Impacts of Selenium Supplementation on Soil Mercury Speciation, Soil Properties and Mercury-Resistant Microorganisms and Resistant Genes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Se- and Hg-Contaminated Soil
2.2. Experimental Design
2.3. Chemical Analysis
2.3.1. Soil Properties
2.3.2. Soil Hg and Se Contents
2.3.3. Sequential Extraction of Hg and Se in Soil
2.4. Hg-Resistant Microorganisms and Mer Operon Quantification
2.5. Data Analysis
3. Results
3.1. Effects of Se Supplementation on Soil AHg and ASe
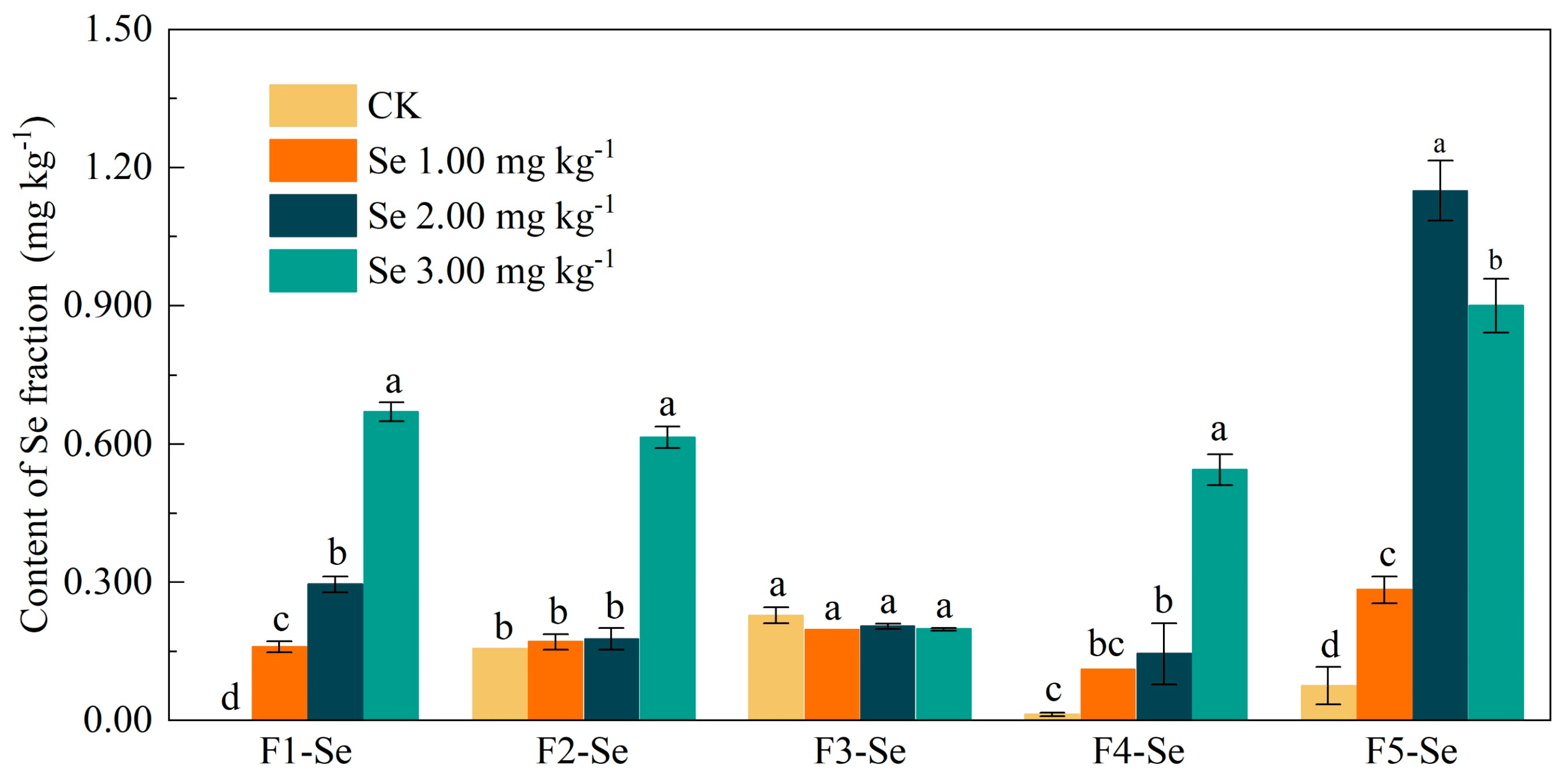
3.2. Effects of Se Supplementation on Soil Hg and Se Speciation
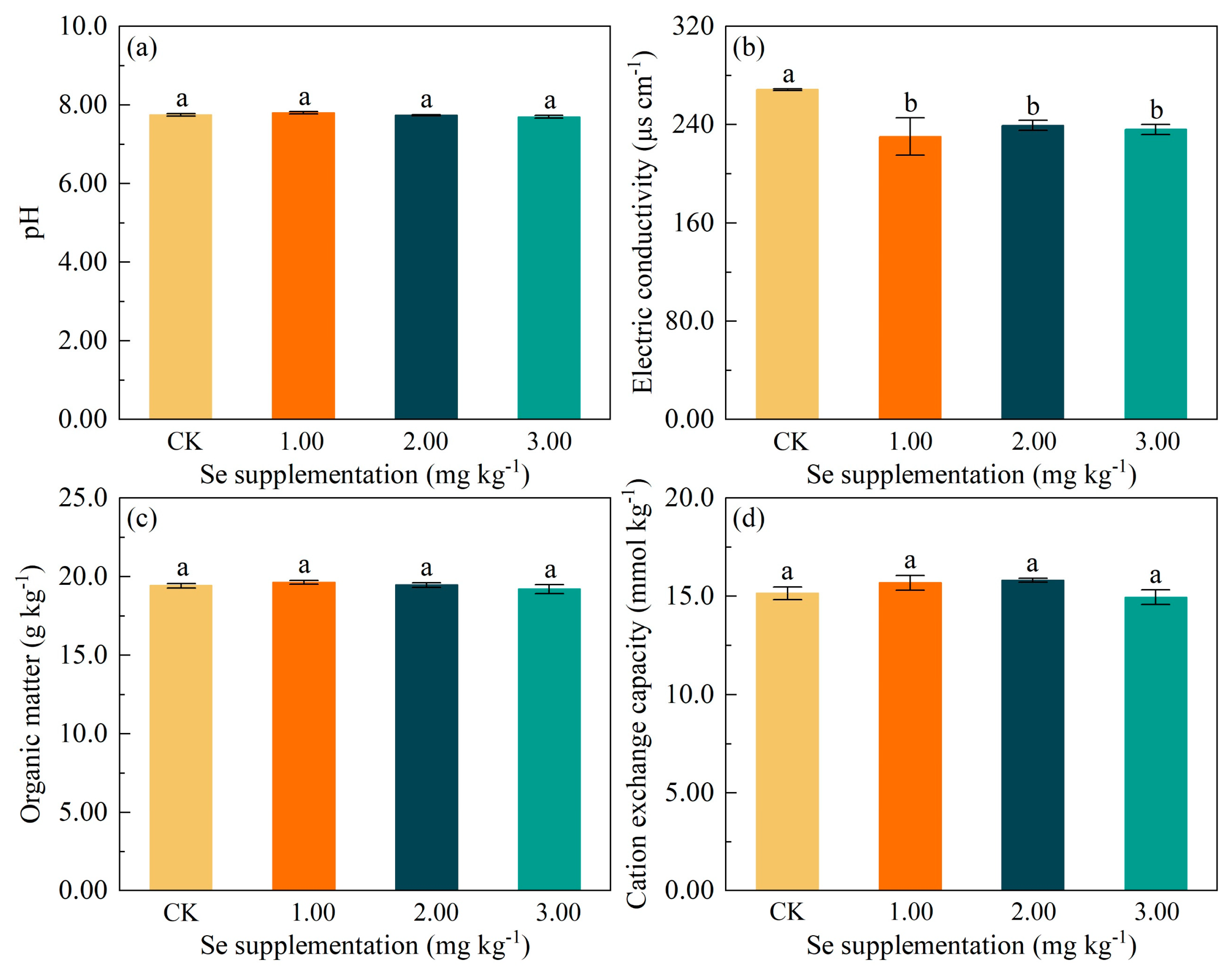
3.3. Effects of Se Supplementation on Soil Physicochemical Properties
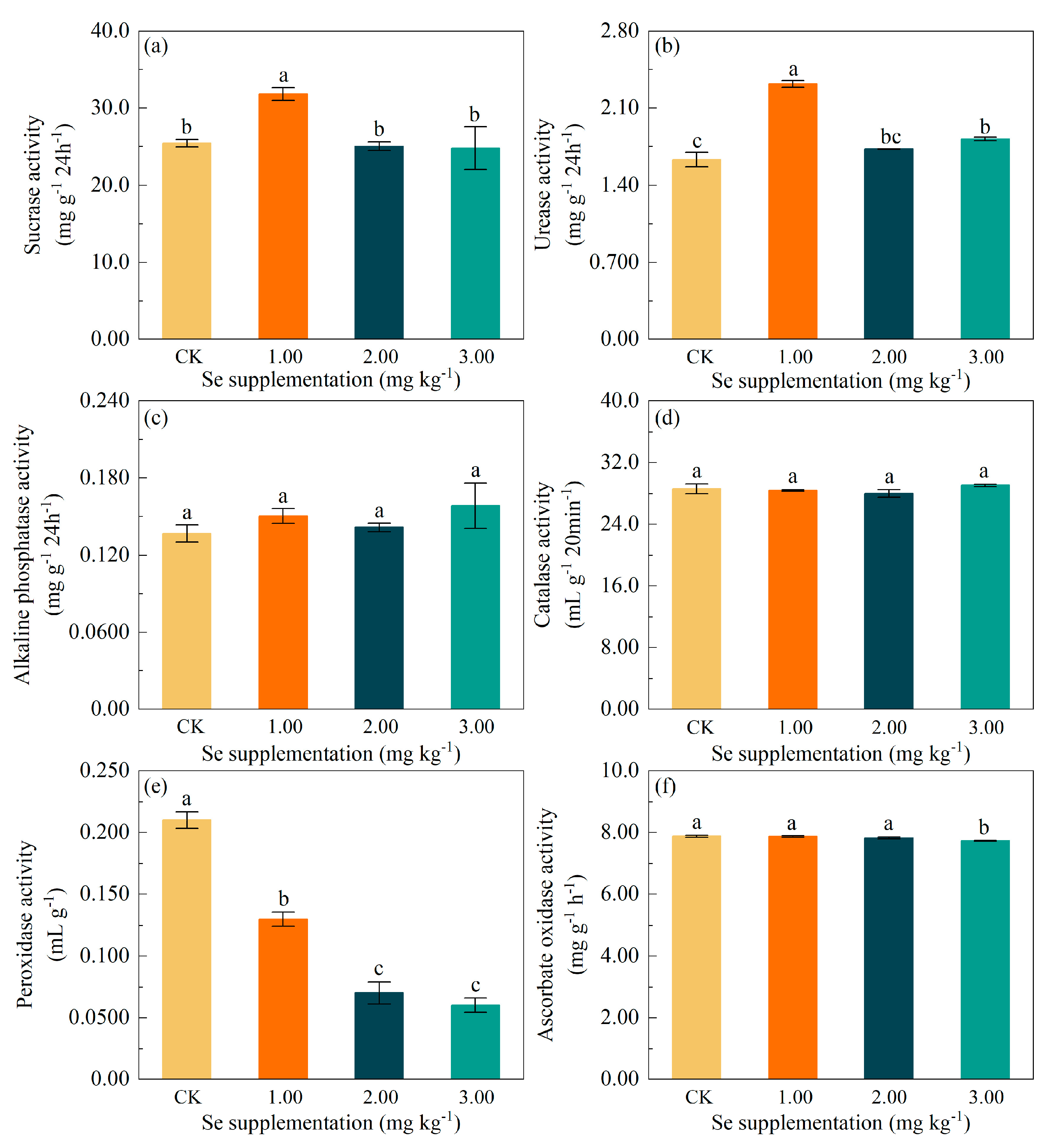
3.4. Effects of Se Supplementation on Soil Enzyme Activities
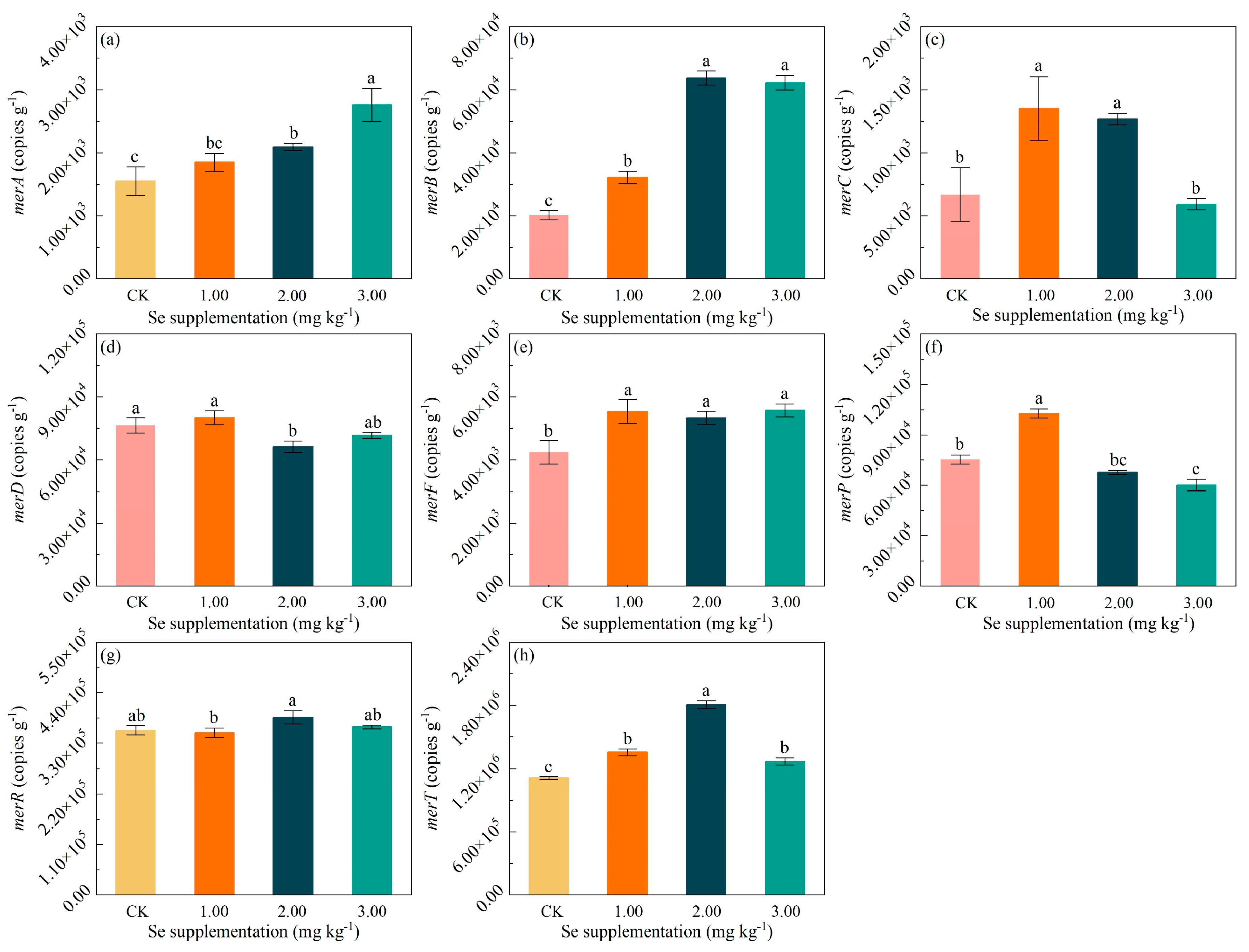
3.5. Effects of Se Supplementation on Hg-Resistant Genes
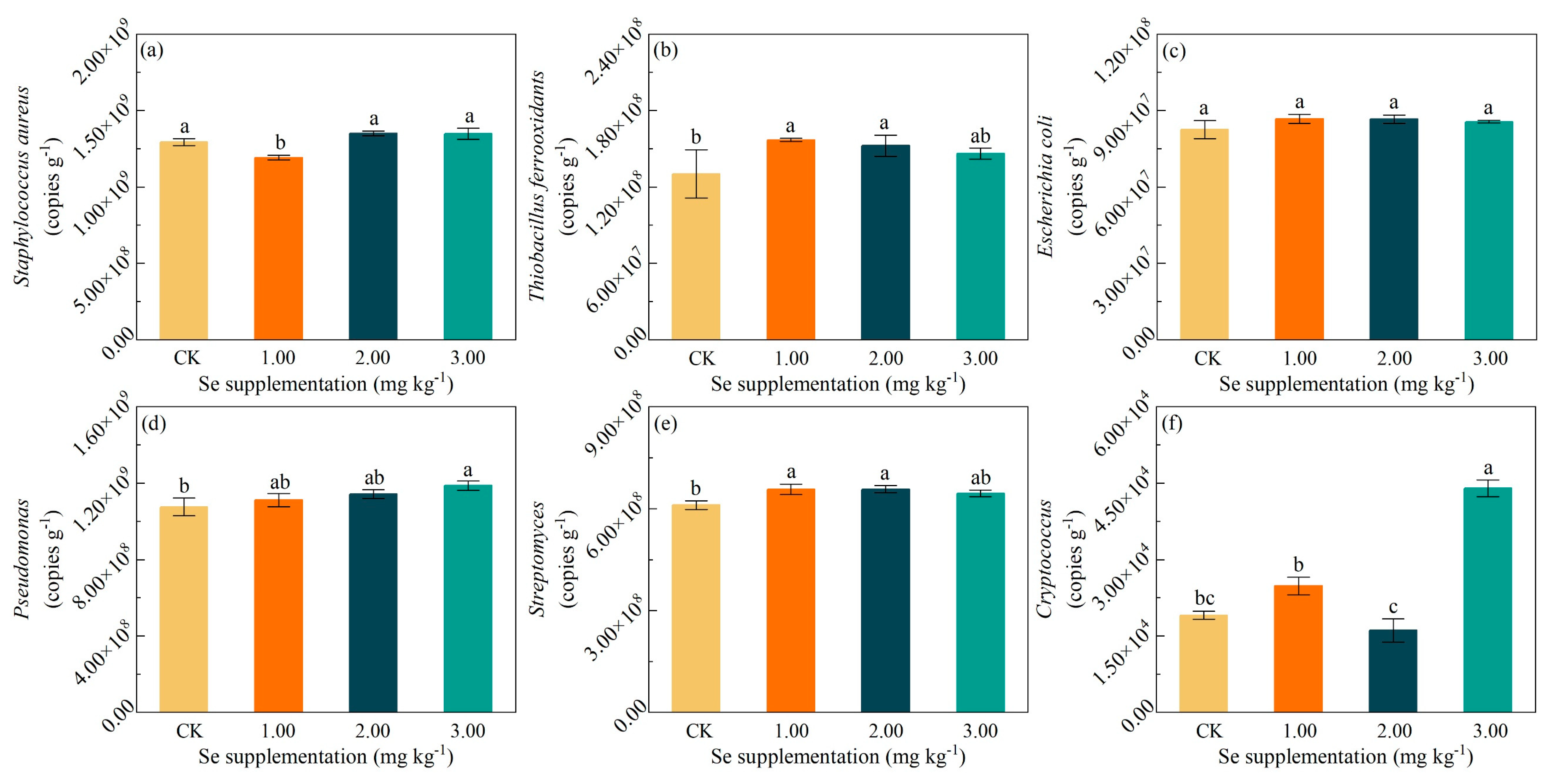
3.6. Effects of Se Supplementation on Hg-Resistant Microorganisms
4. Discussion
4.1. Se Supplementation Promotes the Transformation of Soil Hg and Se Fractions
4.2. Changes in Soil Enzyme Activity under Se Supplementation
4.3. The Impact of Se Supplementation on Microbial Hg-Resistant System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tran, T.A.T.; Dinh, Q.T.; Zhou, F.; Zhai, H.; Xue, M.; Du, Z.; Bañuelos, G.S.; Liang, D. Mechanisms underlying mercury detoxification in soil-plant systems after selenium application: A review. Environ. Sci. Pollut. Res. 2021, 28, 46852–46876. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Wang, F.; Luo, Z.; Guo, S.; Strähle, U. Toxicity of mercury: Molecular evidence. Chemosphere 2020, 245, 125586. [Google Scholar] [CrossRef]
- Ullah, S.; Hussain, S.; Noor, Y.; Khanam, T.; Xia, X.; Darma, A.I.; Feng, Y.; Yang, J. Influencing factors and prediction models of mercury phytoavailability and transference in a soil–lettuce system under Chinese agricultural soils. Agronomy 2024, 14, 1394. [Google Scholar] [CrossRef]
- Wang, J.; Feng, X.; Anderson, C.W.N.; Xing, Y.; Shang, L. Remediation of mercury contaminated sites—A review. J. Hazard. Mater. 2012, 221–222, 1–18. [Google Scholar] [CrossRef]
- Ran, S.; He, T.; Li, S.; Yin, D.; Wu, P.; Xu, Y.; Zhao, J. Selenium/sulfur-modified montmorillonite materials mitigate mercury pollution in farmland. Environ. Pollut. 2023, 329, 121719. [Google Scholar] [CrossRef]
- Meng, W.; Wang, Z.; Hu, B.; Wang, Z.; Li, H.; Goodman, R.C. Heavy metals in soil and plants after long-term sewage irrigation at Tianjin China: A case study assessment. Agric. Water Manag. 2016, 171, 153–161. [Google Scholar] [CrossRef]
- Lin, Y.; Vogt, R.; Larssen, T. Environmental mercury in China: A review. Environ. Toxicol. Chem. 2012, 31, 2431–2444. [Google Scholar] [CrossRef]
- Ha, E.; Basu, N.; Bose-O’Reilly, S.; Dórea, J.G.; McSorley, E.; Sakamoto, M.; Chan, H.M. Current progress on understanding the impact of mercury on human health. Environ. Res. 2017, 152, 419–433. [Google Scholar] [CrossRef]
- Goyer, R.A. Toxic and essential metal interactions. Ann. Rev. Nutr. 1997, 17, 37–50. [Google Scholar] [CrossRef]
- Pařízek, J.; Ošťádalová, I. The protective effect of small amounts of selenite in sublimate intoxication. Experientia 1967, 23, 142–143. [Google Scholar] [CrossRef]
- Khan, M.A.; Wang, F. Mercury-selenium compounds and their toxicological significance: Toward a molecular understanding of the mercury-selenium antagonism. Environ. Toxicol. Chem. 2009, 28, 1567–1577. [Google Scholar] [CrossRef]
- Wang, Y.; Dang, F.; Evans, R.D.; Zhong, H.; Zhao, J.; Zhou, D. Mechanistic understanding of MeHg-Se antagonism in soil-rice systems: The key role of antagonism in soil. Sci. Rep. 2016, 6, 19477. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Y.; Gunn, J.M.; Belzile, N. Selenium and mercury in organisms: Interactions and mechanisms. Environ. Rev. 2008, 16, 71–92. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.; Hintelmann, H.; Tang, W.; Zhong, H. New insights into HgSe antagonism: Minor impact on inorganic Hg mobility while potential impacts on microorganisms. Sci. Total Environ. 2024, 913, 169705. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yan, M.; Liang, L.; Lu, Q.; Han, J.; Liu, L.; Feng, X.; Guo, J.; Wang, Y.; Qiu, G. Impacts of selenium supplementation on soil mercury speciation, and inorganic mercury and methylmercury uptake in rice (Oryza sativa L.). Environ. Pollut. 2019, 249, 647–654. [Google Scholar] [CrossRef]
- Reis, A.T.; Rodrigues, S.M.; Davidson, C.M.; Pereira, E.; Duarte, A.C. Extractability and mobility of mercury from agricultural soils surrounding industrial and mining contaminated areas. Chemosphere 2010, 81, 1369–1377. [Google Scholar] [CrossRef]
- Yu, X.; Li, H.; Pan, K.; Yan, Y.; Wang, W.-X. Mercury distribution, speciation and bioavailability in sediments from the Pearl River Estuary, Southern China. Mar. Pollut. Bull. 2012, 64, 1699–1704. [Google Scholar] [CrossRef]
- Xu, X.; Meng, B.; Zhang, C.; Feng, X.; Gu, C.; Guo, J.; Bishop, K.; Xu, Z.; Zhang, S.; Qiu, G. The local impact of a coal-fired power plant on inorganic mercury and methyl-mercury distribution in rice (Oryza sativa L.). Environ. Pollut. 2017, 223, 11–18. [Google Scholar] [CrossRef]
- Benoit, J.M.; Gilmour, C.C.; Mason, R.P.; Heyes, A. Sulfide controls on mercury speciation and bioavailability to methylating bacteria in sediment pore waters. Environ. Sci. Technol. 1999, 33, 1780. [Google Scholar] [CrossRef]
- Priyadarshanee, M.; Chatterjee, S.; Rath, S.; Dash, H.R.; Das, S. Cellular and genetic mechanism of bacterial mercury resistance and their role in biogeochemistry and bioremediation. J. Hazard. Mater. 2022, 423, 126985. [Google Scholar] [CrossRef]
- Dash, H.R.; Sahu, M.; Mallick, B.; Das, S. Functional efficiency of MerA protein among diverse mercury resistant bacteria for efficient use in bioremediation of inorganic mercury. Biochimie 2017, 142, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Natasha; Shahid, M.; Khalid, S.; Bibi, I.; Bundschuh, J.; Khan Niazi, N.; Dumat, C. A critical review of mercury speciation, bioavailability, toxicity and detoxification in soil-plant environment: Ecotoxicology and health risk assessment. Sci. Total Environ. 2020, 711, 134749. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Pitts, K.; McGarvey, J.A.; Summers, A.O. Bacterial oxidation of mercury metal vapor, Hg(0). Appl. Environ. Microbiol. 1998, 64, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Raymat, H.; Liu, G.; Liriano, C.; Li, Y.; Yin, Y.; Shi, J.; Jiang, G.; Cai, Y. Elemental mercury: Its unique properties affect its behavior and fate in the environment. Environ. Pollut. 2017, 229, 69–86. [Google Scholar] [CrossRef]
- Naik, M.M.; Dubey, S.K. Lead- and mercury-resistant marine bacteria and their application in lead and mercury bioremediation. In Marine Pollution and Microbial Remediation; Naik, M.M., Dubey, S.K., Eds.; Springer: Singapore, 2017; pp. 29–40. [Google Scholar]
- Kaur, A.; Nirwan, J.; Tiwana, A.S.; Singh, A.; Singh, R.P.; Verma, A.; Gupta, S. Sequestration of mercury and cadmium by a heavy metals’ tolerant Bacillus cereus strain JS-3: A microbial preparedness for terrestrial and anthropogenic hazards. Biorem. J. 2022, 26, 198–207. [Google Scholar] [CrossRef]
- Meyer, L.; Guyot, S.; Chalot, M.; Capelli, N. The potential of microorganisms as biomonitoring and bioremediation tools for mercury-contaminated soils. Ecotoxicol. Environ. Saf. 2023, 262, 115185. [Google Scholar] [CrossRef]
- Pereira-García, C.; del Amo, E.H.; Vigués, N.; Rey-Velasco, X.; Rincón-Tomás, B.; Pérez-Cruz, C.; Sanz-Sáez, I.; Hu, H.; Bertilsson, S.; Pannier, A.; et al. Unmasking the physiology of mercury detoxifying bacteria from polluted sediments. J. Hazard. Mater. 2024, 467, 133685. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, V.; Chawla, M.; Thakur, M.; Bhardwaj, R.; Wang, J.; O’Connor, D.; Hou, D.; Rinklebe, J. Bioremediation of mercury contaminated soil and water: A review. Land Degrad. Dev. 2024, 35, 1261–1283. [Google Scholar] [CrossRef]
- Dash, H.R.; Das, S. Bioremediation of mercury and the importance of bacterial mer genes. Int. Biodeterior. Biodegrad. 2012, 75, 207–213. [Google Scholar] [CrossRef]
- Syarif, H.; Edwan, K.; Yos, P.; Qomarudin, H. Application of mercury resistant bacteria isolated from artisanal small-scale gold tailings in biotransformation of mercury (II)-contaminated soil. Int. J. Geomate 2020, 19, 106–114. [Google Scholar]
- Yang, X.; Mao, K.; Chang, C.; Wang, J.; Wu, Q.; Shao, Y.; Huang, J.-H.; Zhang, H.; Feng, X. Interactions of selenium and mercury in soil-plant systems: Characterizations, occurrences, and mechanisms. Crit. Rev. Environ. Sci. Technol. 2024, 1–19. [Google Scholar] [CrossRef]
- Wang, X.; Ye, Z.; Li, B.; Huang, L.; Meng, M.; Shi, J.; Jiang, G. Growing Rice Aerobically markedly decreases mercury accumulation by reducing both hg bioavailability and the production of MeHg. Environ. Sci. Technol. 2014, 48, 1878–1885. [Google Scholar] [CrossRef]
- Lu, R. Soil Agrochemical Analysis Method; China Agricultural Science and Technology Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Bao, D. Soil Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Guan, S. Soil Enzyme and Its Research Method; Agriculture Press: Beijing, China, 1986. (In Chinese) [Google Scholar]
- Tessier, A.; Campbell, P.; Bisson, M. Sequential extraction procedure for the speciation of particular trace elements. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Tang, W.; Dang, F.; Evans, D.; Zhong, H.; Xiao, L. Understanding reduced inorganic mercury accumulation in rice following selenium application: Selenium application routes, speciation and doses. Chemosphere 2017, 169, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tam, N.F.; Fu, S.; Ametkhan, A.; Ouyang, Y.; Ye, Z. Selenium addition alters mercury uptake, bioavailability in the rhizosphere and root anatomy of rice (Oryza sativa). Ann. Bot. 2014, 114, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Guo, J.; Feng, X.; Zhang, C.; Chou, G. Effects of selenium on mercury speciation and its bioavailability in paddy soils. Chin. J. Ecol. 2015, 34, 1402–1406. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Y.; Ahsan, M.Z.; Luo, D.; Panhwar, F.H.; Li, L.; Su, Y.; Jia, X.; Ye, X.; Rongjun, C.; Lihua, L.; et al. Dynamics of selenium-mercury interaction under mercury stress in high and low selenium rice genotypes. Environ. Exp. Bot. 2024, 224, 105822. [Google Scholar] [CrossRef]
- Li, H.-F.; Lombi, E.; Stroud, J.L.; McGrath, S.P.; Zhao, F.-J. Selenium speciation in soil and rice: Influence of water management and Se fertilization. J. Agric. Food. Chem. 2010, 58, 11837–11843. [Google Scholar] [CrossRef]
- Syversen, T.; Kaur, P. The toxicology of mercury and its compounds. J. Trace Elem. Med. Biol. 2012, 26, 215–226. [Google Scholar] [CrossRef]
- Chen, D.; Sun, S. Tiemannite and metacinnabar from mercury belt in western Hunan-Eastern Guizhou. Acta Pet. Mineral. 1991, 10, 58–62. [Google Scholar]
- Chiasson-Gould, S.A.; Blais, J.M.; Poulain, A.J. Dissolved organic matter kinetically controls mercury bioavailability to bacteria. Environ. Sci. Technol. 2014, 48, 3153–3161. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Hu, C.; Wang, X.; Zhao, Y.; Jia, W.; Sun, X.; Elyamine, A.M.; Zhao, X. Selenium induces changes of rhizosphere bacterial characteristics and enzyme activities affecting chromium/selenium uptake by pak choi (Brassica campestris L. ssp. Chinensis Makino) in chromium contaminated soil. Environ. Pollut. 2019, 249, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, J.; Yu, J.; Wang, K.; Li, J.; Cui, Y.; Shangguan, Z.; Deng, L. Soil enzyme activity and stoichiometry in response to precipitation changes in terrestrial ecosystems. Soil Biol. Biochem. 2024, 191, 109321. [Google Scholar] [CrossRef]
- Hassan, Z.; Ali, S.; Farid, M.; Rizwan, M.; Shahid, M. Effect of chromium (Cr) on the microbial and enzymatic activities in the soil: A review. In Biodiversity Conservation in Changing Climate; Abid Ali Khan, M.M., Abid, M., Omar, A.M., Nazeer Haider Zaidi, S., Maheshwari, R.K., Eds.; Lenin Media Private Limited: Delhi, India, 2016; pp. 305–323. [Google Scholar]
- Hu, L.; Zhang, B.J.; Wu, D.S.; Liu, Y.; Gao, G.Q.; Wang, X.L.; Hu, S.M.; Fan, H.B.; Fang, H.Y. Effects of different exogenous selenium on enzyme activities and microorganisms in arsenic contaminated soil. Appl. Ecol. Environ. Res. 2022, 20, 4237–4255. [Google Scholar] [CrossRef]
- Li, J.; Tang, W.; Lu, S.; Wang, Y.; Kuang, Z.; Yuan, J. Application of selenocysteine increased soil nitrogen content, enzyme activity, and microbial quantity in Camellia oleifera Abel. forests. Forests 2023, 14, 982. [Google Scholar] [CrossRef]
- Boyd, E.S.; Barkay, T. The mercury resistance operon: From an origin in a geothermal environment to an efficient detoxification machine. Front. Microbiol. 2012, 3, 349. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, Q.; Mu, K.; Wang, Q.; Liu, K.; Wang, J. A promotion strategy of enhancing the mercury removal in Shewanella oneidensis MR-1 based on the mercury absorption and electronic consumption via mer operon. J. Environ. Chem. Eng. 2024, 12, 112993. [Google Scholar] [CrossRef]
- Singh, A.D.; Khanna, K.; Kour, J.; Dhiman, S.; Bhardwaj, T.; Devi, K.; Sharma, N.; Kumar, P.; Kapoor, N.; Sharma, P.; et al. Critical review on biogeochemical dynamics of mercury (Hg) and its abatement strategies. Chemosphere 2023, 319, 137917. [Google Scholar] [CrossRef]
- Champier, L.; Duarte, V.; Michaud-Soret, I.; Covès, J. Characterization of the MerD protein from Ralstonia metallidurans CH34: A possible role in bacterial mercury resistance by switching off the induction of the mer operon. Mol. Microbiol. 2004, 52, 1475–1485. [Google Scholar] [CrossRef]
- Huang, C.-C.; Narita, M.; Yamagata, T.; Phung, L.T.; Endo, G.; Silver, S. Characterization of two regulatory genes of the mercury resistance determinants from TnMERI1 by luciferase-based examination. Gene 2002, 301, 13–20. [Google Scholar] [CrossRef]
- Powlowski, J.; Sahlman, L. Reactivity of the two essential cysteine residues of the periplasmic mercuric ion-binding protein, MerP. J. Biol. Chem. 1999, 274, 33320–33326. [Google Scholar] [CrossRef]
- Ballatori, N.; Madejczyk, M.S. Transport of nonessential metals across mammalian cell membranes. In Molecular Biology of Metal Homeostasis and Detoxification: From Microbes to Man; Tamas, M.J., Martinoia, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 455–483. [Google Scholar]
- Amin, A.; Naveed, M.; Sarwar, A.; Rasheed, S.; Saleem, H.G.M.; Latif, Z.; Bechthold, A. In vitro and in silico studies reveal Bacillus cereus AA-18 as a potential candidate for bioremediation of mercury-contaminated wastewater. Front. Microbiol. 2022, 13, 847806. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.-Y.; Ma, B.-C.; Hu, S.-Y.; Wu, C. Tailored bacteria tackling with environmental mercury: Inspired by natural mercuric detoxification operons. Environ. Pollut. 2024, 341, 123016. [Google Scholar] [CrossRef]
- Osborn, A.M.; Bruce, K.D.; Strike, P.; Ritchie, D.A. Distribution, diversity and evolution of the bacterial mercury resistance (mer) operon. FEMS Microbiol. Rev. 1997, 19, 239–262. [Google Scholar] [CrossRef] [PubMed]
- Norambuena, J.; Miller, M.; Boyd, J.M.; Barkay, T. Expression and regulation of the mer operon in Thermus thermophilus. Environ. Microbiol. 2020, 22, 1619–1634. [Google Scholar] [CrossRef]
- Trojanska, M.; Rogala, M.; Kowalczyk, A.; Chyc, M.; Latowski, D.; Bojko, M. Is the merA gene sufficient as a molecular marker of mercury bacterial resistance? Acta Biochim. Pol. 2022, 69, 507–512. [Google Scholar] [CrossRef]
- Huang, F.; Chen, L.; Zhou, Y.; Huang, J.; Wu, F.; Hu, Q.; Chang, N.; Qiu, T.; Zeng, Y.; He, H.; et al. Exogenous selenium promotes cadmium reduction and selenium enrichment in rice: Evidence, mechanisms, and perspectives. J. Hazard. Mater. 2024, 476, 135043. [Google Scholar] [CrossRef]
- Somagattu, P.; Chinnannan, K.; Yammanuru, H.; Reddy, U.K.; Nimmakayala, P. Selenium dynamics in plants: Uptake, transport, toxicity, and sustainable management strategies. Sci. Total Environ. 2024, 949, 175033. [Google Scholar] [CrossRef]


| Soil Physicochemical Properties | Value |
|---|---|
| Clay (%) | 5.14 |
| Silt (%) | 41.8 |
| Sand (%) | 53.1 |
| pH | 7.70 |
| Electric conductivity (μs cm−1) | 269 |
| Cation exchange capacity (mmol kg−1) | 15.2 |
| Organic matter (g kg−1) | 19.4 |
| Hg (mg kg−1) | 5.38 |
| Se (mg kg−1) | 0.272 |
| Se Supplementation (mg kg−1) | PAHg (%) | PASe (%) |
|---|---|---|
| CK | 31.4 ± 0.58 a | 33.0 ± 3.63 b |
| 1.00 | 15.3 ± 0.44 b | 32.5 ± 0.63 b |
| 2.00 | 8.28 ± 0.30 c | 32.8 ± 0.36 b |
| 3.00 | 6.79 ± 0.39 d | 44.0 ± 0.59 a |
| Element | Se Supplementation (mg kg−1) | PF1 (%) | PF2 (%) | PF3 (%) | PF4 (%) | PF5 (%) |
|---|---|---|---|---|---|---|
| Hg | CK | 11.9 ± 0.78 a | 16.4 ± 1.20 a | 5.57 ± 0.80 a | 42.6 ± 0.58 c | 23.5 ± 2.09 c |
| 1.00 | 9.22 ± 0.41 b | 4.74 ± 0.28 b | 4.56 ± 0.07 ab | 49.2 ± 0.20 b | 32.3 ± 0.15 b | |
| 2.00 | 7.69 ± 0.31 c | 5.74 ± 0.55 b | 4.35 ± 0.09 ab | 56.7 ± 1.25 a | 25.5 ± 1.18 c | |
| 3.00 | 2.32 ± 0.06 d | 1.91 ± 0.49 c | 4.13 ± 0.11 b | 44.8 ± 1.29 c | 46.9 ±0.84 a | |
| Se | CK | 0.00 ± 0.00 d | 33.1 ± 1.71 a | 49.0 ± 5.62 a | 2.73 ± 0.44 c | 15.2 ± 7.51 c |
| 1.00 | 17.3 ±1.04 b | 18.6 ± 1.94 b | 21.4 ± 0.18 b | 12.0 ±0.12 b | 30.8 ± 2.63 b | |
| 2.00 | 15.0 ± 0.81 c | 9.00 ± 1.08 c | 10.4 ± 0.09 c | 7.32 ± 3.40 bc | 58.4 ± 3.30 a | |
| 3.00 | 22.9 ± 0.42 a | 21.0 ± 1.02 b | 6.77 ± 0.14 c | 18.6 ± 1.00 a | 30.8 ± 1.82 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, G.; Li, Y.; Li, H. Impacts of Selenium Supplementation on Soil Mercury Speciation, Soil Properties and Mercury-Resistant Microorganisms and Resistant Genes. Agronomy 2024, 14, 1928. https://doi.org/10.3390/agronomy14091928
Pei G, Li Y, Li H. Impacts of Selenium Supplementation on Soil Mercury Speciation, Soil Properties and Mercury-Resistant Microorganisms and Resistant Genes. Agronomy. 2024; 14(9):1928. https://doi.org/10.3390/agronomy14091928
Chicago/Turabian StylePei, Guangpeng, Yuxin Li, and Hua Li. 2024. "Impacts of Selenium Supplementation on Soil Mercury Speciation, Soil Properties and Mercury-Resistant Microorganisms and Resistant Genes" Agronomy 14, no. 9: 1928. https://doi.org/10.3390/agronomy14091928





