Type III Secretion Effectors of Xanthomonas oryzae pv. oryzicola: The Arsenal to Attack Equivalent Rice Defense for Invasion
Abstract
1. Introduction
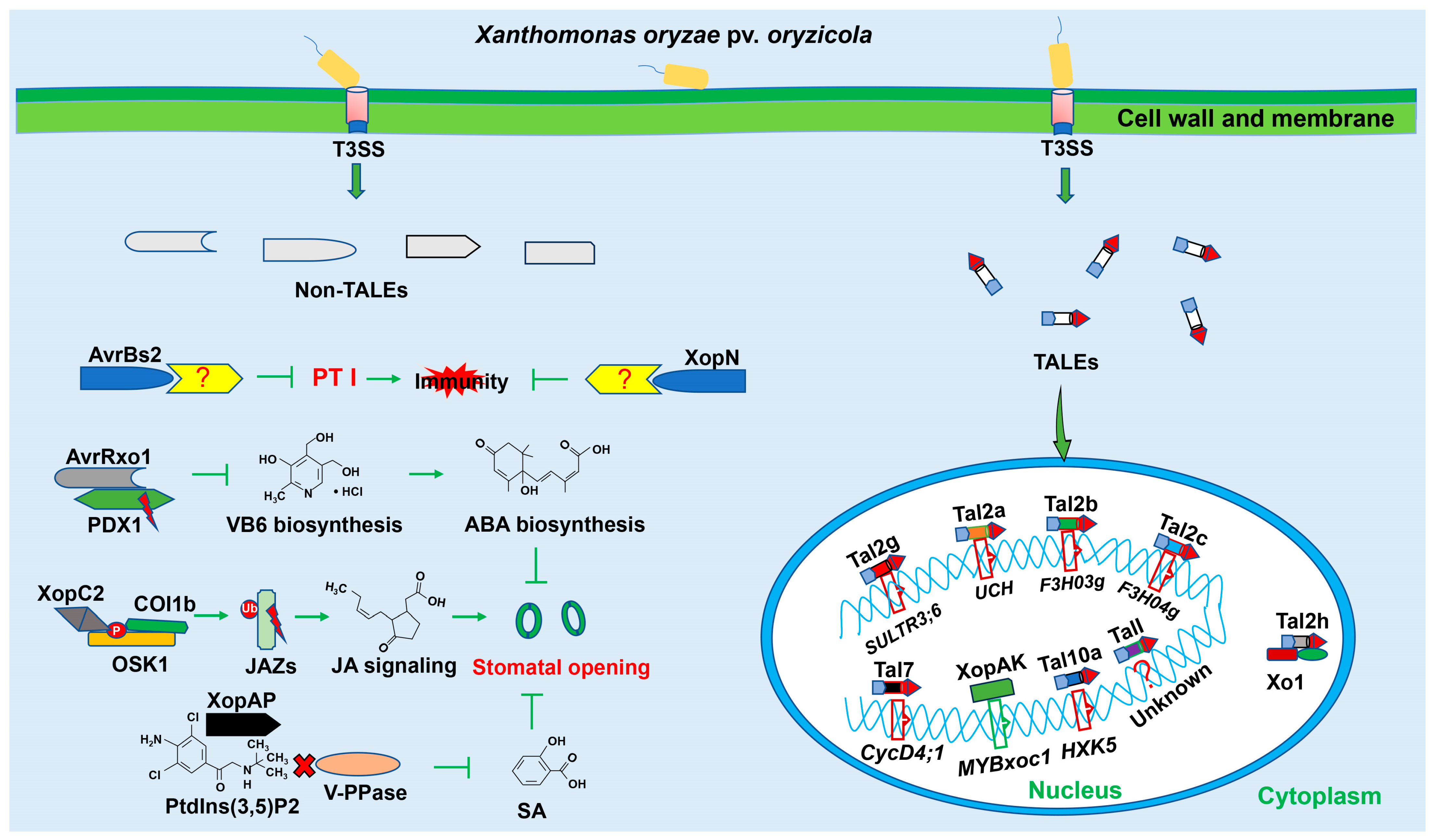
2. Type III Secretion System Is Essential for Virulence of Xanthomonas oryzae pv. oryzicola
2.1. Type III Secretion System of Xoc
2.2. Non-TALEs from Xoc
2.3. TALEs from Xoc
| Effectors | Targets | Function and Mechanism of Effector-Targets | References |
|---|---|---|---|
| Non-TALEs | |||
| AvrRxo1 | NAD; OsPDX1 | Phosphotransferase; cytotoxicity; degradation; suppression of VB6 biosynthesis; activation of stomatal opening | [46,47,48,49,50] |
| AvrBs2 | Unknown | Suppression of PAMP-triggered defense | [52,53] |
| XopN | Unknown | Unknown | [53] |
| XopC2 | OSK1 | Phosphorylation; activation of JA signaling and stomatal opening | [56] |
| XopAP | PtdIns(3,5)P2 | Lipase; binding and competing; suppression of stomatal defense | [57] |
| XopAk | OsMYBxoc1 | Transcriptional suppression; accumulation of iron ions | [58] |
| TALEs | |||
| Tal2g/Tal5d | OsSULTR3;6 | Transcriptional activation | [11,59] |
| Tal2h | Xo1 | Protein–protein interaction; suppression of Xo1-mediated resistance | [60,61] |
| Tal2a | UCH | Transcriptional activation; induction of hypersensitive reaction | [62] |
| Tal7 | CycD4;1 | Transcriptional activation; suppression of avrXa7-Xa7-mediated resistance | [63] |
| Tal10a | OsHXK5 | Transcriptional activation; suppression of PR gene expression and MAPK activation | [64] |
| TalI | Unknown | Transcriptional activation; interference of SA signaling | [65] |
| Tal2b | OsF3H03g | Transcriptional activation; reduction in SA level | [9] |
| Tal2c | OsF3H04g | Transcriptional activation; reduction in SA level | [66] |
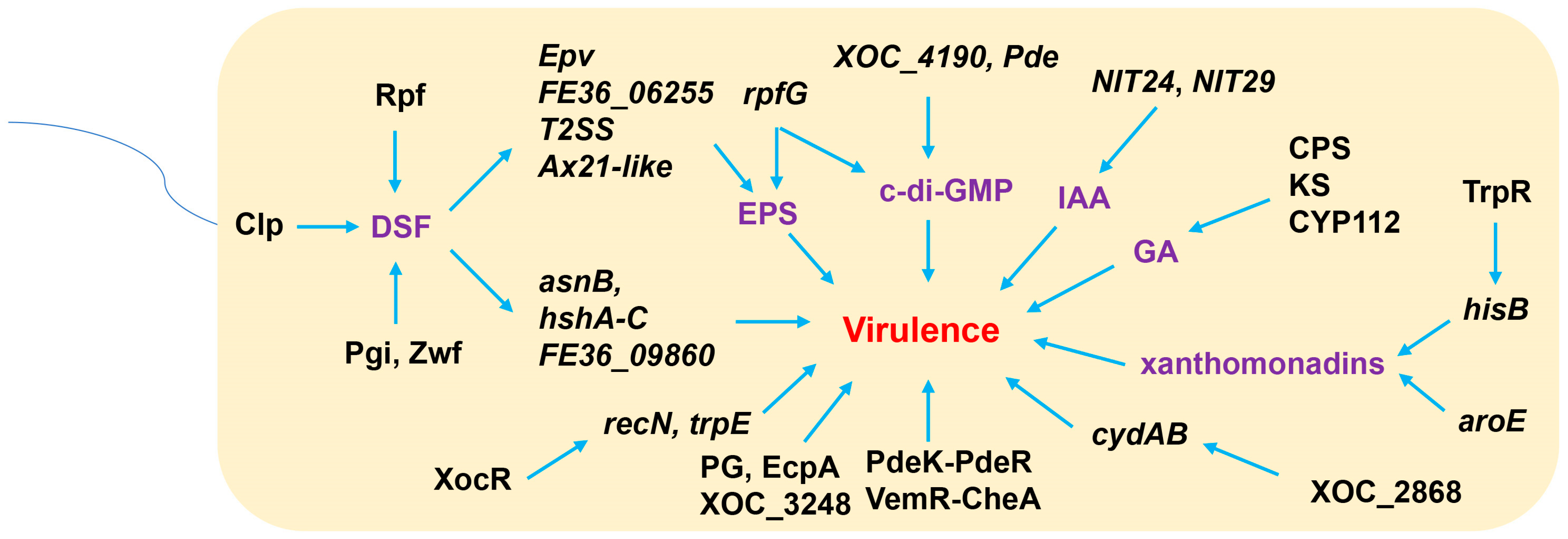
3. Diffusible Signal Factor (DSF)-Mediated Quorum Sensing (QS) and Extracellular Polysaccharides (EPSs) Are Involved in Xoc Virulence
4. Other Factors Affect the Virulence of Xoc
4.1. Molecules
4.2. Two-Component Regulatory System (TCS)
4.3. Other Factors
5. Discussion and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Niño-Liu, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Yan, J.; Liang, Y.; Shi, Y.; He, Z.; Wu, Y.; Zeng, Q.; Liu, X.; Peng, J. Resistance genes and their interactions with bacterial blight/leaf streak pathogens (Xanthomonas oryzae) in rice (Oryza sativa L.)-an updated review. Rice 2020, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.; Bonas, U. How Xanthomonas type III effectors manipulate the host plant. Curr. Opin. Microbiol. 2009, 12, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zou, H.; Che, Y.; Cui, Y.; Guo, W.; Zou, L.; Chatterjee, S.; Biddle, E.M.; Yang, C.; Chen, G. A novel regulatory role of HrpD6 in regulating hrp-hrc-hpa genes in Xanthomonas oryzae pv. oryzicola. Mol. Plant Microbe Interact. 2011, 24, 1086–1101. [Google Scholar] [CrossRef]
- White, F.F.; Potnis, N.; Jones, J.B.; Koebnik, R. The type III effectors of Xanthomonas. Mol. Plant Pathol. 2009, 10, 749–766. [Google Scholar] [CrossRef]
- Boch, J.; Scholze, H.; Schornack, S.; Landgraf, A.; Hahn, S.; Kay, S.; Lahaye, T.; Nickstadt, A.; Bonas, U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science 2009, 326, 1509–1512. [Google Scholar] [CrossRef]
- Ji, Z.; Zakria, M.; Zou, L.; Xiong, L.; Li, Z.; Ji, G.; Chen, G. Genetic diversity of transcriptional activator-like effector genes in Chinese isolates of Xanthomonas oryzae pv. oryzicola. Phytopathology 2014, 104, 672–682. [Google Scholar] [CrossRef][Green Version]
- Wilkins, K.E.; Booher, N.J.; Wang, L.; Bogdanove, A.J. TAL effectors and activation of predicted host targets distinguish Asian from African strains of the rice pathogen Xanthomonas oryzae pv. oryzicola while strict conservation suggests universal importance of five TAL effectors. Front. Plant Sci. 2015, 6, 536. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, H.; Yuan, B.; Liu, H.; Kong, L.; Chu, Z.; Ding, X. Tal2b targets and activates the expression of OsF3H03g to hijack OsUGT74H4 and synergistically interfere with rice immunity. New Phytol. 2022, 233, 1864–1880. [Google Scholar] [CrossRef]
- Zhao, B.; Ardales, E.Y.; Raymundo, A.; Bai, J.; Trick, H.N.; Leach, J.E.; Hulbert, S.H. The avrRxo1 gene from the rice pathogen Xanthomonas oryzae pv. oryzicola confers a nonhost defense reaction on maize with resistance gene Rxo1. Mol. Plant Microbe Interact. 2004, 17, 771–779. [Google Scholar] [CrossRef][Green Version]
- Cernadas, R.A.; Doyle, E.L.; Niño-Liu, D.O.; Wilkins, K.E.; Bancroft, T.; Wang, L.; Schmidt, C.L.; Caldo, R.; Yang, B.; White, F.F.; et al. Code-assisted discovery of TAL effector targets in bacterial leaf streak of rice reveals contrast with bacterial blight and a novel susceptibility gene. PLoS Pathog. 2014, 10, e1003972. [Google Scholar] [CrossRef] [PubMed]
- Büttner, D.; Bonas, U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol. Rev. 2010, 34, 107–133. [Google Scholar] [CrossRef]
- Timilsina, S.; Potnis, N.; Newberry, E.A.; Liyanapathiranage, P.; Iruegas-Bocardo, F.; White, F.F.; Goss, E.M.; Jones, J.B. Xanthomonas diversity, virulence and plant-pathogen interactions. Nat. Rev. Microbiol. 2020, 18, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectr. 2015, 3, 1–30. [Google Scholar] [CrossRef]
- Whitfield, C.; Wear, S.S.; Sande, C. Assembly of bacterial capsular polysaccharides and exopolysaccharides. Annu. Rev. Microbiol. 2020, 74, 521–543. [Google Scholar] [CrossRef] [PubMed]
- Dunger, G.; Relling, V.M.; Tondo, M.L.; Barreras, M.; Ielpi, L.; Orellano, E.G.; Ottado, J. Xanthan is not essential for pathogenicity in citrus canker but contributes to Xanthomonas epiphytic survival. Arch. Microbiol. 2007, 188, 127–135. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, J.G.; Lee, B.M.; Cho, J.Y. Mutational analysis of the gum gene cluster required for xanthan biosynthesis in Xanthomonas oryzae pv. oryzae. Biotechnol. Lett. 2009, 31, 265–270. [Google Scholar] [CrossRef]
- Bianco, M.I.; Toum, L.; Yaryura, P.M.; Mielnichuk, N.; Gudesblat, G.E.; Roeschlin, R.; Marano, M.R.; Ielpi, L.; Vojnov, A.A. Xanthan pyruvilation is essential for the virulence of Xanthomonas campestris pv. campestris. Mol. Plant Microbe Interact. 2016, 29, 688–699. [Google Scholar] [CrossRef]
- Ryan, R.P.; An, S.Q.; Allan, J.H.; McCarthy, Y.; Dow, J.M. The DSF family of cell-cell signals: An expanding class of bacterial virulence regulators. PLoS Pathog. 2015, 11, e1004986. [Google Scholar] [CrossRef]
- Feng, Y.; Long, Z.; Xiang, H.; Ran, J.; Zhou, X.; Yang, S. Research on diffusible signal factor-mediated quorum sensing in Xanthomonas: A mini-review. Molecules 2023, 28, 876. [Google Scholar] [CrossRef]
- Simpson, B.W.; Trent, M.S. Pushing the envelope: LPS modifications and their consequences. Nat. Rev. Microbiol. 2019, 17, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F.; Silipo, A.; Andersen Gersby, L.B.; Palmigiano, A.; Lanzetta, R.; Garozzo, D.; Boyer, C.; Pruvost, O.; Newman, M.A.; Molinaro, A. Xanthomonas citri pv. citri pathotypes: LPS structure and function as microbe-associated molecular patterns. ChemBioChem 2017, 18, 772–781. [Google Scholar] [CrossRef]
- Singh, A.; Bansal, K.; Kumar, S.; Patil, P.B. Deep population genomics reveals systematic and parallel evolution at a lipopolysaccharide biosynthetic locus in Xanthomonas pathogens that infect rice and sugarcane. Appl. Environ. Microbiol. 2022, 88, e0055022. [Google Scholar] [CrossRef]
- Green, E.R.; Mecsas, J. Bacterial secretion systems: An overview. Microbiol. Spectr. 2016, 4, 213–239. [Google Scholar] [CrossRef]
- Kanonenberg, K.; Spitz, O.; Erenburg, I.N.; Beer, T.; Schmitt, L. Type I secretion system-it takes three and a substrate. FEMS Microbiol. Lett. 2018, 365, fny094. [Google Scholar] [CrossRef]
- Meuskens, I.; Saragliadis, A.; Leo, J.C.; Linke, D. Type V secretion systems: An overview of passenger domain functions. Front. Microbiol. 2019, 10, 1163. [Google Scholar] [CrossRef] [PubMed]
- Büttner, D.; He, S.Y. Type III protein secretion in plant pathogenic bacteria. Plant Physiol. 2009, 150, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Puhar, A.; Sansonetti, P.J. Type III secretion system. Curr. Biol. 2014, 24, R784–R791. [Google Scholar] [CrossRef]
- Rossier, O.; Wengelnik, K.; Hahn, K.; Bonas, U. The Xanthomonas Hrp type III system secretes proteins from plant and mammalian bacterial pathogens. Proc. Natl. Acad. Sci. USA 1999, 96, 9368–9373. [Google Scholar] [CrossRef]
- Tampakaki, A.P.; Fadouloglou, V.E.; Gazi, A.D.; Panopoulos, N.J.; Kokkinidis, M. Conserved features of type III secretion. Cell. Microbiol. 2004, 6, 805–816. [Google Scholar] [CrossRef]
- Gürlebeck, D.; Thieme, F.; Bonas, U. Type III effector proteins from the plant pathogen Xanthomonas and their role in the interaction with the host plant. J. Plant Physiol. 2006, 163, 233–255. [Google Scholar] [CrossRef]
- Zou, L.; Wang, X.; Xiang, Y.; Zhang, B.; Li, Y.; Xiao, Y.; Wang, J.; Walmsley, A.R.; Chen, G. Elucidation of the hrp clusters of Xanthomonas oryzae pv. oryzicola that control the hypersensitive response in nonhost tobacco and pathogenicity in susceptible host rice. Appl. Environ. Microbiol. 2006, 72, 6212–6224. [Google Scholar] [CrossRef]
- Li, Y.; Che, Y.; Zou, H.; Cui, Y.; Guo, W.; Zou, L.; Biddle, E.M.; Yang, C.; Chen, G. Hpa2 required by HrpF to translocate Xanthomonas oryzae transcriptional activator-like effectors into rice for pathogenicity. Appl. Environ. Microbiol. 2011, 77, 3809–3818. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Li, Y.; Chen, G. A non-marker mutagenesis strategy to generate poly-hrp gene mutants in the rice pathogen Xanthomonas oryzae pv. oryzicola. Agric. Sci. China 2011, 10, 12. [Google Scholar] [CrossRef]
- Guo, W.; Cui, Y.; Li, Y.; Che, Y.; Yuan, L.; Zou, L.; Zou, H.; Chen, G. Identification of seven Xanthomonas oryzae pv. oryzicola genes potentially involved in pathogenesis in rice. Microbiology 2012, 158, 505–518. [Google Scholar] [CrossRef]
- Cui, Y.; Zou, L.; Zou, H.; Li, Y.; Zakria, M.; Chen, G. HrpE3 is a type III effector protein required for full virulence of Xanthomonas oryzae pv. oryzicola in rice. Mol. Plant Pathol. 2013, 14, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zou, L.; Li, Y.; Zou, H.; Liu, X.; Chen, G. Xoryp_08180 of Xanthomonas oryzae pv. oryzicola, encoding a hypothetical protein, is regulated by HrpG and HrpX and required for full virulence in rice. J. Integr. Agric. 2012, 11, 600–610. [Google Scholar] [CrossRef]
- Guo, W.; Zou, L.; Li, Y.; Cui, Y.; Ji, Z.; Cai, L.; Zou, H.; Hutchins, W.C.; Yang, C.; Chen, G. Fructose-bisphophate aldolase exhibits functional roles between carbon metabolism and the hrp system in rice pathogen Xanthomonas oryzae pv. oryzicola. PLoS ONE 2012, 7, e31855. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Chu, C.; Yang, X.; Fang, Y.; Liu, X.; Chen, G.; Liu, J. Phosphohexose mutase of Xanthomonas oryzae pv. oryzicola is negatively regulated by HrpG and HrpX, and required for the full virulence in rice. Eur. J. Plant Pathol. 2014, 140, 353–364. [Google Scholar] [CrossRef]
- Xue, X.; Zou, L.; Ma, W.; Liu, Z.; Chen, G. Identification of 17 HrpX-regulated proteins including two novel type III effectors, XOC_3956 and XOC_1550, in Xanthomonas oryzae pv. oryzicola. PLoS ONE 2014, 9, e93205. [Google Scholar] [CrossRef]
- Wang, L.; Makino, S.; Subedee, A.; Bogdanove, A.J. Novel candidate virulence factors in rice pathogen Xanthomonas oryzae pv. oryzicola as revealed by mutational analysis. Appl. Environ. Microbiol. 2007, 73, 8023–8027. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Vinogradov, E.V.; Bogdanove, A.J. Requirement of the lipopolysaccharide O-chain biosynthesis gene wxocB for type III secretion and virulence of Xanthomonas oryzae pv. oryzicola. J. Bacteriol. 2013, 195, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, S.; Nie, W.; Wang, P.; Fu, L.; Ahmad, I.; Zhu, B.; Chen, G. A key antisense sRNA modulates the oxidative stress response and virulence in Xanthomonas oryzae pv. oryzicola. PLoS Pathog. 2021, 17, e1009762. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ren, P.; Zhang, D.; Cui, P.; Zhu, G.; Xian, X.; Tang, J.; Lu, G. HpaP divergently regulates the expression of hrp genes in Xanthomonas oryzae pathovars oryzae and oryzicola. Mol. Plant Pathol. 2023, 24, 44–58. [Google Scholar] [CrossRef]
- Zhao, B.; Lin, X.; Poland, J.; Trick, H.; Leach, J.; Hulbert, S. A maize resistance gene functions against bacterial streak disease in rice. Proc. Natl. Acad. Sci. USA 2005, 102, 15383–15388. [Google Scholar] [CrossRef]
- Liu, H.; Chang, Q.; Feng, W.; Zhang, B.; Wu, T.; Li, N.; Yao, F.; Ding, X.; Chu, Z. Domain dissection of AvrRxo1 for suppressor, avirulence and cytotoxicity functions. PLoS ONE 2014, 9, e113875. [Google Scholar] [CrossRef]
- Triplett, L.R.; Shidore, T.; Long, J.; Miao, J.; Wu, S.; Han, Q.; Zhou, C.; Ishihara, H.; Li, J.; Zhao, B.; et al. AvrRxo1 is a bifunctional type III secreted effector and toxin-antitoxin system component with homologs in diverse environmental contexts. PLoS ONE 2016, 11, e0158856. [Google Scholar] [CrossRef] [PubMed]
- Schuebel, F.; Rocker, A.; Edelmann, D.; Schessner, J.; Brieke, C.; Meinhart, A. 3′-NADP and 3′-NAADP, two metabolites formed by the bacterial type III effector AvrRxo1. J. Biol. Chem. 2016, 291, 22868–22880. [Google Scholar] [CrossRef]
- Han, Q.; Zhou, C.; Wu, S.; Liu, Y.; Triplett, L.; Miao, J.; Tokuhisa, J.; Deblais, L.; Robinson, H.; Leach, J.E.; et al. Crystal structure of Xanthomonas AvrRxo1-ORF1, a type III effector with a polynucleotide kinase domain, and its interactor AvrRxo1-ORF2. Structure 2015, 23, 1900–1909. [Google Scholar] [CrossRef]
- Liu, H.; Lu, C.; Li, Y.; Wu, T.; Zhang, B.; Liu, B.; Feng, W.; Xu, Q.; Dong, H.; He, S.; et al. The bacterial effector AvrRxo1 inhibits vitamin B6 biosynthesis to promote infection in rice. Plant Commun. 2022, 3, 100324. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K.; Cheng, Q.; Kong, D.; Zhang, X.; Wang, Z.; Wang, Q.; Xie, Q.; Yan, J.; Chu, J.; et al. Cysteine protease RD21A regulated by E3 ligase SINAT4 is required for drought-induced resistance to Pseudomonas syringae in Arabidopsis. J. Exp. Bot. 2020, 71, 5562–5576. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Y.; Wang, S.; Fang, A.; Wang, J.; Liu, L.; Zhang, K.; Mao, Y.; Sun, W. The type III effector AvrBs2 in Xanthomonas oryzae pv. oryzicola suppresses rice immunity and promotes disease development. Mol. Plant Microbe Interact. 2015, 28, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Li, J.; Mo, X.; Ni, Z.; Jiang, W.; He, Y.; Huang, S. Type III effectors xopN and avrBS2 contribute to the virulence of Xanthomonas oryzae pv. oryzicola strain GX01. Res. Microbiol. 2020, 171, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Dahlbeck, D.; Krasileva, K.V.; Fong, R.W.; Staskawicz, B.J. Computational and biochemical analysis of the Xanthomonas effector AvrBs2 and its role in the modulation of Xanthomonas type three effector delivery. PLoS Pathog. 2011, 7, e1002408. [Google Scholar] [CrossRef][Green Version]
- Kim, J.G.; Li, X.; Roden, J.A.; Taylor, K.W.; Aakre, C.D.; Su, B.; Lalonde, S.; Kirik, A.; Chen, Y.; Baranage, G.; et al. Xanthomonas T3S effector XopN suppresses PAMP-triggered immunity and interacts with a Tomato Atypical Receptor-Like Kinase and TFT1. Plant Cell 2009, 21, 1305–1323. [Google Scholar] [CrossRef]
- Wang, S.; Li, S.; Wang, J.; Li, Q.; Xin, X.F.; Zhou, S.; Wang, Y.; Li, D.; Xu, J.; Luo, Z.Q.; et al. A bacterial kinase phosphorylates OSK1 to suppress stomatal immunity in rice. Nat. Commun. 2021, 12, 5479. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Xu, Z.; Chen, H.; Zhang, J.; Manion, B.; Liu, F.; Zou, L.; Fu, Z.Q.; Chen, G. The Xanthomonas type III effector XopAP prevents stomatal closure by interfering with vacuolar acidification. J. Integr. Plant Biol. 2022, 64, 1994–2008. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, B.; Wu, W.; Li, Y.; Yin, Z.; Lu, C.; Zhao, H.; Kong, L.; Ding, X. The MYB transcription factor OsMYBxoc1 regulates resistance to Xoc by directly repressing transcription of the iron transport gene OsNRAMP5 in rice. Plant Commun. 2024, 5, 100859. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Z.; Li, Z.; Zakria, M.; Zou, L.; Chen, G. Increasing resistance to bacterial leaf streak in rice by editing the promoter of susceptibility gene OsSULRT3;6. Plant Biotechnol. J. 2021, 19, 1101–1103. [Google Scholar] [CrossRef]
- Read, A.C.; Rinaldi, F.C.; Hutin, M.; He, Y.Q.; Triplett, L.R.; Bogdanove, A.J. Suppression of Xo1-mediated disease resistance in rice by a truncated, non-DNA-binding TAL effector of Xanthomonas oryzae. Front. Plant Sci. 2016, 7, 1516. [Google Scholar] [CrossRef]
- Read, A.C.; Hutin, M.; Moscou, M.J.; Rinaldi, F.C.; Bogdanove, A.J. Cloning of the rice Xo1 resistance gene and interaction of the Xo1 protein with the defense-suppressing Xanthomonas effector Tal2h. Mol. Plant Microbe Interact. 2020, 33, 1189–1195. [Google Scholar] [CrossRef]
- Hummel, A.W.; Wilkins, K.E.; Wang, L.; Cernadas, R.A.; Bogdanove, A.J. A transcription activator-like effector from Xanthomonas oryzae pv. oryzicola elicits dose-dependent resistance in rice. Mol. Plant Pathol. 2017, 18, 55–66. [Google Scholar] [CrossRef]
- Cai, L.; Cao, Y.; Xu, Z.; Ma, W.; Zakria, M.; Zou, L.; Cheng, Z.; Chen, G. A transcription activator-like effector Tal7 of Xanthomonas oryzae pv. oryzicola activates rice gene Os09g29100 to suppress rice immunity. Sci. Rep. 2017, 7, 5089. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liao, Z.; Jin, X.; Liao, L.; Zhang, Y.; Zhang, R.; Zhao, X.; Qin, H.; Chen, J.; He, Y.; et al. Xanthomonas oryzae pv. oryzicola effector Tal10a directly activates rice OsHXK5 expression to facilitate pathogenesis. Plant J. 2024. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Wang, Z.; Chen, X.; Liu, Y.; Zhang, M.; Song, C.; Dong, H. Identification of a TAL effector in Xanthomonas oryzae pv. oryzicola enhancing pathogen growth and virulence in plants. Physiol. Mol. Plant Pathol. 2021, 114, 101620. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, H.; Bi, Y.; Yu, Y.; Liu, H.; Yang, H.; Yuan, B.; Ding, X.; Chu, Z. Tal2c activates the expression of OsF3H04g to promote infection as a redundant TALE of Tal2b in Xanthomonas oryzae pv. oryzicola. Int. J. Mol. Sci. 2021, 22, 13628. [Google Scholar] [CrossRef]
- Liang, B.; Wang, H.; Yang, C.; Wang, L.; Qi, L.; Guo, Z.; Chen, X. Salicylic acid is required for broad-spectrum disease resistance in rice. Int. J. Mol. Sci. 2022, 23, 1354. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Q.; Gao, S.; Yu, N.; Zhao, L.; Wang, J.; Zhao, J.; Huang, P.; Yao, L.; Wang, M.; et al. Disruption of the primary salicylic acid hydroxylases in rice enhances broad-spectrum resistance against pathogens. Plant Cell Environ. 2022, 45, 2211–2225. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Bi, Y.; Yu, Y.; Zhou, Z.; Yuan, B.; Ding, X.; Zhang, Q.; Chen, X.; Yang, H.; Liu, H.; et al. Activated expression of rice DMR6-like gene OsS3H partially explores the susceptibility to bacterial leaf streak mediated by knock-out OsF3H04g. Int. J. Mol. Sci. 2023, 24, 13263. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Y.; Yao, W.; Yin, Z.; Wang, Y.; Huang, Z.; Zhou, J.Q.; Liu, J.; Lu, X.; Wang, F.; et al. CRISPR/Cas9-mediated simultaneous mutation of three salicylic acid 5-hydroxylase (OsS5H) genes confers broad-spectrum disease resistance in rice. Plant Biotechnol. J. 2023, 21, 1873–1886. [Google Scholar] [CrossRef]
- Gupta, P.K.; Balyan, H.S.; Gautam, T. SWEET genes and TAL effectors for disease resistance in plants: Present status and future prospects. Mol. Plant Pathol. 2021, 22, 1014–1026. [Google Scholar] [CrossRef]
- Wang, X.; Ju, Y.; Wu, T.; Kong, L.; Yuan, M.; Liu, H.; Chen, X.; Chu, Z. The clade III subfamily of OsSWEETs directly suppresses rice immunity by interacting with OsHMGB1 and OsHsp20L. Plant Biotechnol. J. 2024, 22, 2186–2200. [Google Scholar] [CrossRef]
- Shi, X.; Xie, X.; Guo, Y.; Zhang, J.; Gong, Z.; Zhang, K.; Mei, J.; Xia, X.; Xia, H.; Ning, N.; et al. A fungal core effector exploits the OsPUX8B.2-OsCDC48-6 module to suppress plant immunity. Nat. Commun. 2024, 15, 2559. [Google Scholar] [CrossRef] [PubMed]
- Mole, B.M.; Baltrus, D.A.; Dangl, J.L.; Grant, S.R. Global virulence regulation networks in phytopathogenic bacteria. Trends Microbiol. 2007, 15, 363–371. [Google Scholar] [CrossRef]
- Slater, H.; Alvarez-Morales, A.; Barber, C.E.; Daniels, M.J.; Dow, J.M. A two-component system involving an HD-GYP domain protein links cell-cell signalling to pathogenicity gene expression in Xanthomonas campestris. Mol. Microbiol. 2000, 38, 986–1003. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Sonti, R.V. rpfF mutants of Xanthomonas oryzae pv. oryzae are deficient for virulence and growth under low iron conditions. Mol. Plant Microbe Interact. 2002, 15, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qian, G.; Yin, F.; Fan, J.; Zhai, Z.; Liu, C.; Hu, B.; Liu, F. Proteomic analysis of the regulatory function of DSF-dependent quorum sensing in Xanthomonas oryzae pv. oryzicola. Microb. Pathog. 2011, 50, 48–55. [Google Scholar] [CrossRef]
- Rai, R.; Javvadi, S.; Chatterjee, S. Cell-cell signalling promotes ferric iron uptake in Xanthomonas oryzae pv. oryzicola that contribute to its virulence and growth inside rice. Mol. Microbiol. 2015, 96, 708–727. [Google Scholar] [CrossRef]
- Song, Z.; Zhao, Y.; Zhou, X.; Wu, G.; Zhang, Y.; Qian, G.; Liu, F. Identification and characterization of two novel DSF-controlled virulence-associated genes within the nodB-rhgB locus of Xanthomonas oryzae pv. oryzicola Rs105. Phytopathology 2015, 105, 588–596. [Google Scholar] [CrossRef]
- Song, Z.; Zhao, Y.; Qian, G.; Odhiambo, B.O.; Liu, F. Novel insights into the regulatory roles of gene hshB in Xanthomonas oryzae pv. oryzicola. Res. Microbiol. 2017, 168, 165–173. [Google Scholar] [CrossRef]
- Qian, G.; Zhang, Y.; Zhou, Y.; Liu, C.; Zhao, Y.; Song, Z.; Fan, J.; Hu, B.; Liu, F. epv, encoding a hypothetical protein, is regulated by DSF-mediating quorum sensing as well as global regulator Clp and is required for optimal virulence in Xanthomonas oryzae pv. oryzicola. Phytopathology 2012, 102, 841–847. [Google Scholar] [CrossRef][Green Version]
- Qian, G.; Liu, C.; Wu, G.; Yin, F.; Zhao, Y.; Zhou, Y.; Zhang, Y.; Song, Z.; Fan, J.; Hu, B.; et al. AsnB, regulated by diffusible signal factor and global regulator Clp, is involved in aspartate metabolism, resistance to oxidative stress and virulence in Xanthomonas oryzae pv. oryzicola. Mol. Plant Pathol. 2013, 14, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Zhou, Y.; Zhao, Y.; Song, Z.; Wang, S.; Fan, J.; Hu, B.; Venturi, V.; Liu, F. Proteomic analysis reveals novel extracellular virulence-associated proteins and functions regulated by the diffusible signal factor (DSF) in Xanthomonas oryzae pv. oryzicola. J. Proteome Res. 2013, 12, 3327–3341. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Ahmed, W.; Yang, J.; Meng, H.; Wei, L.; Ji, G. Identification and characterization of two transcriptional regulators in Xanthomonas oryzae pv. oryzicola YM15. Physiol. Mol. Plant Pathol. 2023, 124, 101964. [Google Scholar] [CrossRef]
- Guo, W.; Zou, L.; Ji, Z.; Cai, L.; Chen, G. Glucose 6-phosphate isomerase (Pgi) is required for extracellular polysaccharide biosynthesis, DSF signals production and full virulence of Xanthomonas oryzae pv. oryzicola in rice. Physiol. Mol. Plant Pathol. 2017, 100, 209–219. [Google Scholar] [CrossRef]
- Salazar-Cerezo, S.; Martínez-Montiel, N.; García-Sánchez, J.; Pérez-Y-Terrón, R.; Martínez-Contreras, R.D. Gibberellin biosynthesis and metabolism: A convergent route for plants, fungi and bacteria. Microbiol. Res. 2018, 208, 85–98. [Google Scholar] [CrossRef]
- Lu, X.; Hershey, D.M.; Wang, L.; Bogdanove, A.J.; Peters, R.J. An ent-kaurene-derived diterpenoid virulence factor from Xanthomonas oryzae pv. oryzicola. New Phytol. 2015, 206, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Nagel, R.; Peters, R.J. Investigating the phylogenetic range of gibberellin biosynthesis in bacteria. Mol. Plant Microbe Interact. 2017, 30, 343–349. [Google Scholar] [CrossRef]
- Nagel, R.; Turrini, P.C.; Nett, R.S.; Leach, J.E.; Verdier, V.; Van Sluys, M.A.; Peters, R.J. An operon for production of bioactive gibberellin A4 phytohormone with wide distribution in the bacterial rice leaf streak pathogen Xanthomonas oryzae pv. oryzicola. New Phytol. 2017, 214, 1260–1266. [Google Scholar] [CrossRef]
- Zhang, H.; Rong, Z.; Li, Y.; Yin, Z.; Lu, C.; Zhao, H.; Kong, L.; Meng, L.; Ding, X. NIT24 and NIT29-mediated IAA synthesis of Xanthomonas oryzae pv. oryzicola suppresses immunity and boosts growth in rice. Mol. Plant Pathol. 2024, 25, e13409. [Google Scholar] [CrossRef]
- Dow, J.M.; Fouhy, Y.; Lucey, J.F.; Ryan, R.P. The HD-GYP domain, cyclic di-GMP signaling, and bacterial virulence to plants. Mol. Plant Microbe Interact. 2006, 19, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, C.; Jiang, W.; Wang, L.; Li, C.; Wang, Y.; Dow, J.M.; Sun, W. The HD-GYP domain protein RpfG of Xanthomonas oryzae pv. oryzicola regulates synthesis of extracellular polysaccharides that contribute to biofilm formation and virulence on rice. PLoS ONE 2013, 8, e59428. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Jiang, W.; Zhao, M.; Ling, J.; Zeng, X.; Deng, J.; Jin, D.; Dow, J.M.; Sun, W. A systematic analysis of the role of GGDEF-EAL domain proteins in virulence and motility in Xanthomonas oryzae pv. oryzicola. Sci. Rep. 2016, 6, 23769. [Google Scholar] [CrossRef]
- Wan, X.; Yang, J.; Ahmed, W.; Liu, Q.; Wang, Y.; Wei, L.; Ji, G. Functional analysis of pde gene and its role in the pathogenesis of Xanthomonas oryzae pv. oryzicola. Infect. Genet. Evol. 2021, 94, 105008. [Google Scholar] [CrossRef]
- Su, P.; Song, Z.; Wu, G.; Zhao, Y.; Zhang, Y.; Wang, B.; Qian, G.; Fu, Z.Q.; Liu, F. Insights into the roles of two genes of the histidine biosynthesis operon in pathogenicity of Xanthomonas oryzae pv. oryzicola. Phytopathology 2018, 108, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Jacob-Dubuisson, F.; Mechaly, A.; Betton, J.M.; Antoine, R. Structural insights into the signalling mechanisms of two-component systems. Nat. Rev. Microbiol. 2018, 16, 585–593. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, M.; Xu, L.; Niu, X.; Qin, H.; Li, Y.; Li, M.; Jiang, Z.; Yang, X.; Huang, G.; et al. Genome-wide screening for novel candidate virulence related response regulator genes in Xanthomonas oryzae pv. oryzicola. Front. Microbiol. 2018, 9, 1789. [Google Scholar] [CrossRef]
- Wei, C.; Wang, S.; Liu, P.; Cheng, S.T.; Qian, G.; Wang, S.; Fu, Y.; Qian, W.; Sun, W. The PdeK-PdeR two-component system promotes unipolar localization of FimX and pilus extension in Xanthomonas oryzae pv. oryzicola. Sci. Signal. 2021, 14, eabi9589. [Google Scholar] [CrossRef]
- Cai, L.; Ma, W.; Zou, L.; Xu, X.; Xu, Z.; Deng, C.; Qian, W.; Chen, X.; Chen, G. Xanthomonas oryzae pv. oryzicola response regulator VemR is co-opted by the sensor kinase CheA for phosphorylation of multiple pathogenicity-related targets. Front. Microbiol. 2022, 13, 928551. [Google Scholar] [CrossRef]
- Zou, H.; Song, X.; Zou, L.; Yuan, L.; Li, Y.; Guo, W.; Che, Y.; Zhao, W.; Duan, Y.; Chen, G. EcpA, an extracellular protease, is a specific virulence factor required by Xanthomonas oryzae pv. oryzicola but not by X. oryzae pv. oryzae in rice. Microbiology 2012, 158, 2372–2383. [Google Scholar] [CrossRef]
- Kalunke, R.M.; Tundo, S.; Benedetti, M.; Cervone, F.; De Lorenzo, G.; D’Ovidio, R. An update on polygalacturonase-inhibiting protein (PGIP), a leucine-rich repeat protein that protects crop plants against pathogens. Front. Plant Sci. 2015, 6, 146. [Google Scholar] [CrossRef]
- Davidsson, P.; Broberg, M.; Kariola, T.; Sipari, N.; Pirhonen, M.; Palva, E.T. Short oligogalacturonides induce pathogen resistance-associated gene expression in Arabidopsis thaliana. BMC Plant Biol. 2017, 17, 19. [Google Scholar] [CrossRef]
- Wu, T.; Peng, C.; Li, B.; Wu, W.; Kong, L.; Li, F.; Chu, Z.; Liu, F.; Ding, X. OsPGIP1-mediated resistance to bacterial leaf streak in rice is beyond responsive to the polygalacturonase of Xanthomonas oryzae pv. oryzicola. Rice 2019, 12, 90. [Google Scholar] [CrossRef]
- Cai, L.; Zou, L.; Ling, G.; Xue, X.; Zou, H.; Chen, G. An inner membrane protein (Imp) of Xanthomonas oryzae pv. oryzicola functions in carbon acquisition, EPS production, bacterial motility and virulence in rice. J. Integr. Agric. 2014, 13, 2656–2668. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, Y.; Qian, G.; Liu, F. XocR, a LuxR solo required for virulence in Xanthomonas oryzae pv. oryzicola. Front. Cell. Infect. Microbiol. 2015, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wang, H.; Liu, X.; Xin, D.; Rao, Y.; Zhu, B. Transcriptome analysis of Xanthomonas oryzae pv. oryzicola exposed to H2O2 reveals horizontal gene transfer contributes to its oxidative stress response. PLoS ONE 2019, 14, e0218844. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.; Nie, W.; Wu, Y.; Iftikhar, A.; Ayizekeranmu, Y.; Huang, J.; Chen, G.; Zhu, B. A transferred regulator that contributes to Xanthomonas oryzae pv. oryzicola oxidative stress adaptation and virulence by regulating the expression of cytochrome bd oxidase genes. J. Integr. Agric. 2022, 21, 1673–1682. [Google Scholar] [CrossRef]
- Arroyo-Velez, N.; González-Fuente, M.; Peeters, N.; Lauber, E.; Noël, L.D. From effectors to effectomes: Are functional studies of individual effectors enough to decipher plant pathogen infectious strategies? PLoS Pathog. 2020, 16, e1009059. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, X.; Gong, Q.; Li, Z.; Li, Y.; Wang, S.; Yang, Y.; Ma, W.; Liu, L.; Zhu, B.; et al. Engineering broad-spectrum bacterial blight resistance by simultaneously disrupting variable TALE-binding elements of multiple susceptibility genes in rice. Mol. Plant 2019, 12, 1434–1446. [Google Scholar] [CrossRef]
- Oliva, R.; Ji, C.; Atienza-Grande, G.; Huguet-Tapia, J.C.; Perez-Quintero, A.; Li, T.; Eom, J.S.; Li, C.; Nguyen, H.; Liu, B.; et al. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 2019, 37, 1344–1350. [Google Scholar] [CrossRef]
- Ni, Z.; Cao, Y.; Jin, X.; Fu, Z.; Li, J.; Mo, X.; He, Y.; Tang, J.; Huang, S. Engineering resistance to bacterial blight and bacterial leaf streak in rice. Rice 2021, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fang, Y.; Wu, H.; Zhao, N.; Guo, X.; Mackon, E.; Peng, H.; Huang, S.; He, Y.; Qin, B.; et al. Improvement of resistance to rice blast and bacterial leaf streak by CRISPR/Cas9-mediated mutagenesis of Pi21 and OsSULTR3;6 in rice (Oryza sativa L.). Front. Plant Sci. 2023, 14, 1209384. [Google Scholar] [CrossRef] [PubMed]
- Scinto-Madonich, N.J.; Baruah, S.; Young, S.; Vignona, K.; Read, A.C.; Carpenter, S.C.D.; Wang, L.; Shi, X.; Chang, G.; Piñeros, M.A.; et al. Initial characterization of a bacterial leaf streak susceptibility gene suggests it encodes a membrane transporter that influences seed nutrition and germination. Physiol. Mol. Plant Pathol. 2023, 126, 102031. [Google Scholar] [CrossRef]
- Shafique, M.S.; Liu, Y.; Li, M.; Wang, H.; Su, R.; Wang, C.; Ji, Z. Coevolution unveiled: Sulfate transporters mediate rice resistance and susceptibility to Xanthomonas oryzae pv. oryzicola. Plant Biotechnol. J. 2024, 22, 2632–2634. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, N.; Huang, Y.; Miao, W.; Zhang, Q.; Wu, T. Type III Secretion Effectors of Xanthomonas oryzae pv. oryzicola: The Arsenal to Attack Equivalent Rice Defense for Invasion. Agronomy 2024, 14, 1881. https://doi.org/10.3390/agronomy14091881
Tan N, Huang Y, Miao W, Zhang Q, Wu T. Type III Secretion Effectors of Xanthomonas oryzae pv. oryzicola: The Arsenal to Attack Equivalent Rice Defense for Invasion. Agronomy. 2024; 14(9):1881. https://doi.org/10.3390/agronomy14091881
Chicago/Turabian StyleTan, Nawei, Yechao Huang, Weiguo Miao, Qingxia Zhang, and Tao Wu. 2024. "Type III Secretion Effectors of Xanthomonas oryzae pv. oryzicola: The Arsenal to Attack Equivalent Rice Defense for Invasion" Agronomy 14, no. 9: 1881. https://doi.org/10.3390/agronomy14091881
APA StyleTan, N., Huang, Y., Miao, W., Zhang, Q., & Wu, T. (2024). Type III Secretion Effectors of Xanthomonas oryzae pv. oryzicola: The Arsenal to Attack Equivalent Rice Defense for Invasion. Agronomy, 14(9), 1881. https://doi.org/10.3390/agronomy14091881






