Impact of Bio-Organic Fertilizer Incorporation on Soil Nutrients, Enzymatic Activity, and Microbial Community in Wheat–Maize Rotation System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Description
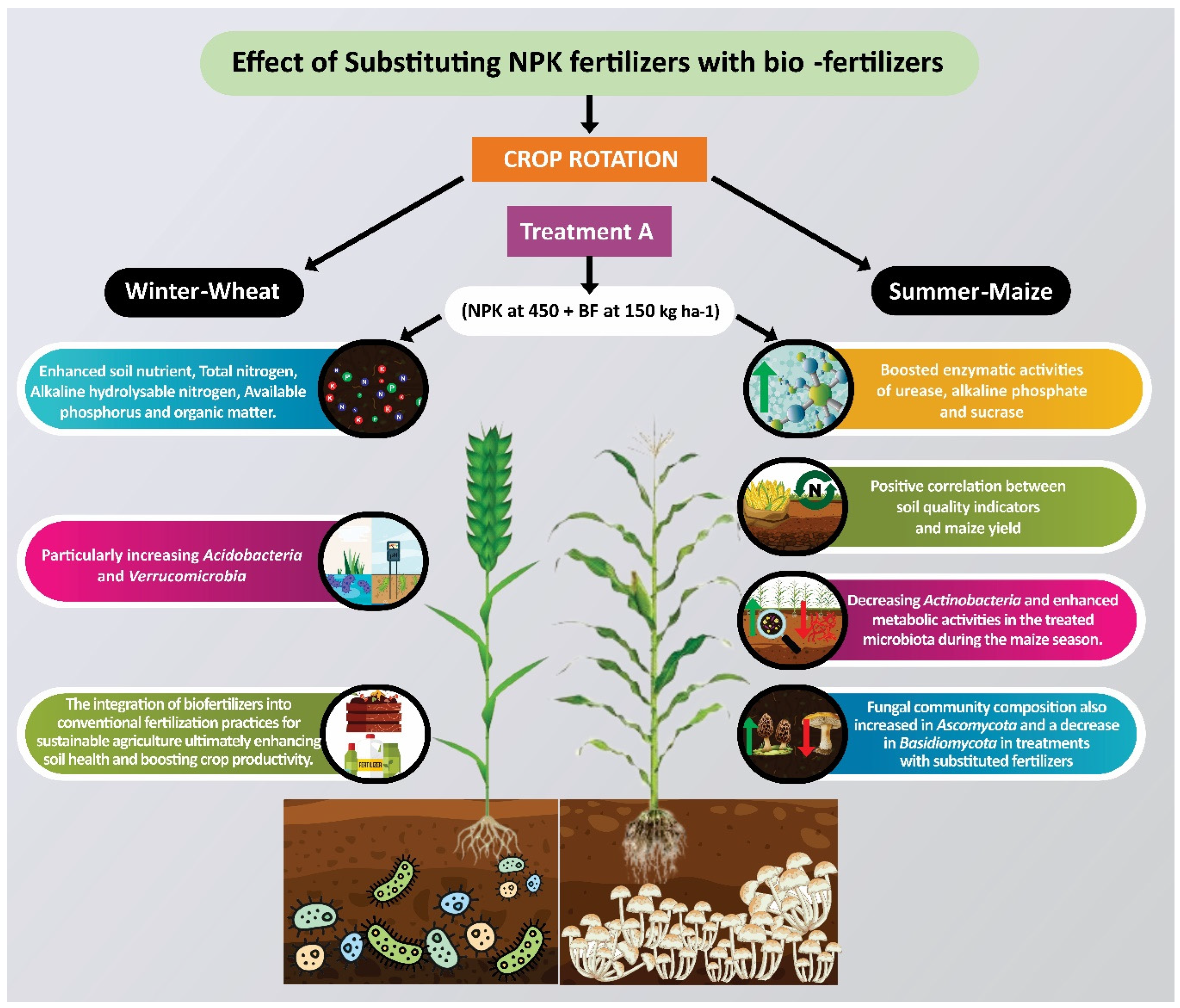
2.2. Experimental Design and Treatments
2.3. Field Sampling and Processing
2.4. Soil Characteristics and Microbial Enzymatic Activities
2.5. Soil DNA Extraction, Quantitative Real-Time PCR (q-PCR), and Illumina Sequencing
2.6. Sequencing Data Processing
2.7. Statistical Analyses
3. Results
3.1. Impact of Various Fertilization Treatments on Soil Nutrient Levels in Wheat during Different Stages of Growth
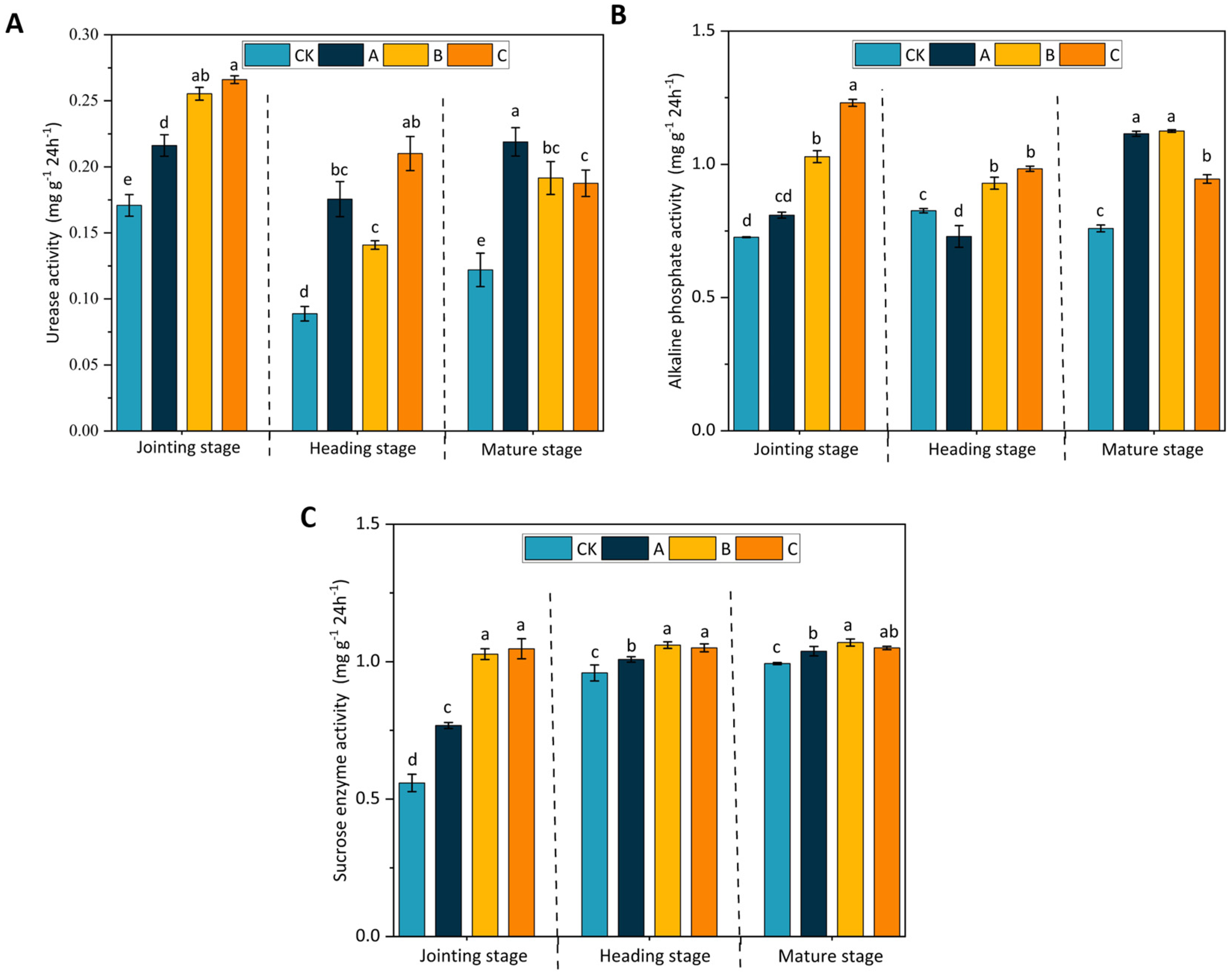
3.2. Soil Enzymatic Activities at Different Stages of Wheat Growth
3.3. Changes in Soil Nutrient Contents under Different Fertilization Treatments during Various Growth Stages in Maize
3.4. Alteration in Soil Enzymatic Activities during Different Developmental Phases in Maize
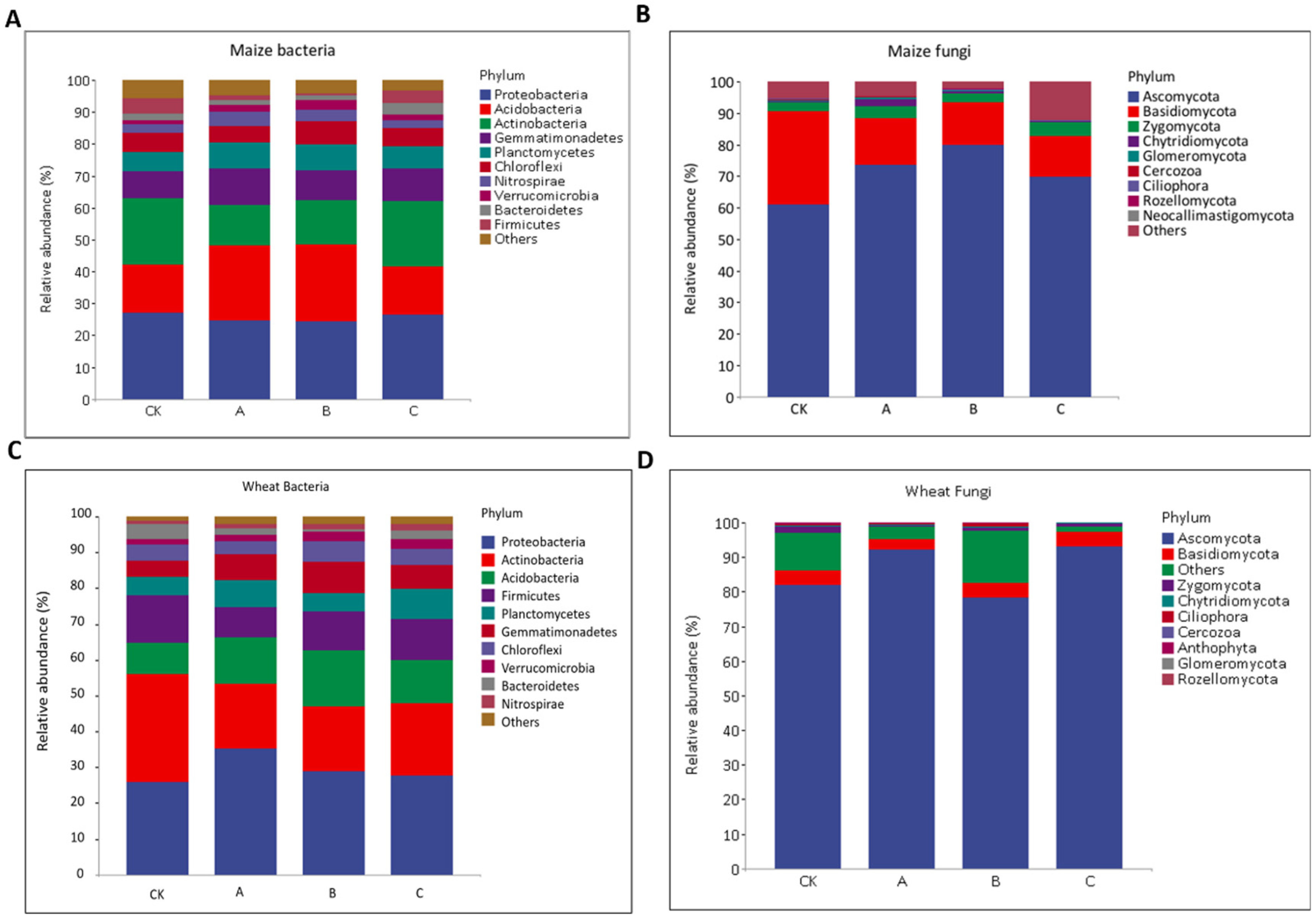
3.5. Composition and Relative Abundance of Bacterial and Fungal Communities in Soils in Response to Different Fertilization Treatments at Mature the Stage
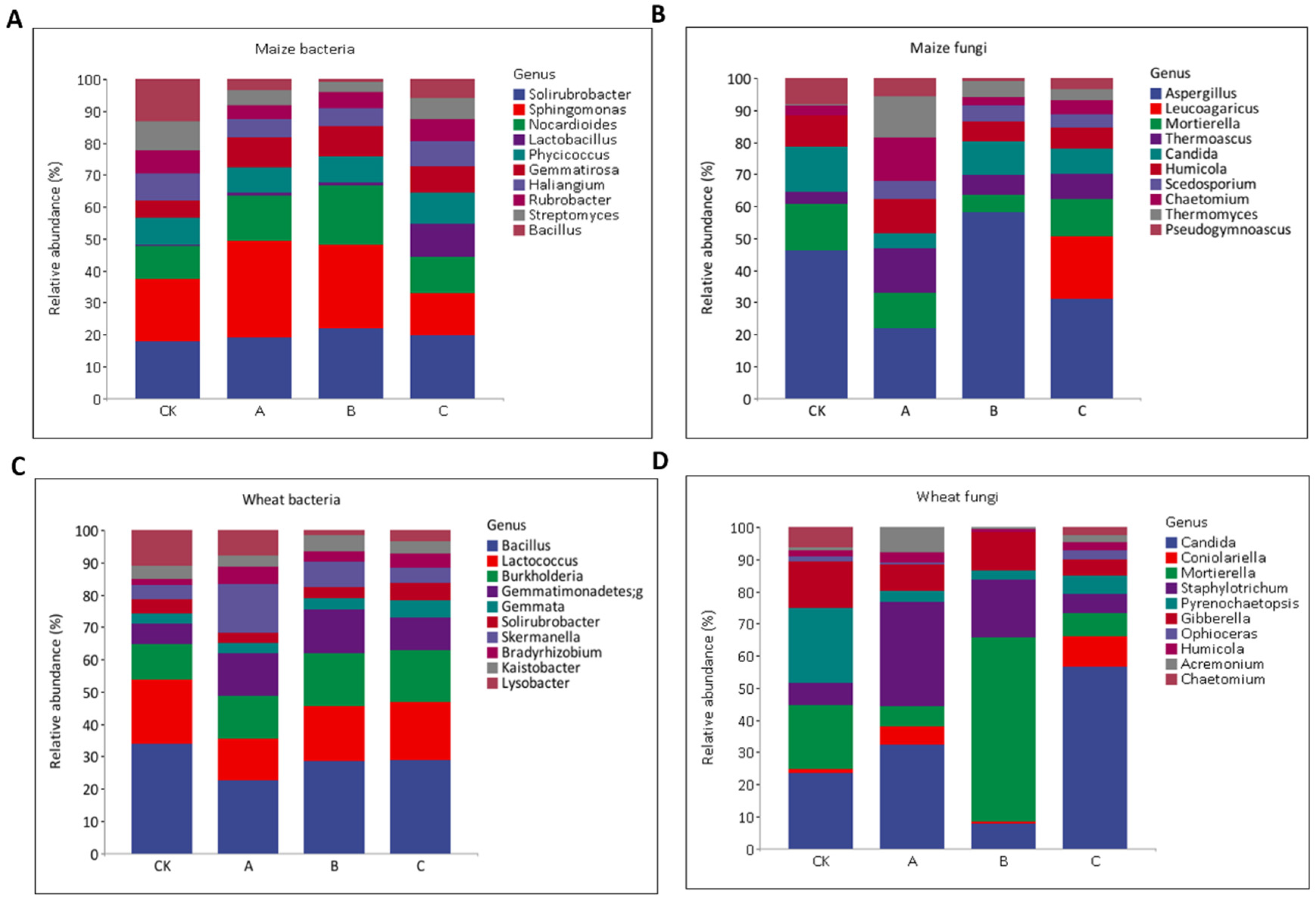
3.6. Changes in the Relative Abundance of Bacterial and Fungal Composition at Genus Level under Different Bio-Fertilizer Treatments in Wheat–Maize across the Seasons
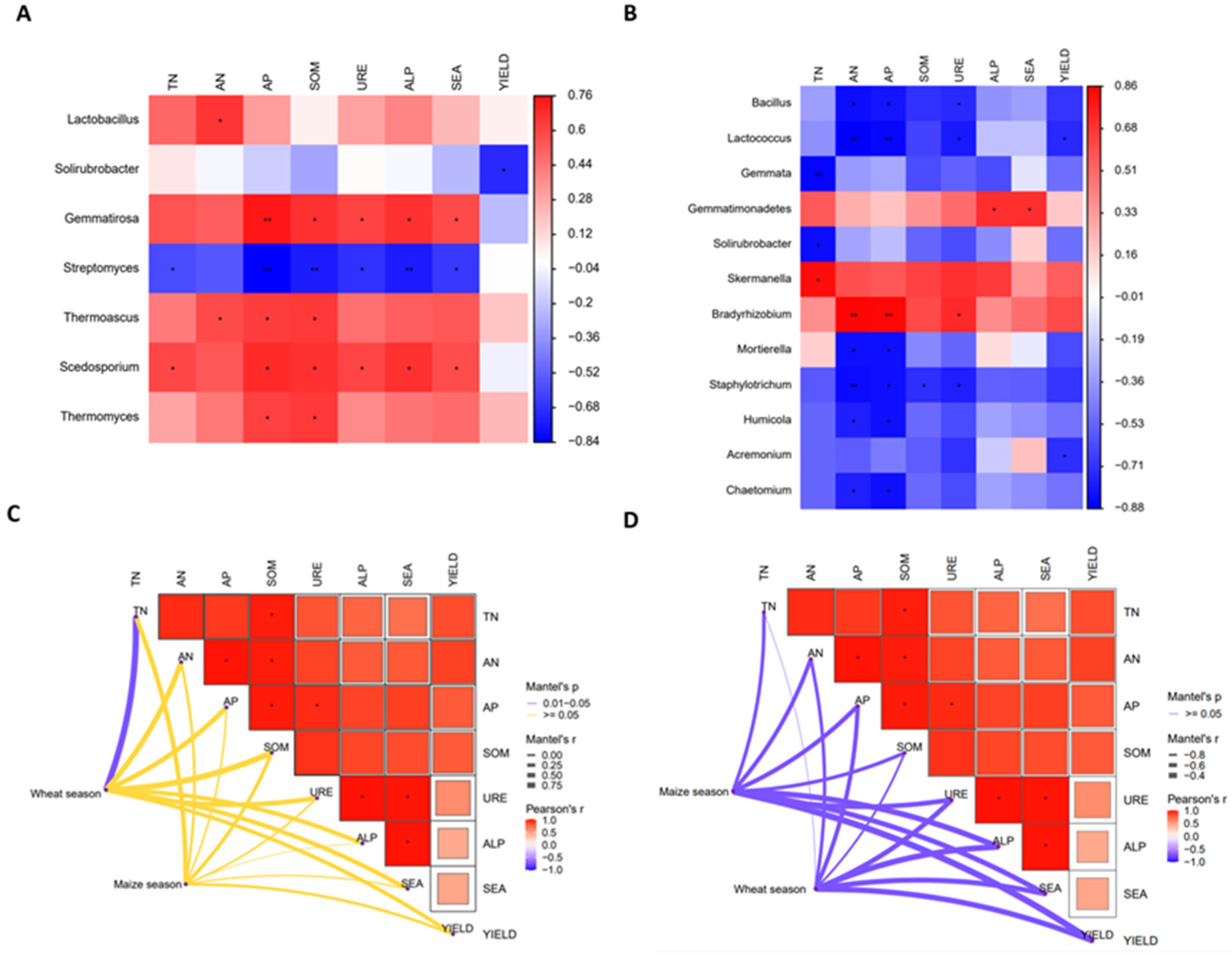
3.7. Correlation Analysis between Enzymes and Genes Related to Phyla and Genera and Soil Nutrients in Wheat–Maize under Different Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cui, Z.; Dou, Z.; Chen, X.; Ju, X.; Zhang, F. Managing agricultural nutrients for food security in China: Past, present, and future. Agron. J. 2014, 106, 191–198. [Google Scholar] [CrossRef]
- Chen, A.; Lei, B.; Lu, Y.; Mao, Y.; Zhang, D. Study on nitrogen application rate tolerance based on rice yield and ecological security in hilly areas of South China. Trans. Chin. Soc. Agric. Eng. 2013, 29, 131–139. [Google Scholar]
- Zhao, J.; Luo, Q.; Deng, H.; Yan, Y. Opportunities and challenges of sustainable agricultural development in China. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 893–904. [Google Scholar] [CrossRef]
- Chao, G.; Bo, S.; Zhang, T.-L. Sustainable nutrient management in Chinese agriculture: Challenges and perspective. Pedosphere 2006, 16, 253–263. [Google Scholar]
- Chen, F.; Xiao, T.; Zhu, Z.; Yang, X.; Ran, W.; Shen, Q. Effect of bio-organic fertilizers on root-knot nematode of muskmelon in field. Plant Nutr. Fertil. Sci. 2011, 17, 1262–1267. [Google Scholar]
- Syed, S.; Wang, X.; Prasad, T.N.; Lian, B. Bio-organic mineral fertilizer for sustainable agriculture: Current trends and future perspectives. Minerals 2021, 11, 1336. [Google Scholar] [CrossRef]
- Fan, T.; Stewart, B.; Yong, W.; Junjie, L.; Guangye, Z. Long-term fertilization effects on grain yield, water-use efficiency and soil fertility in the dryland of Loess Plateau in China. Agric. Ecosyst. Environ. 2005, 106, 313–329. [Google Scholar] [CrossRef]
- Juan, L.; Zhao, B.-Q.; Li, X.-Y.; Jiang, R.-B.; Bing, S.H. Effects of long-term combined application of organic and mineral fertilizers on microbial biomass, soil enzyme activities and soil fertility. Agric. Sci. China 2008, 7, 336–343. [Google Scholar]
- Mi, W.; Wu, L.; Ma, Q.; Zhang, X.; Liu, Y. Combined application of organic materials and inorganic fertilizers improving rice yield and soil fertility of yellow clayey paddy soil. Trans. Chin. Soc. Agric. Eng. 2016, 32, 103–108. [Google Scholar]
- Arikan, Ş.; Pirlak, L. Effects of plant growth promoting rhizobacteria (PGPR) on growth, yield and fruit quality of sour cherry (Prunus cerasus L.). Erwerbs-Obstbau 2016, 58, 221–226. [Google Scholar] [CrossRef]
- Han, Z.; Hou, H.; Yao, X.; Qian, X.; Zhou, M. Substituting Partial Chemical Fertilizers with Bio-Organic Fertilizers to Reduce Greenhouse Gas Emissions in Water-Saving Irrigated Rice Fields. Agronomy 2024, 14, 544. [Google Scholar] [CrossRef]
- Zhai, L.; Wang, Z.; Zhai, Y.; Zhang, L.; Zheng, M.; Yao, H.; Lv, L.; Shen, H.; Zhang, J.; Yao, Y. Partial substitution of chemical fertilizer by organic fertilizer benefits grain yield, water use efficiency, and economic return of summer maize. Soil Tillage Res. 2022, 217, 105287. [Google Scholar] [CrossRef]
- He, H.; Peng, M.; Ru, S.; Hou, Z.; Li, J. A suitable organic fertilizer substitution ratio could improve maize yield and soil fertility with low pollution risk. Front. Plant Sci. 2022, 13, 988663. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sharma, V.; Shukla, A.K.; Verma, V.; Kaur, M.; Singh, P.; Gaber, A.; Hossain, A. Effect of addition of organic manures on basmati yield, nutrient content and soil fertility status in north-western India. Heliyon 2023, 9, e14514. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sharma, S.; Sharma, V.; Shukla, A.K.; Walia, S.S.; Alhomrani, M.; Gaber, A.; Toor, A.S.; Verma, V.; Randhawa, M.K. Long-term integrated nutrient management in the maize–wheat cropping system in alluvial soils of North-Western India: Influence on soil organic carbon, microbial activity and nutrient status. Agronomy 2021, 11, 2258. [Google Scholar] [CrossRef]
- Curtin, D.; Campbell, C.; Jalil, A. Effects of acidity on mineralization: pH-dependence of organic matter mineralization in weakly acidic soils. Soil Biol. Biochem. 1998, 30, 57–64. [Google Scholar] [CrossRef]
- Murphy, B. Impact of soil organic matter on soil properties—A review with emphasis on Australian soils. Soil Res. 2015, 53, 605–635. [Google Scholar] [CrossRef]
- Reeve, J.R.; Hoagland, L.A.; Villalba, J.J.; Carr, P.M.; Atucha, A.; Cambardella, C.; Davis, D.R.; Delate, K. Organic farming, soil health, and food quality: Considering possible links. Adv. Agron. 2016, 137, 319–367. [Google Scholar]
- Yadav, K.K.; Sarkar, S. Biofertilizers, impact on soil fertility and crop productivity under sustainable agriculture. Environ. Ecol. 2019, 37, 89–93. [Google Scholar]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.S.; Saxena, A.K. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal. Agric. Biotechnol. 2020, 23, 101487. [Google Scholar] [CrossRef]
- Kumar, D.; Dalal, R.P.S.; Arora, I. Bio-fertilizers a Future Prospect Towards Sustainable Agricultural Development. In Innovative Approaches for Sustainable Development: Theories and Practices in Agriculture; Springer: Berlin/Heidelberg, Germany, 2022; pp. 91–105. [Google Scholar]
- Jing, W.; Sajnani, S.; Zhou, M.; Zhu, H.; Xu, Y. Evaluating the Ecological Impact of Wastewater Discharges on Microbial and Contaminant Dynamics in Rivers. Water 2024, 16, 377. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Olanrewaju, O.S.; Babalola, O.O. Plant growth promoting rhizobacterial mitigation of drought stress in crop plants: Implications for sustainable agriculture. Agronomy 2019, 9, 712. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, D.; Huang, B.; Song, Z.; Ren, L.; Hao, B.; Liu, J.; Zhu, J.; Fang, W.; Yan, D. Organic fertilizer improves soil fertility and restores the bacterial community after 1, 3-dichloropropene fumigation. Sci. Total Environ. 2020, 738, 140345. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yuan, L.; Liu, Y.; Ji, J.; Hou, H. Long-term application of manures plus chemical fertilizers sustained high rice yield and improved soil chemical and bacterial properties. Eur. J. Agron. 2017, 90, 34–42. [Google Scholar] [CrossRef]
- Zhang, L.-S.; Ma, Y.-A.; Li, B.-J.; Zhu, J.; Sun, S.-C. Effect of organic and inorganic fertilizers combined application on increase yield of winter wheat. J. Plant Nutr. Fertil. 2003, 9, 503–505. [Google Scholar]
- Jiang, D.; Hengsdijk, H.; Ting-Bo, D.; Qi, J.; Wei-Xing, C. Long-term effects of manure and inorganic fertilizers on yield and soil fertility for a winter wheat-maize system in Jiangsu, China. Pedosphere 2006, 16, 25–32. [Google Scholar] [CrossRef]
- Kennedy, A.C.; Stubbs, L. Soil microbial communities as indicators of soil health. Ann. Arid Zone 2006, 45. [Google Scholar]
- Regmi, A.; Ladha, J.; Pathak, H.; Pasuquin, E.; Bueno, C.; Dawe, D.; Hobbs, P.; Joshy, D.; Maskey, S.; Pandey, S. Yield and soil fertility trends in a 20-year rice–rice–wheat experiment in Nepal. Soil Sci. Soc. Am. J. 2002, 66, 857–867. [Google Scholar] [CrossRef]
- Cai, Z.; Qin, S. Dynamics of crop yields and soil organic carbon in a long-term fertilization experiment in the Huang-Huai-Hai Plain of China. Geoderma 2006, 136, 708–715. [Google Scholar] [CrossRef]
- Manna, M.C.; Swarup, A.; Wanjari, R.; Mishra, B.; Shahi, D. Long-term fertilization, manure and liming effects on soil organic matter and crop yields. Soil Tillage Res. 2007, 94, 397–409. [Google Scholar] [CrossRef]
- Ren, J.; Liu, X.; Yang, W.; Yang, X.; Li, W.; Xia, Q.; Li, J.; Gao, Z.; Yang, Z. Rhizosphere soil properties, microbial community, and enzyme activities: Short-term responses to partial substitution of chemical fertilizer with organic manure. J. Environ. Manag. 2021, 299, 113650. [Google Scholar] [CrossRef] [PubMed]
- Dadenko, E.; Kazeev, K.S.; Kolesnikov, S.; Val’kov, V. Changes in the enzymatic activity of soil samples upon their storage. Eurasian Soil Sci. 2009, 42, 1380–1385. [Google Scholar] [CrossRef]
- Guangming, L.; Xuechen, Z.; Xiuping, W.; Hongbo, S.; Jingsong, Y.; Xiangping, W. Soil enzymes as indicators of saline soil fertility under various soil amendments. Agric. Ecosyst. Environ. 2017, 237, 274–279. [Google Scholar] [CrossRef]
- Li, P.; Lang, M.; Wang, X.X.; Zhang, T.L. Sorption and desorption of copper and cadmium in a contaminated soil affected by soil amendments. CLEAN–Soil Air Water 2016, 44, 1547–1556. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, Z.; Zhang, B.; Du, S.; Liu, G. The effects of agricultural management on selected soil properties of the arable soils in Tibet, China. Catena 2012, 93, 1–8. [Google Scholar] [CrossRef]
- Chen, J.; Arafat, Y.; Wu, L.; Xiao, Z.; Li, Q.; Khan, M.A.; Khan, M.U.; Lin, S.; Lin, W. Shifts in soil microbial community, soil enzymes and crop yield under peanut/maize intercropping with reduced nitrogen levels. Appl. Soil Ecol. 2018, 124, 327–334. [Google Scholar] [CrossRef]
- Raiesi, F.; Salek-Gilani, S. The potential activity of soil extracellular enzymes as an indicator for ecological restoration of rangeland soils after agricultural abandonment. Appl. Soil Ecol. 2018, 126, 140–147. [Google Scholar] [CrossRef]
- Stpniewska, Z.; Wolińska, A.; Ziomek, J. Response of soil catalase activity to chromium contamination. J. Environ. Sci. 2009, 21, 1142–1147. [Google Scholar] [CrossRef]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.; McGarrell, D.M.; Marsh, T.; Garrity, G.M. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37, D141–D145. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, P.; Yu, T.; Zang, H.; Zeng, Z.; Yang, Y. Manure replacement of chemical fertilizers can improve soil quality in the wheat-maize system. Appl. Soil Ecol. 2024, 200, 105453. [Google Scholar] [CrossRef]
- Mondal, S.; Kumar, R.; Mishra, J.S.; Dass, A.; Kumar, S.; Vijay, K.V.; Kumari, M.; Khan, S.R.; Singh, V.K. Grain nitrogen content and productivity of rice and maize under variable doses of fertilizer nitrogen. Heliyon 2023, 9, e17321. [Google Scholar] [CrossRef] [PubMed]
- Ai, C.; Zhang, S.; Zhang, X.; Guo, D.; Zhou, W.; Huang, S. Distinct responses of soil bacterial and fungal communities to changes in fertilization regime and crop rotation. Geoderma 2018, 319, 156–166. [Google Scholar] [CrossRef]
- Bonanomi, G.; De Filippis, F.; Zotti, M.; Idbella, M.; Cesarano, G.; Al-Rowaily, S.; Abd-ElGawad, A. Repeated applications of organic amendments promote beneficial microbiota, improve soil fertility and increase crop yield. Appl. Soil Ecol. 2020, 156, 103714. [Google Scholar] [CrossRef]
- Kazem, B.M.; Hussein, H.J.; Hussein, A.A. The Effect of adding Fertilizer NPK-Chitosan is Growing and Productive (Zea mays L). Univ. Thi-Qar J. Agric. Res. 2024, 13, 146–152. [Google Scholar]
- Dincă, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and soil microbial community: A review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Futa, B.; Kraska, P.; Andruszczak, S.; Gierasimiuk, P.; Jaroszuk-Sierocińska, M. Impact of subsurface application of compound mineral fertilizer on soil enzymatic activity under reduced tillage. Agronomy 2021, 11, 2213. [Google Scholar] [CrossRef]
- Novianti, F.; Syaiful, S.; Dachlan, A.; Fadhli, N. Efficiency of Fertilizing Maize Plants Through the Application of Slow Release NPK Tablet Fertilizer with Biofertilizer. Indones. J. Agric. Res. 2023, 6, 223–236. [Google Scholar] [CrossRef]
- Mushalin, I.P.; Putri, H.F.N.; Fajri, H.A.M.; Yuniarda, S.; Khumairah, F.H.; Hibatullah, F.H.; Ambarita, D.; Nurbaity, A.; Herdiyantoro, D.; Kamaluddin, N.N. Current Status and the Role of Biofilm Biofertilizer for Improving the Soil Health and Agronomic Efficiency of Maize on Marginal Soil. Int. J. Life Sci. Agric. Res. 2024, 3, 596–602. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Yao, T.; Su, M.; Li, J.; Liu, Z.; Xin, Y.; Wang, L.; Chen, J.; Gun, S. Effects of microbial inoculation on enzyme activity, available nitrogen content, and bacterial succession during pig manure composting. Bioresour. Technol. 2020, 306, 123167. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Wang, T.; Liu, Y.; Jia, S.; Gao, Y.; Liu, S. Effects of different fertilizer treatments on rhizosphere soil microbiome composition and functions. Land 2020, 9, 329. [Google Scholar] [CrossRef]
- Liu, J.; Shu, A.; Song, W.; Shi, W.; Li, M.; Zhang, W.; Li, Z.; Liu, G.; Yuan, F.; Zhang, S. Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 2021, 404, 115287. [Google Scholar] [CrossRef]
- Tahat, M.M.; Alananbeh, K.M.; Othman, Y.A.; Leskovar, D.I. Soil health and sustainable agriculture. Sustainability 2020, 12, 4859. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Rinkes, Z.L.; Sinsabaugh, R.L.; Weintraub, M.N. Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: Informing enzyme-based decomposition models. Front. Microbiol. 2013, 4, 56126. [Google Scholar] [CrossRef]
- Qaswar, M.; Chai, R.; Ahmed, W.; Jing, H.; Han, T.; Liu, K.; Ye, X.; Xu, Y.; Anthonio, C.K.; Zhang, H. Partial substitution of chemical fertilizers with organic amendments increased rice yield by changing phosphorus fractions and improving phosphatase activities in fluvo-aquic soil. J. Soils Sediments 2020, 20, 1285–1296. [Google Scholar] [CrossRef]
- Siczek, A.; Frąc, M.; Kalembasa, S.; Kalembasa, D. Soil microbial activity of faba bean (Vicia faba L.) and wheat (Triticum aestivum L.) rhizosphere during growing season. Appl. Soil Ecol. 2018, 130, 34–39. [Google Scholar] [CrossRef]
- Wu, W.; Ma, B. Integrated nutrient management (INM) for sustaining crop productivity and reducing environmental impact: A review. Sci. Total Environ. 2015, 512, 415–427. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Wu, J.; Zhang, Y.; Li, Q.; Liu, S.; Gao, Y. The effects of localized plant–soil–microbe interactions on soil nitrogen cycle in maize rhizosphere soil under long-term fertilizers. Agronomy 2023, 13, 2114. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Liu, X.; Yang, W.; Li, W.; Chen, J.; Qiao, Y.; Gao, Z.; Yang, Z. Impact of Bio-Organic Fertilizer Incorporation on Soil Nutrients, Enzymatic Activity, and Microbial Community in Wheat–Maize Rotation System. Agronomy 2024, 14, 1942. https://doi.org/10.3390/agronomy14091942
Ali A, Liu X, Yang W, Li W, Chen J, Qiao Y, Gao Z, Yang Z. Impact of Bio-Organic Fertilizer Incorporation on Soil Nutrients, Enzymatic Activity, and Microbial Community in Wheat–Maize Rotation System. Agronomy. 2024; 14(9):1942. https://doi.org/10.3390/agronomy14091942
Chicago/Turabian StyleAli, Aamir, Xiaoli Liu, Wenping Yang, Wenguang Li, Jie Chen, Yuejing Qiao, Zhiqiang Gao, and Zhenping Yang. 2024. "Impact of Bio-Organic Fertilizer Incorporation on Soil Nutrients, Enzymatic Activity, and Microbial Community in Wheat–Maize Rotation System" Agronomy 14, no. 9: 1942. https://doi.org/10.3390/agronomy14091942




