Effectiveness of Anaerobic Soil Disinfestation for Weed and Nematode Management in Organic Sweetpotato Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Greenhouse Experiments
2.2. Experimental Setup
2.3. Treatment Setup and ASD Initiation
2.4. Data Collection
2.5. Data Analysis
3. Results and Discussion
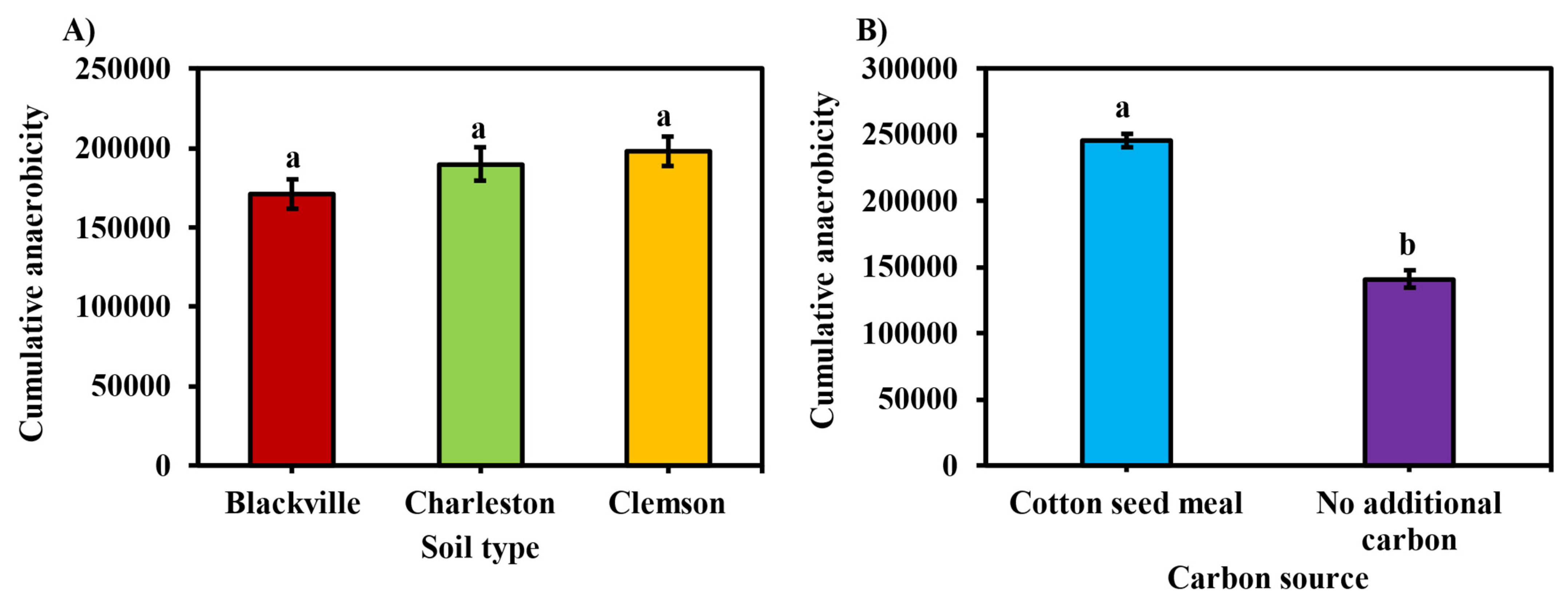
3.1. Soil Redox Potential
3.2. Impact of ASD on Weeds
3.2.1. Weed Counts
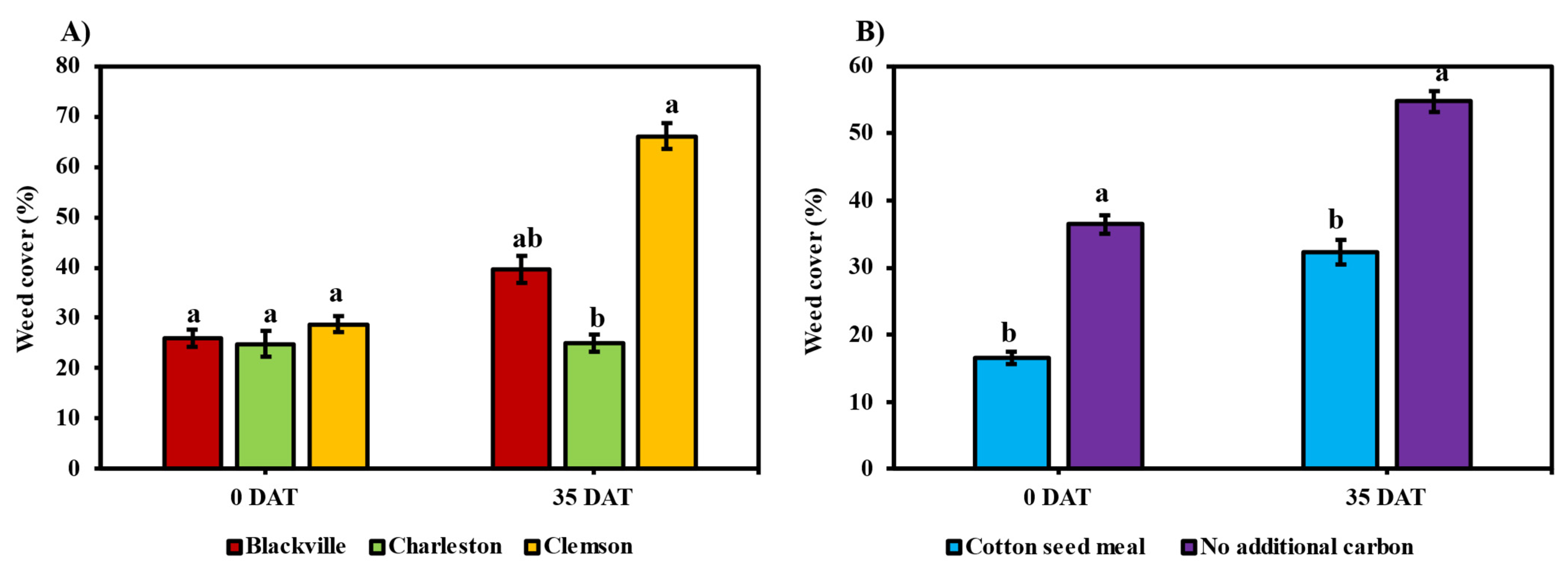
3.2.2. Percent Weed Cover
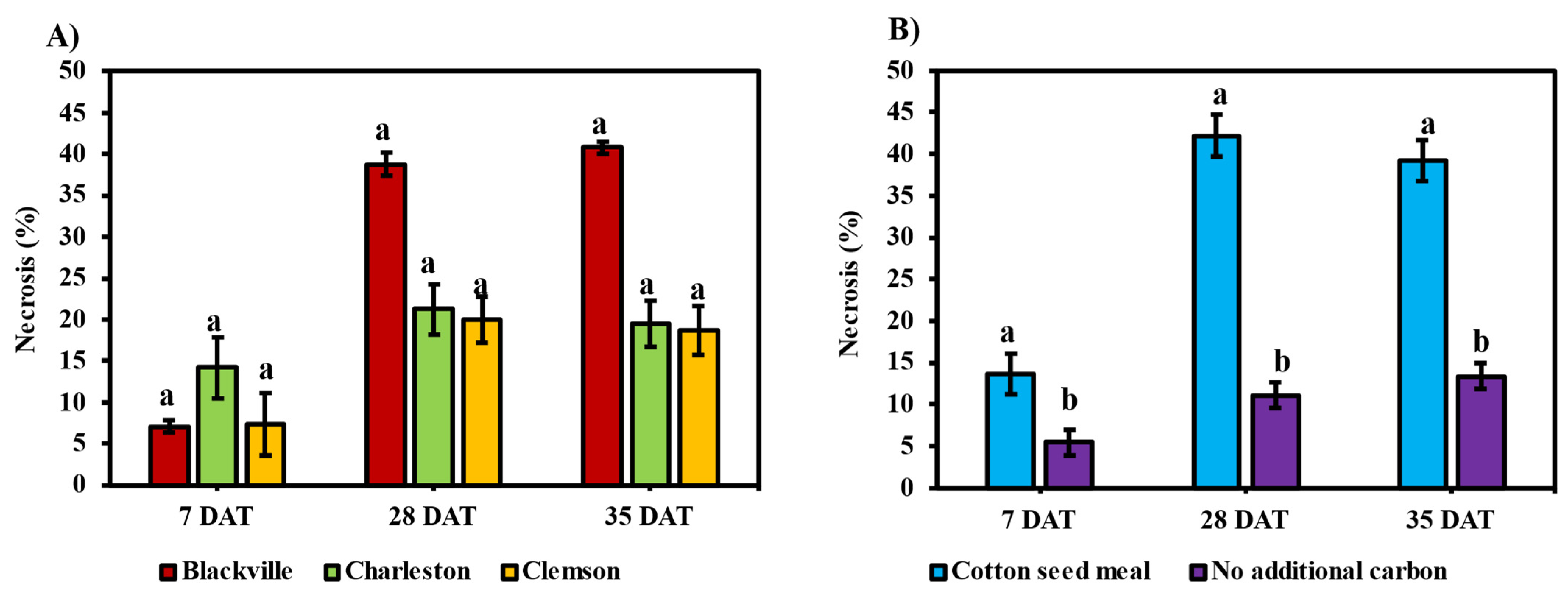
3.3. Necrosis on Sweetpotato Plants
3.4. Nematode Reproduction and Gall Index
3.4.1. Nematode Soil Population Density
3.4.2. Nematode Egg Production
3.4.3. Root Gall Index
3.5. Sweetpotato Biomass
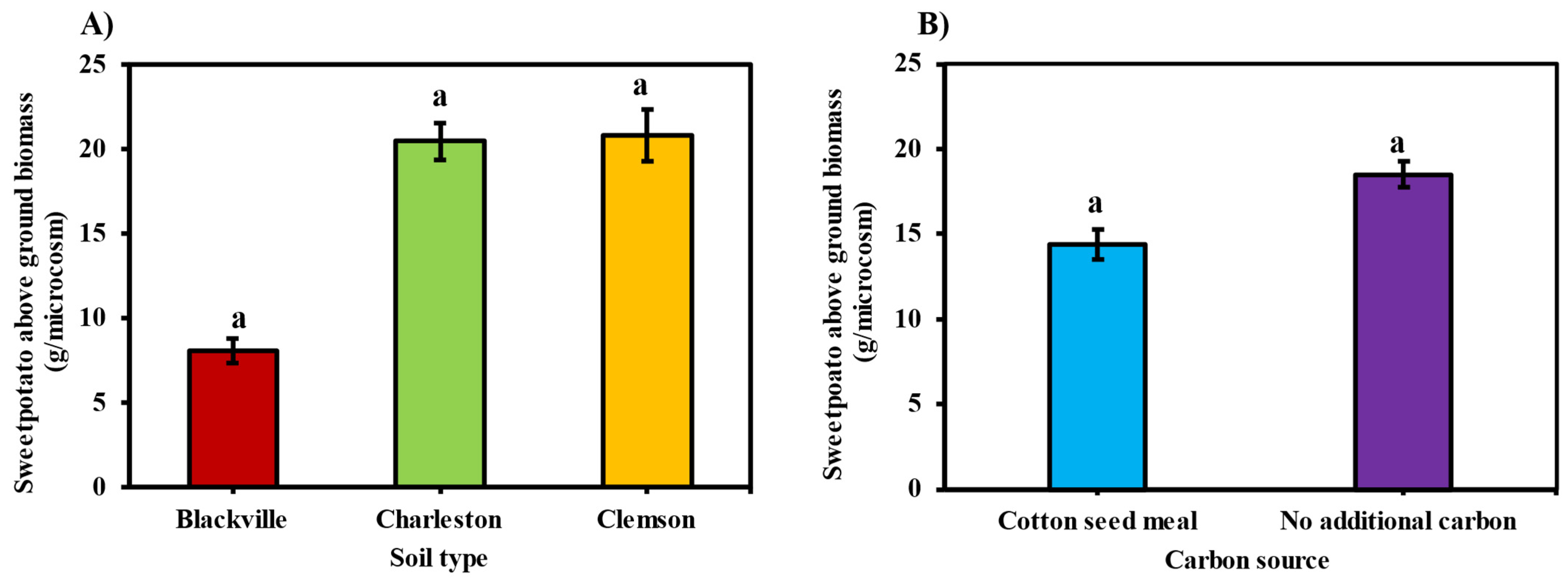
3.5.1. Sweetpotato Above-Ground Biomass
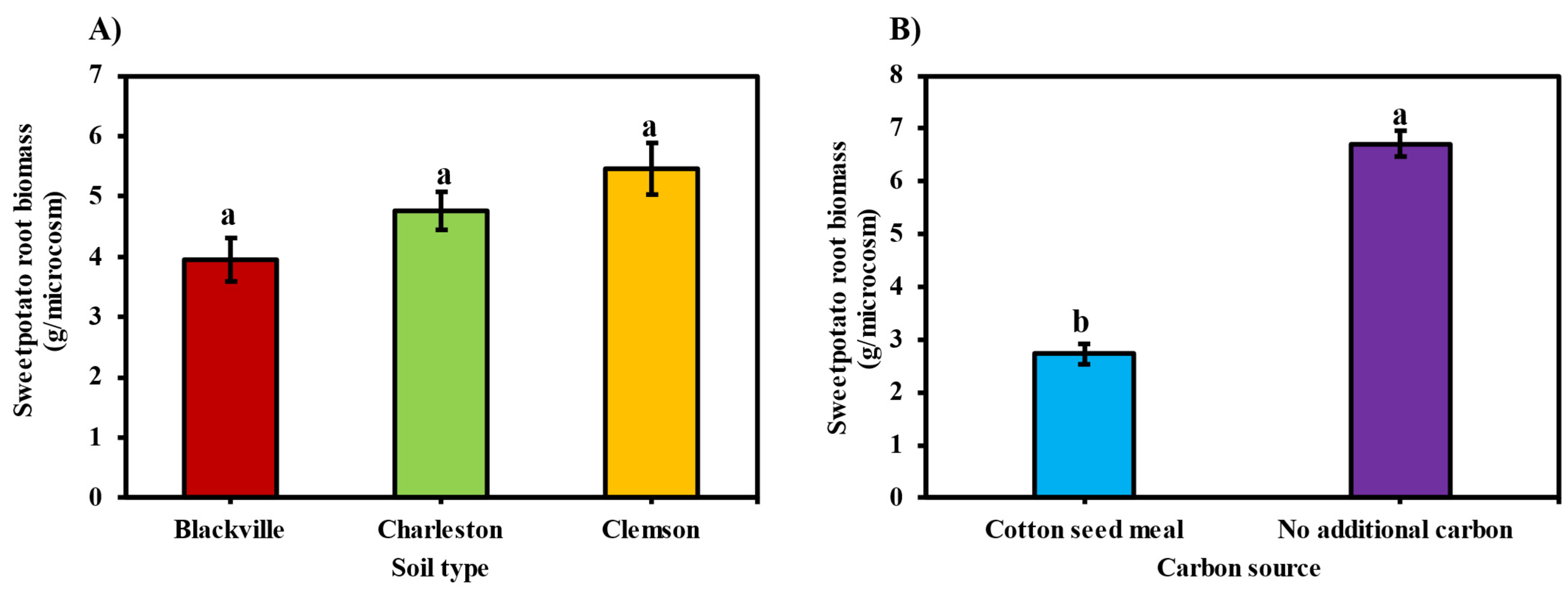
3.5.2. Sweetpotato Root Biomass
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Behera, S.; Chauhan, V.B.S.; Pati, K.; Bansode, V.; Nedunchezhiyan, M.; Verma, A.K.; Monalisa, K.; Naik, P.K.; Naik, S.K. Biology and biotechnological aspect of sweetpotato (Ipomoea batatas L.): A commercially important tuber crop. Planta 2022, 256, 40. [Google Scholar] [CrossRef]
- Harrison, H.F.; Jackson, D.M. Response of Two Sweetpotato Cultivars to Weed Interference. Crop Prot. 2011, 30, 1291–1296. [Google Scholar] [CrossRef]
- Ray, R.C.; Ravi, V. Post harvest spoilage of sweetpotato in Tropics and control measures. Crit. Rev. Food Sci. Nutr. 2005, 45, 623–644. [Google Scholar] [CrossRef]
- Tavva, S.; Nedunchezhiyan, M. Global status of sweetpotato cultivation. Fruit Veg. Cereal Sci. Biotechnol. 2012, 6, 143–147. [Google Scholar]
- Werle, I.S.; Noguera, M.M.; Karaikal, S.K. Integrating weed-suppressive cultivar and cover crops for weed management in organic sweetpotato production. Weed Sci. 2023, 71, 255–264. [Google Scholar] [CrossRef]
- USDA-NASS, The United States Department of Agriculture-National Agricultural Statistics Service. 2022. Available online: https://www.nass.usda.gov/Surveys/Guide_to_NASS_Surveys/Organic_Production/ (accessed on 7 March 2024).
- Nwosisi, S.; Illukpitiya, P.; Nandwani, D.; Arebi, I.T.; Nwosisi, O. Organic and conventional sweetpotato production in the Southeastern of United States: A comparative analysis. Agric. Food Secur. 2021, 10, 27. [Google Scholar] [CrossRef]
- Werle, I.S. Evaluation, Characterization, and Utilization of Weed-Suppressive Sweetpotato Cultivars for Sustainable Weed Management. Master’s Thesis, University of Arkansas, Fayetteville, NC, USA, 2022. [Google Scholar]
- Meyers, S.L.; Jennings, K.M.; Schultheis, J.R.; Monks, D.W. Interference of Palmer Amaranth (Amaranthus palmeri) in sweetpotato. Weed Sci. 2010, 58, 199–203. [Google Scholar] [CrossRef]
- Meyers, S.L.; Shankle, M.W. Postemergence Yellow Nutsedge management in sweetpotato. Weed Technol. 2016, 30, 148–153. [Google Scholar] [CrossRef]
- Monks, D.W.; Jennings, K.M.; Meyers, S.L.; Smith, T.P.; Korres, N.E. Sweetpotato: Important weeds and sustainable weed management. In Weed Control: Sustainability, Hazards, and Risks in Cropping Systems Worldwide; CREC Press: Boca Raton, FL, USA, 2018; Volume 1, pp. 580–597. [Google Scholar]
- Coleman, L.B. Stale Seedbed Manipulation, Increased Rates of Flumioxazin, and Wick-Applied Herbicides for Palmer Amaranth Control in ‘Covington’ Sweetpotato. Master’s Thesis, North Carolina State University, Raleigh, NC, USA, 2014. [Google Scholar]
- Tregeagle, D.; Washburn, D. Sweet Potato Enterprise Budget; North Carolina State University: Raleigh, NC, USA, 2020; Available online: https://cals.ncsu.edu/are-extension/business-planning-and-operations/enterprise-budgets/ (accessed on 23 November 2023).
- Van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef]
- Sikandar, A.; Zhang, M.Y.; Zhu, X.F.; Wang, Y.Y.; Ahmed, M.; Iqbal, M.F.; Javeed, A.; Xuan, Y.H.; Fan, H.Y.; Liu, X.Y.; et al. Efficacy of Penicillium Chrysogenum Strain Snef1216 against Root-Knot nematodes (Meloidogyne incognita) in cucumber (Cucumis sativus L.) under greenhouse conditions. Appl. Ecol. Environ. Res. 2019, 17, 12451–12464. [Google Scholar] [CrossRef]
- Youssef, R.M.; Kim, K.H.; Haroon, S.A.; Matthews, B.F. Post-Transcriptional gene silencing of the gene encoding aldolase from soybean cyst nematode by transformed soybean roots. Exp. Parasitol. 2013, 134, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Dareus, R.; Porto, A.C.M.; Bogale, M.; DiGennaro, P.; Chase, C.A.; Rios, E.F. Resistance to Meloidogyne enterolobii and Meloidogyne incognita in cultivated and wild cowpea. HortScience 2021, 56, 460–468. [Google Scholar] [CrossRef]
- Abad, P.; Favery, B.; Rosso, M.N.; Castagnone-Sereno, P. Root-knot nematode parasitism and host response: Molecular basis of a sophisticated interaction. Mol. Plant Pathol. 2003, 4, 217–224. [Google Scholar] [CrossRef]
- Lawrence, G.W.; Clark, C.A.; Wright, V.L. Influence of Meloidogyne incognita on resistant and susceptible sweetpotato cultivars. J. Nematol. 1986, 18, 59–65. [Google Scholar] [PubMed]
- Brito, J.A.; Stanley, J.D.; Mendes, M.L.; Cetintas, R.; Dickson, D.W. Host status of selected cultivated plants to Meloidogyne mayaguensis in Florida. Nematropica 2007, 37, 65–71. [Google Scholar]
- Ye, W.M.; Koenning, S.R.; Zhuo, K.; Liao, J.L. First report of Meloidogyne enterolobii on cotton and soybean in North Carolina, United States. Plant Dis. 2013, 97, 1262. [Google Scholar] [CrossRef] [PubMed]
- Rutter, W.B.; Skantar, A.M.; Handoo, Z.A.; Mueller, J.D.; Aultman, S.P.; Agudelo, P. Meloidogyne enterolobii found infecting Root-Knot nematode resistant sweetpotato in South Carolina, United States. Plant Dis. 2019, 103, 775. [Google Scholar] [CrossRef]
- Rutter, W.B.; Wadl, P.A.; Mueller, J.D.; Agudelo, P. Identification of sweetpotato germplasm resistant to pathotypically distinct isolates of Meloidogyne enterolobii from the carolinas. Plant Dis. 2021, 105, 3147–3153. [Google Scholar] [CrossRef]
- Alam, M.S.; Khanal, C.; Rutter, W.; Roberts, J. Non-fumigant nematicides are promising alternatives to fumigants for the management of Meloidogyne enterolobii in Tobacco. J. Nematol. 2022, 54, 20220045. [Google Scholar] [CrossRef]
- Onkendi, E.M.; Kariuki, G.M.; Marais, M.; Moleleki, L.N. The threat of Root-Knot nematodes (Meloidogyne spp.) in Africa: A Review. Plant Pathol. 2014, 63, 727–737. [Google Scholar] [CrossRef]
- Blok, W.J.; Lamers, J.G.; Termorshuizen, A.J.; Bollen, G.J. Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathology 2000, 90, 253–259. [Google Scholar] [CrossRef]
- Messiha, N.A.S.; Van Diepeningen, A.D.; Wenneker, M.; Van Beuningen, A.R.; Janse, J.D.; Coenen, T.G.C.; Termorshuizen, A.J.; Van Bruggen, A.H.C.; Blok, W.J. Biological soil disinfestation (BSD), a new control method for potato brown rot, Caused by Ralstonia solanacearum Race 3 Biovar 2. Eur. J. Plant Pathol. 2007, 117, 403–415. [Google Scholar] [CrossRef]
- Momma, N. Biological soil disinfestation (BSD) of soilborne pathogens and Its possible mechanisms. Jpn. Agric. Res. Q. 2008, 42, 7–12. [Google Scholar] [CrossRef]
- Momma, N.; Yamamoto, K.; Simandi, P.; Shishido, M. Role of organic acids in the mechanisms of biological soil disinfestation (BSD). J. Gen. Plant Pathol. 2006, 72, 247–252. [Google Scholar] [CrossRef]
- Momma, N.; Kobara, Y.; Uematsu, S.; Kita, N.; Shinmura, A. Development of biological soil disinfestations in Japan. Appl. Microbiol. Biotechnol. 2013, 97, 3801–3809. [Google Scholar] [CrossRef]
- Butler, D.M.; Kokalis-Burelle, N.; Muramoto, J.; Shennan, C.; McCollum, T.G.; Rosskopf, E.N. Impact of anaerobic soil disinfestation combined with soil solarization on plant-parasitic nematodes and Introduced inoculum of soilborne plant pathogens in raised-bed vegetable production. Crop Prot. 2012, 39, 33–40. [Google Scholar] [CrossRef]
- Butler, D.M.; Kokalis-Burelle, N.; Albano, J.P.; McCollum, T.G.; Muramoto, J.; Shennan, C.; Rosskopf, E.N. Anaerobic soil disinfestation (ASD) combined with soil solarization as a Methyl Bromide alternative: Vegetable crop performance and soil nutrient dynamics. Plant Soil. 2014, 378, 365–381. [Google Scholar] [CrossRef]
- Strauss, S.L.; Kluepfel, D.A. Anaerobic soil disinfestation: A chemical-independent approach to pre-plant control of plant pathogens. J. Integr. Agric. 2015, 14, 2309–2318. [Google Scholar] [CrossRef]
- Hasith Priyashantha, A.K.; Attanayake, R.N. Can Anaerobic soil disinfestation (ASD) be a game changer in tropical agriculture? Pathogens 2021, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Ward, B.K.; Wechter, W.P.; Katawczik, M.L.; Farmaha, B.S.; Suseela, V.; Cutulle, M.A. Assessment of agro-industrial wastes as a carbon source in anaerobic disinfestation of soil contaminated with weed seeds and phytopathogenic bacterium (Ralstonia solanacearum) in Tomato (Solanum lycopersicum). ACS Agric. Sci. Technol. 2022, 2, 769–779. [Google Scholar] [CrossRef]
- Butler, D.M.; Rosskopf, E.N.; Kokalis-Burelle, N. Exploring warm-season cover crops as carbon sources for anaerobic soil disinfestation (ASD). Plant Soil. 2012, 355, 149–165. [Google Scholar] [CrossRef]
- Singh, G.; Ward, B.; Levi, A.; Cutulle, M. Weed management by in situ cover crops and anaerobic soil disinfestation in plasticulture. Agronomy 2022, 12, 2754. [Google Scholar] [CrossRef]
- Liu, D.; Samtani, J.B.; Johnson, C.S.; Butler, D.M.; Derr, J. Weed control assessment of various carbon sources for anaerobic soil disinfestation. Int. J. Fruit Sci. 2020, 20, 1005–1018. [Google Scholar] [CrossRef]
- Fiedler, S.; Vepraskas, M.J.; Richardson, J.L. Soil redox potential: Importance, field measurements, and observations. Adv. Agron. 2007, 94, 1–54. [Google Scholar] [CrossRef]
- Rabenhorst, M.C.; Castenson, K.L. Temperature effects on iron reduction in a hydric soil. Soil Sci. 2005, 170, 734–742. [Google Scholar] [CrossRef]
- Wang, H.; Liu, W.; Zhao, K.; Yu, H.; Zhang, J.; Wang, J. Evaluation of weed control efficacy and crop safety of the new HPPD-inhibiting herbicide-QYR301. Sci. Rep. 2018, 8, 7910. [Google Scholar] [CrossRef]
- Andújar, D.; Ribeiro, A.; Carmona, R.; Fernández-Quintanilla, C.; Dorado, J. An assessment of the accuracy and consistency of human perception of weed cover. Weed Res. 2010, 50, 638–647. [Google Scholar] [CrossRef]
- Hoyle, J.A.; Yelverton, F.H.; Gannon, T.W. Evaluating Multiple Rating Methods Utilized in Turfgrass Weed Science. Weed Technol. 2013, 27, 362–368. [Google Scholar] [CrossRef]
- Meyers, S.L.; Jennings, K.M.; Monks, D.W. Sweetpotato Response to Simulated Glyphosate Wick Drip. Weed Technol. 2017, 31, 130–135. [Google Scholar] [CrossRef]
- Caputo, G.A.; Wadl, P.A.; McCarty, L.; Adelberg, J.; Saski, C.; Cutulle, M. Impact of tank mixing plant hormones with bentazon and mesotrione on sweetpotato injury and weed control. Agrosyst. Geosci. Environ. 2021, 4, e20185. [Google Scholar] [CrossRef]
- Jenkins, W.R. A rapid centrifugal-flotation technique for separating nematodes. Plant Dis. Rep. 1964, 48, 692. [Google Scholar]
- Thies, J.A.; Merrill, S.B.; Corley, E.L. Red food coloring stain: New, safer procedures for staining nematodes in roots and egg masses on root surfaces. J. Nematol. 2002, 34, 179–181. [Google Scholar] [PubMed]
- Schwarz, T.R.; Li, L.; Yencho, C.G.; Pecota, K.V.; Heim, C.R.; Davis, E.L. Screening Sweetpotato Genotypes for Resistance to a North Carolina Isolate of Meloidogyne enterolobii. Plant Dis. 2020, 105, 1101–1107. [Google Scholar] [CrossRef]
- Shrestha, U.; Augé, R.M.; Butler, D.M. A Meta-Analysis of the Impact of anaerobic soil disinfestation on pest suppression and yield of horticultural crops. Front. Plant Sci. 2016, 7, 1254. [Google Scholar] [CrossRef] [PubMed]
- Hewavitharana, S.S.; Mazzola, M. Carbon source-dependent effects of anaerobic soil disinfestation on soil microbiome and suppression of Rhizoctonia solani AG-5 and Pratylenchus penetrans. Phytopathology 2016, 106, 1015–1028. [Google Scholar] [CrossRef]
- Feys, J.; Reheul, D.; De Smet, W.; Clercx, S.; Palmans, S.; Van de Ven, G.; De Cauwer, B. Effect of Anaerobic Soil Disinfestation on Tuber Vitality of Yellow Nutsedge (Cyperus esculentus). Agriculture 2023, 13, 1547. [Google Scholar] [CrossRef]
- Oleg, D.; Maren, J.M. Barriers prevent emergence of yellow nutsedge (Cyperus esculentus) in annual plasticulture strawberry (Fragaria × Ananassa). Weed Technol. 2010, 24, 478–482. [Google Scholar] [CrossRef]
- Meyers, S.L.; Shankle, M.W. Interference of Yellow Nutsedge (Cyperus esculentus) in Beauregard Sweetpotato (Ipomoea batatas). Weed Technol. 2015, 29, 854–860. [Google Scholar] [CrossRef]
- Singh, G.; Wechter, W.P.; Farmaha, B.S.; Cutulle, M. Integration of halosulfuron and anaerobic soil disinfestation for weed control in tomato. HortTechnology 2022, 32, 401–414. [Google Scholar] [CrossRef]
- Shrestha, U.; Rosskopf, E.N.; Butler, D.M. Effect of anaerobic soil disinfestation amendment type and C:N ratio on Cyperus esculentus tuber sprouting, growth and reproduction. Weed Res. 2018, 58, 379–388. [Google Scholar] [CrossRef]
- Díaz-Hernández, S.; Gallo-Llobet, L.; Domínguez-Correa, P.; Rodríguez, A. Effect of Repeated Cycles of Soil Solarization and Biosolarization on Corky Root, Weeds and Fruit Yield in Screen-House Tomatoes under Subtropical Climate Conditions in the Canary Islands. Crop Prot. 2017, 94, 20–27. [Google Scholar] [CrossRef]
- Gilardi, G.; Pugliese, M.; Gullino, M.L.; Garibaldi, A. Evaluation of different carbon sources for anaerobic soil disinfestation against Rhizoctonia solani on lettuce in controlled production systems. Phytopathol. Mediterr. 2020, 59, 77–96. [Google Scholar] [CrossRef]
- Muramoto, J.; Shennan, C.; Zavatta, M.; Baird, G.; Toyama, L.; Mazzola, M. Effect of Anaerobic Soil Disinfestation and Mustard Seed Meal for Control of Charcoal Rot in California Strawberries. Int. J. Fruit Sci. 2016, 16, 59–70. [Google Scholar] [CrossRef]
- Achmon, Y.; Harrold, D.R.; Claypool, J.T.; Stapleton, J.J.; Van der Gheynst, J.S.; Simmons, C.W. Assessment of tomato and wine processing solid wastes as soil amendments for biosolarization. Waste Manag. 2016, 48, 156–164. [Google Scholar] [CrossRef]
- Khanal, C.; Land, J. Study on two nematode species suggests climate change will inflict greater crop damage. Sci. Rep. 2023, 13, 14185. [Google Scholar] [CrossRef]
- Di Gioia, F.; Ozores-Hampton, M.; Hong, J.; Kokalis-Burelle, N.; Albano, J.; Zhao, X.; Black, Z.; Gao, Z.; Moore, K.; Swisher, M.; et al. The effects of anaerobic soil disinfestation on weed and nematode control, fruit yield, and quality of Florida fresh-market tomato. HortScience 2016, 51, 703–711. [Google Scholar] [CrossRef]
- Katase, M.; Kubo, C.; Ushio, S.; Ootsuka, E.; Takeuchi, T.; Mizukubo, T. Nematicidal Activity of volatile fatty acids generated from wheat bran in reductive soil disinfestation. Jpn. J. Nematol. 2009, 39, 53–62. [Google Scholar] [CrossRef]
- Testen, A.L.; Miller, S.A. Carbon source and soil origin shape soil microbiomes and tomato soilborne pathogen populations during anaerobic soil disinfestation. Phytobiomes J. 2018, 2, 138–150. [Google Scholar] [CrossRef]
- Oka, Y. Mechanisms of nematode suppression by organic soil amendments-A review. Appl. Soil Ecol. 2010, 44, 101–115. [Google Scholar] [CrossRef]
- Conn, K.L.; Tenuta, M.; Lazarovits, G. Liquid swine manure can kill Verticillium dahliae microsclerotia in soil by volatile fatty acid, nitrous acid, and ammonia toxicity. Phytopathology 2005, 95, 28–35. [Google Scholar] [CrossRef]
- López-Robles, J.; Olalla, C.; Rad, C.; Díez-Rojo, M.A.; López-Pérez, J.A.; Rodríguez-Kábana, R. The use of liquid swine manure for the control of potato cyst nematode through soil disinfestation in laboratory conditions. Crop Protect. 2013, 49, 1–7. [Google Scholar] [CrossRef]
- Mazzola, M.; Brown, J.; Izzo, A.D.; Cohen, M.F. Mechanism of action and efficacy of seed meal induced pathogen suppression differ in a Brassicaceae species and time-dependent manner. Phytopathology 2007, 97, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Bose, B.; Srivastava, H.S.; Mathur, S.N. Effect of some nitrogenous salts on nitrogen transfer and protease activity in germinating Zea mays L. seeds. Biol. Plant. 1982, 24, 89–95. [Google Scholar] [CrossRef]
- Rice, A.R.; Johnson-Maynard, J.L.; Thill, D.C.; Morra, M.J. Vegetable crop emergence and weed control following amendment with different Brassicaceae seed meals. Renew. Agric. Food Syst. 2007, 22, 204–212. [Google Scholar] [CrossRef]

| Soil Source Origin | Soil Texture | Organic Matter (%) | Soil pH | P lbs/A | K lbs/A | Ca lbs/A | Mg lbs/A | Zn lbs/A | Mn lbs/A | Cu lbs/A | B lbs/A | Na lbs/A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blackville, SC | Wagram sand | 0.75 | 6.1 | 96 | 89 | 698 | 101 | 5.6 | 12 | 0.9 | 0.6 | 5 |
| Charleston, SC | Sandy loam | 2 | 6.7 | 218 | 138 | 1090 | 177 | 5 | 27 | 2.8 | 0.3 | 11 |
| Clemson, SC | Clay | 7 | 3.0 | 26 | 133 | 1490 | 222 | 4.9 | 49 | 1.8 | 2.7 | 13 |
| Soil Type | Carbon Source | Weed Population/Microcosm | |||||
|---|---|---|---|---|---|---|---|
| Yellow Nutsedge | Crabgrass | Carpet Weed | Yellow Woodsorrel | ||||
| Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | ||||
| Blackville | Cotton seed meal | 0.33 a | 0.0 b | 1.0 b | 0.7 a | 12.5 a | 3.3 a |
| No additional carbon | 3.33 a | 2.0 b | 3.7 ab | 2.7 a | 21.0 a | 2.8 a | |
| Charleston | Cotton seed meal | 0.66 a | 0.0 b | 1.7 ab | 1.0 a | 1.5 a | 0.8 a |
| No additional carbon | 3.33 a | 20.0 a | 1.7 ab | 5.0 a | 9.5 a | 8.5 a | |
| Clemson | Cotton seed meal | 0.66 a | 0.0 b | 6.7 a | 12.0 a | 8.3 a | 10.0 a |
| No additional carbon | 3.66 a | 2.3 b | 3.0 ab | 6.3 a | 16.1 a | 6.5 a | |
| p-value | |||||||
| Soil type | 0.9462 | 0.0037 * | 0.0273 * | 0.0262 * | 0.0721 | 0.0992 | |
| Carbon source | 0.0041 * | 0.0014 * | 0.7076 | 0.9580 | 0.0440 * | 0.5322 | |
| Soil type × carbon source | 0.9817 | 0.0037 * | 0.0353 * | 0.1730 | 0.9973 | 0.0662 | |
| Soil Type | Carbon Source | Gall Index/Root System | Egg Mass/Root System | ||
|---|---|---|---|---|---|
| Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | ||
| Blackville | Cotton seed meal | 36.6 a | 0.0 b | 70.3 a | 0.0 c |
| No additional carbon | 13.3 a | 80.0 a | 20.4 a | 225.0 a | |
| Charleston | Cotton seed meal | 13.3 a | 0.0 b | 66.6 a | 0.0 c |
| No additional carbon | 23.3 a | 30.0 b | 21.0 a | 100.3 b | |
| Clemson | Cotton seed meal | 0.0 a | 0.0 b | 0.0 a | 0.3 c |
| No additional carbon | 5.0 a | 10.0 b | 16.6 a | 5.3 c | |
| p-value | |||||
| Soil type | 0.3683 | 0.0028 * | 0.5229 | 0.0013 * | |
| Carbon source | 0.8311 | <0001 * | 0.9377 | <0001 * | |
| Soil type × carbon source | 0.5338 | 0.0028 * | 0.5926 | 0.0005 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Rutter, W.; Wadl, P.A.; Campbell, H.T.; Khanal, C.; Cutulle, M. Effectiveness of Anaerobic Soil Disinfestation for Weed and Nematode Management in Organic Sweetpotato Production. Agronomy 2024, 14, 1935. https://doi.org/10.3390/agronomy14091935
Singh S, Rutter W, Wadl PA, Campbell HT, Khanal C, Cutulle M. Effectiveness of Anaerobic Soil Disinfestation for Weed and Nematode Management in Organic Sweetpotato Production. Agronomy. 2024; 14(9):1935. https://doi.org/10.3390/agronomy14091935
Chicago/Turabian StyleSingh, Simardeep, William Rutter, Phillip A. Wadl, Harrison Tyler Campbell, Churamani Khanal, and Matthew Cutulle. 2024. "Effectiveness of Anaerobic Soil Disinfestation for Weed and Nematode Management in Organic Sweetpotato Production" Agronomy 14, no. 9: 1935. https://doi.org/10.3390/agronomy14091935






