Effects of Different Living Grass Mulching on Soil Carbon and Nitrogen in an Apple Orchard on Loess Plateau
Abstract
:1. Introduction
2. Materials and Methods
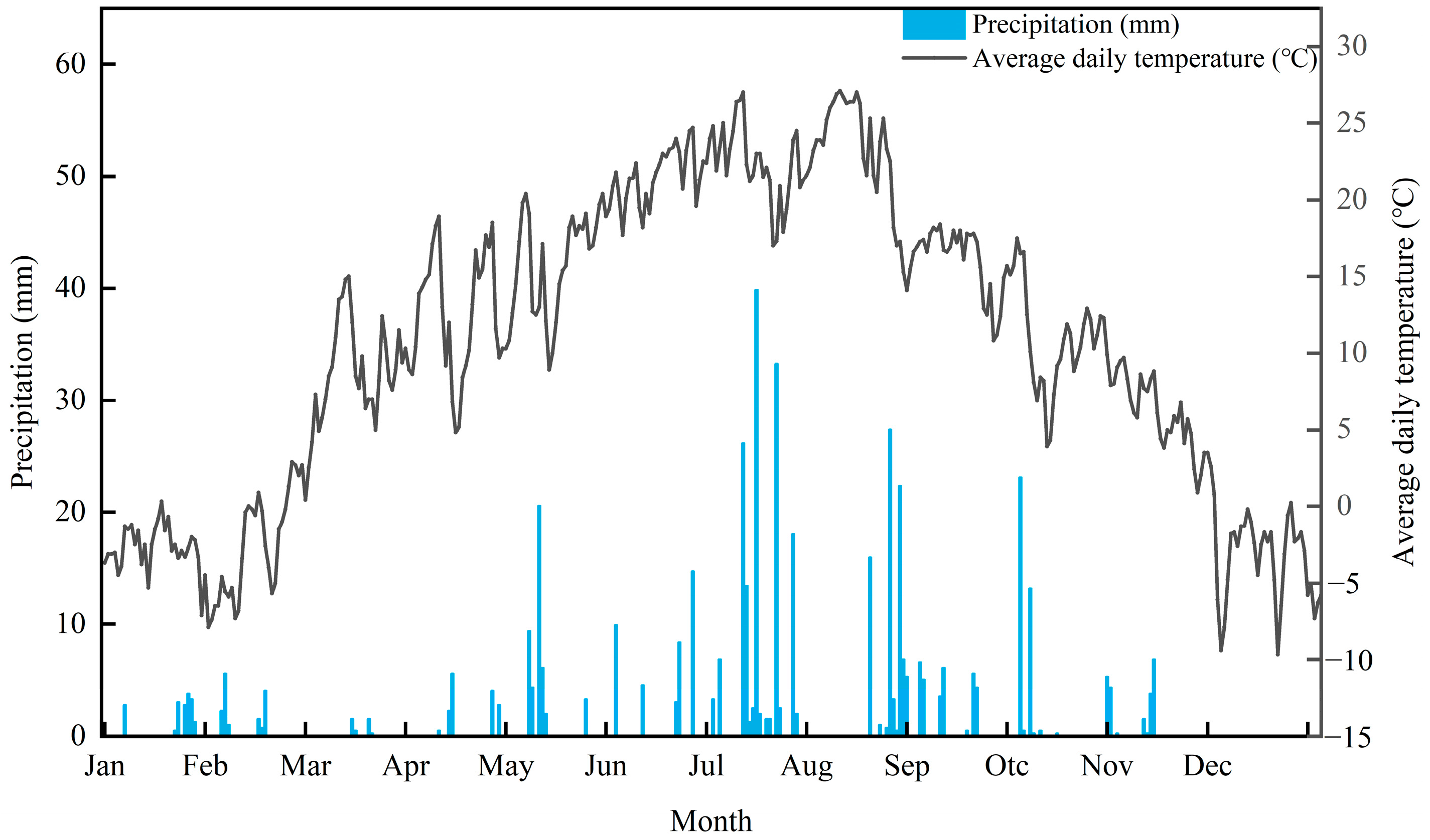
2.1. Study Area
2.2. Experimental Design
2.3. Soil Sample Acquisition and Measurement Methods
2.4. Data Processing
3. Results
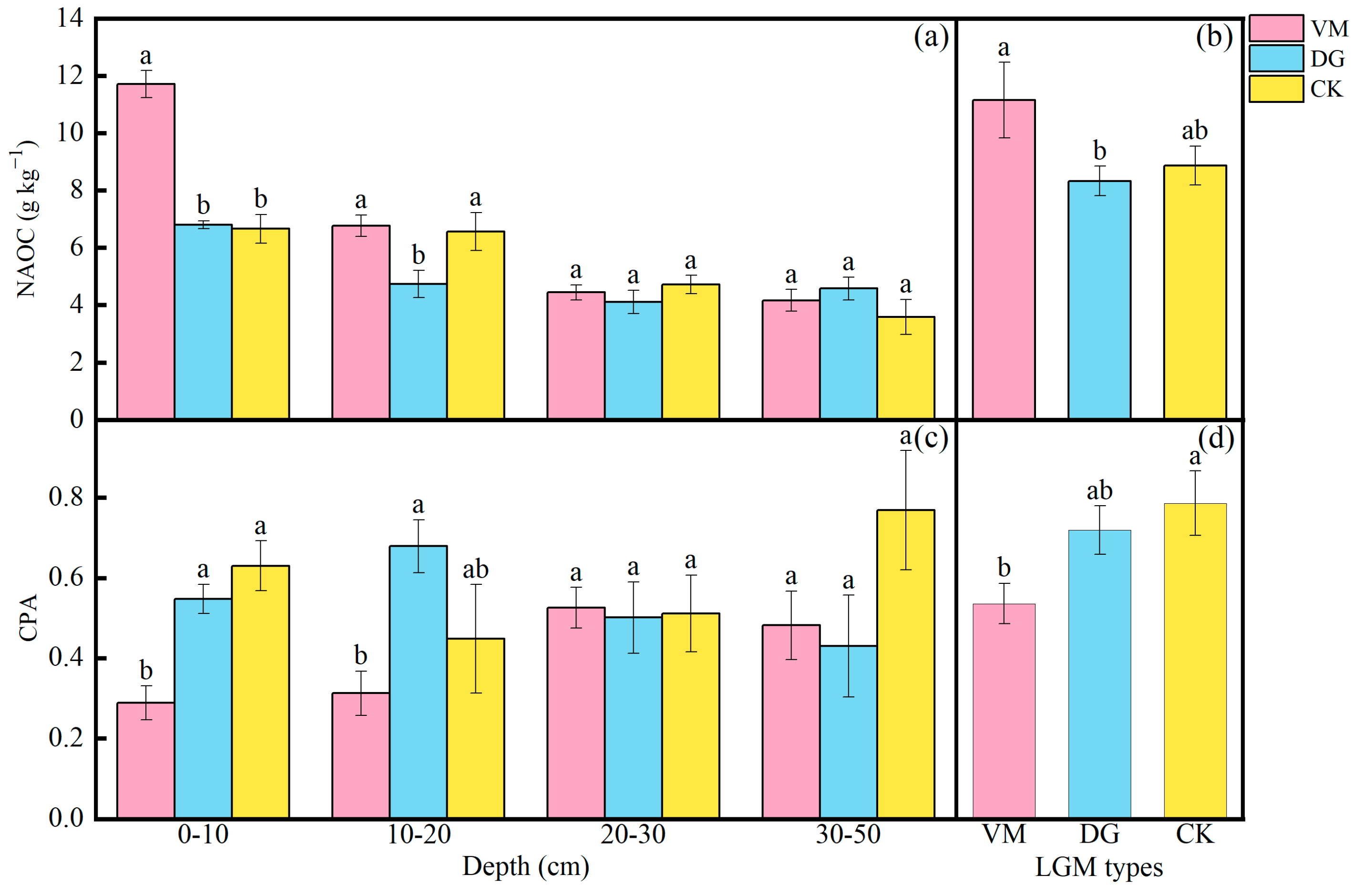
3.1. Effects of Soil Organic Carbon and Its Components in an Apple Orchard Covered with Different Species of Living Grass
3.2. Effects of Soil Nitrogen Content in Apple Orchard Covered with Different Species of Living Grass
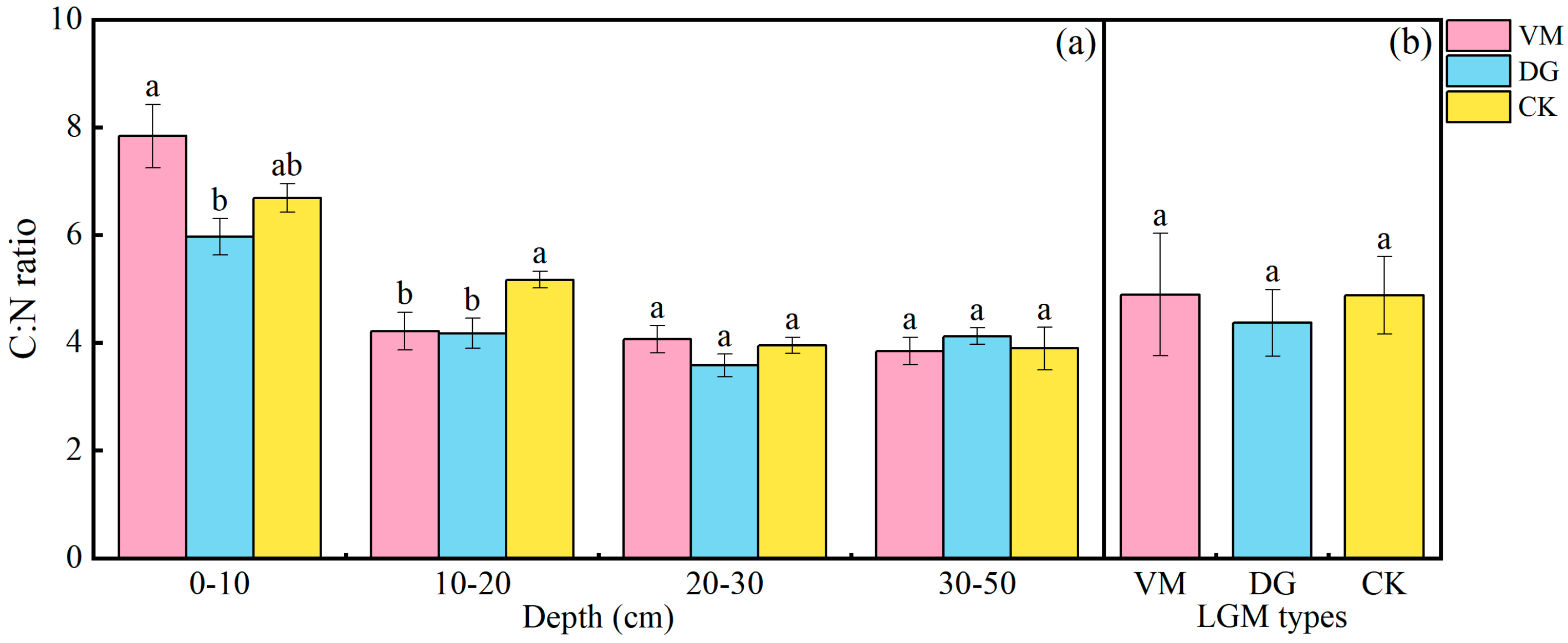
3.3. Effects of Soil C-N Ratio of the Apple Orchard Covered with Different Species of Living Grass
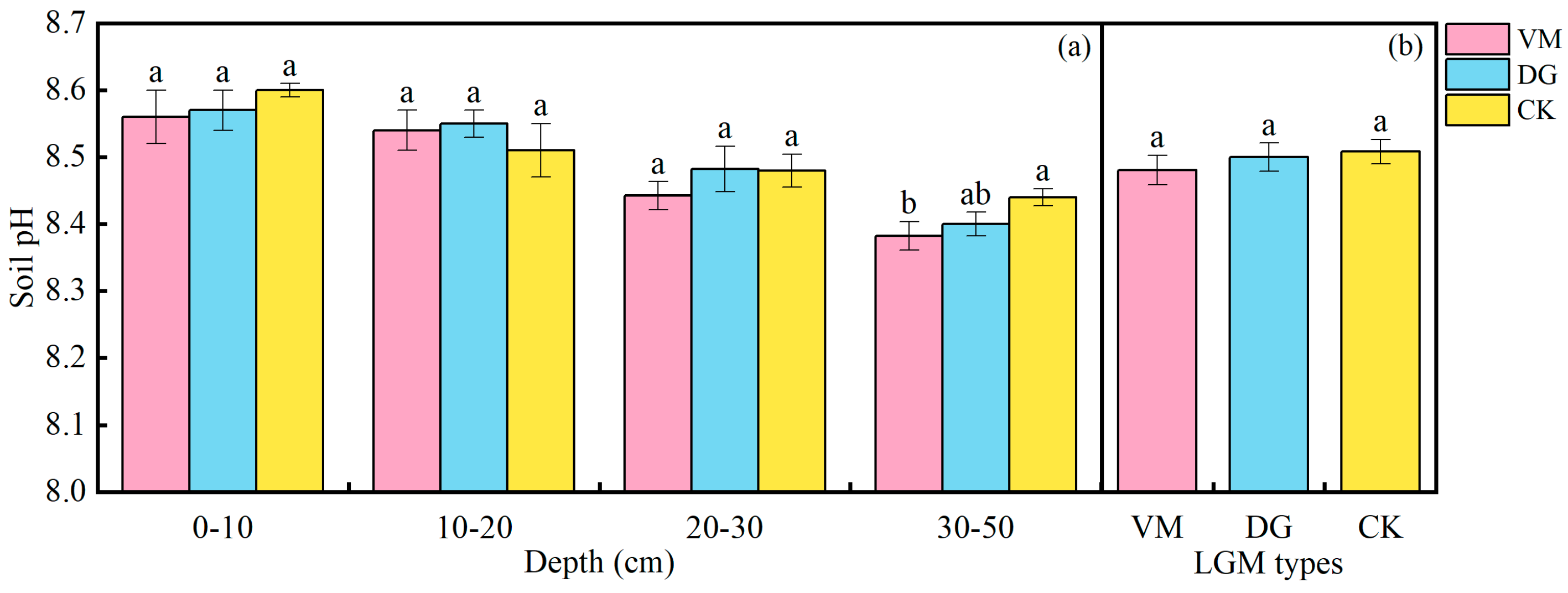
3.4. Effects of Soil pH of the Apple Orchard Covered with Different Species of Living Grass
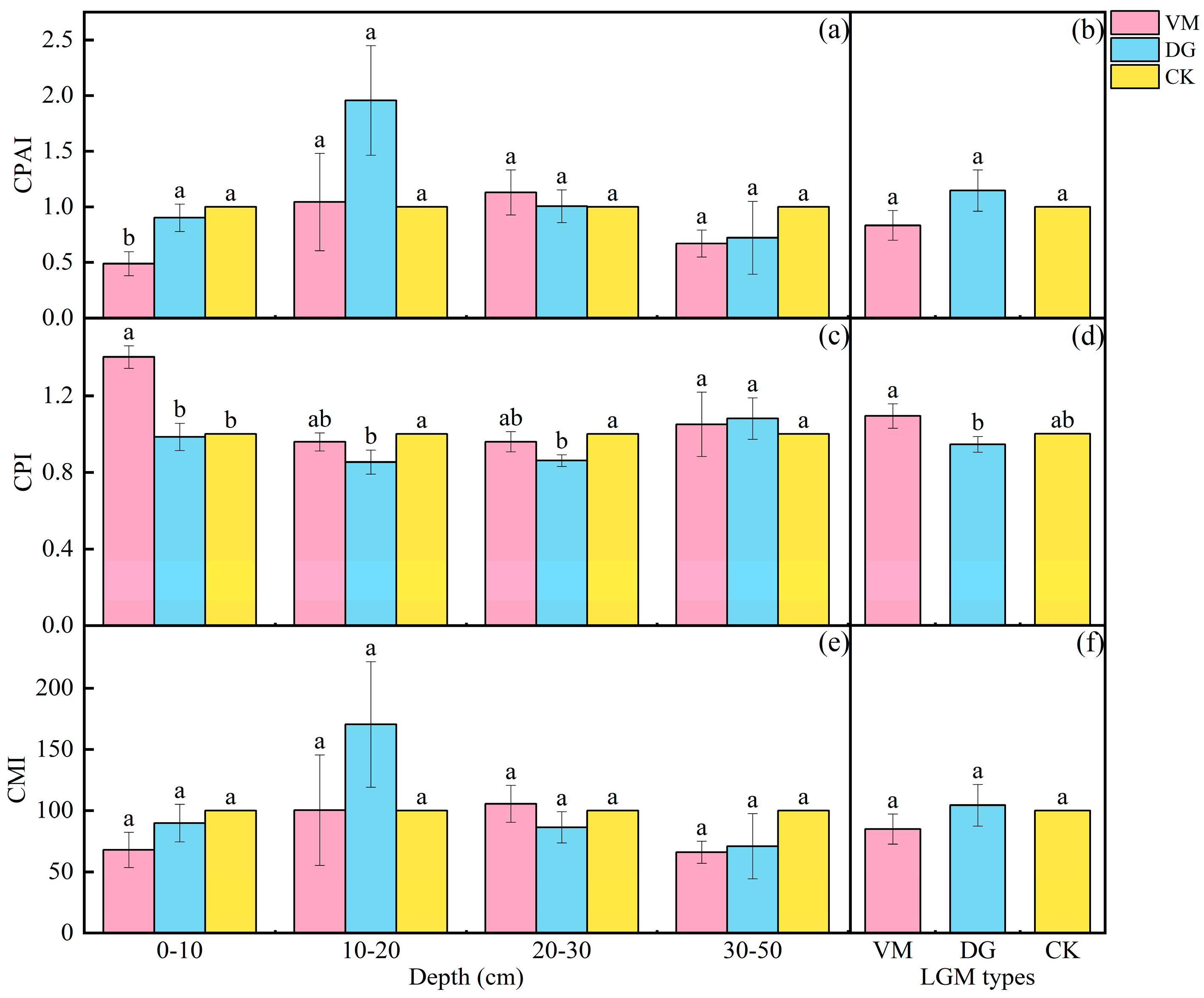
3.5. Effect of Stability of Carbon Pool in the Apple Orchard Covered with Different Species of Living Grass
4. Discussion
4.1. Effects of LMG on Soil Organic Carbon and Its Components and Stability of Carbon Pool in Apple Orchard
4.2. Effect of LMG on Soil Nitrogen Content in an Apple Orchard
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, W.; Abdulai, A. Linking apple farmers to markets. China Agric. Econ. Rev. 2016, 8, 2–21. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Benbrook, C.M. The Dollars and Cents of Soil Health. In Global Soil Security; Field, D.J., Morgan, C.L.S., McBratney, A.B., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 219–226. [Google Scholar] [CrossRef]
- Chen, G.; Liu, S.; Xiang, Y.; Tang, X.; Liu, H.; Yao, B.; Luo, X. Impact of living mulch on soil C:N:P stoichiometry in orchards across China: A meta-analysis examining climatic, edaphic, and biotic dependency. Pedosphere 2020, 30, 181–189. [Google Scholar] [CrossRef]
- van Keulen, G. Microbiology challenges and opportunities in soil health. Microbiology 2021, 167, 001001. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Hu, Y.; Zhang, J.; Jia, R.; Huang, Q.; Gao, H.; Awasthi, M.K.; Li, H.; Zhao, Z. The spatio-temporal change in soil P and P-solubilizing bacteria under clover mulching in apple orchards of Loess Plateau. Chemosphere 2022, 304, 135334. [Google Scholar] [CrossRef]
- Tang, W.; Yang, H.; Wang, W.; Wang, C.; Pang, Y.; Chen, D.; Hu, X. Effects of Living Grass Mulch on Soil Properties and Assessment of Soil Quality in Chinese Apple Orchards: A Meta-Analysis. Agronomy 2022, 12, 1974. [Google Scholar] [CrossRef]
- Griffiths, M.; Delory, B.M.; Jawahir, V.; Wong, K.M.; Bagnall, G.C.; Dowd, T.G.; Nusinow, D.A.; Miller, A.J.; Topp, C.N. Optimisation of root traits to provide enhanced ecosystem services in agricultural systems: A focus on cover crops. Plant Cell Environ. 2022, 45, 751–770. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Merwin, I.A.; Bird, G.W.; Abawi, G.S.; Thies, J.E. Orchard floor management practices that maintain vegetative or biomass groundcover stimulate soil microbial activity and alter soil microbial community composition. Plant Soil 2005, 271, 377–389. [Google Scholar] [CrossRef]
- Qian, X.; Gu, J.; Pan, H.; Zhang, K.; Sun, W.; Wang, X.; Gao, H. Effects of living mulches on the soil nutrient contents, enzyme activities, and bacterial community diversities of apple orchard soils. Eur. J. Soil Biol. 2015, 70, 23–30. [Google Scholar] [CrossRef]
- Hill, N.S.; Levi, M.; Basinger, N.; Thompson, A.; Cabrera, M.; Wallace, J.; Saikawa, E.; Avramov, A.; Mullican, J. White clover living mulch enhances soil health vs. annual cover crops. Agron. J. 2021, 113, 3697–3707. [Google Scholar] [CrossRef]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef]
- Bhaskar, V.; Westbrook, A.S.; Bellinder, R.R.; DiTommaso, A. Integrated management of living mulches for weed control: A review. Weed Technol. 2021, 35, 856–868. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agricultural Press: Beijing, China, 2000; pp. 355–356. [Google Scholar]
- Deflandre, B.; Gagné, J.P. Estimation of dissolved organic carbon (DOC) concentrations in nanoliter samples using UV spectroscopy. Water Res. 2001, 35, 3057–3062. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.; Lise, L. Soil Carbon Fractions Based on their Degree of Oxidation, and the Development of a Carbon Management Index for Agricultural Systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Particulate Soil Organic-Matter Changes across a Grassland Cultivation Sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K.; Elliott, E.T.; Combrink, C. Soil structure and organic matter: I. Distribution of aggregate-size classes and aggregate-associated carbon. Soil Sci. Soc. Am. J. 2000, 64, 681–689. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.L.; Cambardella, C.A. The soil management assessment framework: A quantitative soil quality evaluation method. Soil Sci. Soc. Am. J. 2004, 68, 1945–1962. [Google Scholar] [CrossRef]
- Siddique, I.A.; Grados, D.; Chen, J.; Lærke, P.E.; Jørgensen, U. Soil organic carbon stock change following perennialization: A meta-analysis. Agron. Sustain. Dev. 2023, 43, 58. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, Y.; Liu, Y.; Zhang, S.; Yue, X.; Yao, B.; Xue, J.; Lv, W.; Zhang, L.; Xu, X.; et al. Factors shaping soil organic carbon stocks in grass covered orchards across China: A meta-analysis. Sci. Total Environ. 2022, 807, 150632. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Craig, M.E.; Mayes, M.A.; Sulman, B.N.; Walker, A.P. Biological mechanisms may contribute to soil carbon saturation patterns. Glob. Chang. Biol. 2021, 27, 2633–2644. [Google Scholar] [CrossRef]
- Ashagrie, Y.; Zech, W.; Guggenberger, G.; Mamo, T. Soil aggregation, and total and particulate organic matter following conversion of native forests to continuous cultivation in Ethiopia. Soil Tillage Res. 2007, 94, 101–108. [Google Scholar] [CrossRef]
- Sokol, N.W.; Sanderman, J.; Bradford, M.A. Pathways of mineral-associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry. Glob. Chang. Biol. 2019, 25, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Kalbitz, K.; Solinger, S.; Park, J.H.; Michalzik, B.; Matzner, E. Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- McDowell, W.H.; Zsolnay, A.; Aitkenhead-Peterson, J.A.; Gregorich, E.G.; Jones, D.L.; Joedemann, D.; Kalbitz, K.; Marschner, B.; Schwesig, D. A comparison of methods to determine the biodegradable dissolved organic carbon from different terrestrial sources. Soil Biol. Biochem. 2006, 38, 1933–1942. [Google Scholar] [CrossRef]
- Nelson, P.N.; Dictor, M.C.; Soulas, G. Availability of organic carbon in soluble and particle-size fractions from a soil profile. Soil Biol. Biochem. 1994, 26, 1549–1555. [Google Scholar] [CrossRef]
- Zsolnay, A. Dissolved organic matter: Artefacts, definitions, and functions. Geoderma 2003, 113, 187–209. [Google Scholar] [CrossRef]
- Whalen, J.K.; Parmelee, R.; McCartney, D.A.; Vanarsdale, J.L. Movement of N from decomposing earthworm tissue to soil, microbial and plant N pools. Soil Biol. Biochem. 1999, 31, 487–492. [Google Scholar] [CrossRef]
- Bu, X.; Ding, J.; Wang, L.; Yu, X.; Huang, W.; Ruan, H. Biodegradation and chemical characteristics of hot-water extractable organic matter from soils under four different vegetation types in the Wuyi Mountains, southeastern China. Eur. J. Soil Biol. 2011, 47, 102–107. [Google Scholar] [CrossRef]
- Yang, X.; Meng, J.; Lan, Y.; Chen, W.; Yang, T.; Yuan, J.; Liu, S.; Han, J. Effects of maize stover and its biochar on soil CO2 emissions and labile organic carbon fractions in Northeast China. Agric. Ecosyst. Environ. 2017, 240, 24–31. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.Y.H.; Chen, X.; Wang, J.; Chen, B.; Wang, D.; Guan, Q. Soil labile organic carbon and carbon-cycle enzyme activities under different thinning intensities in Chinese fir plantations. Appl. Soil Ecol. 2016, 107, 162–169. [Google Scholar] [CrossRef]
- Biederbeck, V.O.; Janzen, H.H.; Campbell, C.A.; Zentner, R.P. Labile soil organic matter as influenced by cropping practices in an arid environment. Soil Biol. Biochem. 1994, 26, 1647–1656. [Google Scholar] [CrossRef]
- Priess, J.A.; de Koning, G.H.J.; Veldkamp, A. Assessment of interactions between land use change and carbon and nutrient fluxes in Ecuador. Agric. Ecosyst. Environ. 2001, 85, 269–279. [Google Scholar] [CrossRef]
- Näsholm, T.; Ekblad, A.; Nordin, A.; Giesler, R.; Högberg, M.; Högberg, P. Boreal forest plants take up organic nitrogen. Nature 1998, 392, 914–916. [Google Scholar] [CrossRef]


| Source of Variation | SOC | POC | DOC | EOC | ||||
|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | |
| T | 18.347 | *** | 12.897 | *** | 1.603 | NS | 2.9 | ^ |
| D | 153.697 | *** | 73.29 | *** | 11.927 | *** | 104.26 | *** |
| T × D | 12.392 | *** | 4.999 | *** | 1.125 | NS | 3.043 | ** |
| Source of Variation | C/N | TN | pH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | |
| T | 4.132 | * | 3.312 | * | 4.016 | * | 0.186 | NS | 0.635 | NS |
| D | 13.009 | *** | 51.401 | *** | 1.281 | NS | 1.048 | NS | 20.06 | *** |
| T × D | 2.263 | ^ | 3.487 | ** | 0.405 | NS | 0.954 | NS | 0.469 | NS |
| Source of Variation | NAOC | CPA | CPAI | CPI | CMI | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | |
| T | 17.333 | *** | 4.635 | * | 1.826 | NS | 4.594 | * | 0.79 | NS |
| D | 59.889 | *** | 0.479 | NS | 3.629 | * | 5.231 | ** | 2.199 | NS |
| T × D | 11.398 | *** | 2.532 | * | 1.881 | NS | 3.162 | * | 1.227 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Q.; Ma, T.; Wang, X.; Yang, Q.; Lv, L.; Wang, R.; Li, J.; Ma, J. Effects of Different Living Grass Mulching on Soil Carbon and Nitrogen in an Apple Orchard on Loess Plateau. Agronomy 2024, 14, 1917. https://doi.org/10.3390/agronomy14091917
Xiang Q, Ma T, Wang X, Yang Q, Lv L, Wang R, Li J, Ma J. Effects of Different Living Grass Mulching on Soil Carbon and Nitrogen in an Apple Orchard on Loess Plateau. Agronomy. 2024; 14(9):1917. https://doi.org/10.3390/agronomy14091917
Chicago/Turabian StyleXiang, Qian, Tao Ma, Xianzhi Wang, Qian Yang, Long Lv, Ruobing Wang, Jiaxuan Li, and Jingyong Ma. 2024. "Effects of Different Living Grass Mulching on Soil Carbon and Nitrogen in an Apple Orchard on Loess Plateau" Agronomy 14, no. 9: 1917. https://doi.org/10.3390/agronomy14091917




