The Effects of Struvite on Biomass and Soil Phosphorus Availability and Uptake in Chinese Cabbage, Cowpea, and Maize
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and Materials
2.2. Experimental Design
2.3. Sample Collection and Analysis
2.4. Data Analysis and Statistics
3. Results
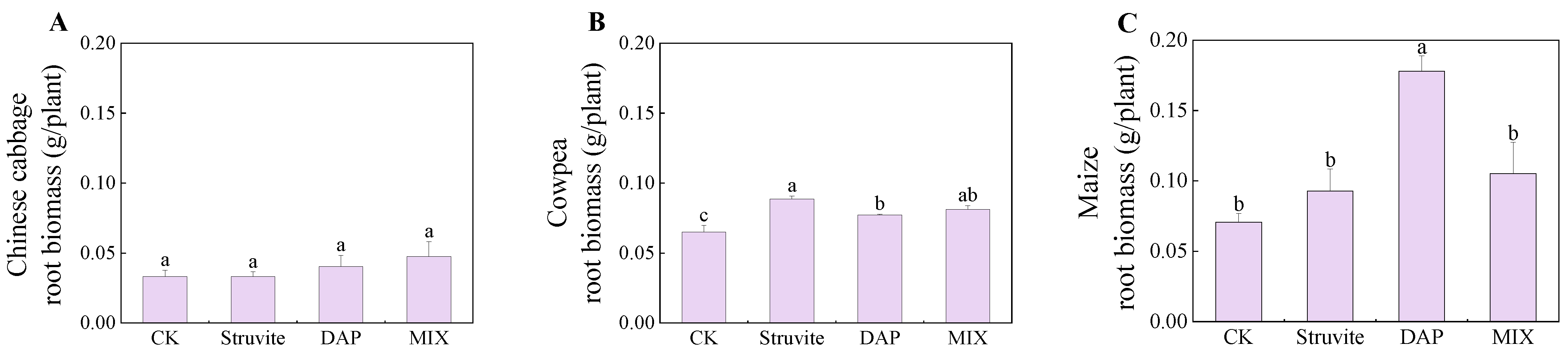
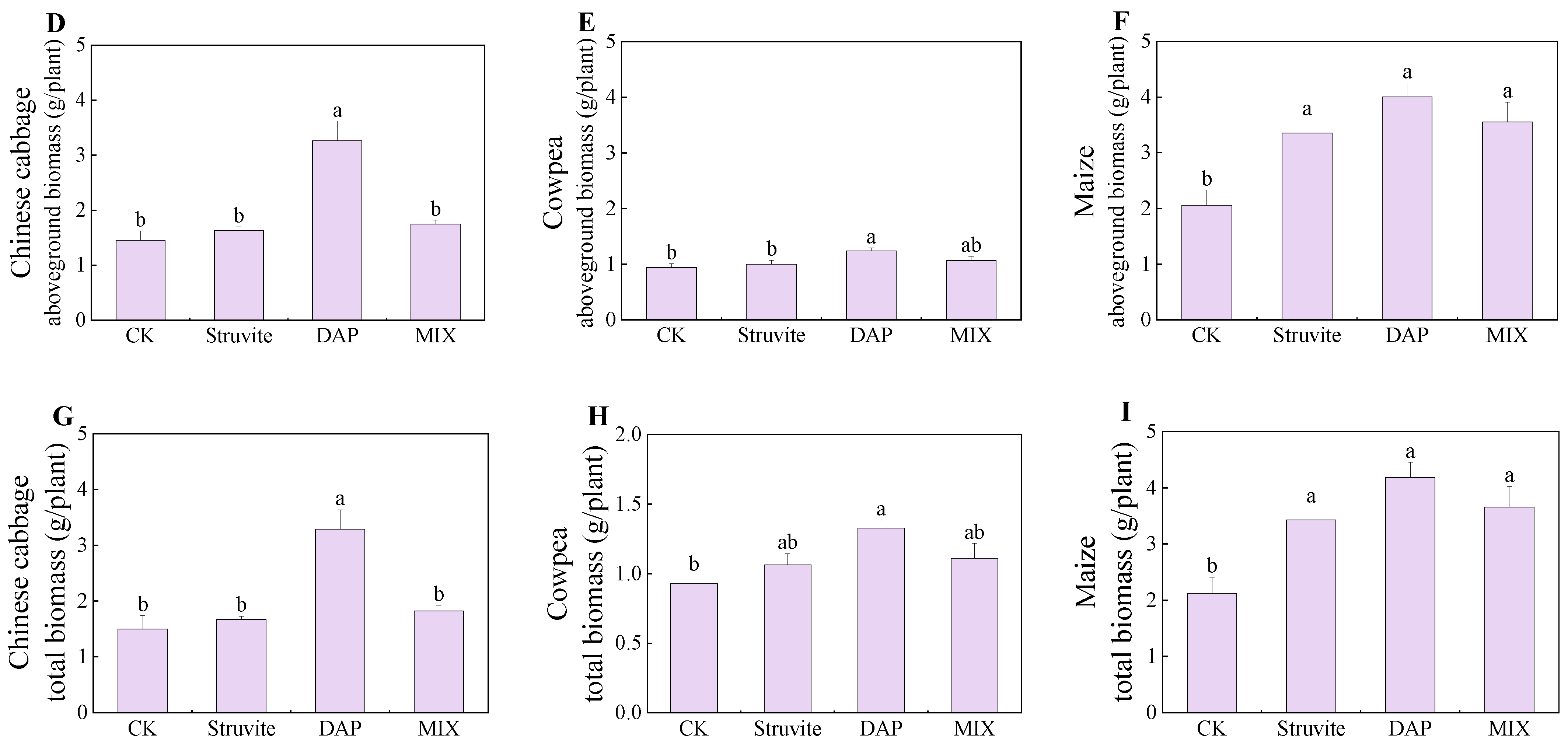
3.1. Crop Biomass
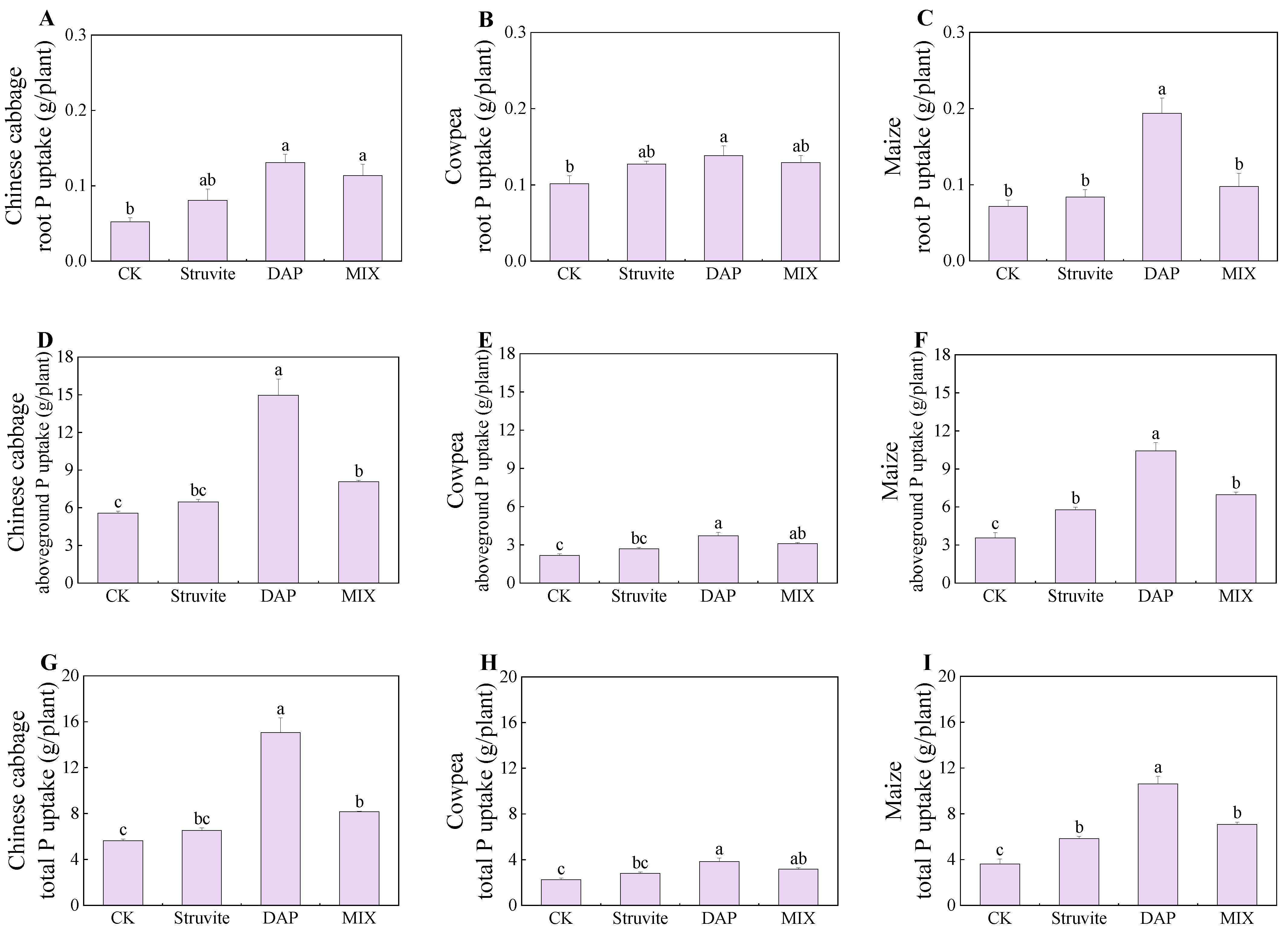
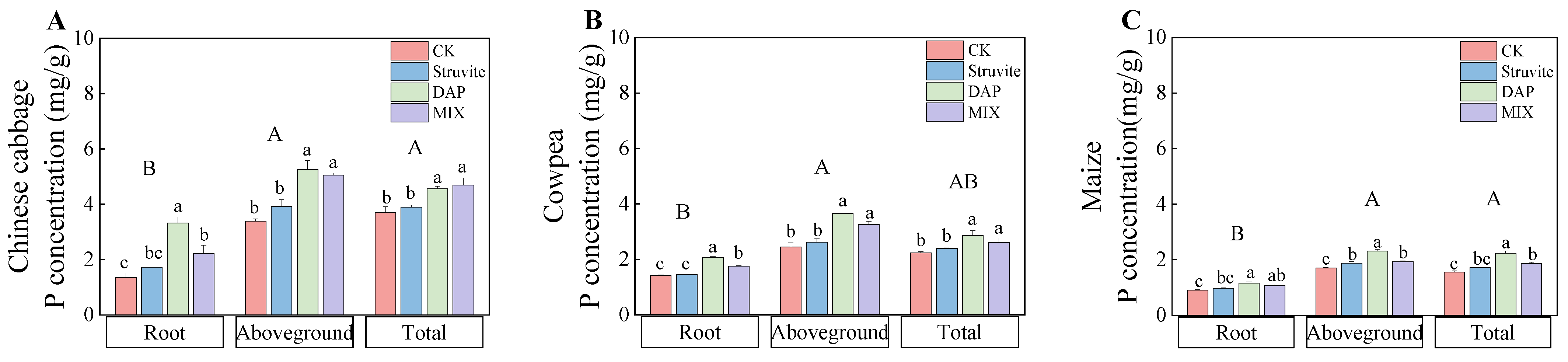
3.2. Crop P Uptake
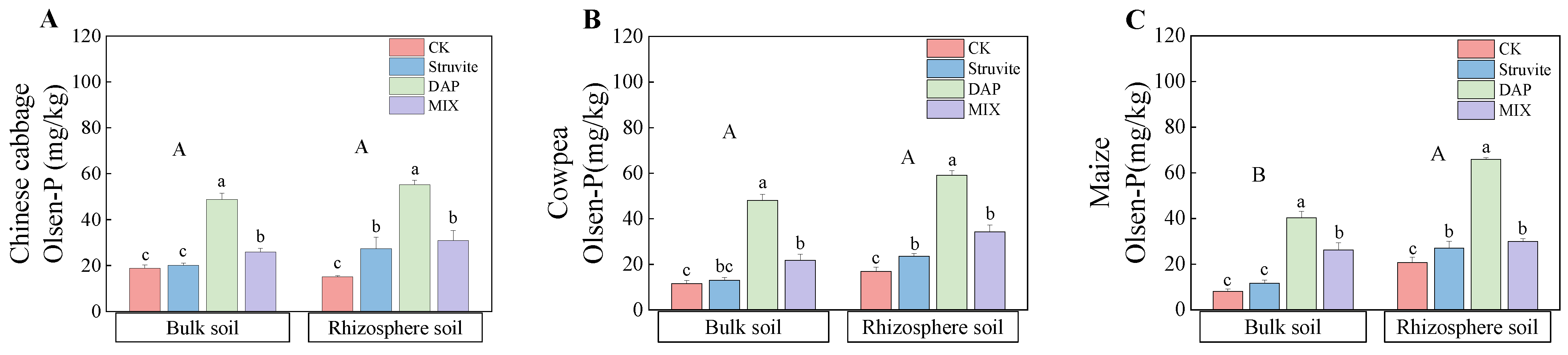
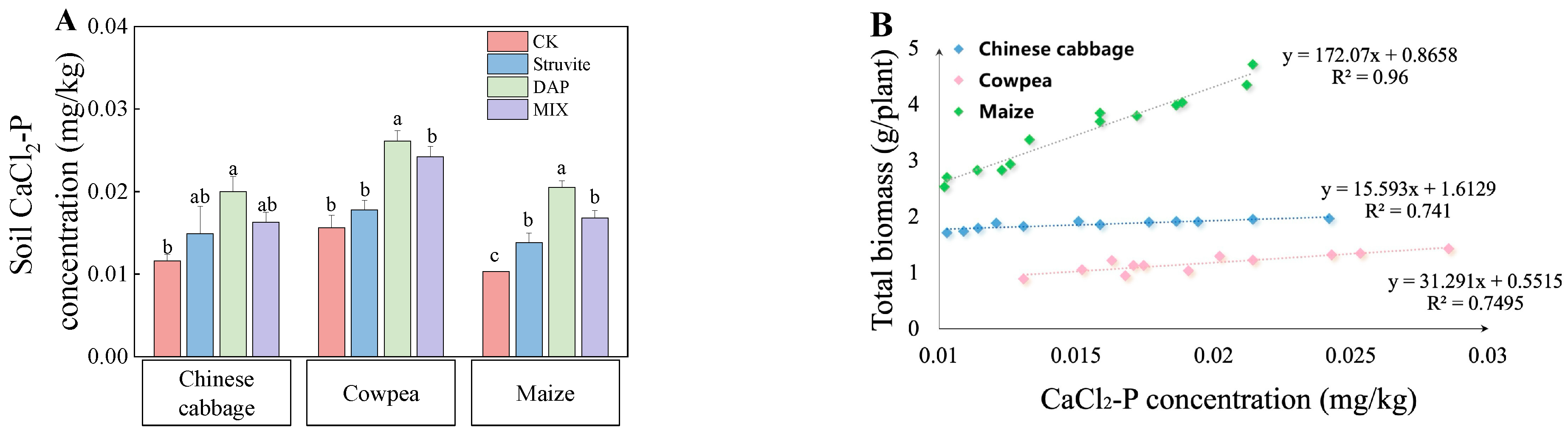
3.3. Soil Olsen-P and P Fractions
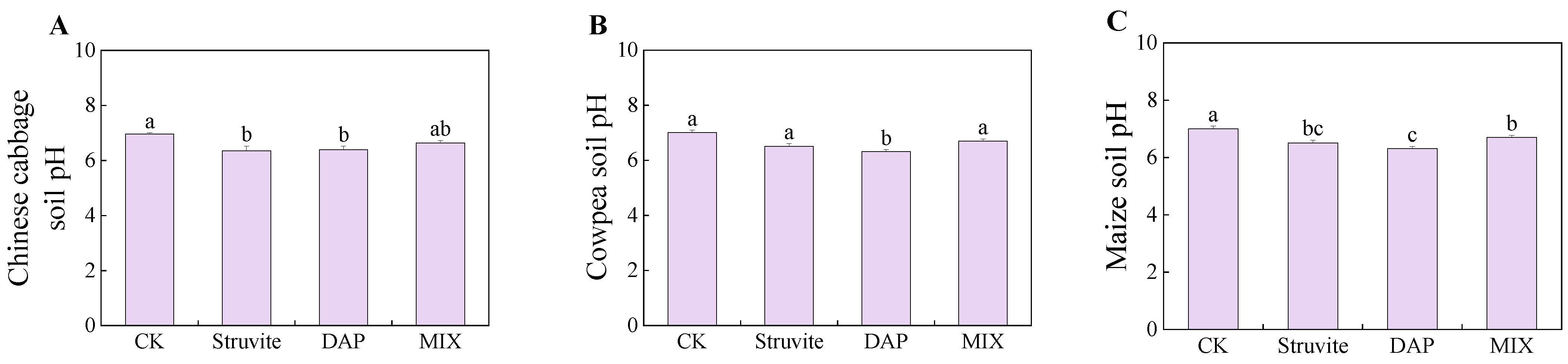
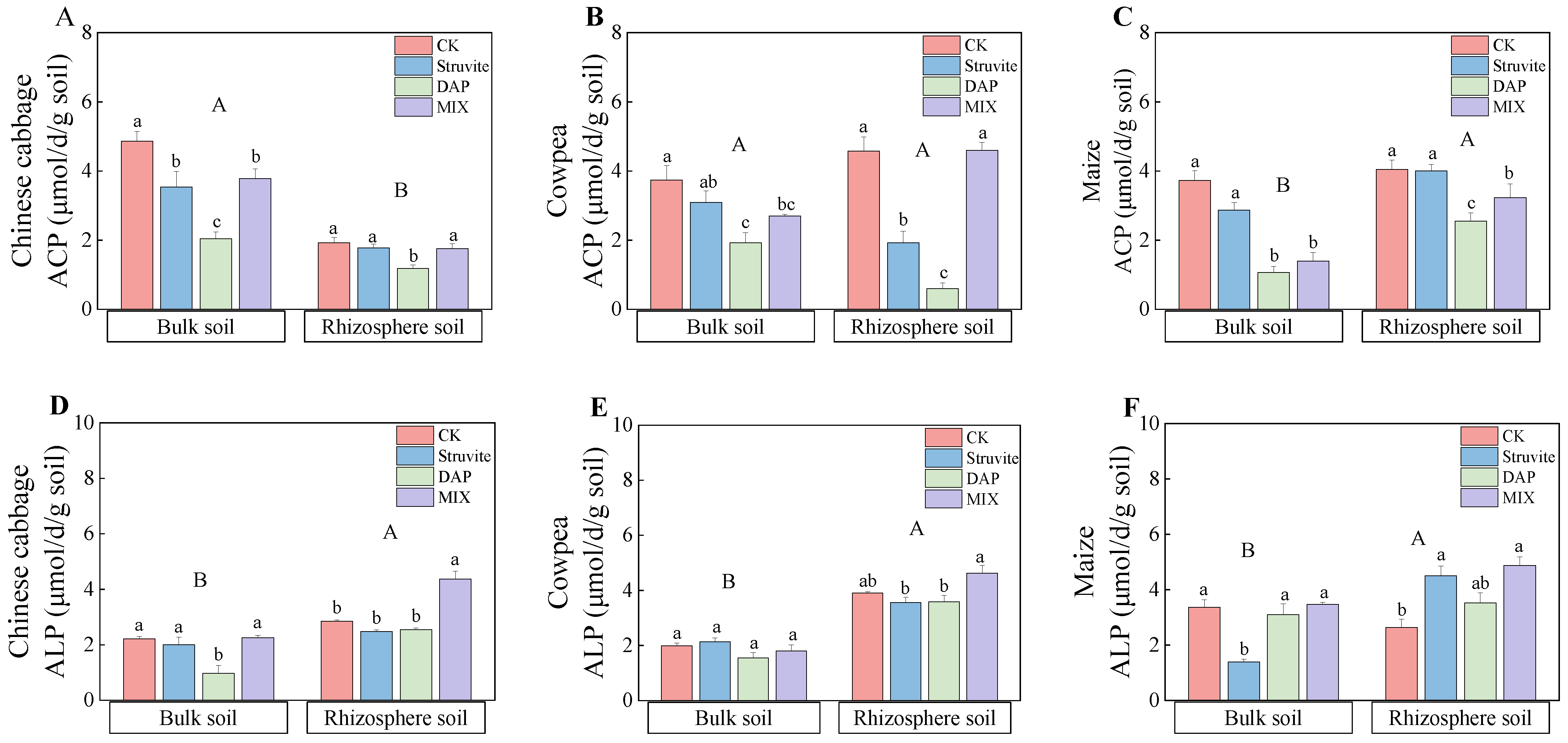
3.4. Soil pH and Soil Phosphatase Activity
4. Discussion
4.1. Crop Biomass and Phosphorus Accumulation
4.2. Soil P and P Fractions
4.3. Effect of Different Phosphate Fertilizers on Soil pH and Soil Phosphatase Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Langhans, C.; Beusen, A.H.W.; Mogollon, J.M.; Bouwman, A.F. Phosphorus for sustainable development goal target of doubling smallholder productivity. Nat. Sustain. 2022, 5, 57–63. [Google Scholar]
- Cordell, D.; Neset, T.S.S. Phosphorus vulnerability: A qualitative framework for assessing the vulnerability of national and regional food systems to the multidimensional stressors of phosphorus scarcity. Glob. Environ. Change 2014, 24, 108–122. [Google Scholar]
- MacDonald, G.K.; Bennett, E.M.; Potter, P.A.; Ramankutty, N. Agronomic phosphorus imbalances across the world’s croplands. Proc. Natl. Acad. Sci. USA 2011, 108, 3086–3091. [Google Scholar]
- Yu, X.; Keitel, C.; Dijkstra, F.A. Global analysis of phosphorus fertilizer use efficiency in cereal crops. Glob. Food Secur. Agric. 2021, 29, 100545. [Google Scholar]
- Gilbert, N. Environment: The disappearing nutrient. Nature 2009, 461, 716–718. [Google Scholar]
- Sheldrick, W.F.; Lingard, J. The use of nutrient audits to determine nutrient balances in Africa. Food Policy 2004, 29, 61–98. [Google Scholar]
- Robles-Aguilar, A.A.; Pang, J.; Postma, J.A.; Schrey, S.D.; Lambers, H.; Jablonowski, N.D. The effect of pH on morphological and physiological root traits of Lupinus angustifolius treated with struvite as a recycled phosphorus source. Plant Soil 2019, 434, 65–78. [Google Scholar]
- Carreras-Sempere, M.; Guivernau, M.; Caceres, R.; Biel, C.; Noguerol, J.; Vinas, M.; Pereira, J.L.S. Effect of fertigation with struvite and ammonium nitrate on substrate microbiota and N2O emissions in a tomato crop on soilless culture system. Agronomy 2024, 14, 119. [Google Scholar] [CrossRef]
- Martens, J.R.T.; Entz, M.H.; Schneider, K.D.; Zvomuya, F.; Wilson, H.F. Response of organic grain and forage crops to struvite application in an alkaline soil. Agron. J. 2022, 114, 795–810. [Google Scholar]
- Szymanska, M.; Sosulski, T.; Bozetka, A.; Dawidowicz, U.; Was, A.; Szara, E.; Malak-Rawlikowska, A.; Sulewski, P.; van Pruissen, G.W.; Cornelissen, R.L. Evaluating the struvite recovered from anaerobic digestate in a farm bio-refinery as a slow-release fertiliser. Energies 2020, 13, 5342. [Google Scholar] [CrossRef]
- Arcas-Pilz, V.; Parada, F.; Rufi-Salis, M.; Stringari, G.; Gonzalez, R.; Villalba, G.; Gabarrell, X. Extended use and optimization of struvite in hydroponic cultivation systems. Resour. Conserv. Recy. 2022, 179, 106130. [Google Scholar]
- Antonini, S.; Arias, M.A.; Eichert, T.; Clemens, J. Greenhouse evaluation and environmental impact assessment of different urine-derived struvite fertilizers as phosphorus sources for plants. Chemosphere 2012, 89, 1202–1210. [Google Scholar] [PubMed]
- Min, K.J.; Kim, D.; Lee, J.; Lee, K.; Park, K.Y. Characteristics of vegetable crop cultivation and nutrient releasing with struvite as a slow-release fertilizer. Environ. Sci. Pollut. R. 2019, 26, 34332–34344. [Google Scholar]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.R.; Jones, D.L.; Withers, P.J.A. Struvite: A slow-release fertiliser for sustainable phosphorus management? Plant Soil 2016, 401, 109–123. [Google Scholar]
- Dechassa, N.; Schenk, M.K. Exudation of organic anions by roots of cabbage, carrot, and potato as influenced by environmental factors and plant age. J. Plant Nutr. Soil Sc. 2004, 167, 623–629. [Google Scholar]
- Rech, I.; Withers, P.J.A.; Jones, D.L.; Pavinato, P.S. Solubility, diffusion and crop uptake of phosphorus in three different struvites. Sustainability 2019, 11, 11010134. [Google Scholar]
- Iqbal, S.; Akhtar, J.; Naz, T.; Riaz, U.; Hussain, S.; Mazhar, Z.; Iqbal, M.M. Root morphological adjustments of crops to improve nutrient use efficiency in limited environments. Commun. Soil Sci. Plan. 2020, 51, 2452–2465. [Google Scholar]
- Yu, B.G.; Chen, X.X.; Cao, W.Q.; Liu, Y.M.; Zou, C.Q. Responses in zinc uptake of different mycorrhizal and non-mycorrhizal crops to varied levels of phosphorus and zinc applications. Front. Plant Sci. 2020, 11, 606472. [Google Scholar]
- Alamzeb, M.; Inamullah. Management of phosphorus sources in combination with rhizobium and phosphate solubilizing bacteria improve nodulation, yield and phosphorus uptake in chickpea. Gesunde Pflanz. 2023, 75, 549–564. [Google Scholar]
- Ulmer, M.G.; Swenson, L.J.; Patterson, D.D.; Dahnke, W.C. Organic-carbon determination by the Walkley-Black, UDY DYE, and fry combustion methods for sellected North-Dakota soils. Commun. Soil Sci. Plan. 1992, 23, 417–429. [Google Scholar]
- Houba, V.J.G.; Temminghoff, E.J.M.; Gaikhorst, G.A.; van Vark, W. Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun. Soil Sci. Plan. 2000, 31, 1299–1396. [Google Scholar]
- Johnston, A.E.; Poulton, P.R.; Fixen, P.E.; Curtin, D. Phosphorus: Its Efficient Use in Agriculture. Adv. Agron. 2014, 123, 177–228. [Google Scholar]
- Zharikova, E.A.; Golodnaya, O.M. Available potassium in volcanic soils of Kamchatka. Eurasian Soil Sci. 2009, 42, 850–860. [Google Scholar]
- Zhang, Y.; Gao, W.; Luan, H.; Tang, J.; Li, R.; Li, M.; Zhang, H.; Huang, S. Long-term organic substitution management affects soil phosphorus speciation and reduces leaching in greenhouse vegetable production. J. Clean. Prod. 2021, 327, 129464. [Google Scholar]
- Tiessen, H.J.W.B. Characterization of available P by sequential extraction. Soil Sampl. Methods Anal. 1993, 3, 5–229. [Google Scholar]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in inorganic and organic soil- phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar]
- Jamal, A.; Saeed, M.F.F.; Mihoub, A.; Hopkins, B.G.G.; Ahmad, I.; Naeem, A. Integrated use of phosphorus fertilizer and farmyard manure improves wheat productivity by improving soil quality and P availability in calcareous soil under subhumid conditions. Front. Plant Sci. 2023, 14, 1034421. [Google Scholar]
- Hertzberger, A.; Pittelkow, C.M.; Harmel, R.D.; Christianson, L.E. Analysis of the manage drain concentration database to evaluate agricultural management effects on drainage water nutrient concentrations. Trans. Asabe 2019, 62, 929–939. [Google Scholar]
- Hertzberger, A.J.; Cusick, R.D.; Margenot, A.J. A review and meta-analysis of the agricultural potential of struvite as a phosphorus fertilizer. Soil Sci. Soc. Am. J. 2020, 84, 653–671. [Google Scholar]
- Hertzberger, A.J.; Cusick, R.D.; Margenot, A.J. Maize and soybean response to phosphorus fertilization with blends of struvite and monoammonium phosphate. Plant Soil 2021, 461, 547–563. [Google Scholar]
- Wen, G.; Huang, L.; Zhang, X.; Hu, Z. Uptake of nutrients and heavy metals in struvite recovered from a mixed wastewater of human urine and municipal sewage by two vegetables in calcareous soil. Environ. Technol. Inno. 2019, 15, 100384. [Google Scholar]
- Ryu, H.D.; Lim, C.S.; Kang, M.K.; Lee, S.I. Evaluation of struvite obtained from semiconductor wastewater as a fertilizer in cultivating Chinese cabbage. J. Hazard. Mater. 2012, 221, 248–255. [Google Scholar]
- Cakmak, I.; Hengeler, C.; Marschner, H. Partitioning of shoot and root dry-matter and carbohydrates in bean-plants suffering from phosphorus, potassium and magnesium-deficiency. J. Exp. Bot. 1994, 45, 1245–1250. [Google Scholar]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D.C. Phosphorus recovery as struvite: Recent concerns for use of seed, alternative Mg source, nitrogen conservation and fertilizer potential. Resour. Conserv. Recy. 2016, 107, 142–156. [Google Scholar]
- Lynch, J.P. Root phenotypes for improved nutrient capture: An underexploited opportunity for global agriculture. New Phytol. 2019, 223, 548–564. [Google Scholar]
- Ylagan, S.; Brye, K.R.; Greenlee, L. Corn and soybean response to wastewater-recovered and other common phosphorus fertilizers. Agrosystems Geosci. Environ. 2020, 3, 20086. [Google Scholar]
- Wang, Y.-L.; Gao, Z.; Wang, Y.; Zhang, Y.-H.; Zhuang, X.-Y.; Zhang, H. Phosphorus Availability and Transformation as Affected by Repeated Phosphorus Additions in an Ultisol. Commun. Soil Sci. Plan. 2015, 46, 1922–1933. [Google Scholar]
- Xiaoying, Z.H.O.N.G.; Xiaorong, Z.H.A.O.; Huajun, B.A.O.; Haohao, L.; Qimei, L. The evaluation of phosphorus leaching risk of 23 Chinese soils. I. Leaching criterion. Acta Ecol. Sin. 2004, 10, 2275–2280. [Google Scholar]
- Heckrath, G.; Brookes, P.C.; Poulton, P.R.; Goulding, K.W.T. Phosphorus leaching from soil containing different phosphorus concentrationds in the broadbalk experiment. J. Environ. Qual. 1995, 24, 904–910. [Google Scholar]
- Hesketh, N.; Brookes, P.C. Development of an indicator for risk of phosphorus leaching. J. Environ. Qual. 2000, 29, 105–110. [Google Scholar]
- Liao, D.; Zhang, C.; Lambers, H.; Zhang, F. Changes in soil phosphorus fractions in response to long-term phosphate fertilization under sole cropping and intercropping of maize and faba bean on a calcareous soil. Plant Soil 2021, 463, 589–600. [Google Scholar]
- Margalef, O.; Sardans, J.; Fernandez-Martinez, M.; Molowny-Horas, R.; Janssens, I.A.; Ciais, P.; Goll, D.; Richter, A.; Obersteiner, M.; Asensio, D.; et al. Global patterns of phosphatase activity in natural soils. Sci. Rep. 2017, 7, 1–13. [Google Scholar]
- Thabet, O.B.D.; Gtari, M.; Sghaier, H. Microbial diversity in phosphate rock and phosphogypsum. Waste Biomass Valorization 2017, 8, 2473–2483. [Google Scholar]
- DeForest, J.L.; Smemo, K.A.; Burke, D.J.; Elliott, H.L.; Becker, J.C. Soil microbial responses to elevated phosphorus and pH in acidic temperate deciduous forests. Biogeochemistry 2012, 109, 189–202. [Google Scholar]
- Johnson, J.F.; Allan, D.L.; Vance, C.P.; Weiblen, G. Root carbon dioxide fixation by phosphorus-deficient Lupinus albus—Contribution to organic acid exudation by proteoid roots. Plant Physiol. 1996, 112, 19–30. [Google Scholar] [PubMed]
- Mndzebele, B.; Ncube, B.; Fessehazion, M.; Mabhaudhi, T.; Amoo, S.; du Plooy, C.; Venter, S.; Modi, A. Effects of cowpea-amaranth intercropping and fertiliser application on soil phosphatase activities, available soil phosphorus, and crop growth response. Agronomy 2020, 10, 79. [Google Scholar] [CrossRef]

| Treatment | Chinese Cabbage | Cowpea | Maize |
|---|---|---|---|
| CK | 0.023 | 0.069 | 0.034 |
| struvite | 0.020 | 0.088 | 0.028 |
| DAP | 0.012 | 0.062 | 0.044 |
| MIX | 0.027 | 0.076 | 0.030 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Wei, B.; Wu, D.; Sun, K.; Jiao, J.; Zhang, W. The Effects of Struvite on Biomass and Soil Phosphorus Availability and Uptake in Chinese Cabbage, Cowpea, and Maize. Agronomy 2024, 14, 1852. https://doi.org/10.3390/agronomy14081852
Sun L, Wei B, Wu D, Sun K, Jiao J, Zhang W. The Effects of Struvite on Biomass and Soil Phosphorus Availability and Uptake in Chinese Cabbage, Cowpea, and Maize. Agronomy. 2024; 14(8):1852. https://doi.org/10.3390/agronomy14081852
Chicago/Turabian StyleSun, Linglulu, Bingli Wei, Dongxun Wu, Kai Sun, Jiabin Jiao, and Wei Zhang. 2024. "The Effects of Struvite on Biomass and Soil Phosphorus Availability and Uptake in Chinese Cabbage, Cowpea, and Maize" Agronomy 14, no. 8: 1852. https://doi.org/10.3390/agronomy14081852





