Chemical and Biological Investigation on the Potential Ornamental Plant Ophiorrhiza chinensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
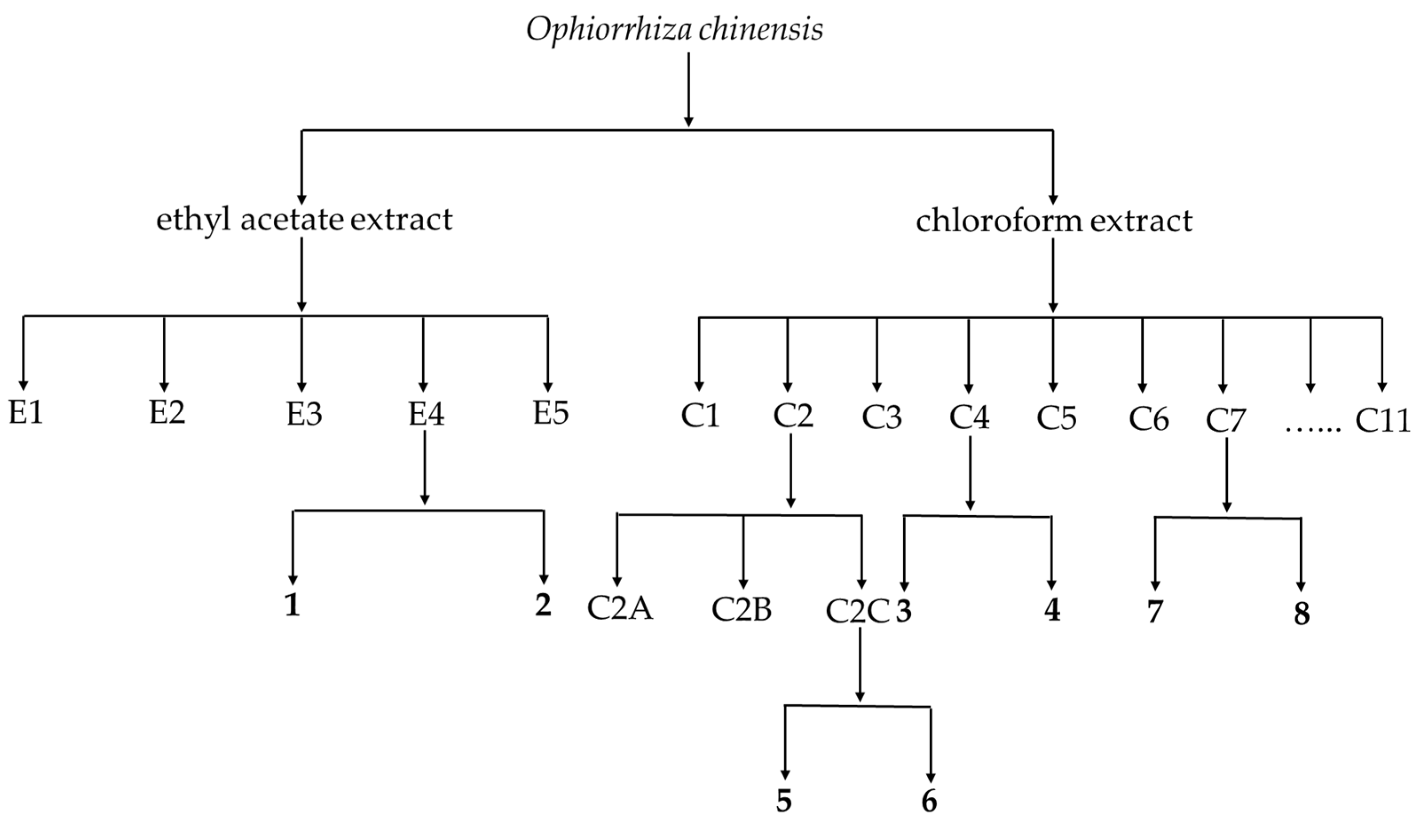
2.3. Extraction and Isolation
2.4. Characteristic 1H and 13C NMR Spectral Data of Isolates
2.5. In Vitro Tyrosinase Inhibitory Activity Assay
2.6. Molecular Docking Calculations
3. Results and Discussion
3.1. Structure Elucidation of the Isolates
3.2. Chemotaxonomic Significance
3.3. Tyrosinase Inhibitory Bioassay
3.4. Preliminary Analysis of Structure–Activity Relationships for Tyrosinase Inhibition
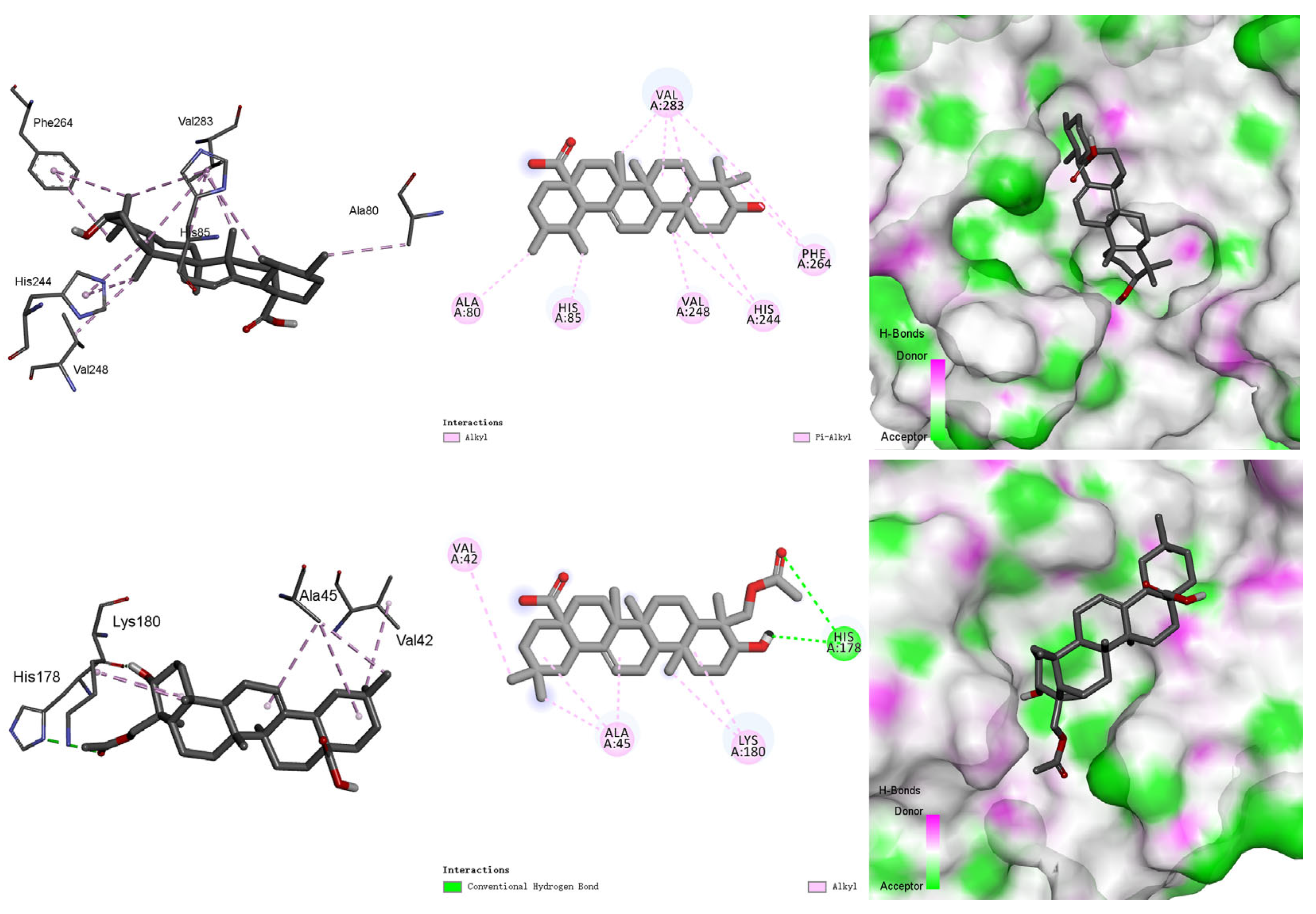
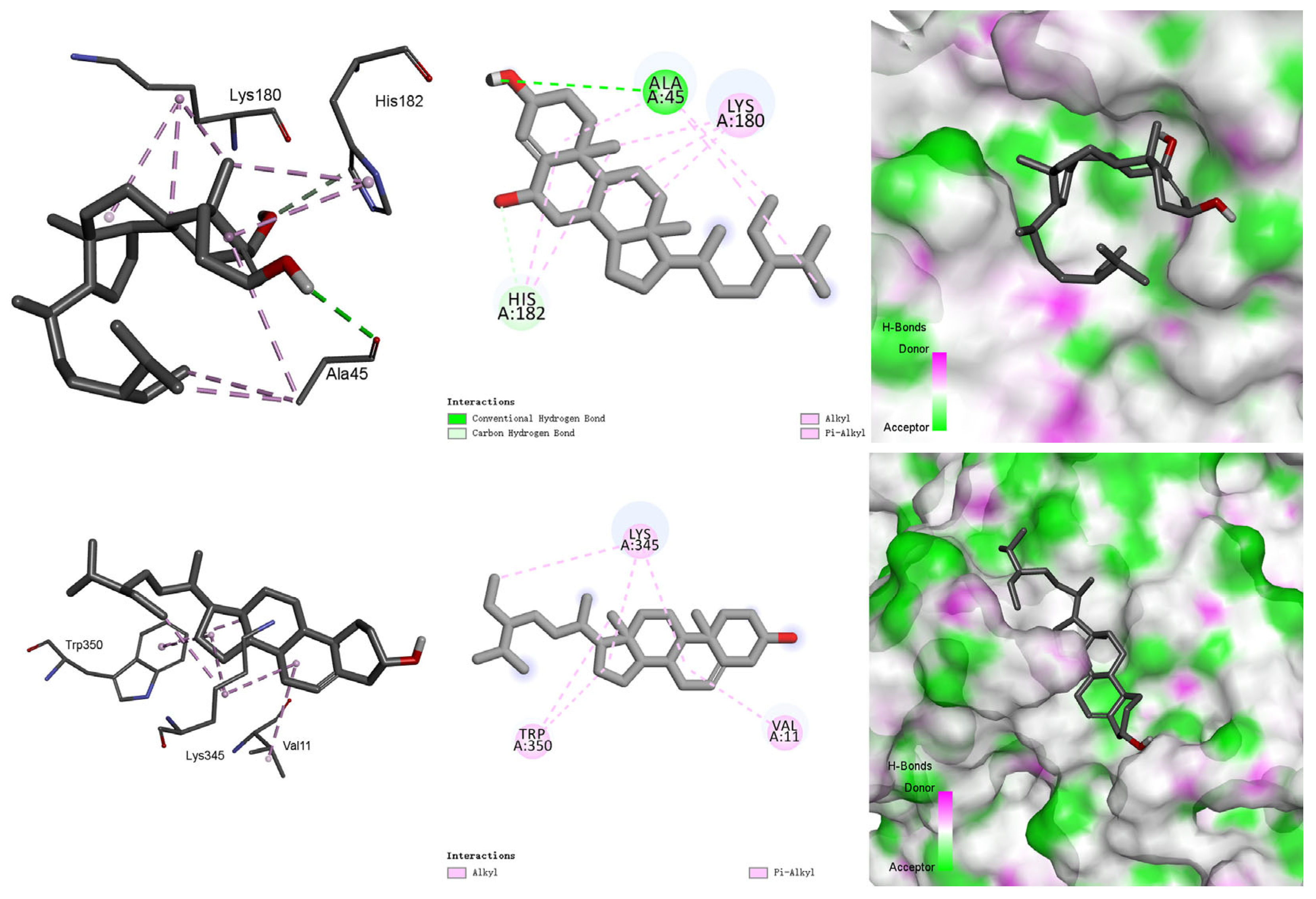
3.5. Molecular Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, H. The valuable utilizationand reproductive cultivation techniques of Ophiorrhiza japonica. S. China Agric. 2021, 15, 24–25. [Google Scholar] [CrossRef]
- Chinese Materia Medica Committee of the State Administration of Traditional Chinese Medicine. Zhushacao, Shengencao, Duanxiaoshegencao, Diguicao. In Chinese Materia Medica; Shanghai Science and Technology Press: Shanghai, China, 1999; Volume 18, pp. 457–460. [Google Scholar]
- Zhong, L.-J.; Liang, L.-F.; Chen, J.-K.; Bu, Q.; Xu, M.-J. Research progress on chemical constituents and pharmacological activities from the genus Ophiorrhiza. Chin. Pharm. J. 2022, 57, 1221–1231. [Google Scholar] [CrossRef]
- Anil, J.J.; Renjith, R.; Sabulal, B. Secondary metabolites from Ophiorrhiza. Nat. Prod. J. 2018, 8, 248–267. [Google Scholar] [CrossRef]
- Taher, M.; Shaari, S.S.; Susanti, D.; Arbain, D.; Zakaria, Z.A. Genus Ophiorrhiza: A review of its distribution, traditional uses, phytochemistry, biological activities and propagation. Molecules 2020, 25, 2611. [Google Scholar] [CrossRef]
- Flora of China Editorial Committee. Ophiorrhiza Chinensis H. S. Lo. In Flora of China; Science Press: Beijing, China, 1999; Volume 71, p. 168. [Google Scholar]
- Shi, J.; Xu, M.; Zhou, C.; Zou, Q.; Zhang, X. Comparative study on the content of chlorogenic acid in Ophiorrhiza chinensis from different organs. Hunan Zhongyiyao Daxue Xuebao 2016, 36, 28–30. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, J.; Zhou, A.; Zhou, Y.; Ye, W.; Cao, D.-S.; Yang, R. Substrate-photocaged enzymatic fluorogenic probe enabling sequential activation for light-controllable monitoring of intracellular tyrosinase activity. Anal. Chem. 2020, 92, 7194–7199. [Google Scholar] [CrossRef]
- Parvez, S.; Kang, M.; Chung, H.-S.; Bae, H. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother. Res. 2007, 21, 805–816. [Google Scholar] [CrossRef]
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Baber, M.A.; Crist, C.M.; Devolve, N.L.; Patrone, J.D. Tyrosinase inhibitors: A perspective. Molecules 2023, 28, 5762. [Google Scholar] [CrossRef]
- Kim, H.-D.; Choi, H.; Abekura, F.; Park, J.-Y.; Yang, W.-S.; Yang, S.-H.; Kim, C.-H. Naturally-occurring tyrosinase inhibitors classified by enzyme kinetics and copper chelation. Int. J. Mol. Sci. 2023, 24, 8226. [Google Scholar] [CrossRef]
- Ullah, S.; Son, S.; Yun, H.Y.; Kim, D.H.; Chun, P.; Moon, H.R. Tyrosinase inhibitors: A patent review (2011-2015). Expert. Opin. Ther. Pat. 2016, 26, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Bonesi, M.; Xiao, J.; Tundis, R.; Aiello, F.; Sicari, V.; Loizzo, R.M. Advances in the tyrosinase inhibitors from plant source. Curr. Med. Chem. 2019, 26, 3279–3299. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Peng, X.; Li, S.; Liu, B.; Chu, C. Recent discovery of tyrosinase inhibitors in traditional Chinese medicines and screening methods. J. Ethnopharmacol. 2023, 303, 115951. [Google Scholar] [CrossRef]
- Bu, Q.; Jin, Y.; Xu, M.-J.; Wu, L.; Liang, L.-F. Structurally diverse metabolites from the Ophiorrhiza japonica Bl. and their antioxidant activities in vitro and PPARα agonistic activities in silico. Molecules 2022, 27, 5301. [Google Scholar] [CrossRef] [PubMed]
- Si, X.-y.; Wang, Y.-f.; He, R.-j.; Yang, B.-y.; Huang, Y.-l. Chemical constituents from leaves of Castanopsis lamontii and its tyrosinase inhibitory activities. Chin. Tradit. Herb. Drugs 2023, 54, 6214–6219. [Google Scholar]
- Souza, P.M.; Elias, S.T.; Simeoni, L.A.; de Paula, J.E.; Gomes, S.M.; Guerra, E.N.S.; Fonseca, Y.M.; Silva, E.C.; Silveira, D.; Magalhães, P.O. Plants from Brazilian Cerrado with potent tyrosinase inhibitory activity. PLoS ONE 2012, 7, e48589. [Google Scholar] [CrossRef]
- Luo, P.; Deng, Z.; Wang, J.; Shen, Z.; Pan, W. Chemical constituents from Primulina linearifolia (W. T. Wang) Yin Z. Wang. Guangxi Sci. 2023, 30, 883–890. [Google Scholar] [CrossRef]
- Pereda-Miranda, R.; Delgado, G.; de Vivar, A.R. New triterpenoids from Salvia nicolsoniana. J. Nat. Prod. 1986, 49, 225–230. [Google Scholar] [CrossRef]
- Kalinovskii, A.I. 13C NMR spectroscopy of oleanane derivatives. Chem. Nat. Compd. 1992, 28, 1–22. [Google Scholar] [CrossRef]
- Wang, J.; Fei, J.-d.; Ding, G.; Li, R.-t.; Yu, C.-y.; Zou, Z.-m. Chemical constituents in the petroleum ether extract of the whole herb of Flickingeria fimbriata. Chin. Pharm. J. 2013, 48, 1343–1346. [Google Scholar]
- Kim, J.S.; Kim, J.C.; Shim, S.H.; Lee, E.J.; Jin, W.Y.; Bae, K.; Son, K.H.; Kim, H.P.; Kang, S.S.; Chang, H.W. Chemical constituents of the root of Dystaenia takeshimana and their anti-inflammatory activity. Arch. Pharm. Res. 2006, 29, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-s.; Zhang, K.; Tan, G.-s.; Zeng, G.-y.; Zhou, Y.-j. Studies on constituents of Oldenladia diffusa. Chin. Pharm. J. 2005, 40, 817–819. [Google Scholar]
- Fraga, B.M.; Quintana, N.; Díaz, C.E. Anthraquinones from natural and transformed roots of Plocama pendula. Chem. Biodivers. 2009, 6, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Mahibalan, S.; Rao, P.C.; Khan, R.; Basha, A.; Siddareddy, R.; Masubuti, H.; Fujimoto, Y.; Begum, A.S. Cytotoxic constituents of Oldenlandia umbellata and isolation of a new symmetrical coumarin dimer. Med. Chem. Res. 2016, 25, 466–472. [Google Scholar] [CrossRef]
- Ying, Y.-M.; Shan, W.-G.; Liu, W.-H.; Zhan, Z.-J. Alkaloids and nucleoside derivatives from a fungal endophyte of Huperzia serrata. Chem. Nat. Compd. 2013, 49, 184–186. [Google Scholar] [CrossRef]
- Li, Y.-H.; Luo, F.; Peng, S.-L.; Liang, J.; Ding, L.-S. A new dihydroisocoumarin from the rhizomes of Notopterygium forbesii. Nat. Prod. Res. 2006, 20, 860–865. [Google Scholar] [CrossRef]
- Mahato, S.B.; Kundu, A.P. 13C NMR spectra of pentacyclic triterpenoids—A compilation and some salient features. Phytochemistry 1994, 37, 1517–1575. [Google Scholar] [CrossRef]
- Kitajima, M.; Fujii, N.; Yoshino, F.; Sudo, H.; Saito, K.; Aimi, N.; Takayama, H. Camptothecins and two new monoterpene glucosides from Ophiorrhiza liukiuensis. Chem. Pharm. Bull. 2005, 53, 1355–1358. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Das, S.; Mandal, A.B.; Arunachalam, G.; Bhattacharya, S.K. Evaluation of analgesic and anti-inflammatory activity of Ophiorrhiza nicobarica, an ethnomedicine from Nicobar Islands, India. Orient. Pharm. Exp. Med. 2007, 7, 395–408. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Xiang, W.; Song, Q.-S. Chemical constituents of Ophiorrhiza rosea. Chin. Tradit. Herb. Drugs 2009, 40, 519–521. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Li, J.; Xiang, W.; Song, Q.-S. Chemical constituents of Ophiorrhiza austroyunnanensis. Nat. Prod. Res. Dev. 2011, 23, 652–654. [Google Scholar] [CrossRef]
- Agarwal, K.P.; Dhar, M.M. Chemical examination of Ophiorrhiza mungos. J. Sci. Ind. Res. 1959, 18B, 114–115. [Google Scholar]
- Fujita, E.; Sumi, A. Studies on the constituents of Ophiorrhiza japonica Bl. Yakugaku Zasshi 1967, 87, 1153–1155. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-F.; Song, Q.-S.; Xiang, W.; Wang, Y.-L. Study on chemical constituents and antibacterial activity of Ophiorrhiza cantoniensis Hance. Nat. Prod. Res. Dev. 2014, 26, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Nakamura, M.; Takayama, H.; Saito, K.; Stöckigt, J.; Aimi, N. Constituents of regenerated plants of Ophiorrhiza pumila; formation of a new glycocamptothecin and predominant formation of (3R)-deoxypumiloside over (3S)-congener. Tetrahedron Lett. 1997, 38, 8997–9000. [Google Scholar] [CrossRef]
- Aimi, N.; Murakami, H.; Tsuyuki, T.; Nishiyama, T.; Sakai, S.-i.; Haginiwa, J. Hydrolytic degradation of β-carboline-type monoterpenoid glucoindole alkaloids: A possible mechanism for harman formation in Ophiorrhiza and related rubiaceous plants. Chem. Pharm. Bull. 1986, 34, 3064–3066. [Google Scholar] [CrossRef]
- Hamzah, A.S.; Arbain, D.; Mahyudin; Sargent, M.V.; Lajis, N.H. The alkaloids of Ophiorrhiza communis and O. tomentosa. Pertanika J. Sci. Technol. 1994, 2, 33–38. [Google Scholar]
- Nonato, M.G.; Truscott, R.J.W.; Carver, J.A.; Hemling, M.E.; Garson, M.J. Glucoindole alkaloids from Ophiorrhiza acuminata. Planta Med. 1995, 61, 278–280. [Google Scholar] [CrossRef]
- Ashraf, Z.; Rafiq, M.; Nadeem, H.; Hassan, M.; Afzal, S.; Waseem, M.; Afzal, K.; Latip, J. Carvacrol derivatives as mushroom tyrosinase inhibitors; synthesis, kinetics mechanism and molecular docking studies. PLoS ONE 2017, 12, e0178069. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef]


| Compounds | Structural Types | Names | References |
|---|---|---|---|
| 1 | ursane-type triterpene | ursolic acid | [19] |
| 2 | oleane-type triterpene | 24-acetoxy-3α-hydroxyolean-12-en-28-oic | [20,21] |
| 3 | stigmastane-type steroid | stigmast-4-ene-3β,6β-diol | [22] |
| 4 | stigmastane-type steroid | β-stiosterol | [23] |
| 5 | anthraquinone | anthragallol 1,3-dimethyl ether | [24,25] |
| 6 | anthraquinone | anthragallol 1,2-dimethyl ether | [26] |
| 7 | aromatic alkaloid | harmane | [27] |
| 8 | coumarin | scopoletin | [28] |
| Compounds | IC50 (μM) |
|---|---|
| 1 | 43.8 ± 0.1 |
| 2 | 25.7 ± 1.3 |
| 3 | 35.5 ± 2.0 |
| 4 | 65.8 ± 7.8 |
| 5 | 68.1 ± 1.4 |
| 6 | 67.4 ± 4.4 |
| 1 Vitamin C | 3.6 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, Q.; Ge, Z.-Y.; Liang, L.-F. Chemical and Biological Investigation on the Potential Ornamental Plant Ophiorrhiza chinensis. Agronomy 2024, 14, 1872. https://doi.org/10.3390/agronomy14081872
Bu Q, Ge Z-Y, Liang L-F. Chemical and Biological Investigation on the Potential Ornamental Plant Ophiorrhiza chinensis. Agronomy. 2024; 14(8):1872. https://doi.org/10.3390/agronomy14081872
Chicago/Turabian StyleBu, Qing, Zeng-Yue Ge, and Lin-Fu Liang. 2024. "Chemical and Biological Investigation on the Potential Ornamental Plant Ophiorrhiza chinensis" Agronomy 14, no. 8: 1872. https://doi.org/10.3390/agronomy14081872





