [Google Scholar] [CrossRef]
- Chen, X.; Ran, Z.; Li, R.; Duan, W.; Song, Z.; Fang, L.; Guo, L.; Zhou, J. Biochar reduces the cadmium content of Panax quinquefolium L. by improving rhizosphere microecology. Sci. Total Environ. 2024, 915, 170005. [Google Scholar] [CrossRef] [PubMed]



| DSE | Cd | DSE × Cd | ||||
|---|---|---|---|---|---|---|
| F | ηp2 | F | ηp2 | F | ηp2 | |
| Height | 60.34 *** | 0.85 | 31.21 *** | 0.75 | 6.44 *** | 0.64 |
| Leaf number | 151.10 *** | 0.93 | 35.11 *** | 0.77 | 21.15 *** | 0.86 |
| Branch | 61.46 *** | 0.85 | 13.46 *** | 0.56 | 3.05 *** | 0.46 |
| SPAD | 56.29 *** | 0.84 | 48.74 *** | 0.82 | 22.85 *** | 0.87 |
| Shoot biomass | 126.73 *** | 0.92 | 143.78 *** | 0.93 | 17.10 *** | 0.83 |
| Root biomass | 93.06 *** | 0.90 | 144.15 *** | 0.93 | 22.38 *** | 0.86 |
| Total biomass | 146.31 *** | 0.93 | 221.79 *** | 0.95 | 15.45 *** | 0.81 |
| Root–shoot ratio | 45.73 *** | 0.81 | 17.03 *** | 0.61 | 20.54 *** | 0.85 |
| IAA | 56.32 *** | 0.84 | 3.97 * | 0.27 | 13.45 *** | 0.79 |
| Root length | 270.15 *** | 0.96 | 91.23 *** | 0.90 | 85.37 *** | 0.96 |
| Root volume | 80.27 *** | 0.88 | 83.87 *** | 0.89 | 39.49 *** | 0.92 |
| Calycosin-7-O-glucoside | 304.85 *** | 0.97 | 139.96 *** | 0.93 | 19.96 *** | 0.85 |
| Formononetin | 614.60 *** | 0.98 | 955.70 *** | 0.99 | 156.82 *** | 0.98 |
| Shoot Cd | 277.98 *** | 0.96 | 1037.17 *** | 0.99 | 28.91 *** | 0.89 |
| Root Cd | 271.20 *** | 0.96 | 1782.58 *** | 0.99 | 51.45 *** | 0.94 |
| Translocation factor | 282.29 *** | 0.96 | 17.59 *** | 0.62 | 19.25 *** | 0.84 |
| SOC | 205.64 *** | 0.95 | 203.76 *** | 0.95 | 13.72 *** | 0.79 |
| SAN | 142.78 *** | 0.93 | 37.64 *** | 0.78 | 11.95 *** | 0.77 |
| SAP | 147.34 *** | 0.93 | 1185.99 *** | 0.99 | 83.49 *** | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Han, L.; He, C.; Li, X.; He, X. The Promotion of Dark Septate Endophytes on the Performance and Active Ingredients Accumulation of Astragalus mongholicus under Cadmium Stress. Agronomy 2024, 14, 1801. https://doi.org/10.3390/agronomy14081801
Li M, Han L, He C, Li X, He X. The Promotion of Dark Septate Endophytes on the Performance and Active Ingredients Accumulation of Astragalus mongholicus under Cadmium Stress. Agronomy. 2024; 14(8):1801. https://doi.org/10.3390/agronomy14081801
Chicago/Turabian StyleLi, Min, Li Han, Chao He, Xia Li, and Xueli He. 2024. "The Promotion of Dark Septate Endophytes on the Performance and Active Ingredients Accumulation of Astragalus mongholicus under Cadmium Stress" Agronomy 14, no. 8: 1801. https://doi.org/10.3390/agronomy14081801






