Abstract
Plant organ biomass allocation and morphological characteristics are important functional traits. The responses of plant root, stem, and leaf traits to heterogeneous habitats in floodplain wetlands are highly important for understanding the ecological adaptation strategies of riparian plants. However, the patterns of these responses remain unclear. In a floodplain wetland in the middle reaches of the Heihe River, we studied the responses of the root, stem, and leaf morphological traits and biomass allocation of Leymus secalinus to varying habitat conditions. We measured these traits in three sample plots, delineated based on distance from the riverbank: plot I (near the riparian zone, 50–150 m from the riverbank), plot II (middle riparian zone, 200–300 m from the riverbank), and plot III (far riparian zone, 350–450 m from the riverbank). The results showed that in plot I, L. secalinus tended to have slender roots and stems and small leaves, with a biomass allocation strategy that maximized the root–shoot ratio (RSR). In plot II, L. secalinus had thick stems and moderate leaf and root patterns, and the RSR values were between those of plot I and plot III. In plot III, L. secalinus had thin and short stems and large leaves; furthermore, among the root morphological structures, plot III had the shortest Rhizome length (RL) and longest Rhizome diameter (RD), and the RSR was the lowest. Moreover, there was a significant correlation between organ biomass and leaf thickness, stem length, RD, and RL in the three habitats (p < 0.05). By balancing the biomass allocation among organs, wetland plants in floodplains balance changes in root, stem, and leaf morphological characteristics to improve their environmental adaptation.
1. Introduction
Riparian floodplains harbor high biodiversity [] and provide an exceptionally broad range of ecosystem functions and services []. The riparian zone is an important ecological transition zone for material, energy, and information exchange between rivers and terrestrial ecosystems []. Riparian zone ecosystems, including coastal vegetation, animals, and microorganisms, and their environments, are significantly different from other ecosystems in terms of structure and function. The particularity, complexity, and spatial heterogeneity of their habitat affect plant community resource acquisition and spatial distribution in the riparian zone and are influenced by community biomass allocation and phenotypic characteristics [,,].
The differences in temporal and spatial variations in the river hydrological cycle makes riparian zones form habitat types with different resources, determine the uniqueness of riparian plant adaptation, and form specific combinations of plant traits []. To maintain necessary physiological activity and achieve normal growth, plants must balance biomass allocation to leaves, stems, and roots [,,] and optimize the allocation of resources to improve their environmental adaptation []. Therefore, the allocation of plant biomass among organs is closely related to the phenotypic characteristics of plants [,]. With limited resources, plants must develop phenotypic traits adapted to the environment through the plasticity of root, stem, and leaf morphological structures []. Plant functional traits can not only reflect the adaptability of plants to external habitats [,] but also predict the response of other plants to environmental disturbances []. Leaf traits are important functional traits that reflect the competition between plants for light resources and aboveground living space [,]. Leaves are important sites for plants to synthesize carbon through photosynthesis [], while stems mainly play a role in physical support and resource transport. Stems and leaves are important for resource production and transportation of water and nutrients among plant organs and are closely related to plant survival, growth, and reproduction. The morphological characteristics of plants directly determine their biomass allocation pattern and photosynthetic efficiency and have an important influence on their carbon acquisition and light capture abilities [,]. Plant roots play a key role in the absorption [], storage, and transport of water and nutrients in plants []. The root system is not only the main plant organ used to obtain the resources needed for competitive survival but also one of the most sensitive organs to environmental changes. Plant variation at the root level contributes to a better understanding of the environmental adaptation mechanism of plants [], and leaves, stems, and roots are the most important plant organs used to obtain resources. The changes in their characteristics with environmental changes reflect the ability of plants to use light resources, absorb water and nutrients, and adapt to changes in the surrounding environment []. There is broadly coordinated interspecific variation in leaf and root biomass allocation [] and in aboveground and belowground plant morphology [,]; these relationships can be largely modified by plant phenotypic adjustments to variable environmental conditions [,,]. Therefore, under conditions of resource scarcity or environmental stress, plants will balance the allocation of biomass among organs to improve their adaptability to the environment [] and show high plasticity in terms of morphology and structure. This phenotypic plasticity is a key mechanism for successful development of species and the ability of plants to produce different phenotypes under different environmental conditions []. However, there are few studies on the response mechanisms of riparian plant root, stem, and leaf morphological traits and organ biomass allocation to habitats.
Leymus secalinus (Georgi) Tzvelev (Figure 1) is a perennial rhizomatous grass belonging to the family Poaceae and is widely distributed in sandy land, plain oasis, and riparian areas of inland rivers in China, Mongolia, Japan, and Korea [,,,]. The roots and stems of L. secalinus exhibit strong cloning and reproduction abilities, drought resistance [], salt and alkali resistance [], cold resistance [], strong adaptability, and other important characteristics. Its developed underground root and stem network has important ecological value for wind prevention and sand fixation, water conservation, soil erosion prevention, saline-alkali land improvement, ecosystem restoration, etc. []; it can also be inserted into the genetic value in wheat hybridization []. In recent years, studies on L. secalinus have focused mainly on drought tolerance [], saline-alkali stress resistance [], clonal ramet expansion, and clonal configuration [,]. However, there are few studies on the correlation between the aboveground and belowground functional traits of riparian plants, especially the relationship between resource allocation patterns and morphological traits of L. secalinus, the dominant plant in the riparian zone of the Heihe River floodplain wetlands, which remains unclear.

Figure 1.
Leymus secalinus.
Given this information, this paper investigated L. secalinus, the dominant plant species in the riparian zone of the Heihe River, and attempted to answer the following questions: (1) How does the pattern of L. secalinus organ biomass allocation change with changes with riparian habitat conditions? (2) How do the morphological traits of L. secalinus roots, stems, and leaves change under the accompanying habitat conditions? (3) What are the response mechanisms of L. secalinus root, stem, and leaf morphology to different habitats? The aim of this study was to elucidate the resource allocation strategies and ecological adaptation mechanisms of the riparian plant Leymus secalinus in inland river wetlands.
2. Materials and Methods
2.1. Study Sites
The study area is located in a floodplain wetland on a gentle slope of the main stem of the Heihe River in the Ganzhou district of Zhangye city, Gansu Province, China (38.56°–38.59° N, 100.24°–100.26° E) (Figure 2), at an elevation of 1482 m. This area has a temperate continental climate, with an annual average temperature of 7.8 °C, an accumulated temperature ≥ 0 °C of 2734 °C, an average annual precipitation of 132.6 mm, with precipitation mainly falling from June to September, an average annual evaporation of 1986.5 mm, and an annual sunshine duration of 3077 h []. The special hydrological processes and the diversity of the soil types in riparian zones determine the formation of xerophytic, halophytic, and hygrophytic plant communities. The distribution of vegetation in the study area has obvious horizontal differentiation characteristics, with hygrophytes and halophytes as the ecological categories. The dominant plant species are L. secalinus, Achnatherum splendens, Phragmites australis, Agropyron cristatum, Equisetum ramosissimum, Sophora alopecuroides, Myricaria bracteata, Tamarix ramosissima, Salix matsudana, and Elaeagnus angustifolia [].
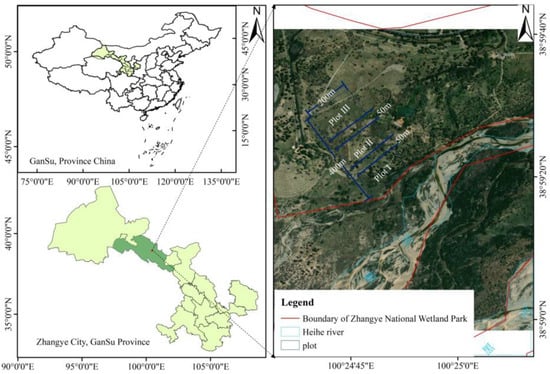
Figure 2.
Study area and locations of the sampling plots.
2.2. Experimental Method and Design
The main stem of the Heihe River enters the Hexi Corridor from Yingluoxia, forming broad sloping plains along both sides of the river. Influenced by seasonal floods and underground flows, a large floodplain wetland dominated by the herbs L. secalinus, A. splendens, P. australis, and S. alopecuroides and trees such as T. ramosissima, E. angustifolia, and S. matsudana has developed along the edges of the riverbank. Affected by the depth of the groundwater, the vegetation in the study area shows obvious zonal distribution characteristics. Based on field observations, a rectangular transect (450 m in length × 200 m in width) from the riverbank to the junction of the wetland and desert was selected as the study site and was sampled from 15 to 27 August 2021. The transect was dominated by L. secalinus, accompanied by plants such as P. australis, A. splendens, T. ramosissima, and E. angustifolia.
First, the soil was removed with a shovel at intervals of 50 m from near the riverbank in each transect, and the groundwater depth was measured after the water level stabilized. Second, according to the distance from the riverbank and the variation in groundwater depth, the transects were divided into three plots. Plot I was near the riparian zone, with a distance from the riverbank of 50–150 m and a groundwater depth of 0.5–1.0 m. Affected by seasonal and intermittent floods, the soil moisture in plot I was saturated at almost all times of the year. The main plants were L. secalinus, P. australis, A. splendens, M. bracteate, and T. ramosissima. Plot II was in the middle riparian zone, with a distance from the riverbank of 200–300 m and an underground water depth of 1.0–1.5 m; it was seasonally flooded, with the soil moisture being seasonally saturated. The main plants in this area were L. secalinus, A. splendens, S. alopecuroides, S. matsudana, etc. Plot III was the far riparian zone, with a distance from the riverbank of 350–450 m and an underground water depth of 1.5–2.0 m; it was generally not flooded, with soil that was minimally affected by the river water level, so the soil moisture did not meet the growth needs of most plants, which mainly included L. secalinus and A. splendens.
The sampling was carried out in the period when there was generally no obvious precipitation, and the soil water and salt conditions were relatively stable. Six 1 m × 1 m quadrats were selected from each plot, for an overall total of 18 quadrats. First, the characteristics of the plant communities in each quadrat, including height, coverage, and density, were measured. Second, six well-growing and intact L. secalinus plants were selected randomly from each quadrat; after measuring plant height with a tape measure, the aboveground portion was cut off, put into numbered envelopes, and transported to the laboratory for stem and leaf trait measurements. Then, the roots of each L. secalinus plant were completely removed by whole-root excavation after the root depth was measured via the trench method. The rhizome of L. secalinus were placed on a mesh screen (aperture = 0.25 mm), and an adjacent water source was used to clean the soil and debris attached to the rhizome. The rhizome were placed in a numbered self-sealing bag and transported to the laboratory together with the aboveground parts to measure the biomass and other root traits. Finally, the aboveground parts of all plants in each habitat were cut and transported to the laboratory.
2.2.1. Measurement of the Leaf and Stem Traits of L. secalinus
The leaf area (LA) was measured using a portable leaf area meter (CI-202, Walz, Camas, WA, USA) by scanning each blade, and the mean LA of five replicate measurements–Leaf thickness (LT), stem length (SL) and stem diameter (SD)–were measured by Vernier Calipers (accuracy of 0.01 mm), and the mean LT, SL, and SD of five replicate measurements were determined. Then, the blades were packed into an envelope and dried in an oven at 80 °C for 48 h, and the leaf dry weight (LDW) was recorded (to 0.0001 g accuracy). The specific leaf area (SLA) was expressed as the ratio of LA to LDW [].
2.2.2. Measurement of the Root Morphological Traits and Organ Biomass of L. secalinus
In the laboratory, L. secalinus roots were placed on a tray in the opening of the positioning system on a scanner (Epson perfection V700 photo, J221A, Seiko Epson Corporation, Tokyo, Japan). After scanning, the root images were stored at a 600 dpi resolution in a graphics file, and the rhizome diameter (RD), rhizome length (RL), and root surface area (RSA) were measured with WIN-Rhizo (Pro 5.0) software (Regent Instruments, Québec City, QC, Canada). Then, the L. secalinus roots and leaves were placed in numbered envelopes and placed in an oven. After drying in the oven at 80 °C for 48 h, the root–shoot ratio (RSR is a basic form of plasticity in plant allocation patterns []), specific root length (SRL reflects the adaptation characteristics of plants to different community environments), stem biomass percentage (PSB is the proportion of the biomass input of the supporting stems in the total biomass), leaf biomass percentage (PLB is the proportion of plant biomass input to photosynthetic organ leaves in total biomass), rhizome biomass percentage (PRB depicts the relative investment of biomass to specific belowground parts []), and total biomass (TB is the biomass investment of the plant in the organs of roots, stems, and leaves) were calculated.
where AB is the aboveground biomass, RM is the rhizome dry weight, SM is the stem dry weight, LM is the leaf dry weight, and TRL is the total rhizome length [].
RSR = RM/AB
SRL = TRL/RM
PSB = SM/TB
PLB = LM/TB
PRB = RM/TB
TB = RM + LM + SM
2.2.3. Measurement of Soil Properties (Moisture Content, Soil Bulk Density, Soil Salinity, and pH)
In the wetland community survey, soil samples were randomly collected from the 1 m × 1 m × 0.5 m (length × width × depth) soil profile using a ring knife (200 cm3). Five soil layers were collected, each at a depth of 10 cm, with three replicates per layer. The replicate soil samples at each layer were mixed while fresh and then oven dried at 105 °C for 12 h. The soil moisture content (SMC) and soil bulk density (SBD) were calculated for each soil layer [,,].
The salt content of the soil samples was measured using the electrical conductivity (EC) method. A portable conductivity meter (DDS-11C, Shanghai Lei Magnetic Instrument Factory, Shanghai, China) and portable soil pH meter (ST3100, Ohaus Instruments Co., Ltd., Shanghai, China) were used to measure the EC and pH of the leachate, respectively, with three replicates per sample, and the averages were calculated [,,].
2.3. Statistical Analysis
Microsoft Excel 2019 was used to organize all the original experimental data, and the root–shoot ratio, stem mass ratio, leaf mass ratio, and rhizome mass ratio of various L. secalinus plants were calculated. The biological traits and soil properties of the wetland communities in the three habitats were statistically analyzed. One-way ANOVA was used to compare the rhizome, stem, and leaf morphological traits, organ biomass, and population traits of L. secalinus among the different habitats (α = 0.05). Based on the rhizome, stem, and leaf morphological traits, organ biomass, and environment data, after all the data were standardized using the statistical option in SPSS 22.0, redundancy analysis (RDA, type II scaling) was performed with the software CANOCO4.5 and was used to determine the responses of the most significant trait variables to environmental factors []. Then, Origin 2022 software was used for correlation analysis.
3. Results
3.1. Soil Properties of Each Habitat
The soil properties significantly differed among the different habitats (p < 0.05, Figure 3, Table S1). The soil moisture content (SMC) and soil bulk density (SBD) were highest in plot I and lowest in plot III (Figure 3A,B). The soil pH did not differ significantly between plots (Figure 3C). The EC was lowest in plot I and highest in plot III (Figure 3D). With the change in habitat conditions from plot I to plot III, the soil moisture content and bulk density decreased by 51% and 19%, respectively. The EC increased 2.15-fold from plot I to plot III.
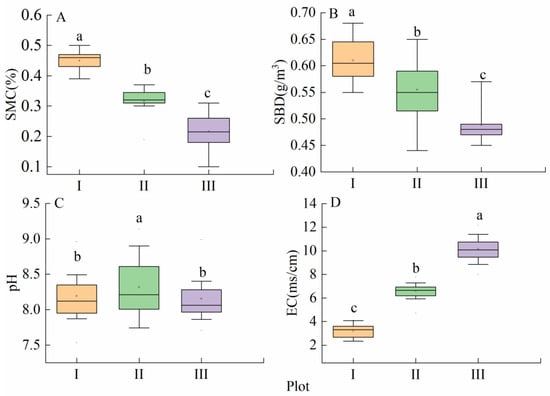
Figure 3.
Soil properties of each habitat. I, near riparian zone; II, middle riparian zone; and III, far riparian zone. Different lowercase letters indicate significant differences among plots (p < 0.05). SMC (soil moisture content); SBD (soil bulk density); EC (electrical conductivity).
3.2. Plant Community Traits in Each Habitat
For the plant community traits in the different habitats (p < 0.05, Table 1), the coverage and density values were highest in plot I and lowest in plot III. As the habitat conditions changed from plot I to plot III, the coverage and density decreased by 52% and 52%, respectively. The average height first decreased and then increased, with an overall decrease of 40.9%. In addition, the main species gradually changed from P. australis, L. secalinus, and A. splendens to L. secalinus and A. splendens to L. secalinus (Table 1).

Table 1.
Plant community traits in different habitats (mean ± SE).
3.3. Population Traits of L. secalinus
The population traits significantly differed among the different habitats (p < 0.05, Figure 4; Table S2). As the habitat conditions changed from plot I to plot III, the average height of the L. secalinus population decreased by 29.80% (Figure 4C). The coverage, density, and root depth showed increasing trends (Figure 4A,B,D) and increased 0.55-, 6.14-, and 0.82-fold, respectively (Figure 4A,B,D, Table S2).
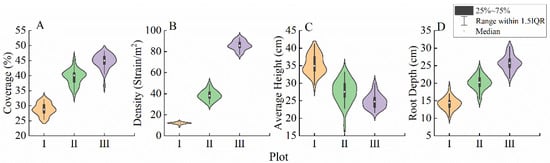
Figure 4.
Population traits of Leymus secalinus in each plot. I, near riparian zone; II, middle riparian zone; and III, far riparian zone. Different lowercase letters indicate significant differences among plots (p < 0.05). n = 36, represents the number of L. secalinus counted by each habitat gradient.
3.4. Analysis of the Biomass Ratio of L. secalinus Roots, Stems and Leaves
In the different habitats, the proportions of L. secalinus organ biomass significantly differed (p < 0.05, Figure 5). With the change in habitat from plot I to plot III, the L. secalinus leaf biomass percentage (PLB) significantly increased (p < 0.05), increasing from 34% in plot I to 53% in plot III, with a 0.56-fold overall increase. The stem biomass percentage (PSB) and rhizome biomass percentage (PRB) significantly decreased (p < 0.05) from 13% and 53% in plot I to 7% and 40% in plot III, with overall decreases of 46.15% and 24.53%, respectively.
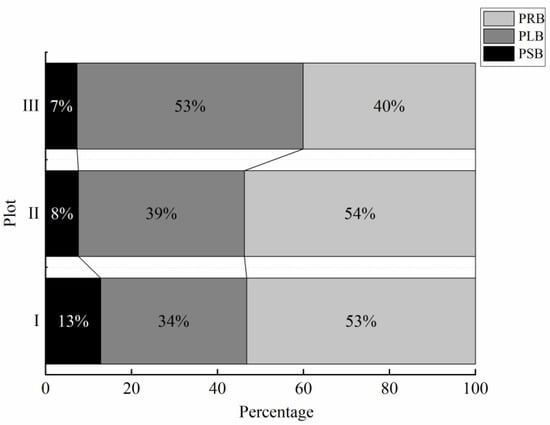
Figure 5.
Comparisons of the shoot biomass percentage and root biomass percentage in different plots of Leymus secalinus. I, near riparian zone; II, middle riparian zone; and III, far riparian zone. PSB, stem biomass percentage; PLB, leaf biomass percentage; PRB, root biomass percentage.
3.5. Functional Traits of L. secalinus in Each Habitat
The functional traits of L. secalinus significantly differed among the different habitats (p < 0.05, Table 2). With the change in habitat from plot I to plot III, leaf area, rhizome diameter, and leaf biomass exhibited increasing trends, increasing 0.26-, 0.78-, and 0.49-fold, respectively. In contrast, leaf thickness, specific leaf area, stem length, stem biomass, specific root length, and the root–shoot ratio exhibited decreasing trends, with decreases of 22.92%, 14.14%, 67.43%, 48.72%, 24.77%, and 68.57%, respectively. In addition, the stem diameter, rhizome length, and rhizome biomass first increased and then decreased, with overall decreases of 3.19%, 45.86%, and 35.71%, respectively; the root surface area first increased and then decreased, with an overall increase of 4.30%.

Table 2.
Main functional traits of Leymus secalinus in each plot (mean ± SE).
3.6. Effects of Environmental Factors on L. secalinus Functional Traits
The RDA showed that sequence axes 1 and 2 explained 98.3% and 1.3%, respectively, of the ecological information (Figure 6). EC had the highest correlation with axis 1, followed by the soil moisture content and soil bulk density (Figure 6). Figure 6 shows that the EC gradually increased from left to right with RDA axis 1, and the soil moisture content and soil bulk density gradually decreased from left to right with RDA axis 1. Leaf area, stem diameter, leaf biomass, root diameter, and root surface area are influenced by the environmental characteristics that make up axis 1. However, SLA, root length, specific Rhizome length, leaf thickness, Rhizome biomass, stem length, stem biomass, and the root–shoot ratio were mainly affected by soil moisture content and bulk density. Moreover, the pH was less strongly correlated with axis 1, which indicated that the functional traits of L. secalinus were less affected by soil pH. Moreover, Figure 6 shows that the SLA, leaf area, and stem diameter of the L. secalinus leaves were less affected by soil moisture content, soil bulk density, EC, and pH. Overall, leaf biomass, stem length, rhizome diameter, stem biomass, specific root length, leaf thickness, rhizome biomass, rhizome length, and root surface area were the main functional characteristics strongly affected by environmental factors (Figure 6).
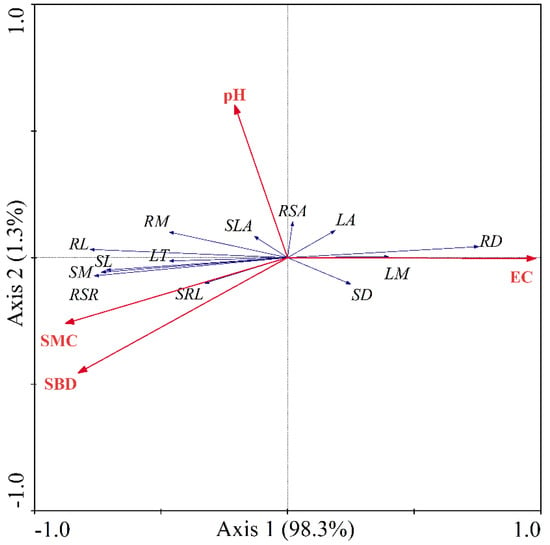
Figure 6.
RDA ordination of the functional traits of Leymus secalinus and related environmental fac-tors. SMC (soil moisture content); SBD (soil bulk density); EC (electrical conductivity); LA (leaf area); LT (leaf thickness); SRL (specific root length); SL (stem length); SD (stem diameter); RD (Rhizome diameter); RL (Rhizome length); SRL (specific root length); RSA (root surface area); SM (stem bio-mass); LM (leaf biomass); RM (Rhizome biomass); RSR (root–shoot ratio). The blue arrows represent the root, stem, and leaf morphological traits, and the red arrows represent environmental factors.
3.7. Correlations between the Root, Stem and Leaf Morphological Characteristics and Biomass Allocation of L. secalinus
The correlations between the main morphological characteristics and biomass allocation of L. secalinus and the habitat gradient obtained by RDA are shown in Table S3. Pearson correlation analysis revealed highly positive correlations (p < 0.01, Figure 7, Table S3) between stem biomass and leaf thickness, stem length, rhizome length, and specific root length. Moreover, the same correlations were detected between the root–shoot ratio and leaf thickness, stem length, and rhizome length and between rhizome biomass and stem length and rhizome length. Moreover, there were highly significant negative correlations (p < 0.01, Figure 7, Table S3) between stem biomass, the root–shoot ratio, and rhizome diameter. In addition, the same correlations were detected between leaf biomass and stem length and between rhizome biomass and rhizome diameter and specific root length. Moreover, a less significant positive correlation was found (p < 0.05, Figure 7, Table S3) between leaf thickness and rhizome biomass and between rhizome diameter and leaf biomass, and a less significant negative correlation was observed (p < 0.05, Figure 7, Table S3) between leaf thickness and leaf biomass and between specific rhizome length and leaf biomass. In addition, there was no significant correlation (p > 0.05, Figure 7, Table S3) between the specific root length and root–shoot ratio among the three habitats.
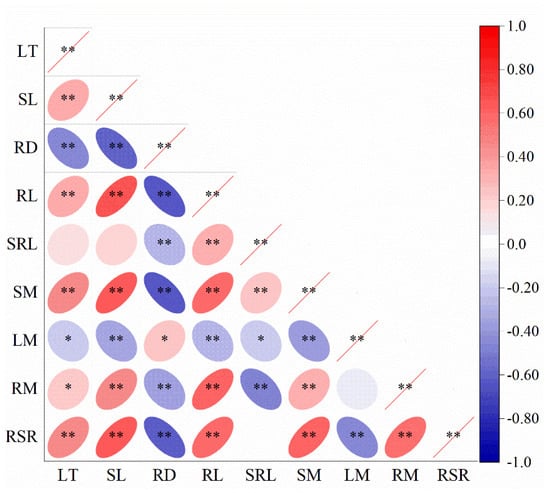
Figure 7.
Correlation coefficients between morphological traits and organ biomass allocation of L. secalinus. LT (leaf thickness); SL (stem length); RD (rhizome diameter); RL (rhizome length); SRL (specific root length); SM (stem biomass); LM (leaf biomass); RM (rhizome biomass); RSR (root-shoot ratio). * p < 0.05 (significant at the 0.05 level, bilateral; the null hypothesis is rejected at the 95% confidence level, and the sample shows a linear correlation); ** p < 0.01 (significant at the 0.01 level, bilateral; the null hypothesis is rejected at the 99% confidence level, and the sample shows a linear correlation). The blue ovals represent negative correlations between the morphological characteris-tics and biomass allocation, and the red ovals represent positive correlations between the morpho-logical characteristics and biomass allocation. The deeper the color is, the more significant the cor-relation. The lighter the color is, the weaker the correlation. I, near riparian zone; II, middle riparian zone; and III, far riparian zone.
4. Discussion
Plant functional traits are closely related to plant colonization, survival, growth, and death []. These plant traits respond to changes in the living environment or have certain effects on ecosystem functions and can reflect the growth status of plants and their adaptability to the external environment [,,]. In this study, we found that the organ biomass allocation and morphological characteristics of L. secalinus roots, stems, and leaves changed significantly with changes in habitat conditions from the near riparian zone (Plot I) to the far riparian zone (Plot III) (Table 2, Figure 5, Table S3); there was a significant correlation between organ biomass and leaf thickness, stem length, RD and RL in the three habitats (p < 0.05; Figure 7).
Plant-available resources are usually related to the physical or geometric crowding effects of neighboring plants, the spatial distributions of which can change []. The study area is part of the natural wetland ecosystem of the Heihe River riparian zone, an inland river in the temperate arid zone of the Hexi Corridor, northwestern China. Soil water and groundwater are the main water resources used by L. secalinus. Our study revealed that the main morphological traits and biomass proportions of organs of L. secalinus significantly differed (p < 0.05, Figure 5, Table 2) under the different habitat conditions. The reasons for these differences are as follows. The first cause was differences in the biological environment, i.e., the community environment. In the near riparian zone, species other than L. secalinus were most abundant, and L. secalinus was an associated species that did not have an advantage in the community (Table 1). Within the community, there is a high degree of resource competition among plant populations []. The second cause was differences in the soil environment. The soil moisture content and bulk density were the highest, and the soil electrical conductivity was the lowest (Figure 3, Table S1). Moreover, L. secalinus had the lowest population coverage, density, and root distribution depth but the greatest height (Figure 4 and Table S2). In this habitat, although L. secalinus was tallest (Figure 4 and Table S2), it grew smaller leaves on slender stems (Table 2), while its roots tended to be distributed in the surface layer (Figure 4 and Table S2). The biomass investment for aboveground stems was greater in plot I than in plot II or plot III. L. secalinus allocated the most biomass to the rhizome, followed by leaves and then stems (Figure 5). The possible reasons for this result are as follows: on the one hand, the construction of slender stems and small leaves is conducive to avoiding the shade created by neighboring plants and obtaining more light resources, allowing L. secalinus to survive in the gaps of tall grass reeds. On the other hand, with limited photosynthetic carbon assimilation products and sufficient soil moisture, L. secalinus does not need to increase the distribution depth of its roots but can expand the horizontal foraging range of its rhizome by increasing the resource investment in underground rhizome, constructing the maximum rhizome length and specific rhizome length (Table 2) and improving the ability to obtain soil nutrients [] to survive in a highly competitive environment.
A change in the root–shoot ratio is a basic form of plasticity in plant allocation patterns []. From the near riparian zone to the middle riparian zone, the soil electrical conductivity increased and the soil moisture content decreased (Figure 3, Table S1). The average height, density, and coverage of the wetland community were between those of the near riparian zone and far riparian zone (Table 1). The population characteristics (height, density, coverage, and root depth) of L. secalinus also changed (Figure 4, Table S2). In this habitat, the stems and leaves of L. secalinus were moderately sized, the rhizome diameter and stem length were greater, the root surface area and rhizome length were at their maximum (Table 2), and L. secalinus allocated the most biomass to the rhizome, followed by leaves and then stems. Compared with those in the near riparian zone, the root shoot ratio and stem biomass decreased and the leaf biomass and rhizome biomass increased (Table 2, Figure 5). The possible reasons for this result are as follows: this habitat belongs to the transition zone marked by increasing dominance of L. secalinus and the transition zone from the near riparian zone to the far riparian zone. With decreasing soil moisture content and bulk density, the soil electrical conductivity gradually increased, and the number of species in the community decreased compared with that in plot I, though L. secalinus was still not dominant in the community. Therefore, it is necessary to balance the biomass allocation of each organ to construct phenotypic traits suitable for the environment []. (1) For the aboveground stems and leaves, the leaf thickness decreased, while the leaf area increased. On the one hand, with limited resource investment, the light capture ability of the leaves was maintained. On the other hand, thick and long stems can maintain high water conductivity and meet the water consumption demand of L. secalinus leaves in a competitive environment, which is beneficial for improving the interspecific competitive ability of the aboveground parts of L. secalinus. (2) For the belowground roots, L. secalinus increased its root surface area and length and expanded its foraging range in the horizontal direction (Table 2), which improved the foraging efficiency of the rhizome and provided sufficient resources for the growth of aboveground stems and leaves, thus helping to improve the resource competitiveness of L. secalinus.
In the far riparian zone, the soil electrical conductivity reached its maximum value, while the soil moisture content and wetland community average coverage and density presented were the smallest (Figure 3, Table S1 and Table 1). However, the coverage, density, and root depth reached their maximum values for the L. secalinus population, but the average height was the lowest (Figure 4, Table S2). In this habitat, L. secalinus tended to be the shortest, with larger leaves growing on short and thick stems (Table 2), and the root distribution depth increased (Figure 4, Table S2). L. secalinus allocated the most biomass to leaves, followed by rhizome and then stems; compared with the other two habitats, the biomass of leaves was the greatest, and that of stems and rhizome was the smallest (Table 2, Figure 5). The possible reasons for these results are as follows: (1) in this habitat, by increasing the root distribution depth, L. secalinus roots can move away from the surface soil with a high salt content, which is conducive to improving the ability of roots to acquire deep soil resources. (2) On the one hand, growing large leaves on thin and short stems can shorten the distance of water transfer to leaves, fulfil the water consumption demand of large leaves, and help to ensure higher aboveground photosynthetic efficiency []. (3) In this habitat, L. secalinus was the dominant species, maintaining an absolute resource advantage, and there was no need to increase the resource allocation of belowground rhizomes. Compared with the near riparian zone and the middle zone, in this plot, L. secalinus developed a relatively compact root network system with the lowest rhizome length and specific root length and a lower root surface area (Table 2). Plant roots with the lowest rhizome length and specific root length, lower root surface area, and greater root depth are beneficial for improving the absorption efficiency of soil resources below the surface can meet plant needs. In addition, a relatively compact root network can help reduce the proportion of root carbon consumption caused by the increase in root depth, maintain high aboveground resource allocation, and improve the accumulation efficiency of photosynthetic carbon assimilation products. With changes in wetland environmental conditions, L. secalinus preferred to invest in rhizome biomass in the near riparian zone and stem biomass and leaf biomass investment in the far riparian zone, which was more consistent with the CS strategy of CSR []. This reflects the ecological adaptation strategy of the riparian plant L. secalinus, which adjusts its resource allocation strategy as the habitat changes to improve its fitness and interspecific competitive ability.
5. Conclusions
This study found that the diversity of the wetland habitats in the riparian floodplain area substantially drives the plant community structure, soil characteristics, and thereby the phenotypic characteristics and biomass allocation patterns. In the near riparian zone, L. secalinus expanded its foraging range for soil resources by increasing its investment in belowground root-level resources and developing slender stems and small leaves to improve interspecific competition. In the far riparian zone, L. secalinus developed short, thin stems and large leaves to improve its photosynthetic efficiency. Moreover, increasing the root distribution depth improved the ability of the plants to acquire deep soil resources. This phenotypic plasticity reflects the ecological adaptation strategy of L. secalinus plants to maintain their survival, growth, and reproduction by balancing biomass allocation and adjusting the morphological characteristics of stems, roots, and leaves in a timely manner. In this study, we investigated the changes in the biomass allocation patterns and morphological characteristics of L. secalinus in different habitats in the riparian zone and investigated the phenotypic plasticity and ecological adaptation strategies of L. secalinus in response to heterogeneous environments. L. secalinus is a typical clonal plant, and the morphological traits of its clonal components also play an important role in coping with differences in hydrology, soil texture, nutrients, and other conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14091899/s1, Table S1: The soil properties of each habitat; Table S2: The population traits of Leymus secalinus in each plot; Table S3: Correlation analysis between morphological traits and organ biomass allocation of Leymus secalinus.
Author Contributions
Conceptualization, Q.L. and C.Z.; Data curation, validation and software, J.W., Q.L. and M.K.; Formal analysis, original draft preparation, J.W. and Q.L.; C.Z. and Q.L., Funding acquisition and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported, in part, National natural science foundation of China (Grant NO. 41461013 and NO. 41861009) and Xichang University Doctoral Start Foundation Program (YBZ202237).
Data Availability Statement
The data used in the present work have been listed in the Supporting information.
Acknowledgments
We would like to acknowledge the professional manuscript services of American Journal Experts (https://secure.authorservices.springernature.com) (accessed on 26 July 2024). Besides, we are very grateful to Xiaoya Li, Min Ma, Xuqian Bai and Zhiwei Zhang for their assistance in the field and laboratory work.
Conflicts of Interest
The authors declare no conflict of interest. The manuscript has been approved by all authors. I declare on behalf of all authors that the work described is original research that has not been published previously and is not under consideration for publication elsewhere, in whole or in part.
Abbreviations
SMC, Soil moisture content; EC, Soil electrical conductivity; SBD, Soil bulk density; LA, Leaf area; LT, Leaf thickness; SL, stem length; SD, stem diameter; RD, Root diameter; RL, Root length; SM, stem biomass; LM, leaf biomass; RM, Root biomass; RSR, Root-shoot ratio; SLA, Specific leaf area; SRL, Specific root length.
References
- Garssen, A.G.; Baattrup-Pedersen, A.; Voesenek, L.A.C.J.; Verhoeven, J.T.A.; Soons, M.B. Riparian plant community responses to increased flooding: A meta-analysis. Glob. Chang. Biol. 2015, 21, 2881–2890. [Google Scholar] [CrossRef]
- Popoff, N.; Jaunatre, R.; Bouteiller, C.L.; Trinquier, M.; Paillet, Y.; Evette, A. Ecological succession and fine sediment accretion influence local patch dynamics of a pioneer riparian species (Typha minima Hoppe). Freshw. Biol. 2021, 66, 2351–2363. [Google Scholar] [CrossRef]
- Han, L.; Wang, H.Z.; Yu, J. Research progress and prospects on riparian zone ecology. Ecol. Environ. Sci. 2013, 22, 879–886. [Google Scholar] [CrossRef]
- Tabacchi, E.; Lambs, L.; Guilloy, H.; Planty-Tabacchi, A.-M.; Muller, E.; Décamps, H. Impacts of riparian vegetation on hydrological processes. Hydrol. Process. 2000, 14, 2959–2976. [Google Scholar] [CrossRef]
- Tikssa, M.; Bekele, T.; Kelbessa, E. Plant community distribution and variation along the Awash river corridor in the main Ethiopian rift. Afr. J. Ecol. 2010, 48, 21–28. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, C.Z.; Wen, J.; Kang, M.P.; Li, X.Y. Fractal analysis of root architecture responses of Saussurea salsa to a gradient of flooding intensity and salinity. Plant Soil 2022, 471, 669–683. [Google Scholar] [CrossRef]
- Liang, S.C.; Liu, R.H.; Rong, C.Y.; Chang, B.; Jiang, Y. Variation and correlation of plant functional traits in the riparian zone of the Lijiang River, Guilin, Southwest China. Chin. J. Plant Ecol. 2019, 43, 16–26. [Google Scholar] [CrossRef]
- Mensah, S.; Glèlè Kakaï, R.; Seifert, T. Patterns of biomass allocation between foliage and woody structure: The effects of tree size and specific functional traits. Ann. For. Res. 2016, 59, 49–60. [Google Scholar] [CrossRef]
- Hecht, V.L.; Temperton, V.M.; Nagel, K.A.; Rascher, U.; Pude, R.; Postma, J.A. Plant density modifies root system architecture in spring barley (Hordeum vulgare L.) through a change in nodal root number. Plant Soil 2019, 439, 179–200. [Google Scholar] [CrossRef]
- Freschet, G.T.; Violle, C.; Bourget, M.Y.; Scherer-Lorenzen, M.; Fort, F. Allocation, morphology, physiology, architecture: The multiple facets of plant above- and below-ground responses to resource stress. New Phytol. 2018, 219, 1338–1352. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, C.Z.; Kang, M.P.; Li, X.Y. The relationship of the main root-shoot morphological characteristics and biomass allocation of Saussurea salsa under different habitat conditions in Sugan lake wetland on the northern margin of the Qinghai-Tibet Plateau. Ecol. Indic. 2021, 128, 107836. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Pallas, B.; Da Silva, D.; Valsesia, P.; Yang, W.; Guillaume, O.; Lauri, P.-E.; Vercambre, G.; Génard, M.; Costes, E. Simulation of carbon allocation and organ growth variability in apple tree by connecting architectural and source-sink models. Ann. Bot. 2016, 118, 317–330. [Google Scholar] [CrossRef]
- Lavorel, S.; Grigulis, K.; Lamarque, P.; Colace, M.P.; Garden, D.; Girel, J.; Pellet, G.; Douzet, R. Using plant functional traits to understand the landscape distribution of multiple ecosystem services. J. Ecol. 2011, 99, 135–147. [Google Scholar] [CrossRef]
- Moor, H.; Rydin, H.; Hylander, K.; Nilsson, M.B.; Lindborg, R.; Norberg, J. Towards a trait based ecology of wetland vegetation. J. Ecol. 2017, 105, 1623–1635. [Google Scholar] [CrossRef]
- Dawson, S.K.; Warton, D.I.; Kingsford, R.T.; Berney, P.; Keith, D.A.; Catford, J.A. Plant traits of propagule banks and standing vegetation reveal flooding alleviates impacts of agriculture on wetland restoration. J. Appl. Ecol. 2017, 54, 1907–1918. [Google Scholar] [CrossRef]
- Fu, H.; Yuan, G.X.; Cao, T.; Ni, L.Y.; Zhang, X.L. Clonal growth and foraging behavior of a submerged macrophyte Vallisneria natans in response to water depth gradient. J. Lake Sci. 2012, 24, 705–711. [Google Scholar] [CrossRef][Green Version]
- Hanke, J.M.; Ludewig, K.; Jensen, K. Effects of water level and competition on the endangered river corridor plant Cnidium dubium in the context of climate change. Wetl. Ecol. Manag. 2015, 23, 215–226. [Google Scholar] [CrossRef]
- Deng, J.M.; Li, T.; Wang, G.X.; Liu, J.; Yu, Z.L.; Zhao, C.M.; Ji, M.F.; Zhang, Q.; Liu, J.Q. Trade-offs between the metabolic rate and population density of plants. PLoS ONE 2008, 3, e1799. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Garnier, E.; Navas, M.L. A trait-based approach to comparative functional plant ecology: Concepts, methods and applications for agroecology: A review. Agron. Sustain. Dev. 2012, 32, 365–399. [Google Scholar] [CrossRef]
- Plaza-Bonilla, D.; Álvaro-Fuentes, J.; Hansen, N.C.; Lampurlanés, J.; Cantero-Martínez, C. Winter cereal root growth and aboveground-belowground biomass ratios as affected by site and tillage system in dry land Mediterranean conditions. Plant Soil 2014, 374, 925–939. [Google Scholar] [CrossRef]
- Zhong, F.; Wang, H.C.; Li, J.N.; Duan, Z.H. Influence of Water-Heat Condition on Distribution of Platycladus orientalis (L)Franco Roots in Southern and Northern Mountains of Lanzhou City. J. Desert Res. 2006, 26, 559–563. [Google Scholar]
- Baastrup-Spohr, L.; Sand-Jensen, K.; Nicolajsen, S.V.; Bruun, H.H. From soaking wet to bone dry: Predicting plant community composition along a steep hydrological gradient. J. Veg. Sci. 2015, 26, 619–630. [Google Scholar] [CrossRef]
- Zhao, G.S.; Liu, M.; Shi, P.L.; Zong, N.; Zhang, X.; Zhang, X.Z. Variation of leaf and root traits and ecological adaptive strategies along a precipitation gradient on Changtang Plateau. Acta Ecol. Sin. 2020, 40, 295–309. [Google Scholar] [CrossRef]
- Enquist, B.J.; Niklas, K.J. Global allocation rules for patterns of biomass partitioning in seed plants. Science 2002, 295, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Freschet, G.T.; Cornelissen, J.H.C.; van Logtestijn, R.S.P.; Aerts, R. Evidence of the ‘plant economics spectrum’ in a subarctic flora. J. Ecol. 2010, 98, 362–373. [Google Scholar] [CrossRef]
- Laughlin, D.C.; Leppert, J.J.; Moore, M.M.; Sieg, C.H. A multi-trait test of the leaf-height-seed plant strategy scheme with 133 species from a pine forest flora. Funct. Ecol. 2010, 24, 493–501. [Google Scholar] [CrossRef]
- Liu, G.; Freschet, G.T.; Pan, X.; Cornelissen, J.H.C.; Li, Y.; Dong, M. Coordinated variation in leaf and root traits across multiple spatial scales in Chinese semi-arid and arid ecosystems. New Phytol. 2010, 188, 543–553. [Google Scholar] [CrossRef]
- Freschet, G.T.; Bellingham, P.J.; Lyver, P.O.B.; Bonner, K.I.; Wardle, D.A. Plasticity in above- and belowground resource acquisition traits in response to single and multiple environmental factors in three tree species. Ecol. Evol. 2013, 3, 1065–1078. [Google Scholar] [CrossRef]
- Pigliucci, M.; Murren, C.J.; Schlichting, C.D. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 2006, 209, 2362–2367. [Google Scholar] [CrossRef] [PubMed]
- Dong, M. Effect of severing Rhizome on clonal growth in rhizomatous grass species Psammochloa villosa and Leymus secalinus. Acta Bot. Sin. 1999, 41, 194–198. [Google Scholar]
- Du, L.X.; Zhu, H.L.; Dong, K.H.; Fan, P.P.; Sun, J.P. Effects of salt stress on the ions uptake and transport of Leymus secalinus seeding. Acta Agrestia Sin. 2015, 23, 510–516. [Google Scholar]
- Zhu, Y.J.; Ye, X.H.; Chu, Y.; Gao, S.Q.; Dong, M. Effects of precipitation on clonal plant distribution on Ordos Plateau. Acta Ecol. Sin. 2020, 40, 952–963. [Google Scholar] [CrossRef]
- Liu, L.; Bai, Y.X.; Qiao, Y.G.; Miao, C.; She, W.W.; Qin, S.G.; Zhang, Y.Q. Water-use characteristics of two dominant plant species in different community types in the Mu Us Desert. CATENA 2023, 221, 106803. [Google Scholar] [CrossRef]
- Yi, J.; Gu, A.L.; Jia, G.H.; Wu, X. Studies on the drought hardiness in seeding of Leymus Hochst. J. Arid Land Resour. Environ. 2001, 15, 41–46. [Google Scholar]
- Qi, B.J.; Yi, J.; Gu, A.L.; Yuan, J.Z. Studies on the salt hardiness in seeds and seeding of Leymus Hochst. J. Arid Land Resour. Environ. 2001, 15, 41–46. [Google Scholar]
- Yi, J.; Li, Q.F.; Tian, R.H. Seed dormancy and hormone control of germination in Leymus Hochst. Acta Agreatia Sin. 1997, 5, 93–100. [Google Scholar] [CrossRef]
- Yang, Y.F.; Zhang, B.T. Clone growth and its age structure of Leymus secalimus modules in the Songnen Plain of China. Chin. J. Appl. Ecol. 2004, 11, 2109–2112. [Google Scholar] [CrossRef]
- Sha, L.N.; Liang, X.; Tang, Y.; Xu, J.Q.; Chen, W.J.; Cheng, Y.R.; Wu, D.D.; Zhang, Y.; Wang, Y.; Kang, H.Y.; et al. Evolutionary patterns of plastome resolve multiple origins of the Ns-containing polyploid species in Triticeae. Mol. Phylogenetics Evol. 2022, 175, 107591. [Google Scholar] [CrossRef]
- Zhang, W.H.; Liu, D.L.; Miao, Y.J.; Xu, Y.M.; Chen, M.H.; Shao, J. Comparison of drought resistance of wild Leymus secalinus and Elymus nutans in Tibet. Pratacultural Sci. 2017, 34, 1255–1263. [Google Scholar] [CrossRef]
- Han, L.; Zhao, C.Z.; Feng, W.; Zheng, H.L.; Duan, B.B. Trade-off relationship between vein density and vein diameter of Achnatherum splendens in response to habitat changes in Zhangye wetland. Chin. J. Plant Ecol. 2017, 41, 872–881. [Google Scholar] [CrossRef]
- Geng, H.L.; Wang, Y.H.; Wang, F.Y.; Jia, B.R. The dynamics of root-shoot ratio and its environmental effective factors of recovering Leymus chinensis steppe vegetation in Inner Mongolia. Acta Ecol. Sin. 2008, 28, 4629–4634. [Google Scholar] [CrossRef]
- Li, Q.; Jun, W.; Zhao, C.Z.; Zhao, L.C.; Dan, K. The relationship between the main leaf traits and photosynthetic physiological characteristics of Phragmites australis under different habitats of a salt marsh at Qinwangchuan, China. AoB Plants 2022, 14, plac054. [Google Scholar] [CrossRef]
- Garnier, E.; Stahl, U.; Laporte, M.A.; Kattge, J.; Mougenot, I.; Kühn, I.; Laporte, B.; Amiaud, B.; Ahrestani, F.S.; Bönisch, G.; et al. Towards a thesaurus of plant characteristics: An ecological contribution. J. Ecol. 2017, 105, 298–309. [Google Scholar] [CrossRef]
- Meng, T.T.; Ni, J.; Wang, G.H. Plant functional traits, enviroments and ecosystem functioning. J. Plant Ecol. 2007, 31, 150–165. [Google Scholar]
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Kattge, J.; Diaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Bönisch, G.; Garnier, E.; Westoby, M.; Reich, P.B.; Wright, I.J.; et al. TRY—A global database of plant traits. Glob. Chang. Biol. 2011, 17, 2905–2935. [Google Scholar] [CrossRef]
- Kenney, A.M.; McKay, J.K.; Richards, J.H.; Juenger, T.E. Direct and indirect selection on flowering time, water-use efficiency (WUE, δ13C), and WUE plasticity to drought in Arabidopsis thaliana. Ecol. Evol. 2014, 4, 4505–4521. [Google Scholar] [CrossRef]
- Roumet, C.; Birouste, M.; Picon-Cochard, C.; Ghestem, M.; Osman, N.; Vrignon-Brenas, S.; Stokes, A. Root structure-function relationships in 74 species: Evidence of a root economics spectrum related to carbon economy. New Phytol. 2016, 210, 815–826. [Google Scholar] [CrossRef]
- Kleyer, M.; Minden, V. Why functional ecology should consider all plant organs: An allocation-based perspective. Basic Appl. Ecol. 2015, 16, 1–9. [Google Scholar] [CrossRef]
- Dang, J.J.; Zhao, C.Z.; Li, Y.; Hou, Z.J.; Dong, X.G. G Variations with slope in stem and leaf traits of Melica przewalskyi in alpine grassland. Chin. J. Plant Ecol. 2014, 38, 1307–1314. [Google Scholar]
- Pierce, S.; Negreiros, D.; Cerabolini, B.E.L.; Kattge, J.; Díaz, S.; Kleyer, M.; Tampucci, D. A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Funct. Ecol. 2016, 31, 444–457. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).