Preparation, Characterization, and Testing of Compost Tea Derived from Seaweed and Fish Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Marine Residues
2.2. Compost Preparation
2.3. Compost Tea Preparation
2.4. Compost Tea Analysis
2.5. Compost Tea Testing
2.6. Data Processing
3. Results and Discussion
3.1. Compost Tea Characterization
- -
- the mean values of EC, K, Ca, Mg, and Zn obtained in this study were significantly higher than those reported by Zaccardelli et al. [26] for aerated CT prepared from two types of compost derived from agro-industrial residual materials (wood, artichoke, fennel, and escarole residues), whereas the values of pH, Cu, and Mn were similar;
- -
- the mean values of EC, N, P, K, Ca, Mg, and Fe were significantly higher than those found by Samet et al. [13] for aerated CT obtained from compost produced from olive mill wastewater, olive pomace, and coffee grounds, whereas the values of pH were similar;
- -
- compared to aerated CT prepared by Morales-Corts et al. [31] from garden waste-based compost, the mean values of EC, Ca, Mg, Fe, Mn, and Zn obtained in this study were significantly higher, that of Cu was significantly lower, whereas the values of pH were similar;
- -
- -
- the mean values of EC, pH, and N were significantly higher than those found by Jarboui et al. [2] for non-aerated CT prepared from food waste-derived compost;
- -
- the mean values of P and K were significantly higher than those obtained by Xu et al. [4] for non-aerated CTs derived from compost based on pig manure and rice straw, whereas those of N were similar.
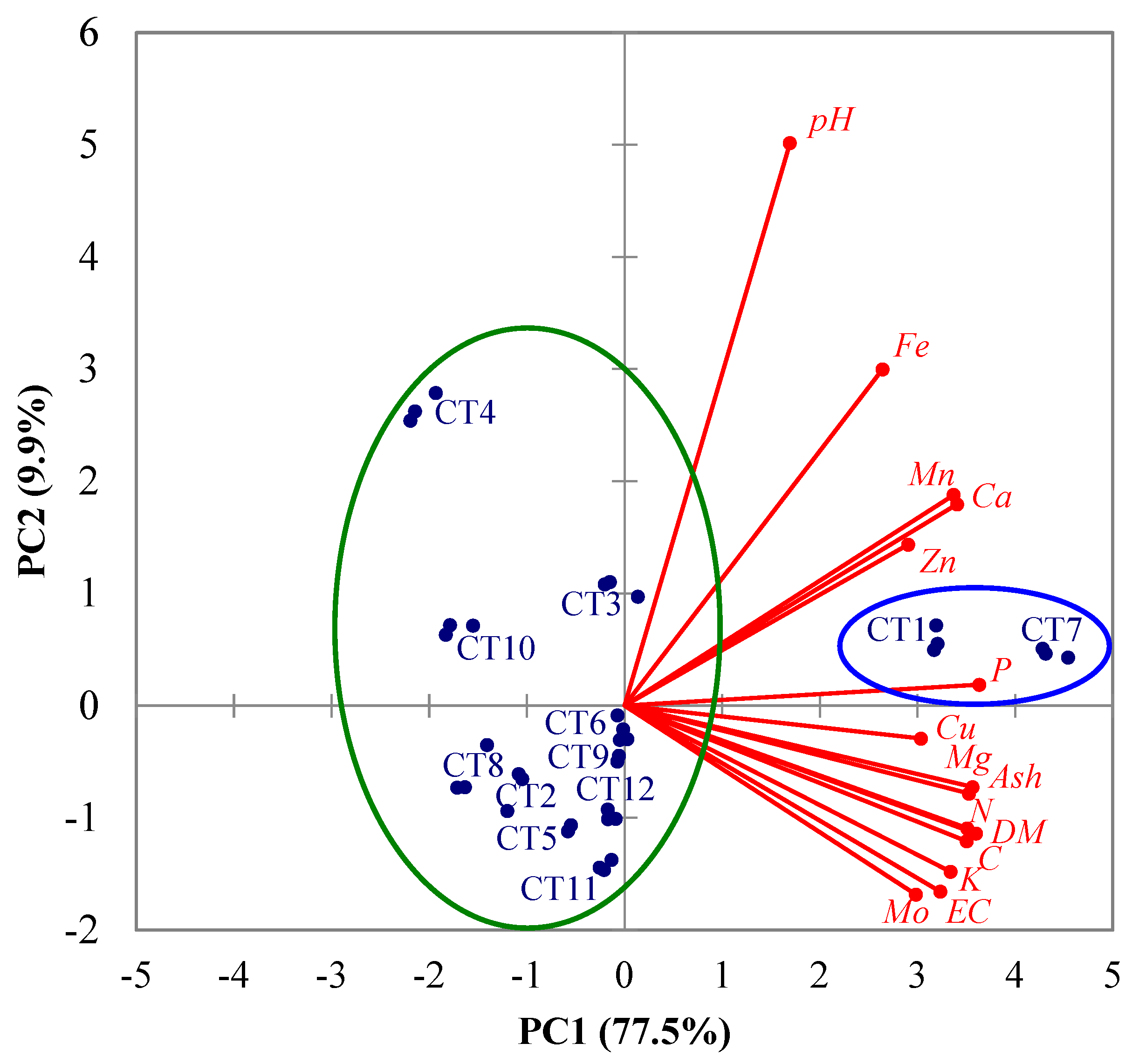
3.2. Results of PCA
- -
- depending on significant levels of factor loadings (highlighted in bold in Table 4), the most important variables are EC, DM, Ash, C, N, P, K, Ca, Mg, Cu, Fe, Mn, Mo, and Zn for PC1 as well as pH for PC2;
- -
- CT1 and CT7 samples obtained in the experimental runs 1 (X1 = −1 and X2 = −1) and 7 (X1 = −1.414 and X2 = 0) have higher values of EC, DM, Ash, C, N, P, K, Ca, Mg, Cu, Fe, Mn, Mo, and Zn than the other samples [discrimination on PC1 between CT1 and CT7 (inside the blue ellipse in Figure 6) and the other samples (inside the green ellipse), especially CT2 (X1 = −1 and X2 = 1), CT4 (X1 = 1 and X2 = 1), CT8 (X1 = 1.414 and X2 = 0), and CT10 (X1 = 0 and X2 = 1.414)];
- -
- CT4 samples obtained in the experimental run 4 (X1 = 1 and X2 = 1) have higher values of pH than CT2, CT3, CT5, CT6, CT8–CT12 samples (discrimination on PC2 between CT4 and the other samples inside the green ellipse in Figure 6);
- -
- EC, DM, Ash, C, N, P, K, Ca, Mg, Cu, Fe, Mn, Mo, and Zn are directly correlated and the corresponding correlation coefficients (0.394 ≤ r ≤ 0.999) are significant at α = 0.05 (Table 5); pH is directly correlated with Ash, P, Ca, Cu, Fe, Mn, and Zn, with the corresponding correlation coefficients (0.358 ≤ r ≤ 0.667) being significant at α = 0.05.
3.3. Prediction of Process Responses
- -
- pHpr and Mnpr do not vary significantly with X1, X12, X2, X22, or X1X2 (0.360 ≤ Rj2 ≤ 0.693, 0.675 ≤ Fj ≤ 2.708, and 0.129 ≤ pj ≤ 0.658 for j = 2, 14);
- -
- ECpr, DMpr, Ashpr, Cpr, Npr, Ppr, Kpr, Capr, Mgpr, Fepr, Mopr, and Znpr vary significantly with at least one of X1, X12, X2, X22, and X1X2, and there is a good agreement between experimental and predicted values of process responses (0.809 ≤ Rj2 ≤ 0.979, 5.080 ≤ Fj ≤ 54.87, and 0.0001 ≤ pj ≤ 0.036 for j = 1, 3, …, 10, 12, 13, 15); ECpr increases significantly with an increase in X12 and a decrease in X1, X2, and X22; DMpr, Cpr, Npr, Ppr, Kpr, and Mgpr increase significantly with a decrease in X1 and X2; Ashpr and Znpr increase significantly with an increase in X12 and a decrease in X1 and X2; Capr increases significantly with an increase in X12 and a decrease in X1; Fepr increases significantly with an increase in X1X2; Mopr increases significantly with a decrease in X1, X2, and X22;
- -
- Cupr increases significantly with a decrease in X2, but the statistical model defined by Equation (7) for j = 11 is statistically non-significant (F = 2.326 and p = 0.167).
3.4. Optimization of Fermentation Process Conditions
3.5. Validation of Statistical Models
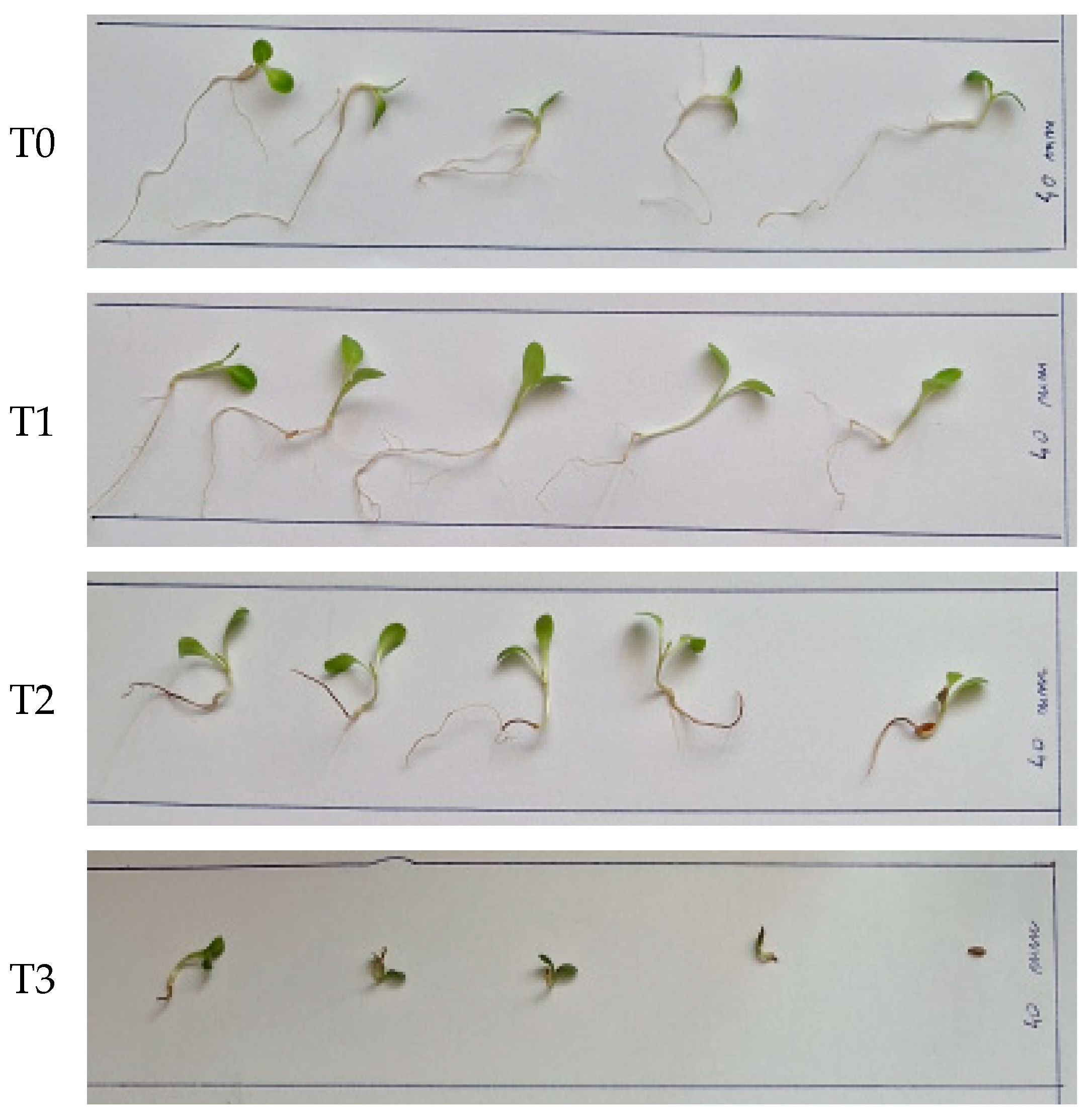
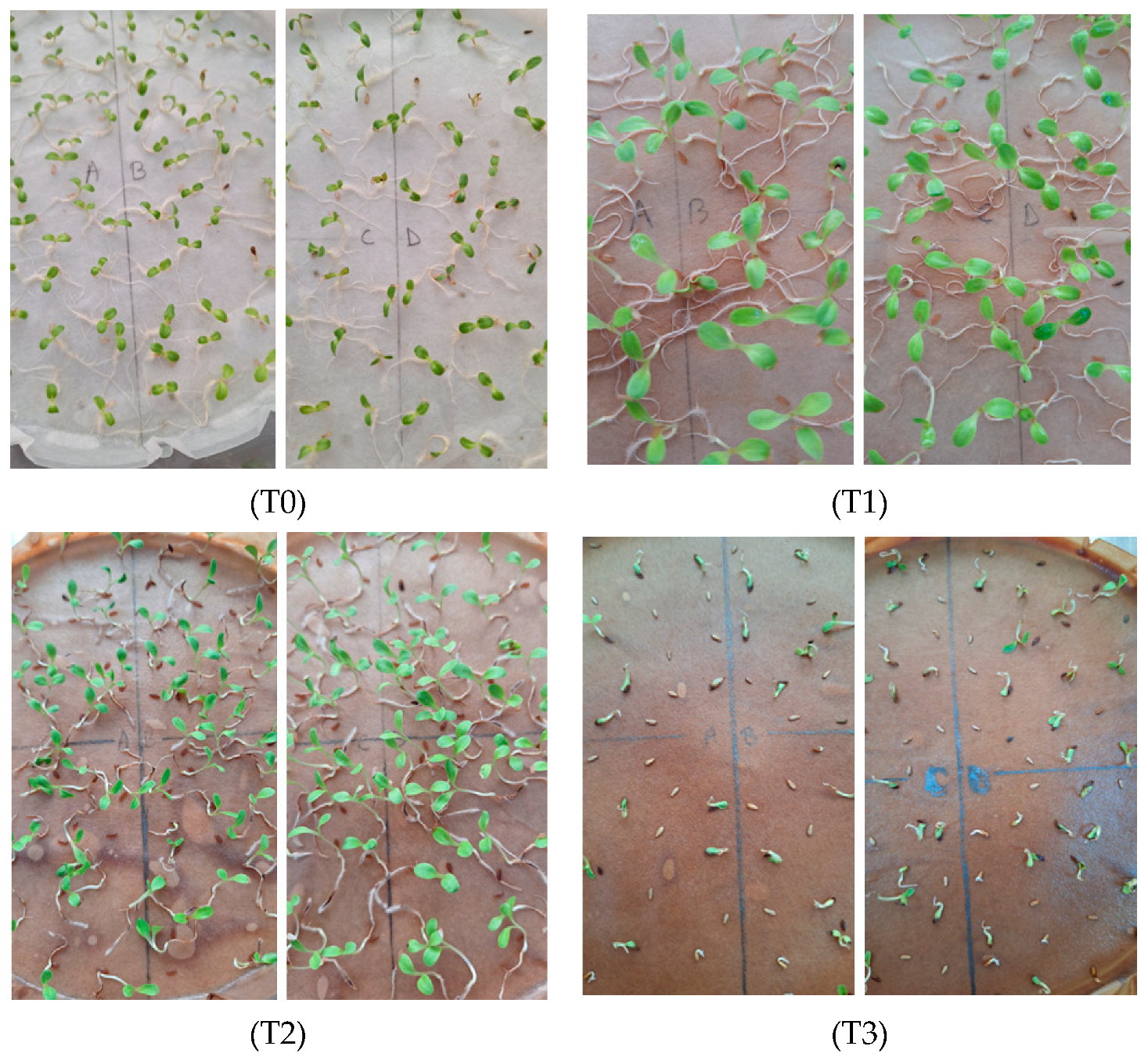
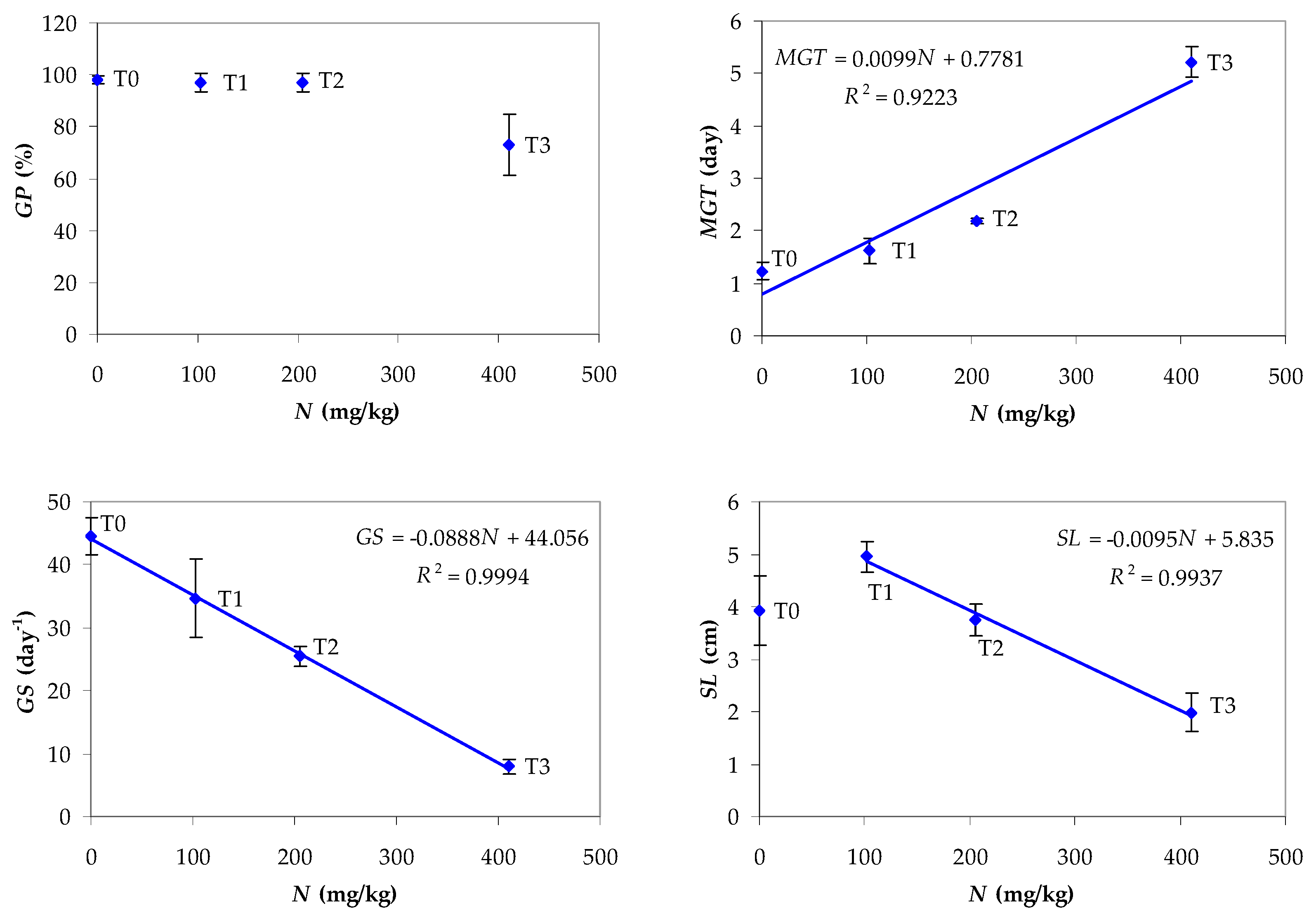
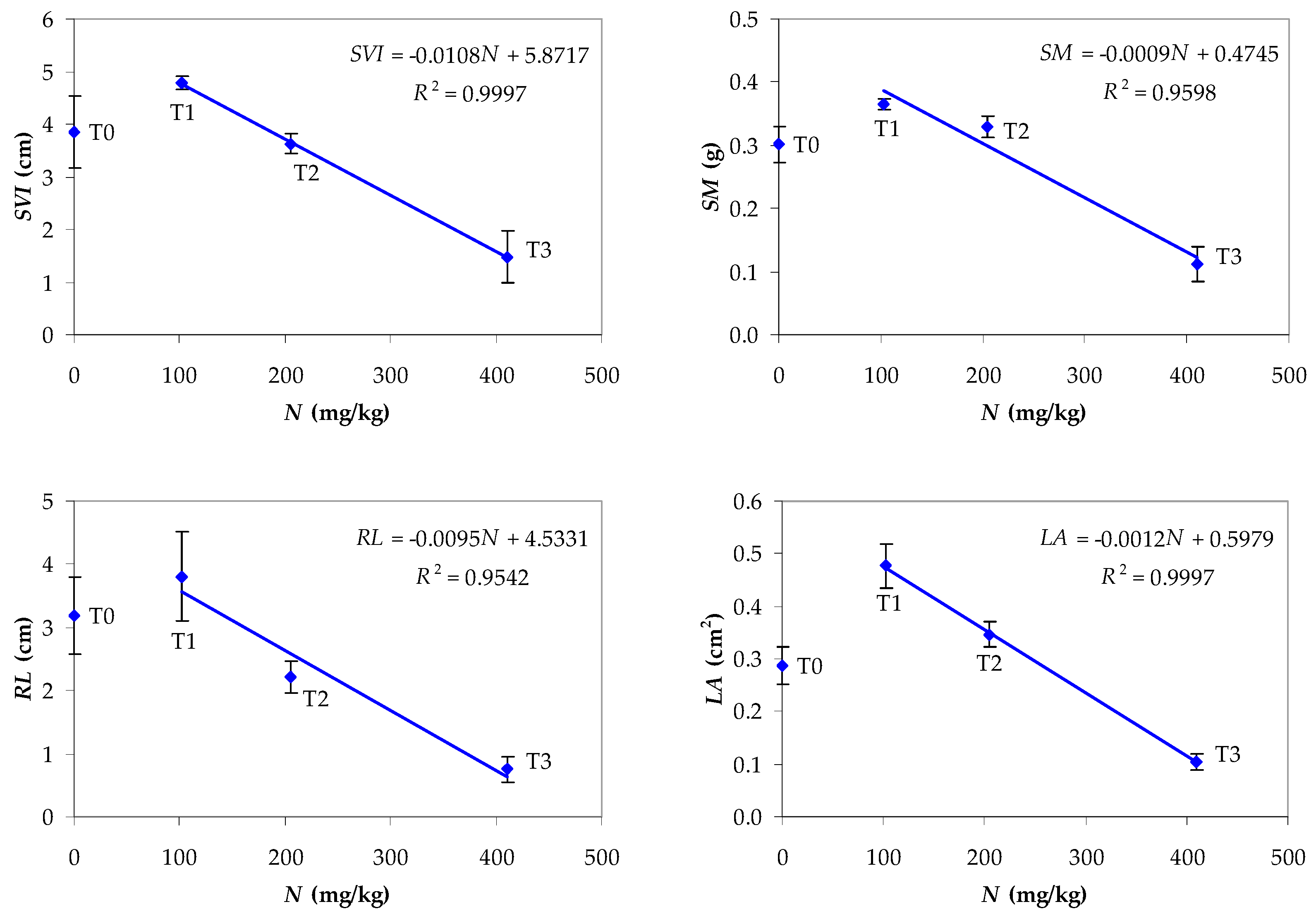
3.6. Testing Compost Tea for Lettuce Seed Germination and Seedling Growth
- -
- the value of GP for N = 410.0 mg/kg (treatment T3 with undiluted CT), i.e., 73%, is significantly lower than the values obtained by applying the other treatments (N = 0–205.0 mg/kg), which are almost equal (GP = 97–98%);
- -
- MGT increases linearly with N (R2 = 0.9223) and GS decreases linearly with N (R2 = 0.9994) for values of N ranging from 0 (treatment T0) to 410.0 mg/kg (treatment T3);
- -
- SL, SVI, SM, RL, and LA decrease linearly with N (R2 = 0.9542–0.9997) for values of N ranging from 102.5 (treatment T1) to 410.0 mg/kg (treatment T3).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ingham, E.R. The Compost Tea Brewing Manual, 5th ed.; Soil Foodweb Incorporated: Corvallis, OR, USA, 2005. [Google Scholar]
- Jarboui, R.; Dhouib, B.; Ammar, E. Effect of food waste compost (FWC) and its non-aerated fermented extract (NFCE) on seeds germination and plant growth. Open J. Soil Sci. 2021, 11, 122–138. [Google Scholar] [CrossRef]
- Pant, A.P.; Radovich, T.J.K.; Hue, N.V.; Paull, R.E. Biochemical properties of compost tea associated with compost quality and effects on pak choi growth. Sci. Hortic. 2012, 148, 138–146. [Google Scholar] [CrossRef]
- Xu, D.B.; Wang, Q.J.; Wu, Y.C.; Yu, G.H.; Shen, Q.R.; Huang, Q.W. Humic-like substances from different compost extracts could significantly promote cucumber growth. Pedosphere 2012, 22, 815–824. [Google Scholar] [CrossRef]
- Litterick, A.M.; Harrier, L.; Wallace, P.; Watson, C.A.; Wood, M. The role of uncomposted materials, composts, manures, and compost extracts in reducing pest and disease incidence and severity in sustainable temperate agricultural and horticultural crop production—A review. Crit. Rev. Plant Sci. 2004, 23, 453–479. [Google Scholar] [CrossRef]
- Pant, A.P.; Radovich, T.J.K.; Hue, N.V.; Talcott, S.T.; Krenek, K.A. Vermicompost extracts influence growth, mineral nutrients, phytonutrients and antioxidant activity in pak choi (Brassica rapa cv. Bonsai, Chinensis group) grown under vermicompost and chemical fertiliser. J. Sci. Food Agric. 2009, 89, 2383–2392. [Google Scholar] [CrossRef]
- Pilla, N.; Tranchida-Lombardo, V.; Gabrielli, P.; Aguzzi, A.; Caputo, M.; Lucarini, M.; Durazzo, A.; Zaccardelli, M. Effect of compost tea in horticulture. Horticulturae 2023, 9, 984. [Google Scholar] [CrossRef]
- Scheuerell, S.; Mahaffee, W. Compost tea: Principles and prospects for plant disease control. Compost Sci. Util. 2002, 10, 313–338. [Google Scholar] [CrossRef]
- Scheuerell, S.J.; Mahaffee, W.F. Variability associated with suppression of gray mold (Botrytis cinerea) on geranium by foliar applications of nonaerated and aerated compost teas. Plant Dis. 2006, 90, 1201–1208. [Google Scholar] [CrossRef]
- St. Martin, C.C.G.; Brathwaite, R.A.I. Compost and compost tea: Principles and prospects as substrates and soil-borne disease management strategies in soil-less vegetable production. Biol. Agric. Hortic. 2012, 28, 1–33. [Google Scholar] [CrossRef]
- Pane, C.; Palese, A.M.; Celano, G.; Zaccardelli, M. Effects of compost tea treatments on productivity of lettuce and kohlrabi systems under organic cropping management. Ital. J. Agron. 2014, 9, 153–156. [Google Scholar] [CrossRef]
- Pane, C.; Palese, A.M.; Spaccini, R.; Piccolo, A.; Celano, G.; Zaccardelli, M. Enhancing sustainability of a processing tomato cultivation system by using bioactive compost teas. Sci. Hortic. 2016, 202, 117–124. [Google Scholar] [CrossRef]
- Samet, M.; Charfeddine, M.; Kamoun, L.; Nouri-Ellouze, O.; Gargouri-Bouzid, R. Effect of compost tea containing phosphogypsum on potato plant growth and protection against Fusarium solani infection. Environ. Sci. Pollut. Res. 2018, 25, 18921–18937. [Google Scholar] [CrossRef] [PubMed]
- Weltzien, H.C. Some effects of composted organic materials on plant health. Agric. Ecosyst. Environ. 1989, 27, 439–446. [Google Scholar] [CrossRef]
- Marín, F.; Diánez, F.; Santos, M.; Carretero, F.; Gea, F.J.; Castañeda, C.; Navarro, M.J.; Yau, J.A. Control of Phytophthora capsici and Phytophthora parasitica on pepper (Capsicum annuum L.) with compost teas from different sources, and their effects on plant growth promotion. Phytopathol. Mediterr. 2014, 53, 216–228. [Google Scholar]
- Meshref, H.A.; Rabie, M.H.; El-Ghamry, A.M.; El-Agamy, M.A. Maximizing utilization of compost addition using foliar compost extract and humic substances in alluvial soil. J. Soil Sci. Agric. Eng. 2010, 1, 957–971. [Google Scholar] [CrossRef]
- Ali, O.A.; El-Tahlawy, Y.A.; Abdel-Gwad, S.A. Impact of compost tea types application on germination, nodulation, morphological characters and yield of two lentil cultivars. Egypt. J. Agron. 2018, 40, 1–19. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Edwards, C.A.; Dick, R.; Dick, L. Vermicompost tea production and plant growth impacts. Biocycle 2007, 48, 51–52. [Google Scholar]
- Naidu, Y.; Meon, S.; Kadir, J.; Siddiqui, Y. Microbial starter for the enhancement of biological activity of compost tea. Int. J. Agric. Biol. 2010, 12, 51–56. [Google Scholar]
- Ros, M.; Hurtado-Navarro, M.; Giménez, A.; Fernández, J.A.; Egea-Gilabert, C.; Lozano-Pastor, P.; Pascual, J.A. Spraying agro-industrial compost tea on baby spinach crops: Evaluation of yield, plant quality and soil health in field experiments. Agronomy 2020, 10, 440. [Google Scholar] [CrossRef]
- Hegazi, A.Z.; Algharib, A.M. Utilizing compost tea as a nutrient amendment in open filed cowpea seed production system. J. Biodivers. Environ. Sci. 2014, 5, 318–328. [Google Scholar]
- Arancon, N.Q.; Pant, A.; Radovich, T.; Hue, N.V.; Potter, J.K.; Converse, C.E. Seed germination and seedling growth of tomato and lettuce as affected by vermicompost water extracts (teas). HortScience 2012, 47, 1722–1728. [Google Scholar] [CrossRef]
- Kim, M.J.; Shim, C.K.; Kim, Y.K.; Hong, S.J.; Park, J.H.; Han, E.J.; Kim, J.H.; Kim, S.C. Effect of aerated compost tea on the growth promotion of lettuce, soybean, and sweet corn in organic cultivation. Plant Pathol. J. 2015, 31, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Y.; Meon, S.; Ismail, R.; Rahmani, M.; Ali, A. Bio-efficiency of compost extracts on the wet rot incidence, morphological and physiological growth of okra (Abelmoschus esculentus [(L.) Moench]). Sci. Hortic. 2008, 117, 9–14. [Google Scholar] [CrossRef]
- González-Hernández, A.I.; Suárez-Fernández, M.B.; Pérez-Sánchez, R.; Gómez-Sánchez, M.Á.; Morales-Corts, M.R. Compost tea induces growth and resistance against Rhizoctonia solani and Phytophthora capsici in pepper. Agronomy 2021, 11, 781. [Google Scholar] [CrossRef]
- Zaccardelli, M.; Pane, C.; Villecco, D.; Palese, A.M.; Celano, G. Compost tea spraying increases yield performance of pepper (Capsicum annuum L.) grown in greenhouse under organic farming system. Ital. J. Agron. 2018, 13, 229–234. [Google Scholar] [CrossRef]
- González-Hernández, A.I.; Pérez-Sánchez, R.; Plaza, J.; Morales-Corts, M.R. Compost tea as a sustainable alternative to promote plant growth and resistance against Rhizoctonia solani in potato plants. Sci. Hortic. 2022, 300, 111090. [Google Scholar] [CrossRef]
- López-Martín, J.J.; Morales-Corts, M.R.; Pérez-Sánchez, R.; Gómez-Sánchez, M.Á. Efficiency of garden waste compost teas on potato growth and its suppressiveness against Rhizoctonia. Agric. For. 2018, 64, 7. [Google Scholar]
- Edwards, C.A.; Arancon, N.Q.; Greytak, S. Effects of vermicompost teas on plant growth and disease. Biocycle 2006, 47, 28–29. [Google Scholar]
- González-Hernández, A.I.; Gómez-Sánchez, M.Á.; Pérez-Sánchez, R.; Morales-Corts, M.R. Garden waste compost tea: A horticultural alternative to promote plant growth and root traits in tomato (Solanum lycopersicum L.) plants. Horticulturae 2023, 9, 1127. [Google Scholar] [CrossRef]
- Morales-Corts, M.R.; Pérez-Sánchez, R.; Gómez-Sánchez, M.A. Efficiency of garden waste compost teas on tomato growth and its suppressiveness against soilborne pathogens. Sci. Agric. 2018, 75, 400–409. [Google Scholar] [CrossRef]
- Dionne, A.; Tweddell, R.J.; Antoun, H.; Avis, T.J. Effect of non-aerated compost teas on damping-off pathogens of tomat. Can. J. Plant Pathol. 2012, 34, 51–57. [Google Scholar] [CrossRef]
- Ramírez-Gottfried, R.I.; Preciado-Rangel, P.; Carrillo, M.G.; García, A.B.; González-Rodríguez, G.; Espinosa-Palomeque, B. Compost tea as organic fertilizer and plant disease control: Bibliometric analysis. Agronomy 2023, 13, 2340. [Google Scholar] [CrossRef]
- Ahuja, I.; Dauksas, E.; Remme, J.F.; Richardsen, R.; Løes, A.K. Fish and fish waste-based fertilizers in organic farming—With status in Norway: A review. Waste Manag. 2020, 115, 95–112. [Google Scholar] [CrossRef]
- Illera-Vives, M.; Labandeira, S.S.; Brito, L.M.; López-Fabal, A.; López-Mosquera, M.E. Evaluation of compost from seaweed and fish waste as a fertilizer for horticultural use. Sci. Hortic. 2015, 186, 101–107. [Google Scholar] [CrossRef]
- Illera-Vives, M.; Seoane Labandeira, S.; Iglesias Loureiro, L.; López-Mosquera, M.E. Agronomic assessment of a compost consisting of seaweed and fish waste as an organic fertilizer for organic potato crops. J. Appl. Phycol. 2017, 29, 1663–1671. [Google Scholar] [CrossRef]
- Moloșag, A.; Pârvulescu, O.C.; Ion, V.A.; Asănică, A.C.; Soane, R.; Moț, A.; Dobrin, A.; Frîncu, M.; Løes, A.-K.; Cabell, J.; et al. Effects of marine residue-derived fertilizers on strawberry growth, nutrient content, fruit yield and quality. Agronomy 2023, 13, 1221. [Google Scholar] [CrossRef]
- Cabell, J.; Løes, A.-K. Blue Biomass Composting Technology; Report, No. 4; Norwegian Centre for Organic Agriculture (NORSØK): Tingvoll, Norway, 2023; Volume 8, Available online: https://orgprints.org/id/eprint/50477/ (accessed on 8 July 2024).
- Løes, A.-K.; Ahuja, I.; de Boer, A.; Rittl, T. Fertilisation Effects of Marine-Derived Residual Materials on Agricultural Crops; Report, No. 13; Norwegian Centre for Organic Agriculture (NORSØK): Tingvoll, Norway, 2022; Volume 7, Available online: https://orgprints.org/id/eprint/45330/ (accessed on 8 July 2024).
- Dima, A.D.; Pârvulescu, O.C.; Mateescu, C.; Dobre, T. Optimization of substrate composition in anaerobic co-digestion of agricultural waste using central composite design. Biomass Bioenerg. 2020, 138, 105602. [Google Scholar] [CrossRef]
- Calcan, S.I.; Pârvulescu, O.C.; Ion, V.A.; Răducanu, C.E.; Bădulescu, L.; Dobre, T.; Egri, D.; Moț, A.; Popa, V.; Crăciun, M.E. Valorization of vine prunings by slow pyrolysis in a fixed-bed reactor. Processes 2022, 10, 37. [Google Scholar] [CrossRef]
- Calcan, S.I.; Pârvulescu, O.C.; Ion, V.A.; Răducanu, C.E.; Bădulescu, L.; Madjar, R.; Dobre, T.; Egri, D.; Moț, A.; Iliescu, L.M.; et al. Effects of biochar on soil properties and tomato growth. Agronomy 2022, 12, 1824. [Google Scholar] [CrossRef]
- Egri, D.; Pârvulescu, O.C.; Ion, V.A.; Răducanu, C.E.; Calcan, S.I.; Bădulescu, L.; Madjar, R.; Orbeci, C.; Dobre, T.; Moț, A.; et al. Vine pruning-derived biochar for agronomic benefits. Agronomy 2022, 12, 2730. [Google Scholar] [CrossRef]
- Sharples, G.C.; Kuehl, R.O. Lettuce seed weight and size in relation to germination and seedling radicle growth. HortScience 1974, 9, 582–584. [Google Scholar] [CrossRef]
- Kader, M.A. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. R. Soc. N. S. W. 2005, 138, 65–75. [Google Scholar] [CrossRef]
- Maguire, J.D. Speed of germination—Aid in selection and evaluation for seedling emergence and vigor. Crop. Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Šerá, B. Methodological contribution on seed germination and seedling initial growth tests in wild plants. Not. Bot. Horti Agrobot. Cluj Napoca 2023, 51, 13164. [Google Scholar] [CrossRef]
- Pasztor, R.; Bala, M.; Sala, F. Flowers quality in relation to planting period in some Hyacinth cultivars. AgroLife Sci. J. 2020, 9, 263–272. [Google Scholar]
- Drăghici-Popa, A.M.; Boscornea, A.C.; Brezoiu, A.M.; Tomas, Ș.T.; Pârvulescu, O.C.; Stan, R. Effects of extraction process factors on the composition and antioxidant activity of blackthorn (Prunus spinosa L.) fruit extracts. Antioxidants 2023, 12, 1897. [Google Scholar] [CrossRef] [PubMed]
- Nasri, N.; Kaddour, R.; Rabhi, M.; Plassard, C.; Lachaal, M. Effect of salinity on germination, phytase activity and phytate content in lettuce seedling. Acta Physiol. Plant. 2011, 33, 935–942. [Google Scholar] [CrossRef]
- Uçarlı, C. Effects of salinity on seed germination and early seedling stage. Abiotic Stress Plants 2020, 211, 105772. [Google Scholar]
- Rosas, J.T.F.; Junior, E.M.; Lorenzoni, R.M.; dos Santos, F.F.L.; Martins, R.N. Effect of salinity on germination of lettuce cultivars produced in Brazil. J. Exp. Agric. Int. 2019, 34, 1–8. [Google Scholar] [CrossRef]
- Ünlükara, A.; Cemek, B.; Karaman, S.; Erşahin, S. Response of lettuce (Lactuca sativa var. crispa) to salinity of irrigation water. N. Z. J. Crop Hortic. Sci. 2008, 36, 265–273. [Google Scholar] [CrossRef]
- Ünlükara, A.; Kurunç, A.; Kesmez, G.D.; Yurtseven, E. Growth and evapotranspiration of okra (Abelmoschus esculentus L.) as influenced by salinity of irrigation water. J. Irrig. Drain. Eng. 2008, 134, 160–166. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rashidi, B.; Behrozi, D. Effect of different treatments on dormancy-breaking and germination of perennial pepperweed (Lepidium latifolium) (Brassicaceae). Aust. J. Agric. Eng. 2011, 2, 50–55. [Google Scholar]
- Martínez-Ballesta, M.D.C.; Egea-Gilabert, C.; Conesa, E.; Ochoa, J.; Vicente, M.J.; Franco, J.A.; Bañon, S.; Martínez, J.J.; Fernández, J.A. The importance of ion homeostasis and nutrient status in seed development and germination. Agronomy 2020, 10, 504. [Google Scholar] [CrossRef]
- Wen, D.; Xu, H.; Xie, L.; He, H.; Hou, H.; Zhang, C. A loose endosperm structure of wheat seed produced under low nitrogen level promotes early germination by accelerating water uptake. Sci. Rep. 2017, 7, 3116. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Veneklaas, E.J. Nature and nurture: The importance of seed phosphorus content. Plant Soil 2012, 357, 1–8. [Google Scholar] [CrossRef]
- Sathiyavani, E.; Prabaharan, N.K.; Surendar, K.K. Role of mineral nutrition on root growth of crop plants–A review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2810–2837. [Google Scholar]
- Xu, X.; Du, X.; Wang, F.; Sha, J.; Chen, Q.; Tian, G.; Zhu, Z.; Ge, S.; Jiang, Y. Effects of potassium levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Front. Plant Sci. 2020, 11, 904. [Google Scholar] [CrossRef]
- Djidonou, D.; Leskovar, D.I. Seasonal changes in growth, nitrogen nutrition, and yield of hydroponic lettuce. HortScience 2019, 54, 76–85. [Google Scholar] [CrossRef]
- Niu, F.; Zhang, D.; Li, Z.; Van Iersel, M.W.; Alem, P. Morphological response of eucalypts seedlings to phosphorus supply through hydroponic system. Sci. Hortic. 2015, 194, 295–303. [Google Scholar] [CrossRef]
- Yap, M.Y.; Lim, M.W.; Brendan, L.K.W. Influence of calcium macronutrient on tip burn occurrence in hydroponically cultivated lettuces. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1257, 012003. [Google Scholar] [CrossRef]
- Jones, J.B., Jr. Hydroponics: A Practical Guide for the Soilless Grower, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Rouphael, Y.; Colla, G.; Bernardo, L.; Kane, D.; Trevisan, M.; Lucini, L. Zinc excess triggered polyamines accumulation in lettuce root metabolome, as compared to osmotic stress under high salinity. Front. Plant Sci. 2016, 7, 842. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Garg, N. Zinc toxicity in plants: A review. Planta 2021, 253, 129. [Google Scholar] [CrossRef] [PubMed]




| Run | RLS (g/g) | t (Days) | X1 | X2 |
|---|---|---|---|---|
| 1 | 5 | 5 | −1 | −1 |
| 2 | 5 | 9 | −1 | 1 |
| 3 | 9 | 5 | 1 | −1 |
| 4 | 9 | 9 | 1 | 1 |
| 5 | 7 | 7 | 0 | 0 |
| 6 | 7 | 7 | 0 | 0 |
| 7 | 4.2 | 7 | −1.414 | 0 |
| 8 | 9.8 | 7 | 1.414 | 0 |
| 9 | 7 | 4.2 | 0 | −1.414 |
| 10 | 7 | 9.8 | 0 | 1.414 |
| 11 | 7 | 7 | 0 | 0 |
| 12 | 7 | 7 | 0 | 0 |
| Variable | Indicator | ||||
|---|---|---|---|---|---|
| Symbol | Units | Minimum Value (MIN) | Maximum Value (MAX) | Mean Value (m) | Standard Deviation (SD) |
| EC | dS/m | 7.386 | 18.08 | 11.74 | 2.781 |
| pH | - | 7.777 | 8.295 | 7.996 | 0.163 |
| DM | % | 0.721 | 2.686 | 1.514 | 0.517 |
| Ash | % | 0.547 | 1.751 | 0.973 | 0.314 |
| C | % | 0.090 | 0.594 | 0.301 | 0.138 |
| N | % | 0.007 | 0.041 | 0.021 | 0.009 |
| C/N | kg/kg | 13.58 | 16.94 | 14.57 | 0.552 |
| P | % | 0.016 | 0.062 | 0.030 | 0.013 |
| K | % | 0.226 | 0.700 | 0.406 | 0.113 |
| Ca | mg/kg | 70.53 | 805.1 | 264.1 | 237.9 |
| Mg | mg/kg | 37.41 | 258.6 | 110.8 | 56.05 |
| Cu | mg/kg | 0.037 | 0.303 | 0.133 | 0.063 |
| Fe | mg/kg | 9.833 | 25.33 | 16.21 | 4.523 |
| Mn | mg/kg | 0.098 | 0.696 | 0.280 | 0.199 |
| Mo | mg/kg | 0.007 | 0.056 | 0.036 | 0.013 |
| Zn | mg/kg | 0.335 | 2.895 | 1.117 | 0.640 |
| Variable | Zaccardelli et al. [26] | Samet et al. [13] | Morales-Corts et al. [31] | González-Hernández et al. [25,27,30] | Jarboui et al. [2] | Xu et al. [4] | This Study |
|---|---|---|---|---|---|---|---|
| EC (dS/m) | 4.778 ± 0.500 | 7.61 | 2.6 ± 0.1 | 1.2 ± 0.1 | 3.12 ± 0.01 | - | 11.74 ± 2.78 |
| pH | 7.60 ± 0.16 | 8.00 | 7.81 ± 0.15 | 7.16 ± 0.15 | 6.93 ± 0.12 | - | 7.80 ± 0.16 |
| N (%) | - | 0.002 ± 0.000 | - | - | 0.006 ± 0.000 | 0.032 | 0.021 ± 0.009 |
| C/N | - | - | - | 7.1 ± 0.2 | - | - | 14.57 ± 0.55 |
| P (mg/kg) | - | 2.170 ± 0.254 | - | - | - | 239 | 301.5 ± 125.2 |
| K (%) | 0.142 ± 0.023 | 0.188 ± 0.018 | - | - | - | 0.108 | 0.406 ± 0.113 |
| Ca (mg/kg) | 21.8 ± 1.9 | 164.5 ± 34.3 | 50 ± 23 | 280 ± 17 | - | - | 264.1 ± 237.9 |
| Mg (mg/kg) | 37.8 ± 3.1 | 87.35 ± 15.59 | 27.5 ± 16.0 | 20 ± 14 | - | - | 110.8 ± 56.1 |
| Cu (mg/kg) | 0.16 ± 0.02 | - | 0.308 ± 0.047 | - | - | - | 0.133 ± 0.063 |
| Fe (mg/kg) | - | 3.746 ± 0.385 | 9.8 ± 2.1 | - | - | - | 16.21 ± 4.52 |
| Mn (mg/kg) | 0.45 ± 0.01 | - | 0.059 ± 0.019 | - | - | - | 0.280 ± 0.199 |
| Zn (mg/kg) | 0.15 ± 0.01 | - | 0.266 ± 0.025 | - | - | - | 1.117 ± 0.640 |
| Compost feedstock | Agro- industrial residues | Olive residues and coffee grounds | Garden waste | Garden waste | Fruit and vegetable waste | Pig manure and rice straw | Rockweed and fish residues |
| Fermentation conditions | t = 7 days with aeration | RLS = 5 g/g t = 7 days with aeration | 20 °C RLS = 5 L/L t = 14 days with aeration | 20 °C RLS = 5 L/L t = 5 days with aeration | 25 °C RLS = 8 g/g t = 7 days without aeration | 20–25 °C RLS = 8 g/g t = 7 days without aeration | 20 °C RLS = 4.2–9.8 g/g t = 4.2–9.8 days without aeration |
| No. | Variable | Principal Component | ||
|---|---|---|---|---|
| Name | Symbol | PC1 | PC2 | |
| 1 | Electrical conductivity | EC | 0.88 | −0.27 |
| 2 | pH | pH | 0.46 | 0.82 |
| 3 | Dry matter concentration | DM | 0.98 | −0.19 |
| 4 | Ash concentration | Ash | 0.96 | −0.13 |
| 5 | Carbon concentration | C | 0.95 | −0.20 |
| 6 | Nitrogen concentration | N | 0.96 | −0.18 |
| 7 | Phosphorus concentration | P | 0.99 | 0.03 |
| 8 | Potassium concentration | K | 0.91 | −0.24 |
| 9 | Calcium concentration | Ca | 0.93 | 0.29 |
| 10 | Magnesium concentration | Mg | 0.97 | −0.12 |
| 11 | Copper concentration | Cu | 0.83 | −0.05 |
| 12 | Iron concentration | Fe | 0.72 | 0.49 |
| 13 | Manganese concentration | Mn | 0.92 | 0.31 |
| 14 | Molybdenum concentration | Mo | 0.81 | −0.27 |
| 15 | Zinc concentration | Zn | 0.79 | 0.23 |
| Variable | EC | pH | DM | Ash | C | N | P | K | Ca | Mg | Cu | Fe | Mn | Mo | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC | 1.000 | 0.230 | 0.930 | 0.970 | 0.821 | 0.812 | 0.895 | 0.989 | 0.749 | 0.862 | 0.626 | 0.504 | 0.682 | 0.680 | 0.598 |
| pH | 0.230 | 1.000 | 0.306 | 0.358 | 0.271 | 0.278 | 0.478 | 0.269 | 0.618 | 0.324 | 0.394 | 0.667 | 0.651 | 0.182 | 0.469 |
| DM | 0.930 | 0.306 | 1.000 | 0.975 | 0.970 | 0.967 | 0.970 | 0.949 | 0.853 | 0.977 | 0.797 | 0.626 | 0.835 | 0.822 | 0.708 |
| Ash | 0.970 | 0.358 | 0.975 | 1.000 | 0.898 | 0.893 | 0.960 | 0.972 | 0.866 | 0.931 | 0.718 | 0.630 | 0.820 | 0.754 | 0.719 |
| C | 0.821 | 0.271 | 0.970 | 0.898 | 1.000 | 0.999 | 0.925 | 0.857 | 0.807 | 0.975 | 0.846 | 0.619 | 0.825 | 0.864 | 0.673 |
| N | 0.812 | 0.278 | 0.967 | 0.893 | 0.999 | 1.000 | 0.928 | 0.849 | 0.816 | 0.975 | 0.844 | 0.634 | 0.832 | 0.859 | 0.684 |
| P | 0.895 | 0.478 | 0.970 | 0.960 | 0.925 | 0.928 | 1.000 | 0.917 | 0.937 | 0.960 | 0.783 | 0.731 | 0.914 | 0.745 | 0.769 |
| K | 0.989 | 0.269 | 0.949 | 0.972 | 0.857 | 0.849 | 0.917 | 1.000 | 0.771 | 0.898 | 0.689 | 0.542 | 0.718 | 0.705 | 0.621 |
| Ca | 0.749 | 0.618 | 0.853 | 0.866 | 0.807 | 0.816 | 0.937 | 0.771 | 1.000 | 0.860 | 0.700 | 0.775 | 0.971 | 0.640 | 0.860 |
| Mg | 0.862 | 0.324 | 0.977 | 0.931 | 0.975 | 0.975 | 0.960 | 0.898 | 0.860 | 1.000 | 0.808 | 0.704 | 0.841 | 0.777 | 0.696 |
| Cu | 0.626 | 0.394 | 0.797 | 0.718 | 0.846 | 0.844 | 0.783 | 0.689 | 0.700 | 0.808 | 1.000 | 0.495 | 0.792 | 0.740 | 0.562 |
| Fe | 0.504 | 0.667 | 0.626 | 0.630 | 0.619 | 0.634 | 0.731 | 0.542 | 0.775 | 0.704 | 0.495 | 1.000 | 0.729 | 0.408 | 0.568 |
| Mn | 0.682 | 0.651 | 0.835 | 0.820 | 0.825 | 0.832 | 0.914 | 0.718 | 0.971 | 0.841 | 0.792 | 0.729 | 1.000 | 0.675 | 0.836 |
| Mo | 0.680 | 0.182 | 0.822 | 0.754 | 0.864 | 0.859 | 0.745 | 0.705 | 0.640 | 0.777 | 0.740 | 0.408 | 0.675 | 1.000 | 0.665 |
| Zn | 0.598 | 0.469 | 0.708 | 0.719 | 0.673 | 0.684 | 0.769 | 0.621 | 0.860 | 0.696 | 0.562 | 0.568 | 0.836 | 0.665 | 1.000 |
| Run | X1 | X2 | Yj,m | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| j = 1 | j = 2 | j = 3 | j = 4 | j = 5 | j = 6 | j = 7 | j = 8 | |||
| ECm (dS/m) | pHm | DMm (%) | Ashm (%) | Cm (%) | Nm (%) | Pm (%) | Km (%) | |||
| 1 | −1 | −1 | 15.16 | 8.078 | 2.312 | 1.460 | 0.502 | 0.035 | 0.052 | 0.532 |
| 2 | −1 | 1 | 13.32 | 7.920 | 1.281 | 1.001 | 0.153 | 0.010 | 0.024 | 0.443 |
| 3 | 1 | −1 | 9.380 | 8.164 | 1.340 | 0.812 | 0.289 | 0.020 | 0.028 | 0.323 |
| 4 | 1 | 1 | 7.412 | 8.225 | 0.725 | 0.553 | 0.093 | 0.007 | 0.017 | 0.233 |
| 5 | 0 | 0 | 11.75 | 7.815 | 1.471 | 0.932 | 0.296 | 0.020 | 0.027 | 0.388 |
| 6 | 0 | 0 | 11.65 | 8.017 | 1.564 | 0.976 | 0.328 | 0.022 | 0.030 | 0.393 |
| 7 | −1.414 | 0 | 18.01 | 8.285 | 2.680 | 1.715 | 0.589 | 0.040 | 0.059 | 0.684 |
| 8 | 1.414 | 0 | 8.615 | 7.785 | 1.085 | 0.675 | 0.229 | 0.016 | 0.016 | 0.296 |
| 9 | 0 | −1.414 | 11.65 | 7.998 | 1.540 | 0.921 | 0.330 | 0.023 | 0.030 | 0.413 |
| 10 | 0 | 1.414 | 10.12 | 7.980 | 1.047 | 0.729 | 0.147 | 0.010 | 0.023 | 0.338 |
| 11 | 0 | 0 | 11.90 | 7.783 | 1.570 | 0.925 | 0.336 | 0.023 | 0.029 | 0.421 |
| 12 | 0 | 0 | 11.93 | 7.897 | 1.558 | 0.972 | 0.325 | 0.022 | 0.028 | 0.412 |
| a0j | 11.81 | 7.878 | 1.541 | 0.951 | 0.321 | 0.022 | 0.028 | 0.403 | ||
| a1j | −3.121 | −0.040 | −0.473 | −0.321 | −0.100 | −0.007 | −0.011 | −0.121 | ||
| a11j | 0.556 | 0.100 | 0.128 | 0.108 | 0.028 | 0.002 | 0.004 | 0.031 | ||
| a2j | −0.746 | −0.015 | −0.293 | −0.124 | −0.100 | −0.007 | −0.006 | −0.036 | ||
| a22j | −0.655 | 0.077 | −0.167 | −0.076 | −0.057 | −0.004 | −0.001 | −0.026 | ||
| a12j | −0.032 | 0.055 | 0.104 | 0.050 | 0.038 | 0.003 | 0.004 | −0.000 | ||
| Rj2 | 0.979 | 0.360 | 0.921 | 0.956 | 0.880 | 0.877 | 0.877 | 0.942 | ||
| Fj | 54.87 | 0.675 | 14.03 | 26.12 | 8.811 | 8.588 | 8.564 | 19.65 | ||
| pj | 0.000 | 0.658 | 0.003 | 0.001 | 0.010 | 0.010 | 0.011 | 0.001 | ||
| Run | X1 | X2 | Yj,m | ||||||
|---|---|---|---|---|---|---|---|---|---|
| j = 9 | j = 10 | j = 11 | j = 12 | j = 13 | j = 14 | j = 15 | |||
| Cam (mg/kg) | Mgm (mg/kg) | Cum (mg/kg) | Fem (mg/kg) | Mnm (mg/kg) | Mom (mg/kg) | Znm (mg/kg) | |||
| 1 | −1 | −1 | 779.7 | 198.1 | 0.179 | 23.71 | 0.658 | 0.053 | 2.509 |
| 2 | −1 | 1 | 141.7 | 56.72 | 0.056 | 10.06 | 0.106 | 0.032 | 1.092 |
| 3 | 1 | −1 | 326.9 | 83.52 | 0.188 | 12.59 | 0.448 | 0.042 | 1.564 |
| 4 | 1 | 1 | 134.3 | 38.63 | 0.050 | 21.00 | 0.149 | 0.020 | 0.770 |
| 5 | 0 | 0 | 150.6 | 108.6 | 0.118 | 13.48 | 0.182 | 0.032 | 0.726 |
| 6 | 0 | 0 | 231.8 | 120.5 | 0.131 | 15.49 | 0.255 | 0.037 | 0.820 |
| 7 | −1.414 | 0 | 759.7 | 240.1 | 0.265 | 25.16 | 0.692 | 0.053 | 2.029 |
| 8 | 1.414 | 0 | 85.64 | 80.34 | 0.096 | 12.57 | 0.116 | 0.031 | 0.967 |
| 9 | 0 | −1.414 | 173.7 | 114.8 | 0.141 | 15.32 | 0.217 | 0.039 | 0.861 |
| 10 | 0 | 1.414 | 154.4 | 64.73 | 0.084 | 14.43 | 0.187 | 0.008 | 0.430 |
| 11 | 0 | 0 | 122.0 | 115.3 | 0.133 | 14.92 | 0.176 | 0.041 | 0.870 |
| 12 | 0 | 0 | 108.2 | 107.9 | 0.153 | 15.77 | 0.178 | 0.043 | 0.761 |
| a0j | 153.2 | 113.1 | 0.134 | 14.91 | 0.198 | 0.038 | 0.794 | ||
| a1j | −176.7 | −44.83 | −0.029 | −2.250 | −0.123 | −0.007 | −0.346 | ||
| a11j | 147.8 | 15.89 | 0.016 | 1.967 | 0.113 | 0.003 | 0.455 | ||
| a2j | −107.3 | −32.14 | −0.043 | −0.812 | −0.112 | −0.011 | −0.353 | ||
| a22j | 18.51 | −19.34 | −0.018 | −0.027 | 0.011 | −0.006 | 0.028 | ||
| a12j | 111.4 | 24.12 | −0.003 | 5.517 | 0.063 | 0.000 | 0.156 | ||
| Rj2 | 0.809 | 0.869 | 0.660 | 0.815 | 0.693 | 0.943 | 0.833 | ||
| Fj | 5.080 | 7.993 | 2.326 | 5.282 | 2.708 | 20.01 | 6.005 | ||
| pj | 0.036 | 0.013 | 0.167 | 0.033 | 0.129 | 0.001 | 0.025 | ||
| j | Variable | Optimal Value | Percentage Prediction Error | ||
|---|---|---|---|---|---|
| Symbol | Units | Predicted | Experimental | ||
| Yj,pr,opt | Yj,m,opt ± SDj | εj (%) | |||
| 1 | EC | dS/m | 17.50 | 16.92 ± 1.51 | −3.4 |
| 2 | pH | - | 8.239 | 8.463 ± 0.359 | 2.7 |
| 3 | DM | % | 2.694 | 2.658 ± 0.227 | −1.3 |
| 4 | Ash | % | 1.721 | 1.755 ± 0.100 | 2.0 |
| 5 | C | % | 0.599 | 0.606 ± 0.032 | 1.2 |
| 6 | N | % | 0.042 | 0.041 ± 0.002 | −1.9 |
| 7 | P | % | 0.059 | 0.060 ± 0.001 | 1.8 |
| 8 | K | % | 0.649 | 0.628 ± 0.038 | −3.3 |
| 9 | Ca | mg/kg | 895.2 | 887.8 ± 14.3 | −0.8 |
| 10 | Mg | mg/kg | 245.5 | 249.0 ± 7.6 | 1.4 |
| 11 | Cu | mg/kg | 0.225 | 0.232 ± 0.042 | 3.0 |
| 12 | Fe | mg/kg | 28.10 | 29.34 ± 3.18 | 4.2 |
| 13 | Mn | mg/kg | 0.746 | 0.720 ± 0.053 | −3.5 |
| 14 | Mo | mg/kg | 0.059 | 0.058 ± 0.002 | −1.8 |
| 15 | Zn | mg/kg | 2.613 | 2.516 ± 0.116 | −3.8 |
| Variable | Treatment | |||
|---|---|---|---|---|
| T0 | T1 | T2 | T3 | |
| Germination percentage, GP (%) | 98 a | 97 a | 97 a | 73 b |
| Mean germination time, MGT (day) | 1.2 d | 1.6 c | 2.2 b | 5.2 a |
| Germination speed, GS (day−1) | 44.5 a | 34.7 b | 25.5 c | 7.9 d |
| Seedling length, SL (cm) | 3.92 b | 4.95 a | 3.75 b | 1.98 c |
| Seedling vigor index (SVI) (cm) | 3.84 b | 4.79 a | 3.63 b | 1.47 c |
| Seedling mass, SM (g) | 0.30 b | 0.37 a | 0.33 b | 0.11 c |
| Root length, RL (cm) | 3.18 a | 3.80 a | 2.21 b | 0.75 c |
| Total leaf surface area, LA (cm2) | 0.29 c | 0.48 a | 0.35 b | 0.10 d |
| No. | Variable | Treatment | |||
|---|---|---|---|---|---|
| Symbol | Units | T1 | T2 | T3 | |
| 1 | N | mg/kg | 102.5 | 205.0 | 410.0 |
| 2 | P | mg/kg | 150.0 | 300.0 | 600.0 |
| 3 | K | mg/kg | 1570 | 3140 | 6280 |
| 4 | Ca | mg/kg | 222.0 | 443.9 | 887.8 |
| 5 | Mg | mg/kg | 62.25 | 124.5 | 249.0 |
| 6 | Cu | mg/kg | 0.058 | 0.116 | 0.232 |
| 7 | Fe | mg/kg | 7.335 | 14.67 | 29.34 |
| 8 | Mn | mg/kg | 0.180 | 0.360 | 0.720 |
| 9 | Mo | mg/kg | 0.015 | 0.029 | 0.058 |
| 10 | Zn | mg/kg | 0.629 | 1.258 | 2.516 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moț, A.; Pârvulescu, O.C.; Ion, V.A.; Moloșag, A.; Dobrin, A.; Bădulescu, L.; Orbeci, C.; Egri, D.; Dobre, T.; Løes, A.-K.; et al. Preparation, Characterization, and Testing of Compost Tea Derived from Seaweed and Fish Residues. Agronomy 2024, 14, 1919. https://doi.org/10.3390/agronomy14091919
Moț A, Pârvulescu OC, Ion VA, Moloșag A, Dobrin A, Bădulescu L, Orbeci C, Egri D, Dobre T, Løes A-K, et al. Preparation, Characterization, and Testing of Compost Tea Derived from Seaweed and Fish Residues. Agronomy. 2024; 14(9):1919. https://doi.org/10.3390/agronomy14091919
Chicago/Turabian StyleMoț, Andrei, Oana Cristina Pârvulescu, Violeta Alexandra Ion, Ailin Moloșag, Aurora Dobrin, Liliana Bădulescu, Cristina Orbeci, Diana Egri, Tănase Dobre, Anne-Kristin Løes, and et al. 2024. "Preparation, Characterization, and Testing of Compost Tea Derived from Seaweed and Fish Residues" Agronomy 14, no. 9: 1919. https://doi.org/10.3390/agronomy14091919





