Genome-Wide Identification, Characterization, and Expression Profile of PDCB Gene Family in Zea mays L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Detection of PDCB Gene Family Members in Maize and Rice
2.2. Developing Phylogenetic Trees, Analyzing Motifs, and Determining Gene Structures
2.3. Chromosome Location and Synteny Analysis for PDCBs
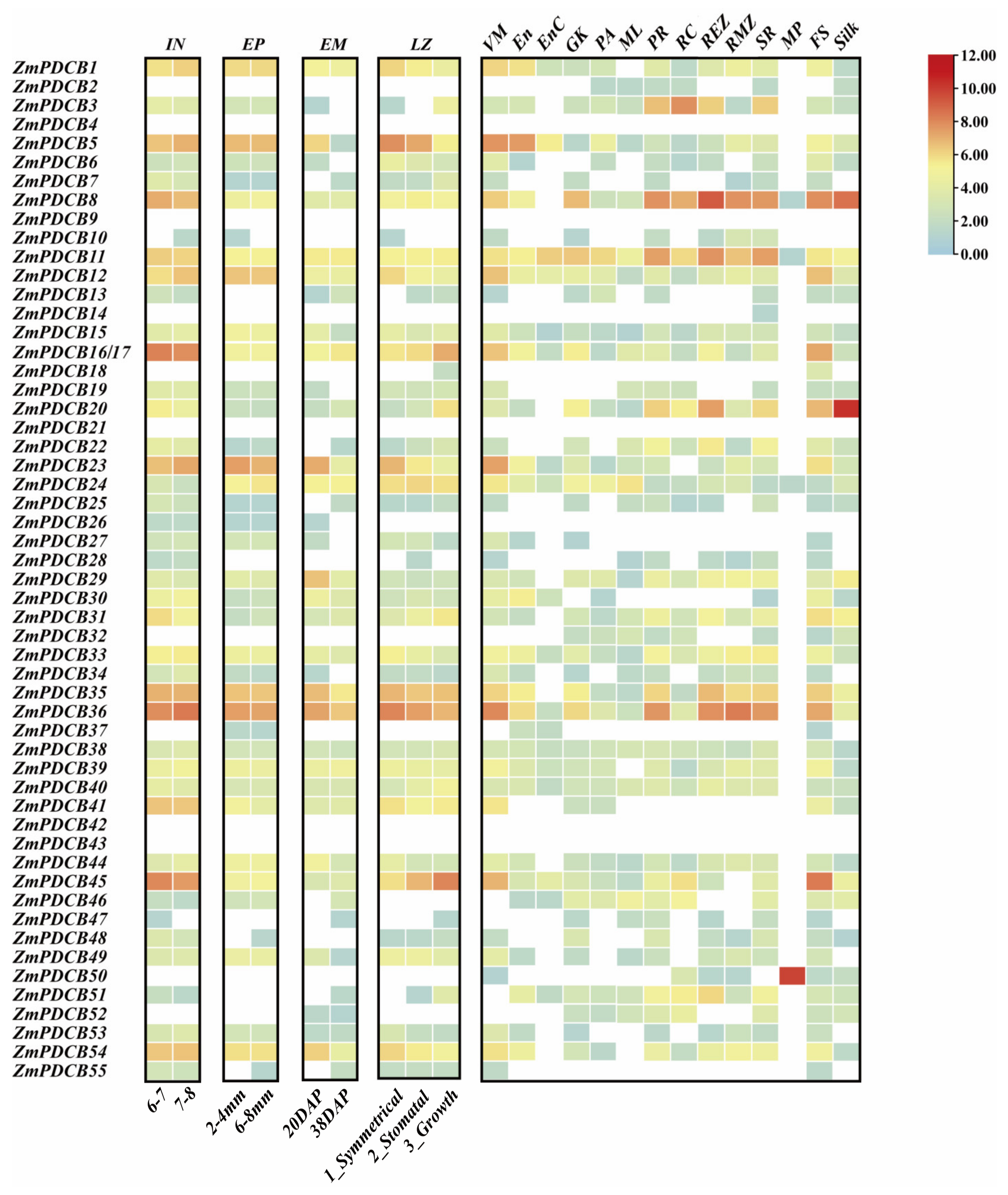
2.4. ZmPDCBs Expression Patterns under Different Tissues
3. Results
3.1. Zea mays PDCB Identification and Characterization
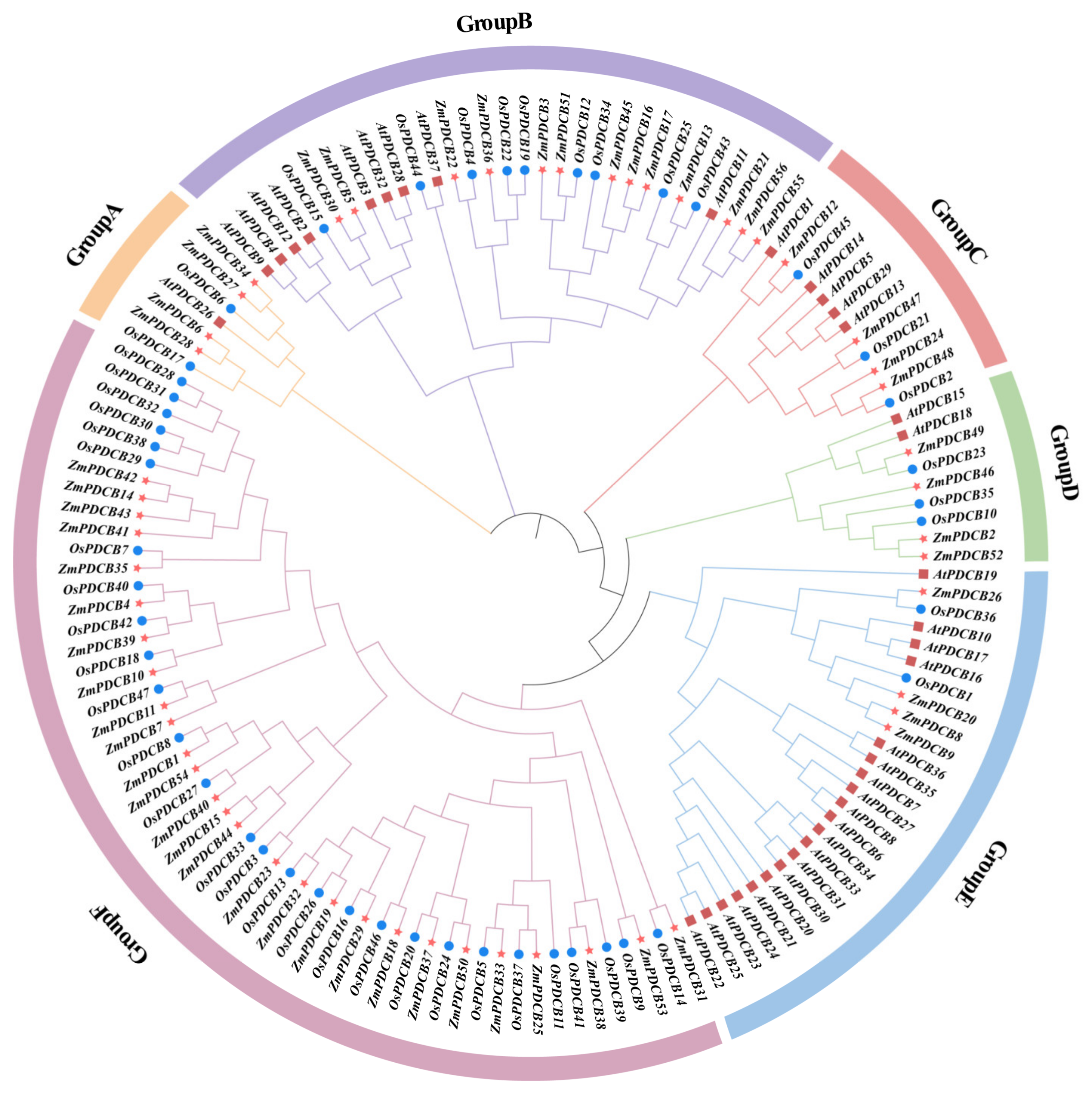
3.2. Phylogenetic Study and Classification of Maize PDCBs
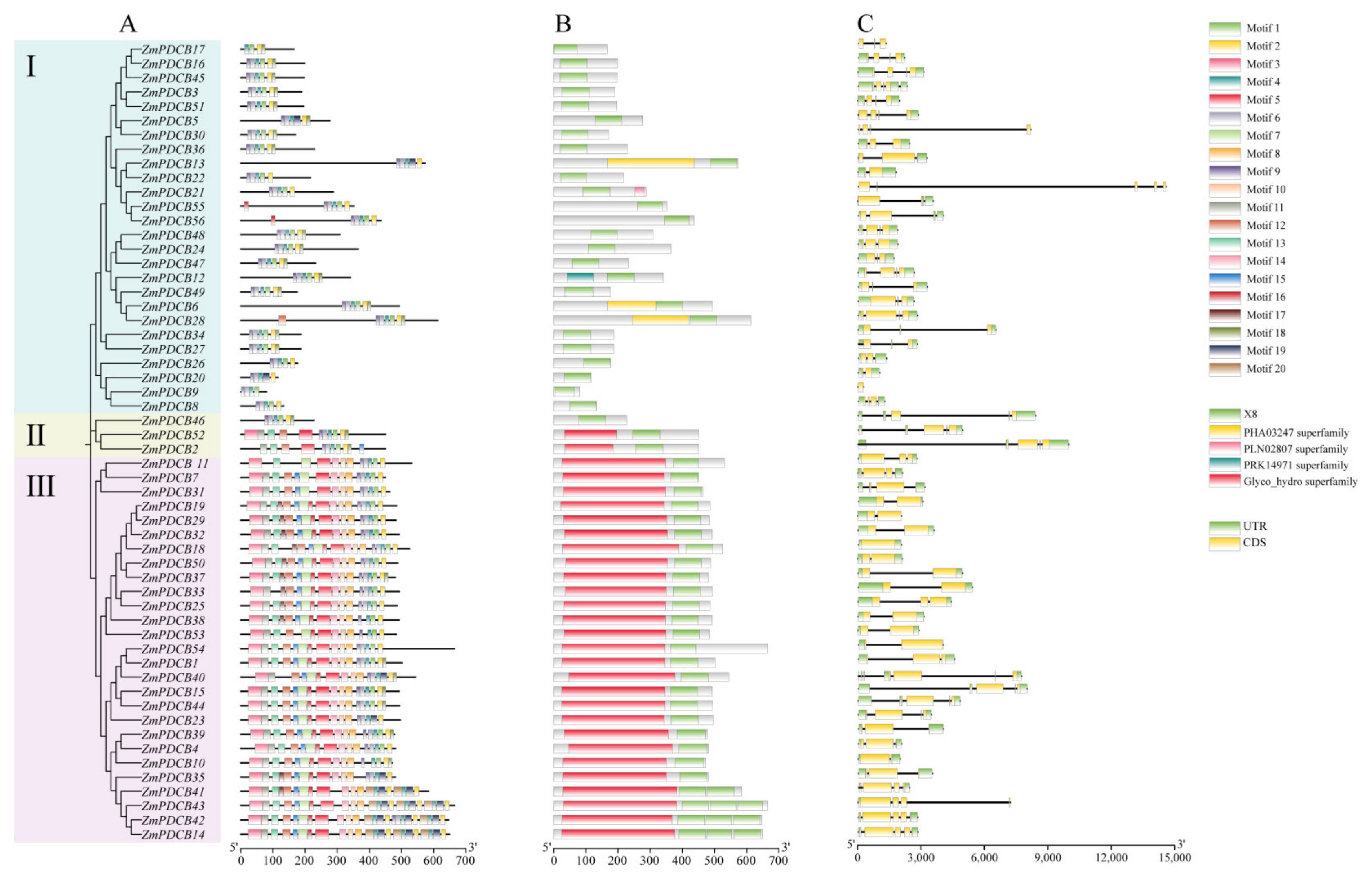
3.3. Gene Structure and Conserved Motif Analysis of ZmPDCB Genes
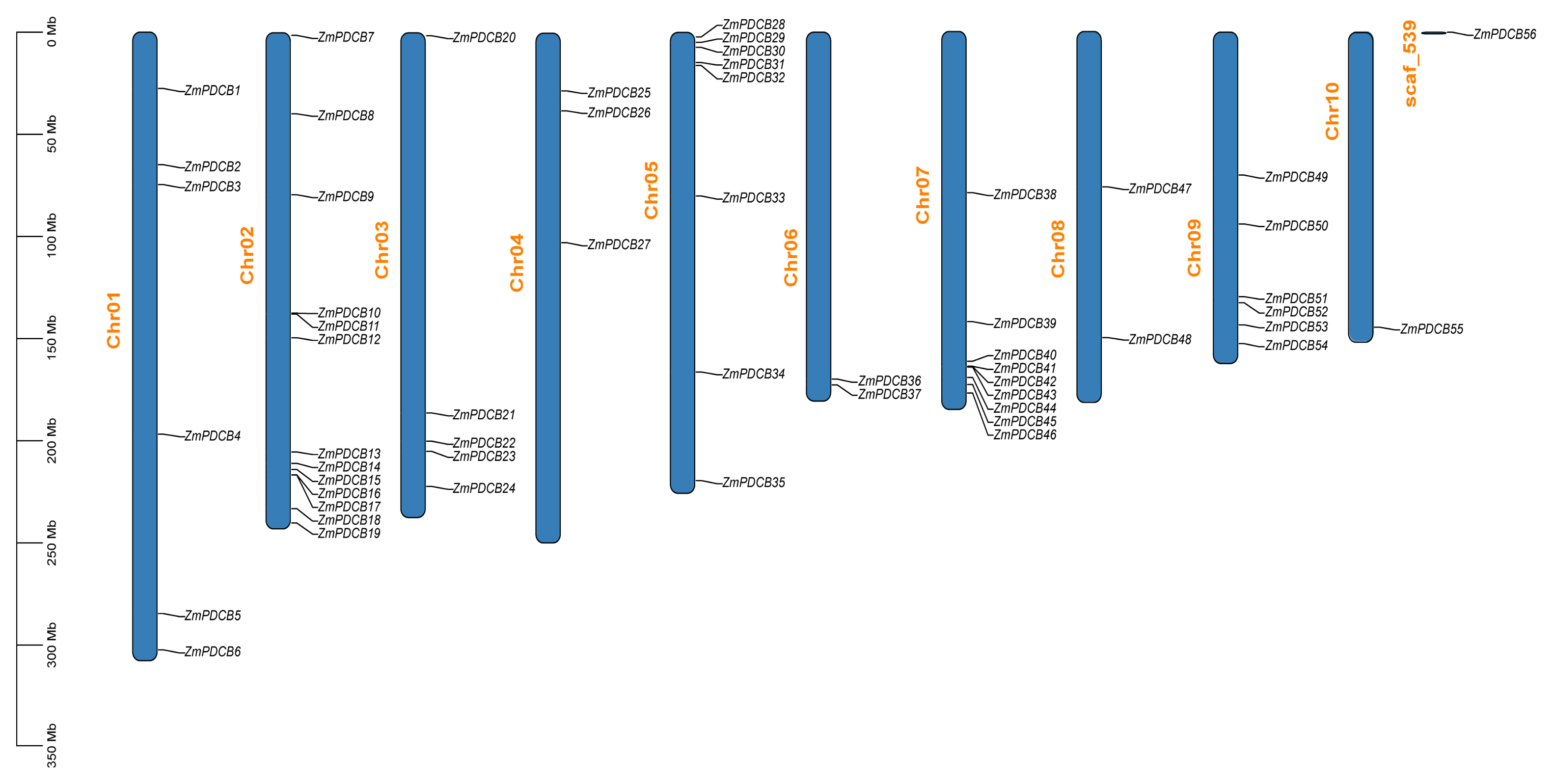
3.4. Chromosomal Location and Synteny Analysis of the PDCBs in Zea mays
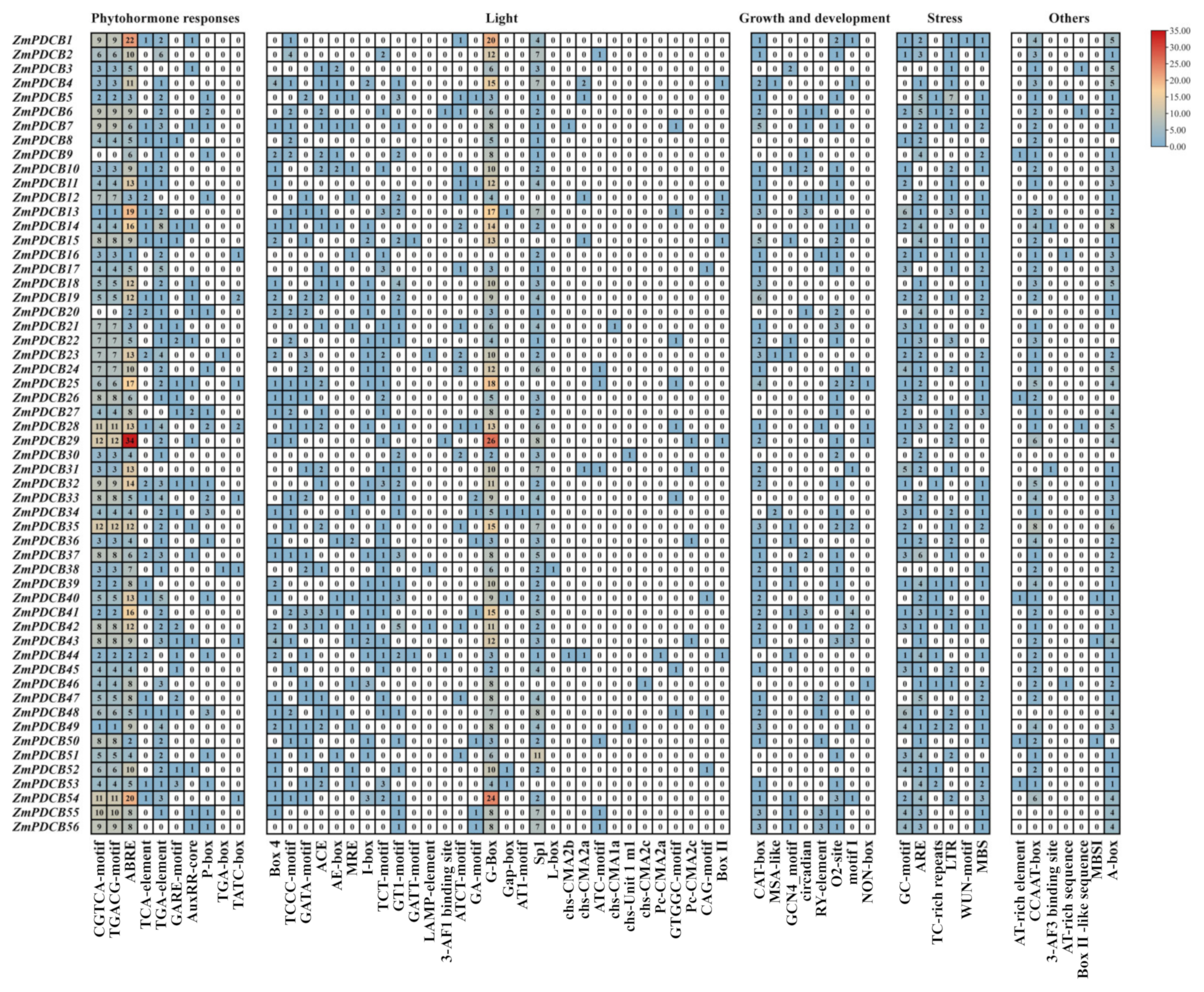
3.5. Cis-Acting Elements in the Promoter of PDCB Genes
3.6. ZmPDCBs Expression Patterns in Various Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ehlers, K.; Kollmann, R. Primary and secondary plasmodesmata: Structure, origin, and functioning. Protoplasma 2001, 216, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Burch-Smith, T.M.; Stonebloom, S.; Xu, M.; Zambryski, P.C. Plasmodesmata during development: Re-examination of the importance of primary, secondary, and branched plasmodesmata structure versus function. Protoplasma 2011, 248, 61–74. [Google Scholar] [CrossRef]
- Sager, R.E.; Lee, J.-Y. Plasmodesmata at a glance. J. Cell Sci. 2018, 131, jcs209346. [Google Scholar] [CrossRef] [PubMed]
- Lucas, W.J.; Ham, B.-K.; Kim, J.-Y. Plasmodesmata—Bridging the gap between neighboring plant cells. Trends Cell Biol. 2009, 19, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Medrano, R.; Xoconostle-Cazares, B.; Kragler, F. The plasmodesmatal transport pathway for homeotic proteins, silencing signals and viruses. Curr. Opin. Plant Biol. 2004, 7, 641–650. [Google Scholar] [CrossRef]
- Evert, R.F. Sieve-Tube Structure in Relation to Function. BioScience 1982, 32, 789–795. [Google Scholar] [CrossRef]
- Dhungana, S.R.; Braun, D.M. Sugar transporters in grasses: Function and modulation in source and storage tissues. J. Plant Physiol. 2021, 266, 153541. [Google Scholar] [CrossRef]
- Heyser, W.; Evert, R.F.; Fritz, E.; Eschrich, W. Sucrose in the Free Space of Translocating Maize Leaf Bundles. Plant Physiol. 1978, 62, 491–494. [Google Scholar] [CrossRef]
- Ayre, B.G. Membrane-Transport Systems for Sucrose in Relation to Whole-Plant Carbon Partitioning. Mol. Plant 2011, 4, 377–394. [Google Scholar] [CrossRef]
- Slewinski, T.L.; Braun, D.M. Current perspectives on the regulation of whole-plant carbohydrate partitioning. Plant Sci. 2010, 178, 341–349. [Google Scholar] [CrossRef]
- Grison, M.S.; Brocard, L.; Fouillen, L.; Nicolas, W.; Wewer, V.; Dörmann, P.; Nacir, H.; Benitez-Alfonso, Y.; Claverol, S.; Germain, V.; et al. Specific Membrane Lipid Composition Is Important for Plasmodesmata Function in Arabidopsis. Plant Cell 2015, 27, 1228–1250. [Google Scholar] [CrossRef] [PubMed]
- Zavaliev, R.; Ueki, S.; Epel, B.L.; Citovsky, V. Biology of callose (β-1,3-glucan) turnover at plasmodesmata. Protoplasma 2011, 248, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. Regulation and Function of Defense-Related Callose Deposition in Plants. Int. J. Mol. Sci. 2021, 22, 2393. [Google Scholar] [CrossRef] [PubMed]
- German, L.; Yeshvekar, R.; Benitez-Alfonso, Y. Callose metabolism and the regulation of cell walls and plasmodesmata during plant mutualistic and pathogenic interactions. Plant Cell Environ. 2022, 46, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-W.; Kumar, R.; Iswanto, A.B.B.; Kim, J.-Y. Callose balancing at plasmodesmata. J. Exp. Bot. 2018, 69, 5325–5339. [Google Scholar] [CrossRef] [PubMed]
- Ohba, Y.; Yoshihara, S.; Sato, R.; Matsuoka, K.; Asahina, M.; Satoh, S.; Iwai, H. Plasmodesmata callose binding protein 2 contributes to the regulation of cambium/phloem formation and auxin response during the tissue reunion process in incised Arabidopsis stem. J. Plant Res. 2023, 136, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Maule, A.J.; Gaudioso-Pedraza, R.; Benitez-Alfonso, Y. Callose deposition and symplastic connectivity are regulated prior to lateral root emergence. Commun. Integr. Biol. 2013, 6, e26531. [Google Scholar] [CrossRef]
- Simpson, C.; Thomas, C.; Findlay, K.; Bayer, E.; Maule, A.J. An Arabidopsis GPI-anchor plasmodesmal neck protein with callose binding activity and potential to regulate cell-to-cell trafficking. Plant Cell 2009, 21, 581–594. [Google Scholar] [CrossRef] [PubMed]
- De Storme, N.; Geelen, D. Callose homeostasis at plasmodesmata: Molecular regulators and developmental relevance. Front. Plant Sci. 2014, 5, 138. [Google Scholar] [CrossRef]
- Li, W.; Yuan, K.; Ren, M.; Xie, Z.; Qi, K.; Gong, X.; Wang, Q.; Zhang, S.; Tao, S. PbPDCB16-mediated callose deposition affects the plasmodesmata blockage and reduces lignification in pear fruit. Plant Sci. 2023, 337, 111876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiao, X.; Li, Z.; Chen, Y.; Li, P.; Peng, R.; Lu, Q.; Wang, Y. Exploring the plasmodesmata callose-binding protein gene family in upland cotton: Unraveling insights for enhancing fiber length. PeerJ 2024, 12, e17625. [Google Scholar] [CrossRef] [PubMed]
- Das Jyoti, S.; Bin Azim, J.; Robin, A.H.K. Genome-wide characterization and expression profiling of EIN3/EIL family genes in Zea mays. Plant Gene 2021, 25, 100270. [Google Scholar] [CrossRef]
- Schnable, P.S.; Ware, D.; Fulton, R.S.; Stein, J.C.; Wei, F.; Pasternak, S.; Liang, C.; Zhang, J.; Fulton, L.; Graves, T.A.; et al. The B73 Maize Genome: Complexity, Diversity, and Dynamics. Science 2009, 326, 1112–1115. [Google Scholar] [CrossRef]
- Xie, J.; Fei, X.; Yan, Q.; Jiang, T.; Li, Z.; Chen, H.; Wang, B.; Chao, Q.; He, Y.; Fan, Z.; et al. The C4 photosynthesis bifunctional enzymes, PDRPs, of maize are co-opted to cytoplasmic viral replication complexes to promote infection of a prevalent potyvirus sugarcane mosaic virus. Plant Biotechnol. J. 2024, 22, 1812–1832. [Google Scholar] [CrossRef]
- Edwards, G.E.; Franceschi, V.R.; Voznesenskaya, E.V. Single-cell C(4) photosynthesis versus the dual-cell (Kranz) paradigm. Annu. Rev. Plant Biol. 2004, 55, 173–196. [Google Scholar] [CrossRef] [PubMed]
- Langdale, J.A. C4 cycles: Past, present, and future research on C4 photosynthesis. Plant Cell 2011, 23, 3879–3892. [Google Scholar] [CrossRef]
- Danila, F.R.; Quick, W.P.; White, R.G.; Furbank, R.T.; von Caemmerer, S. The Metabolite Pathway between Bundle Sheath and Mesophyll: Quantification of Plasmodesmata in Leaves of C3 and C4 Monocots. Plant Cell 2016, 28, 1461–1471. [Google Scholar] [CrossRef]
- Garcia-Hernandez, M.; Berardini, T.; Chen, G.; Crist, D.; Doyle, A.; Huala, E.; Knee, E.; Lambrecht, M.; Miller, N.; Mueller, L.A.; et al. TAIR: A resource for integrated Arabidopsis data. Funct. Integr. Genom. 2002, 2, 239–253. [Google Scholar] [CrossRef]
- Bolser, D.; Staines, D.M.; Pritchard, E.; Kersey, P. Ensembl Plants: Integrating Tools for Visualizing, Mining, and Analyzing Plant Genomics Data; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2020, 49, D412–D419. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, 585–587. [Google Scholar] [CrossRef]
- Shang, L.; He, W.; Wang, T.; Yang, Y.; Xu, Q.; Zhao, X.; Yang, L.; Zhang, H.; Li, X.; Lv, Y.; et al. A complete assembly of the rice Nipponbare reference genome. Mol. Plant 2023, 16, 1232–1236. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Walley, J.W.; Sartor, R.C.; Shen, Z.; Schmitz, R.J.; Wu, K.J.; Urich, M.A.; Nery, J.R.; Smith, L.G.; Schnable, J.C.; Ecker, J.R.; et al. Integration of omic networks in a developmental atlas of maize. Science 2016, 353, 814–818. [Google Scholar] [CrossRef]
- Hufford, M.B.; Seetharam, A.S.; Woodhouse, M.R.; Chougule, K.M.; Ou, S.; Liu, J.; Ricci, W.A.; Guo, T.; Olson, A.; Qiu, Y.; et al. De novo assembly, annotation, and comparative analysis of 26 diverse maize genomes. Science 2021, 373, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Stelpflug, S.C.; Sekhon, R.S.; Vaillancourt, B.; Hirsch, C.N.; Buell, C.R.; De Leon, N.; Kaeppler, S.M. An Expanded Maize Gene Expression Atlas based on RNA Sequencing and its Use to Explore Root Development. Plant Genome 2016, 9. [Google Scholar] [CrossRef]
- Feng, J.; Chen, Y.; Xiao, X.; Qu, Y.; Li, P.; Lu, Q.; Huang, J. Genome-wide analysis of the CalS gene family in cotton reveals their potential roles in fiber development and responses to stress. PeerJ 2021, 9, e12557. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Wang, X.; Sun, Y.; Joseph, P.V.; Paterson, A.H. Detection of colinear blocks and synteny and evolutionary analyses based on utilization of MCScanX. Nat. Protoc. 2024, 19, 2206–2229. [Google Scholar] [CrossRef]
- Alexander, R.D.; Wendelboe-Nelson, C.; Morris, P.C. The barley transcription factor HvMYB1 is a positive regulator of drought tolerance. Plant Physiol. Biochem. 2019, 142, 246–253. [Google Scholar] [CrossRef]
- Cao, L.; Lu, X.; Zhang, P.; Wang, G.; Wei, L.; Wang, T. Systematic Analysis of Differentially Expressed Maize ZmbZIP Genes between Drought and Rewatering Transcriptome Reveals bZIP Family Members Involved in Abiotic Stress Responses. Int. J. Mol. Sci. 2019, 20, 4103. [Google Scholar] [CrossRef]
- Piršelová, B.; Matušíková, I. Callose: The plant cell wall polysaccharide with multiple biological functions. Acta Physiol. Plant. 2013, 35, 635–644. [Google Scholar] [CrossRef]
- Simpson, C. GPI-Anchored Proteins as Potential Plasmodesmal Components; University of East Anglia: Norwich, UK, 2008. [Google Scholar]
- Singh, J.; Garai, S.; Das, S.; Thakur, J.K.; Tripathy, B.C. Role of C4 photosynthetic enzyme isoforms in C3 plants and their potential applications in improving agronomic traits in crops. Photosynth. Res. 2022, 154, 233–258. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Hyun, T.K.; Zhang, M.; Kumar, R.; Koh, E.-J.; Kang, B.-H.; Lucas, W.J.; Kim, J.-Y. Auxin-callose-mediated plasmodesmal gating is essential for tropic auxin gradient formation and signaling. Dev. Cell 2014, 28, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Du, H.; Ding, X.; Zhou, Y.; Xie, P.; Wu, J. Mechanisms of callose deposition in rice regulated by exogenous abscisic acid and its involvement in rice resistance to Nilaparvata lugens Stål (Hemiptera: Delphacidae). Pest. Manag. Sci. 2017, 73, 2559–2568. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Wang, S.; Zhang, M.; Song, X.; Wang, H. Genome-Wide Identification, Characterization, and Expression Profile of PDCB Gene Family in Zea mays L. Agronomy 2024, 14, 1858. https://doi.org/10.3390/agronomy14081858
Guo J, Wang S, Zhang M, Song X, Wang H. Genome-Wide Identification, Characterization, and Expression Profile of PDCB Gene Family in Zea mays L. Agronomy. 2024; 14(8):1858. https://doi.org/10.3390/agronomy14081858
Chicago/Turabian StyleGuo, Jiabao, Shiji Wang, Meichun Zhang, Xiaohan Song, and Hongyan Wang. 2024. "Genome-Wide Identification, Characterization, and Expression Profile of PDCB Gene Family in Zea mays L." Agronomy 14, no. 8: 1858. https://doi.org/10.3390/agronomy14081858




