Plant–Soil Microbial Interaction: Differential Adaptations of Beneficial vs. Pathogenic Bacterial and Fungal Communities to Climate-Induced Drought
Abstract
:1. Introduction
2. Impact of Drought on Microbial Community Structure
3. Adaptive Strategies Used by Beneficial Bacteria and Fungi under Drought
3.1. Effect of Drought on Rhizobacteria
3.2. Adaptive Mechanisms of Rhizobacteria under Drought
3.3. Effect of Drought on Arbuscular Mycorrhizal Fungi
3.4. Adaptive Mechanisms of AMF under Drought
3.5. Comparison of Drought Adaptation Strategies of Rhizobacteria and AMF
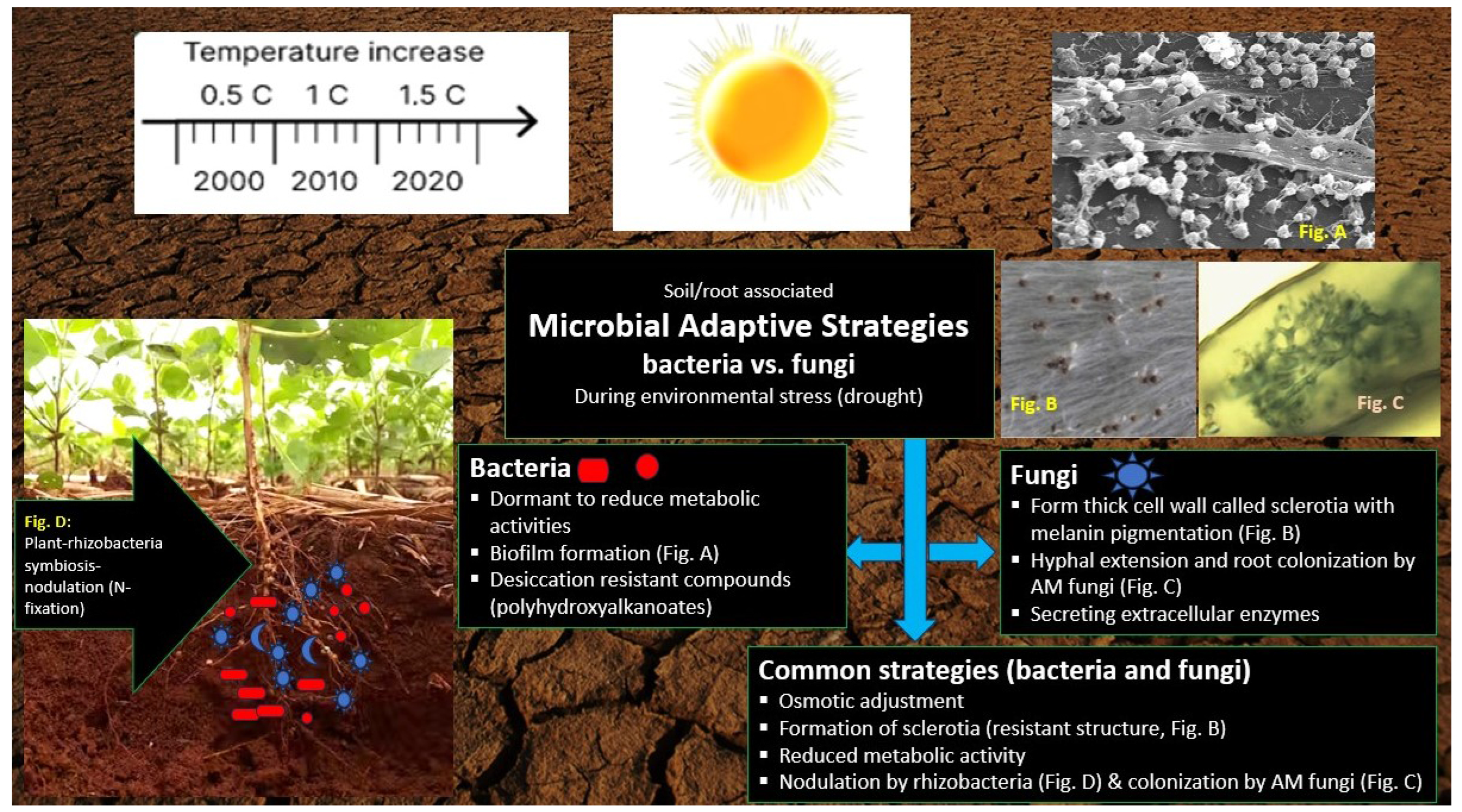
4. Defence Mechanisms of Rhizobacteria and AMF in Plants during Drought Conditions
4.1. Rhizobacteria-Mediated Plant Defence Mechanisms under Drought Conditions
4.2. AMF-Mediated Plant Defence Mechanisms under Drought
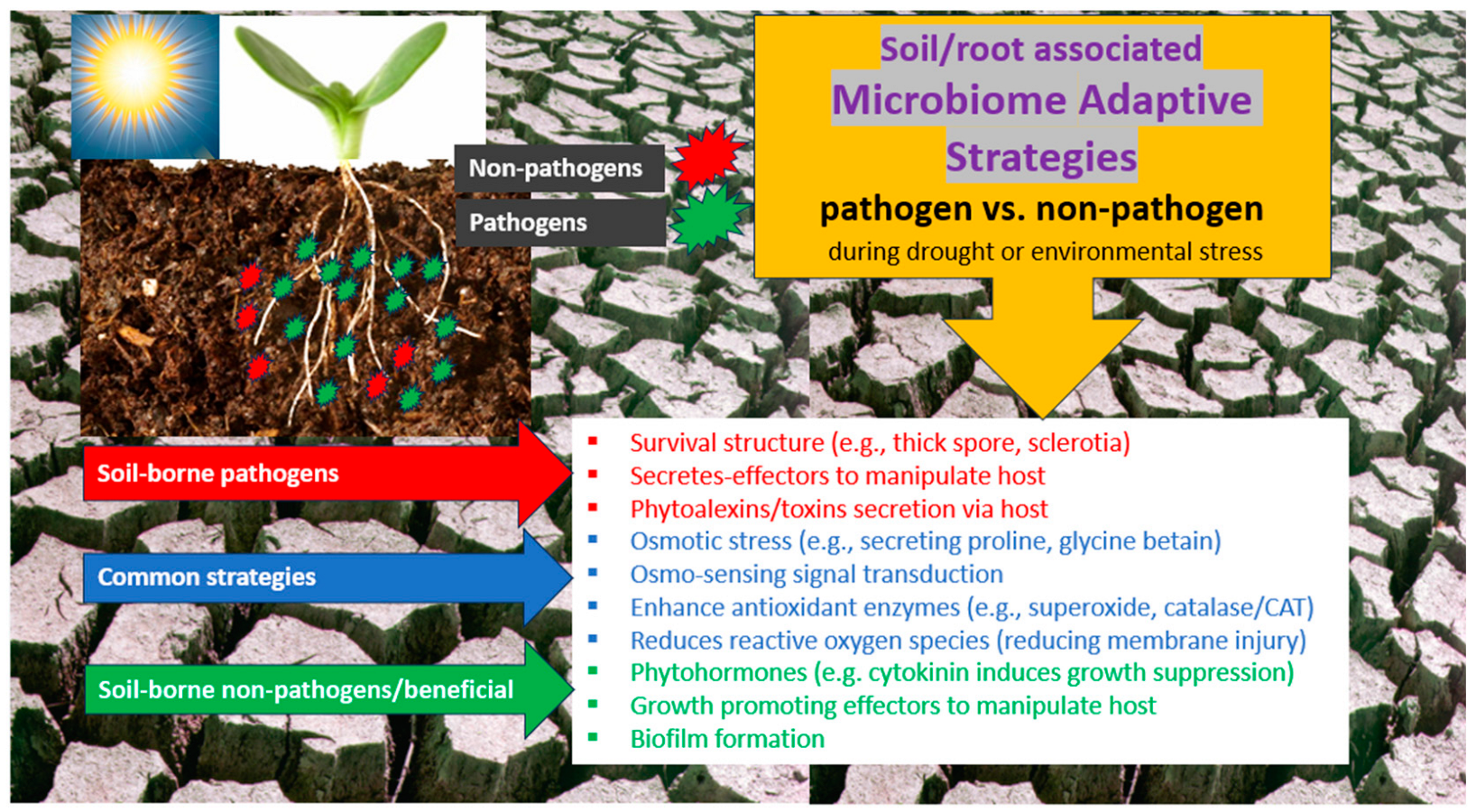
5. Adaptive Strategies during Drought of Pathogenic Bacteria and Fungi and Their Comparison with Adaptive Strategies of Beneficial Microorganisms
5.1. Adaptation of Phytopathogenic Fungi and Bacteria to Drought
5.2. Similarities and Differences in Adaptive Strategies of Pathogenic and Beneficial Microbes to Drought Conditions
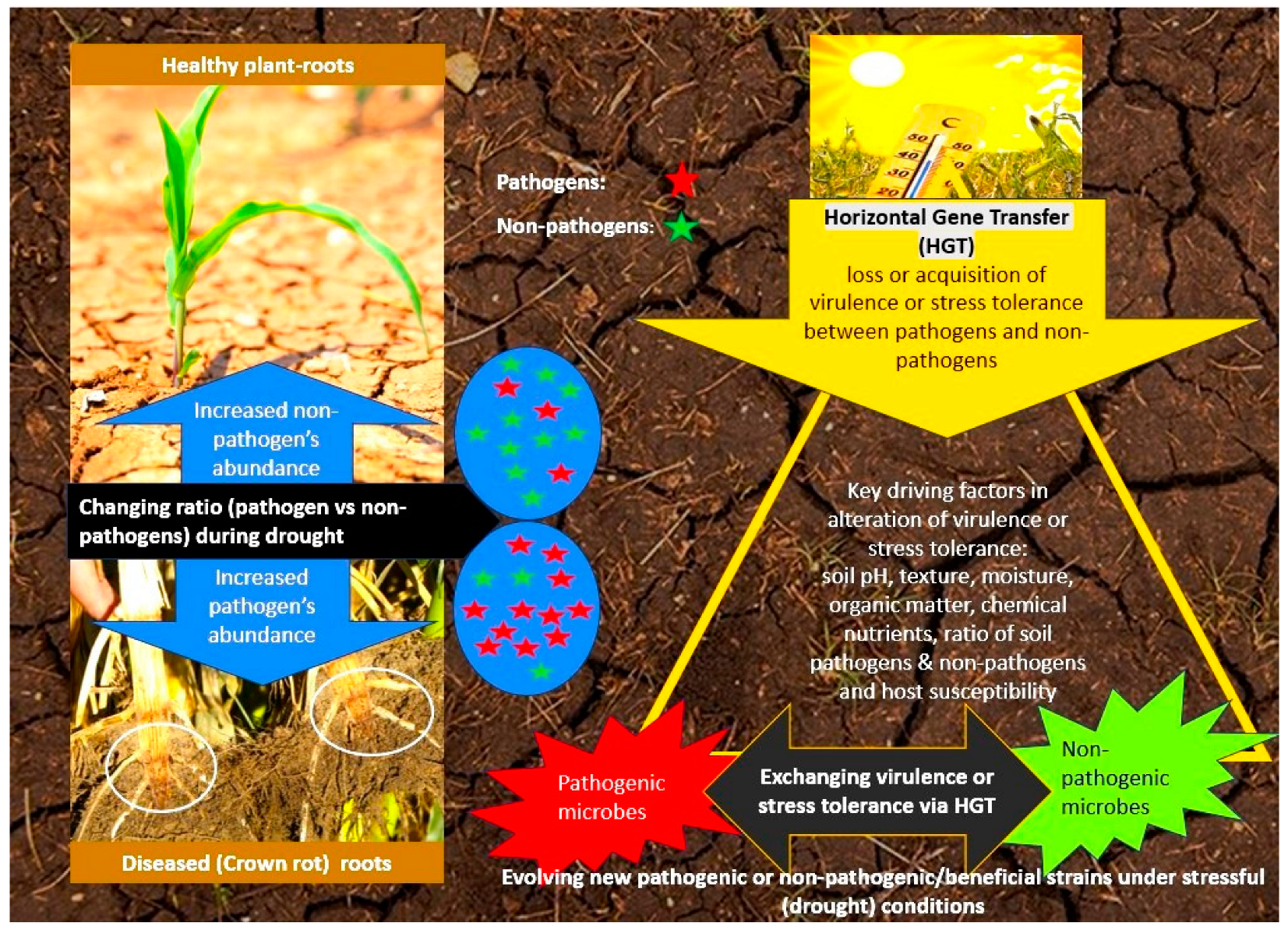
6. Genetic Exchange through Horizontal Gene Transfer (HGT) within Soil–Plant Microbial Ecosystem during Drought
6.1. HGT between Non-Pathogenic and Pathogenic Microorganisms during Drought
6.2. Soil, Plant, and Microbial Interaction Factors Affect HGT under Drought
7. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ault, T.R. On the essentials of drought in a changing climate. Science 2020, 368, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Dean, J.; Maza, F.; Morel, I.; Pulgar, R.; González, M. Microbial communities from arid environments on a global scale. A systematic review. Biol. Res. 2020, 53, 29. [Google Scholar] [CrossRef] [PubMed]
- Naorem, A.; Jayaraman, S.; Dang, Y.P.; Dalal, R.C.; Sinha, N.K.; Rao, C.S.; Patra, A.K. Soil Constraints in an Arid Environment—Challenges, Prospects, and Implications. Agronomy 2023, 13, 220. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Quiring, S.M.; Peña-Gallardo, M.; Yuan, S.; Domínguez-Castro, F. A review of environmental droughts: Increased risk under global warming? Earth-Sci. Rev. 2020, 201, 102953. [Google Scholar] [CrossRef]
- Sahu, P.K.; Singh, D.P.; Prabha, R.; Meena, K.K.; Abhilash, P.C. Connecting microbial capabilities with the soil and plant health: Options for agricultural sustainability. Ecol. Indic. 2019, 105, 601–612. [Google Scholar] [CrossRef]
- Thompson, S.; Levin, S.; Rodriguez-Iturbe, I. Linking plant disease risk and precipitation drivers: A dynamical systems framework. Am. Nat. 2013, 181, E1–E16. [Google Scholar] [CrossRef]
- Leach, J.E.; Triplett, L.R.; Argueso, C.T.; Trivedi, P. Communication in the phytobiome. Cell 2017, 169, 587–596. [Google Scholar] [CrossRef]
- Brettner, L.; Ho, W.C.; Schmidlin, K.; Apodaca, S.; Eder, R.; Geiler-Samerotte, K. Challenges and potential solutions for studying the genetic and phenotypic architecture of adaptation in microbes. Curr. Opin. Genet. Dev. 2022, 75, 101951. [Google Scholar] [CrossRef]
- Esbelin, J.; Santos, T.; Hébraud, M. Desiccation: An environmental and food industry stress that bacteria commonly face. Food Microbiol. 2018, 69, 82–88. [Google Scholar] [CrossRef]
- Manzanera, M. Dealing with water stress and microbial preservation. Environ. Microbiol. 2021, 23, 3351–3359. [Google Scholar] [CrossRef]
- Loiko, N.; Tereshkina, K.; Kovalenko, V.; Moiseenko, A.; Tereshkin, E.; Sokolova, O.S.; Krupyanskii, Y. DNA-binding protein Dps protects Escherichia coli cells against multiple stresses during desiccation. Biology 2023, 12, 853. [Google Scholar] [CrossRef] [PubMed]
- Aminov, R.I. Horizontal gene exchange in environmental microbiota. Front. Microbiol. 2011, 2, 158. [Google Scholar] [CrossRef]
- Mehrabi, R.; Bahkali, A.H.; Abd-Elsalam, K.A.; Moslem, M.; Ben M’Barek, S.; Gohari, A.M.; Jashni, M.K.; Stergiopoulos, I.; Kema, G.H.; de Wit, P.J. Horizontal gene and chromosome transfer in plant pathogenic fungi affecting host range. FEMS Microbiol. Rev. 2011, 35, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Bogati, K.; Walczak, M. The Impact of Drought Stress on Soil Microbial Community, Enzyme Activities and Plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Matthews, K.E.; Facelli, J.M.; Cavagnaro, T.R. Response of soil microbial community structure, carbon and nitrogen cycling to drying and rewetting. Appl. Soil Ecol. 2023, 192, 105099. [Google Scholar] [CrossRef]
- Gillespie, L.M.; Prada-Salcedo, L.D.; Shihan, A.; Fromin, N.; Goldmann, K.; Milcu, A.; Buscot, F.; Buatois, B.; Hättenschwiler, S. Taxonomical and functional responses of microbial communities from forest soils of differing tree species diversity to drying-rewetting cycles. Pedobiologia 2023, 97–98, 150875. [Google Scholar] [CrossRef]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Quero, J.L.; García-Gómez, M.; Gallardo, A.; Ulrich, W. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natl. Acad. Sci. USA 2015, 112, 15684–15689. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.E.; Wallenstein, M.D. Soil microbial community response to drying and rewetting stress: Does historical precipitation regime matter? Biogeochemistry 2012, 109, 101–116. [Google Scholar] [CrossRef]
- Lebre, P.; De Maayer, P.; Cowan, D. Xerotolerant bacteria: Surviving through a dry spell. Nat. Rev. Microbiol. 2017, 15, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Scales, N.C.; Huynh, K.T.; Weihe, C.; Martiny, J.B.H. Desiccation induces varied responses within a soil bacterial genus. Environ. Microbiol. 2023, 25, 3075–3086. [Google Scholar] [CrossRef]
- Breitkreuz, C.; Herzig, L.; Buscot, F.; Reitz, T.; Tarkka, M. Interactions between soil properties, agricultural management and cultivar type drive structural and functional adaptations of the wheat rhizosphere microbiome to drought. Environ. Microbiol. 2021, 23, 5866–5882. [Google Scholar] [CrossRef] [PubMed]
- Chodak, M.; Gołębiewsk, M.; Morawska-Płoskonka, J.; Kuduk, K.; Niklińska, M. Soil chemical properties affect the reaction of forest soil bacteria to drought and rewetting stress. Ann. Microbiol. 2015, 65, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Maisnam, P.; Jeffries, T.C.; Szejgis, J.; Bristol, D.; Singh, B.K.; Eldridge, D.J.; Horn, S.; Chieppa, J.; Nielsen, U.N. Severe prolonged drought favours stress-tolerant microbes in australian drylands. Microb. Ecol. 2023, 86, 3097–3110. [Google Scholar] [CrossRef] [PubMed]
- Metze, D.; Schnecker, J.; Canarini, A.; Fuchslueger, L.; Koch, B.J.; Stone, B.W.; Hungate, B.A.; Hausmann, B.; Schmidt, H.; Schaumberger, A.; et al. Microbial growth under drought is confined to distinct taxa and modified by potential future climate conditions. Nat. Commun. 2023, 14, 5895. [Google Scholar] [CrossRef]
- Jaeger, A.C.; Hartmann, M.; Six, J.; Solly, E.F. Contrasting sensitivity of soil bacterial and fungal community composition to one year of water limitation in Scots pine mesocosms. FEMS Microbiol. Ecol. 2023, 99, fiad051. [Google Scholar] [CrossRef]
- Rosinger, C.; Rousk, J.; Bonkowski, M.; Rethemeyer, J.; Jaeschke, A. Rewetting the hyper-arid Atacama Desert soil reactivates a carbon-starved microbial decomposer community and also triggers archaeal metabolism. Sci. Total Environ. 2023, 892, 164785. [Google Scholar] [CrossRef]
- Chilakala, A.R.; Pandey, P.; Durgadevi, A.; Kandpal, M.; Patil, B.S.; Rangappa, K.; Reddy, P.C.O.; Ramegowda, V.; Senthil-Kumar, M. Drought attenuates plant responses to multiple rhizospheric pathogens: A study on a dry root rot-associated Disease complex in chickpea fields. Field Crops Res. 2023, 298, 108965. [Google Scholar] [CrossRef]
- Sinha, R.; Irulappan, V.; Mohan-Raju, B.; Suganthi, A.; Senthil-Kumar, M. Impact of drought stress on simultaneously occurring pathogen infection in field-grown chickpea. Sci. Rep. 2019, 9, 5577. [Google Scholar] [CrossRef]
- Kaisermann, A.; Maron, P.A.; Beaumelle, L.; Lata, J.C. Fungal communities are more sensitive indicators to non-extreme soil moisture variations than bacterial communities. Appl. Soil Ecol. 2015, 86, 158–164. [Google Scholar] [CrossRef]
- Marasco, R.; Rolli, E.; Ettoumi, B.; Vigani, G.; Mapelli, F.; Borin, S.; Abou-Hadid, A.F.; El-Behairy, U.A.; Sorlini, C.; Cherif, A.; et al. A drought resistance-promoting microbiome is selected by root system under desert farming. PLoS ONE 2012, 7, e48479. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2023, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Siebielec, S.; Siebielec, G.; Klimkowicz-Pawlas, A.; Gałązka, A.; Grządziel, J.; Stuczyński, T. Impact of Water Stress on Microbial Community and Activity in Sandy and Loamy Soils. Agronomy 2020, 10, 1429. [Google Scholar] [CrossRef]
- Barnard, R.L.; Blazewicz, S.J.; Firestone, M.K. Rewetting of soil: Revisiting the origin of soil CO2 emissions. Soil Biol. Biochem. 2020, 147, 107819. [Google Scholar] [CrossRef]
- Liao, Z.; Junliang, F.; Zhenlin, L.; Zhentao, B.; Haidong, W.; Minghui, C.; Fucang, Z.; Zhijun, L. Chapter Three—Response network and regulatory measures of plant-soil-rhizosphere environment to drought stress. Adv. Agron. 2023, 180, 93–196. [Google Scholar] [CrossRef]
- Xu, L.; Dong, Z.; Chiniquy, D.; Pierroz, G.; Deng, S.; Gao, C.; Diamond, S.; Simmons, T.; Wipf, H.M.; Caddell, D.; et al. Genome-resolved metagenomics reveals role of iron metabolism in drought-induced rhizosphere microbiome dynamics. Nat. Commun. 2021, 12, 3209. [Google Scholar] [CrossRef]
- Estrada-González, Á.J.; Medina-De la Rosa, G.; Bautista, E.; Flores, J.; López-Lozano, N.E. Physiological regulations of a highly tolerant cactus to dry season modify its rhizospheric microbial communities. Rhizosphere 2023, 25, 100655. [Google Scholar] [CrossRef]
- Hestrin, R.; Kan, M.; Lafler, M.; Wollard, J.; Kimbrel, J.A.; Ray, P.; Blazewicz, S.J.; Stuart, R.; Craven, K.; Firestone, M.; et al. Plant-associated fungi support bacterial resilience following water limitation. ISME J. 2022, 16, 2752–2762. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Kloepper, J.; Leong, J.; Teintze, M.; Milton, N.S. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Bittencourt, P.P.; Alves, A.F.; Ferreira, M.B.; da Silva Irineu, L.E.S.; Pinto, V.B.; Olivares, F.L. Mechanisms and Applications of Bacterial Inoculants in Plant Drought Stress Tolerance. Microorganisms 2023, 11, 502. [Google Scholar] [CrossRef]
- Amaresan, N.; Kumar, M.S.; Annapurna, K.; Kumar, K.; Sankaranaryanan, N. Beneficial Microbes in Agro-Ecology: Bacteria and Fungi; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Prasad, M.; Srinivasan, R.; Chaudhary, M.; Choudhary, M.; Jat, L.K. Plant growth promoting rhizobacteria (PGPR) for sustainable agriculture: Perspectives and challenges. PGPR Amelior. Sustain. Agric. 2019, 129–157. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of arbuscular mycorrhizal fungi in regulating growth, enhancing productivity, and potentially influencing ecosystems under abiotic and biotic stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Zou, J.; Zhang, B.; Wu, L.; Yang, T.; Huang, Q. Arbuscular mycorrhizal fungi and microbes interaction in rice mycorrhizosphere. Agronomy 2022, 12, 1277. [Google Scholar] [CrossRef]
- Agudelo, M.G.; Ruiz, B.; Capela, D.; Remigi, P. The role of microbial interactions on rhizobial fitness. Front. Plant Sci. 2023, 14, 1277262. [Google Scholar] [CrossRef]
- Manzanera, M.; Garcia de Castro, A.; Tondervik, A.; Rayner-Brandes, M.; Strom, A.R.; Tunnacliffe, A. Hydroxyectoine is superior to trehalose for anhydrobiotic engineering of Pseudomonas putida KT2440. Appl. Environ. Microbiol. 2002, 68, 4328–4333. [Google Scholar] [CrossRef] [PubMed]
- Narváez-Reinaldo, J.J.; Barba, I.; González-López, J.; Tunnacliffe, A.; Manzanera, M. Rapid method for isolation of desiccation-tolerant strains and xeroprotectants. Appl. Environ. Microbiol. 2010, 76, 5254–5262. [Google Scholar] [CrossRef]
- SantaCruz-Calvo, L.; González-López, J.; Manzanera, M. Arthrobacter siccitolerans sp. nov., a highly desiccation-tolerant, xeroprotectant-producing strain isolated from dry soil. Int. J. Syst. Evol. Microbiol. 2013, 63 Pt 11, 4174–4180. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.; Niraula, S.; Parks, D.; Chang, W.S. Draft Genome Sequences of Two Desiccation-Tolerant Strains, Bradyrhizobium japonicum TXVA and TXEA, Isolated from the Root Nodules of Soybean Grown in Texas. Microbiol. Resour. Announc. 2022, 11, e00467-22. [Google Scholar] [CrossRef] [PubMed]
- Pazos-Rojas, L.A.; Cuellar-Sánchez, A.; Romero-Cerón, A.L.; Rivera-Urbalejo, A.; Van Dillewijn, P.; Luna-Vital, D.A.; Muñoz-Rojas, J.; Morales-García, Y.E.; Bustillos-Cristales, M.D.R. The Viable but Non-Culturable (VBNC) State, a Poorly Explored Aspect of Beneficial Bacteria. Microorganisms 2023, 12, 39. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, X.; Guan, D.; Kang, Y.; Li, L.; Cao, F.; Zhao, B.; Ma, M.; Zhao, J.; Li, J. Effects of rehydration on physiological and transcriptional responses of a water-stressed rhizobium. J. Microbiol. 2022, 60, 31–46. [Google Scholar] [CrossRef]
- Muñoz-Rojas, J. Desiccation-tolerant rhizobacteria maintain their plant growth-promoting capability after experiencing extreme water stress. Sci. Fed. J. Appl. Microbiol. 2018, 1, 15–17. [Google Scholar]
- Shankar, A.; Prasad, V. Potential of desiccation-tolerant plant growth-promoting rhizobacteria in growth augmentation of wheat (Triticum aestivum L.) under drought stress. Front. Microbiol. 2023, 14, 1017167. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial inoculants: Reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 2019, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Marasco, H.I.; Rolli, E.; Vigani, G.; Borin, S.; Sorlini, C.; Ouzari, H.; Zocchi, G.; Daffonchio, D. Are drought-resistance promoting bacteria cross-compatible with different plant models? Plant Signal. Behav. 2016, 399, 219–229. [Google Scholar] [CrossRef]
- Naylor, D.; Coleman-Derr, D. Drought stress and root-associated bacterial communities. Front. Plant Sci. 2018, 8, 2223. [Google Scholar] [CrossRef]
- Maryani, Y.; Dewi, W.S.; Yunus, A. Study on osmoprotectant rhizobacteria to improve mung bean growth under drought stress. IOP Conf. Ser. Earth Environ. Sci. 2018, 129, 012014. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Garrido-Oter, R.; Münch, P.C.; Weiman, A.; Dröge, J.; Pan, Y.; McHardy, A.C.; Schulze-Lefert, P. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 2015, 17, 392–403. [Google Scholar] [CrossRef]
- Cytryn, E.J.; Sangurdekar, D.P.; Streeter, J.G.; Franck, W.L.; Chang, W.S.; Stacey, G.; Emerich, D.W.; Joshi, T.; Xu, D.; Sadowsky, M.J. Transcriptional and physiological responses of Bradyrhizobium japonicum to desiccation-induced stress. J. Bacteriol. 2007, 189, 6751–6762. [Google Scholar] [CrossRef]
- Vílchez, J.I.; García-Fontana, C.; Román-Naranjo, D.; González-López, J.; Manzanera, M. Plant drought tolerance enhancement by trehalose production of desiccation-tolerant microorganisms. Front. Microbiol. 2016, 7, 1577. [Google Scholar] [CrossRef]
- Sharma, M.P.; Grover, M.; Chourasiya, D.; Bharti, A.; Agnihotri, R.; Maheshwari, H.S.; Pareek, A.; Buyer, J.S.; Sharma, S.K.; Schütz, L.; et al. Deciphering the role of trehalose in tripartite symbiosis among rhizobia, arbuscular mycorrhizal fungi, and legumes for enhancing abiotic stress tolerance in crop plants. Front. Microbiol. 2020, 11, 509919. [Google Scholar] [CrossRef]
- McIntyre, H.J.; Davies, H.; Hore, T.A.; Miller, S.H.; Dufour, J.P.; Ronson, C.W. Trehalose biosynthesis in Rhizobium leguminosarum bv. trifolii and its role in desiccation tolerance. Appl. Environ. Microbiol. 2007, 73, 3984–3992. [Google Scholar] [CrossRef]
- Iturriaga, G.; Suárez, R.; Nova-Franco, B. Trehalose metabolism: From osmoprotection to signaling. Int. J. Mol. Sci. 2009, 10, 3793–3810. [Google Scholar] [CrossRef]
- Nawaz, M.; Hassan, M.U.; Chattha, M.U.; Mahmood, A.; Shah, A.N.; Hashem, M.; Alamri, S.; Batool, M.; Rasheed, A.; Thabit, M.A.; et al. Trehalose: A promising osmo-protectant against salinity stress-physiological and molecular mechanisms and future prospective. Mol. Biol. Rep. 2022, 49, 11255–11271. [Google Scholar] [CrossRef]
- Dukare, A.; Mhatre, P.; Maheshwari, H.S. Delineation of mechanistic approaches of rhizosphere microorganisms facilitated plant health and resilience under challenging conditions. 3 Biotech 2022, 12, 57. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbiosis. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef]
- Chourasiya, D.; Gupta, M.M.; Sahni, S.; Oehl, F.; Agnihotri, R.; Buade, R.; Maheshwari, H.S.; Prakash, A.; Sharma, M.P. Unraveling the AM fungal community for understanding its ecosystem resilience to changed climate in agroecosystems. Symbiosis 2021, 84, 295–310. [Google Scholar] [CrossRef]
- Öpik, M.; Zobel, M.; Cantero, J.J.; Davison, J.; Facelli, J.M.; Hiiesalu, I.; Jairus, T.; Kalwij, J.M.; Koorem, K.; Leal, M.E.; et al. Global sampling of plant roots expands the described molecular diversity of arbuscular mycorrhizal fungi. Mycorrhiza 2013, 23, 411–430. [Google Scholar] [CrossRef]
- Bennett, A.E.; Groten, K. The costs and benefits of plant–arbuscular mycorrhizal fungal interactions. Annu. Rev. Plant Biol. 2022, 73, 649–672. [Google Scholar] [CrossRef]
- Stahl, P.D.; Christensen, M. Population variation in the mycorrhizal fungus Glomus mosseae: Breadth of environmental tolerance. Mycol. Res. 1991, 95, 300–307. [Google Scholar] [CrossRef]
- Zhang, F.; Ying-Ning, Z.; Wu, Q. Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci. Hortic. 2018, 229, 132–136. [Google Scholar] [CrossRef]
- Liu, X.J.A.; Han, S.; Frey, S.D.; Melillo, J.M.; Zhou, J.; DeAngelis, K.M. Microbial responses to long-term warming differ across soil microenvironments. ISME Commun. 2024, 4, ycae051. [Google Scholar] [CrossRef]
- Millar, N.S.; Bennett, A.E. Stressed out symbiotes: Hypotheses for the influence of abiotic stress on arbuscular mycorrhizal fungi. Oecologia 2016, 182, 625–641. [Google Scholar] [CrossRef]
- Symanczik, S.; Courty, P.E.; Boller, T.; Wiemken, A.; Al-Yahya’ei, M.N. Impact of water regimes on an experimental community of four desert arbuscular mycorrhizal fungal (AMF) species, as affected by the introduction of a non-native AMF species. Mycorrhiza 2015, 25, 639–647. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Cruz, C.; Mahdhi, M.; Mars, M.; Caeiro, M.F. Arbuscular mycorrhizal fungi in soil, roots and rhizosphere of Medicago truncatula: Diversity and heterogeneity under semi-arid conditions. PeerJ 2019, 7, e6401. [Google Scholar] [CrossRef]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef]
- Veresoglou, S.D.; Chen, B.; Rillig, M.C. Arbuscular mycorrhiza and soil nitrogen cycling. Soil Biol. Biochem. 2012, 46, 53–62. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular Mycorrhizal Fungi as Natural Biofertilizers: Let’s Benefit from Past Successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef]
- Moreira-Souza, M.; Trufem, S.F.B.; Gomes-Da-Costa, S.M.; Cardoso, E.J.B.N. Arbuscular mycorrhizal fungi associated with Araucaria angustifolia (Bert.) O. Ktze. Mycorrhiza 2003, 13, 211–215. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Aguilar-Trigueros, C.A.; Roy, J.; Rillig, M.C. Drought induces shifts in soil fungal communities that can be linked to root traits across 24 plant species. New Phytol. 2021, 232, 1917–1929. [Google Scholar] [CrossRef]
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.; Mingsen, Q.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H. Mechanistic Insights into Arbuscular Mycorrhizal Fungi-Mediated Drought Stress Tolerance in Plants. Int. J. Mol. Sci. 2019, 20, 4199. [Google Scholar] [CrossRef]
- Lumini, E.; Vallino, M.; Alguacil, M.M.; Romani, M.; Bianciotto, V. Different farming and water regimes in Italian rice fields affect arbuscular mycorrhizal fungal soil communities. Ecol. Appl. 2011, 21, 1696–1707. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M.; Porcel, R.; Azcón, C.; Aroca, R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: New challenges in physiological and molecular studies. J. Exp. Bot. 2012, 63, 4033–4044. [Google Scholar] [CrossRef]
- He, F.; Zhang, H.; Tang, M. Aquaporin gene expression and physiological responses of Robinia pseudoacacia L. to the mycorrhizal fungus Rhizophagus irregularis and drought stress. Mycorrhiza 2016, 26, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, R.; Nerva, L.; Sillo, F.; Girlanda, M.; Perotto, S. Plant and fungal gene expression in mycorrhizal protocorms of the orchid Serapias vomeracea colonized by Tulasnella calospora. Plant Signal Behav. 2014, 9, e977707. [Google Scholar] [CrossRef] [PubMed]
- Miransari, M. Interactions between arbuscular mycorrhizal fungi and soil bacteria. Appl. Microbiol. Biotechnol. 2011, 89, 917–930. [Google Scholar] [CrossRef]
- Zadworny, M.; Górska, A.; Politycka, B. Arbuscular mycorrhizal fungi alter enzymatic activities in phosphorus-transforming soils. Mycorrhiza 2015, 25, 243–251. [Google Scholar]
- Kuyper, T.W.; Jansa, J. Arbuscular mycorrhiza: Advances and retreats in our understanding of the ecological functioning of the mother of all root symbioses. Plant Soil 2023, 489, 41–88. [Google Scholar] [CrossRef]
- Safari, M.M.R.; Farokhzad, M.; Kaviani, B.; Kulus, D. Endophytic Fungi as Potential Biocontrol Agents against Sclerotium rolfsii Sacc.—The Causal Agent of Peanut White Stem Rot Disease. Cells 2022, 11, 2643. [Google Scholar] [CrossRef]
- Al-Turki, A.; Murali, M.; Omar, A.F.; Rehan, M.; Sayyed, R.Z. Recent advances in PGPR-mediated resilience toward interactive effects of drought and salt stress in plants. Front. Microbiol. 2023, 27, 1214845. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Ali, S.; Tariq, H.; Latif, S.; Yasmin, H.; Mehmood, A.; Shahid, M.A. Water Conservation and Plant Survival Strategies of Rhizobacteria under Drought Stress. Agronomy 2020, 10, 1683. [Google Scholar] [CrossRef]
- Igiehon, O.N.; Babalola, O.O. Rhizobium and mycorrhizal fungal species improved soybean yield under drought stress conditions. Curr. Microbiol. 2021, 78, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Igiehon, N.O.; Babalola, O.O.; Cheseto, X.; Torto, B. Effects of rhizobia and arbuscular mycorrhizal fungi on yield, size distribution and fatty acid of soybean seeds grown under drought stress. Microbiol. Res. 2021, 242, 126640. [Google Scholar] [CrossRef] [PubMed]
- Vaishnav, A.; Kasotia, A.; Choudhary, D.K. Role of Functional Bacterial Phylum Proteobacteria in Glycine max Growth Promotion Under Abiotic Stress: A Glimpse on Case Study. In Silico Approach for Sustainable Agriculture; Choudhary, D., Kumar, M., Prasad, R., Kumar, V., Eds.; Springer: Singapore, 2018; pp. 17–49. [Google Scholar] [CrossRef]
- Nawaz, M.; Ishaq, S.; Ishaq, H.; Khan, N.; Iqbal, N.; Ali, S.; Rizwan, M.; Alsahli, A.A.; Alyemeni, M.N. Salicylic Acid Improves Boron Toxicity Tolerance by Modulating the Physio-Biochemical Characteristics of Maize (Zea mays L.) at an Early Growth Stage. Agronomy 2020, 10, 2013. [Google Scholar] [CrossRef]
- Lin, Y.; Watts, D.B.; Kloepper, J.W.; Feng, Y.; Torbert, H.A. Influence of plant growth-promoting rhizobacteria on corn growth under drought stress. Commun. Soil Sci. Plant Anal. 2020, 51, 250–264. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Santoyo, G.; Yadav, A.N.; Babalola, O.O. Efforts towards overcoming drought stress in crops: Revisiting the mechanisms employed by plant growth-promoting bacteria. Front. Microbiol. 2022, 13, 962427. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.L.; van Groenigen, K.J.; Hungate, B.A. Plant growth promoting rhizobacteria are more effective under drought: A meta-analysis. Plant Soil 2017, 416, 309–323. [Google Scholar] [CrossRef]
- Admassie, M.; Woldehawariat, Y.; Alemu, T.; Gonzalez, E.; Jimenez, J.F. The role of plant growth-promoting bacteria in alleviating drought stress on pepper plants. Agric. Water Manag. 2022, 272, 107831. [Google Scholar] [CrossRef]
- Kohler, J.; Hernández, J.A.; Caravaca, F.; Roldán, A. Plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water-stressed plants. Funct. Plant Biol. 2008, 35, 141–151. [Google Scholar] [CrossRef]
- Amini, R.; Zafarani-Moattar, P.; Shakiba, M.R.; Hasanfard, A. Inoculating moldavian balm (Dracocephalum moldavica L.) with mycorrhizal fungi and bacteria may mitigate the adverse effects of water stress. Sci. Rep. 2023, 13, 16176. [Google Scholar] [CrossRef]
- Mondani, F.; Khani, K.; Honarmand, S.J.; Saeidi, M. Evaluating effects of plant growth-promoting rhizobacteria on the radiation use efficiency and yield of soybean (Glycine max) under water deficit stress condition. Agric. Water Manag. 2019, 213, 707–713. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A. Exopolysaccharide producing rhizobacteria and their impact on growth and drought tolerance of wheat grown under rainfed conditions. PLoS ONE 2019, 14, e0222302. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, M.S.; Raza, M.A.S.; Saleem, M.F.; Erinle, K.O.; Iqbal, R.; Ahmad, S. Effect of rhizobacteria and cytokinins application on wheat growth and yield under normal vs drought conditions. Commun. Soil Sci. Plant Anal. 2019, 50, 2521–2533. [Google Scholar] [CrossRef]
- Muhammad, F.; Raza, M.A.S.; Iqbal, R.; Zulfiqar, F.; Aslam, M.U.; Yong, J.W.H.; Altaf, M.A.; Zulfiqar, F.; Amin, J.; Ibrahim, M.A. Ameliorating drought effects in wheat using an exclusive or co-applied rhizobacteria and ZnO nanoparticles. Biology 2022, 11, 1564. [Google Scholar] [CrossRef]
- de Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Swarnalakshmi, K.; Yadav, V.; Tyagi, D.; Dhar, D.W.; Kannepalli, A.; Kumar, S. Significance of Plant Growth Promoting Rhizobacteria in Grain Legumes: Growth Promotion and Crop Production. Plants 2020, 9, 1596. [Google Scholar] [CrossRef] [PubMed]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Tiepo, A.N.; Hertel, M.F.; Rocha, S.S.; Calzavara, A.K.; Oliveira, A.L.M.D.; Pimenta, J.A.; Oliveira, H.C.; Bianchini, E.; Stolf-Moreira, R. Enhanced drought tolerance in seedlings of Neotropical tree species inoculated with plant growth-promoting bacteria. Plant Physiol. Biochem. 2018, 130, 277–288. [Google Scholar] [CrossRef]
- Siddikee, M.A.; Glick, B.R.; Chauhan, P.S.; Yim, W.J.; Sa, T. Enhancement of growth and salt tolerance of red pepper seedlings (Capsicum annuum L.) by regulating stress ethylene synthesis with halotolerant bacteria containing 1-aminocyclopropane-1-carboxylic acid deaminase activity. Plant Physiol. Biochem. 2011, 49, 427–434. [Google Scholar] [CrossRef]
- Carezzano, M.E.; Alvarez Strazzi, F.B.; Pérez, V.; Bogino, P.; Giordano, W. Exopolysaccharides Synthesized by Rhizospheric Bacteria: A Review Focused on Their Roles in Protecting Plants against Stress. Appl. Microbiol. 2023, 3, 1249–1261. [Google Scholar] [CrossRef]
- Bhargavi, G.; Arya, M.; Jambhulkar, P.P.; Singh, A.; Rout, A.K.; Behera, B.K.; Chaturvedi, S.K.; Singh, A.K. Evaluation of biocontrol efficacy of rhizosphere dwelling bacteria for management of Fusarium wilt and Botrytis gray mold of chickpea. BMC Genom. Data 2024, 25, 7. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, Y.; Yu, Z.; Zhuang, W.; Zeng, Z. Bacillus velezensis BV01 Has Broad-Spectrum Biocontrol Potential and the Ability to Promote Plant Growth. Microorganisms 2023, 11, 2627. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Singh, P.; Qi, Y.; Singh, R.K.; Qin, Q.; Jin, C.; Wang, B.; Fang, W. Pseudomonas aeruginosa Strain 91: A Multifaceted Biocontrol Agent against Banana Fusarium Wilt. J. Fungi 2023, 9, 1047. [Google Scholar] [CrossRef]
- Bouremani, N.; Cherif-Silini, H.; Silini, A.; Bouket, A.C.; Luptakova, L.; Alenezi, F.N.; Baranov, O.; Belbahri, L. Plant growth-promoting rhizobacteria (PGPR): A rampart against the adverse effects of drought stress. Water 2023, 15, 418. [Google Scholar] [CrossRef]
- Kaushal, M.; Wani, S.P. Rhizobacterial-plant interactions: Strategies ensuring plant growth promotion under drought and salinity stress. Agric. Ecosyst. Environ. 2016, 231, 68–78. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 30, 225–246. [Google Scholar] [CrossRef]
- Vu, B.; Chen, M.; Crawford, R.J.; Ivanova, E.P. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef]
- Sato, Y.; Miwa, T.; Inaba, T.; Akachi, T.; Tanaka, E.; Hori, T.; Murofushi, K.; Takagi, H.; Futamata, H.; Aoyagi, T.; et al. Microbially produced fertilizer provides rhizobacteria to hydroponic tomato roots by forming beneficial biofilms. Appl. Microbiol. Biotechnol. 2023, 107, 7365–7374. [Google Scholar] [CrossRef] [PubMed]
- Fadiji, A.E.; Orozco-Mosqueda, M.d.C.; Santos-Villalobos, S.d.l.; Santoyo, G.; Babalola, O.O. Recent Developments in the Application of Plant Growth-Promoting Drought Adaptive Rhizobacteria for Drought Mitigation. Plants 2022, 11, 3090. [Google Scholar] [CrossRef]
- Newman, E.D.; Rowland, J.B.; Hammer, T.G.; Frost, L.A.; Lumibao, C.Y.; Henning, J.A. Trade-Offs in Arbuscular Mycorrhizal Fungal Responses to Drought and Salinity Stress in Panicum amarum (United States Gulf Coast). J. Coast. Res. 2024, 40, 51–63. [Google Scholar] [CrossRef]
- Mitra, D.; Djebaili, R.; Pellegrini, M.; Mahakur, B.; Sarker, A.; Chaudhary, P.; Khoshru, B.; Gallo, M.D.; Kitouni, M.; Barik, D.P.; et al. Arbuscular mycorrhizal symbiosis: Plant growth improvement and induction of resistance under stressful conditions. J. Plant Nutr. 2021, 44, 1993–2028. [Google Scholar] [CrossRef]
- Liu, R.C.; Ding, Y.E.; Wu, Q.S.; Zou, Y.N. Mycorrhizae enhance drought tolerance of trifoliate orange by regulating circadian clock response patterns. Sci. Hortic. 2022, 305, 111426. [Google Scholar] [CrossRef]
- Dowarah, B.; Gill, S.S.; Agarwala, N. Arbuscular mycorrhizal fungi in conferring tolerance to biotic stresses in plants. J. Plant Growth Regul. 2022, 41, 1429–1444. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Hashem, A.; Rasool, S.; Abd_Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D.; Jan, S.; Anjum, N.A.; Ahmad, P. Arbuscular mycorrhizal symbiosis and abiotic stress in plants: A review. J. Plant Biol. 2016, 59, 407–426. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Y.N.; Shu, B.; Wu, Q.S. Deciphering molecular mechanisms regarding enhanced drought tolerance in plants by arbuscular mycorrhizal fungi. Sci. Hortic. 2022, 308, 111591. [Google Scholar] [CrossRef]
- Jajoo, A.; Mathur, S. Role of arbuscular mycorrhizal fungi as an underground saviuor for protecting plants from abiotic stresses. Physiol. Mol. Biol. Plants 2021, 27, 2589–2603. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, Q.; Kuča, K. Unravelling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biol. 2021, 23, 50–57. [Google Scholar] [CrossRef]
- Gholamhoseini, M.; Ghalavand, A.; Dolatabadian, A.; Jamshidi, E.; Khodaei-Joghan, A. Effects of arbuscular mycorrhizal inoculation on growth, yield, nutrient uptake and irrigation water productivity of sunflowers grown under drought stress. Agric. Water Manag. 2013, 117, 106–114. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, J.; Khashi u Rahman, M.; Wu, F. The impact of root exudates, volatile organic compounds, and common mycorrhizal networks on root system architecture in root-root interactions. J. Plant Interact. 2022, 17, 685–694. [Google Scholar] [CrossRef]
- Cheng, H.Q.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Arbuscular mycorrhizal fungi alleviate drought stress in trifoliate orange by regulating H+-ATPase activity and gene expression. Front. Plant Sci. 2021, 12, 659694. [Google Scholar] [CrossRef]
- Zou, Y.; Qin, Q.; Ma, W.; Zhou, L.; Wu, Q.; Xu, Y.; Kuča, K.; Hashem, A.; Al-Arjani, A.F.; Almutairi, K.F.; et al. Metabolomics reveals arbuscular mycorrhizal fungi-mediated tolerance of walnut to soil drought. BMC Plant Biol. 2023, 23, 118. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Elgawad, H.A.; Xiong, Y.; Macovei, A.; Brestic, M.; Skalicky, M.; Shaghaleh, H.; Hamoud, Y.A.; El-Sawah, A.M. Inoculation with Bacillus amyloliquefaciens and mycorrhiza confers tolerance to drought stress and improve seed yield and quality of soybean plant. Physiol. Plant. 2021, 172, 2153–2169. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Ali, D.F.I.; Xiong, Y.C.; Brestic, M.; Skalicky, M.; Hamoud, Y.A.; Ulhassan, Z.; Shaghaleh, H.; AbdElgawad, H.; Farooq, M.; et al. Physiological and biochemical responses of soybean plants inoculated with Arbuscular mycorrhizal fungi and Bradyrhizobium under drought stress. BMC Plant Biol. 2021, 21, 195. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, T.; Wu, Z.; Feng, H.; Yu, M.; Zhang, X.; Chen, B. Arbuscular mycorrhiza enhances drought tolerance of tomato plants by regulating the 14-3-3 genes in the ABA signaling pathway. Appl. Soil Ecol. 2018, 125, 213–221. [Google Scholar] [CrossRef]
- Peng, Y.; Li, S.J.; Yan, J.; Tang, Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Xu, B.L. Research Progress on Phytopathogenic Fungi and Their Role as Biocontrol Agents. Front. Microbiol. 2021, 12, 670135. [Google Scholar] [CrossRef]
- van der Wolf, J.; De Boer, S.H. Phytopathogenic bacteria. In Principles of Plant-Microbe Interactions: Microbes for Sustainable Agriculture; Springer International Publishing: Cham, Switzerland, 2014; pp. 65–77. [Google Scholar]
- Delgado-Baquerizo, M.; Guerra, C.A.; Cano-Díaz, C.; Egidi, E.; Wang, J.T.; Eisenhauer, N.; Singh, B.; Maestre, F. The proportion of soil-borne pathogens increases with warming at the global scale. Nat. Clim. Chang. 2020, 10, 550–554. [Google Scholar] [CrossRef]
- Wakelin, S.A.; Gomez-Gallego, M.; Jones, E.; Smaill, S.; Lear, G.; Lambie, S. Climate change induced drought impacts on plant diseases in New Zealand. Australas. Plant Pathol. 2018, 47, 101–114. [Google Scholar] [CrossRef]
- Rai, A.; Irulappan, V.; Muthappa, S.K. Dry root rot of chickpea: A disease favored by drought. Plant Dis. 2021, 106, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Sharath Chandran, U.S.; Tarafdar, A.; Mahesha, H.S.; Sharma, M. Temperature and Soil Moisture Stress Modulate the Host Defence Response in Chickpea During Dry Root Rot Incidence. Front. Plant Sci. 2021, 12, 653265. [Google Scholar] [CrossRef]
- Chilakala, A.R.; Mali, K.V.; Irulappan, V.; Patil, B.S.; Pandey, P.; Rangappa, K.; Ramegowda, V.; Kumar, M.N.; Puli, C.O.R.; Mohan-Raju, B.; et al. Combined Drought and Heat Stress Influences the Root Water Relation and Determine the Dry Root Rot Disease Development Under Field Conditions: A Study Using Contrasting Chickpea Genotypes. Front. Plant Sci. 2022, 13, 890551. [Google Scholar] [CrossRef] [PubMed]
- Batista, E.; Lopes, A.; Miranda, P.; Alves, A. Can species distribution models be used for risk assessment analyses of fungal plant pathogens? A case study with three Botryosphaeriaceae species. Eur. J. Plant Pathol. 2023, 165, 41–56. [Google Scholar] [CrossRef]
- Oliva, J.; Stenlid, J.; Martínez-Vilalta, J. The effect of fungal pathogens on the water and carbon economy of trees: Implications for drought-induced mortality. New Phytol. 2014, 203, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Ezziyyani, M.; Hamdache, A.; Asraoui, M.; Requena, M.E.; Egea-Gilabert, C.; Candela Castillo, M.E. Effect of Climate Change on Growth, Development and Pathogenicity of Phytopathogenic Telluric Fungi. In Advanced Intelligent Systems for Sustainable Development (AI2SD’2018). AI2SD 2018. Advances in Intelligent Systems and Computing; Ezziyyani, M., Ed.; Springer: Cham, Switzerland, 2019; Volume 911. [Google Scholar] [CrossRef]
- Cubeta, M.A.; Thomas, E.; Dean, R.A. Neotyphodium/Epichloë species endophytes of grasses: Tapping into a rich source of biodiversity. In Advances in Endophytic Research; Springer: New York, NY, USA, 2014; pp. 205–227. [Google Scholar]
- Drogue, B.; Sanguin, H.; Chamam, A.; Mozar, M.; Llauro, C.; Panaud, O.; Prigent-Combaret, C.; Picault, N.; Wisniewski-Dyé, F. Plant root transcriptome profiling reveals a strain-dependent response during Azospirillum–rice cooperation. Front. Plant Sci. 2014, 5, 607. [Google Scholar] [CrossRef]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y.; Yunis, H.; Katan, J. Multiple resistance mechanisms to benzimidazole fungicides in Botrytis cinerea field isolates. Phytopathology 2007, 97, 686–695. [Google Scholar] [CrossRef]
- Büttner, D.; Bonas, U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol. Rev. 2010, 34, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Grenville-Briggs, L.J.; Avrova, A.O.; Bruce, C.R.; Williams, A.; Whisson, S.C.; Birch, P.R.J.; West, P.V. Elevated temperature and CO2 levels affect the interactions between potato and the root pathogen Phytophthora infestans. Glob. Chang. Biol. 2005, 11, 1714–1722. [Google Scholar] [CrossRef]
- El-Abyad, M.S.; Attaby, H.; Abu-Taleb, A.M. Impact of salinity stress on the free amino acid pools of some phytopathogenic fungi. Microbiol. Res. 1994, 149, 309–315. [Google Scholar] [CrossRef]
- Rossier, O.; Vorhölter, F.J. New Insights into the Extracellular Secretion of Xanthomonads. Trends Microbiol. 2019, 27, 605–614. [Google Scholar]
- Bohnert, H.J.; Jensen, R.G. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996, 14, 89–97. [Google Scholar] [CrossRef]
- Keeling, P.J.; Palmer, J.D. Horizontal gene transfer in eukaryotic evolution. Nat. Rev. Genet. 2008, 9, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Kloesges, T.; Popa, O.; Martin, W.; Dagan, T. Networks of gene sharing among 329 proteobacterial genomes reveal differences in lateral gene transfer frequency at different phylogenetic depths. Mol. Biol. Evol. 2011, 28, 1057–1074. [Google Scholar] [CrossRef]
- McDonald, M.C.; Taranto, A.P.; Hill, E.; Schwessinger, B.; Liu, Z.; Simpfendorfer, S.; Milgate, A.; Solomon, P.S. Transposon-Mediated Horizontal Transfer of the Host-Specific Virulence Protein ToxA between Three Fungal Wheat Pathogens. mBio 2019, 10, 01515-19. [Google Scholar] [CrossRef]
- Wintersdorff, C.J.H.; Penders, J.; van Niekerk, J.M.; Mills, N.D.; Majumder, S.; van Alphen, L.B.; Savelkoul, P.H.M.; Wolffs, P.F.G. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front. Microbiol. 2016, 19, 173. [Google Scholar] [CrossRef]
- Saini, A.; Mani, I.; Rawal, M.K.; Verma, C.; Singh, V.; Mishra, S.K. An introduction to microbial genomic islands for evolutionary adaptation and pathogenicity. In Microbial Genomic Islands in Adaptation and Pathogenicity; Mani, I., Singh, V., Alzahrani, K.J., Chu, D.T., Eds.; Springer Nature: Singapore, 2023; pp. 1–15. [Google Scholar] [CrossRef]
- Jang, H.; Gopinath, G.R.; Eshwar, A.; Srikumar, S.; Nguyen, S.; Gangiredla, J.; Patel, I.R.; Finkelstein, S.B.; Negrete, F.; Woo, J.; et al. The secretion of toxins and other exoproteins of Cronobacter: Role in virulence, adaption, and persistence. Microorganisms 2020, 8, 229. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef]
- Poudel, M.; Mendes, R.; Costa, L.A.; Bueno, C.G.; Meng, Y.; Folimonova, S.Y.; Garrett, K.A.; Martins, S.J. The role of plant-associated bacteria, fungi, and viruses in drought stress mitigation. Front. Microbiol. 2021, 12, 743512. [Google Scholar] [CrossRef] [PubMed]
- Drogue, B.; Doré, H.; Borland, S.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Which specificity in cooperation between phytostimulating rhizobacteria and plants? Res. Microbiol. 2012, 163, 500–510. [Google Scholar] [CrossRef]
- Matilla, M.A.; Ramos, J.L. Biosurfactant produced by a Pseudomonas strain growing on polycyclic aromatic hydrocarbons. Appl. Environ. Microbiol. 2007, 73, 1423–1429. [Google Scholar] [CrossRef]
- Newman, K.L.; Almeida, R.P.P.; Purcell, A.H.; Lindow, S.E. Cell-cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proc. Natl. Acad. Sci. USA 2004, 101, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, M.; Abulreesh, H.H.; Khan, M.S.; Ahmad, I.; Pichtel, J. Horizontal gene transfer in soil and the rhizosphere: Impact on ecological fitness of bacteria. In Agriculturally Important Microbes for Sustainable Agriculture: Volume I: Plant-Soil-Microbe Nexus; Meena, V.S., Mishra, P.K., Bisht, J.K., Pattanayak, A., Eds.; Springer: Singapore, 2017; pp. 111–130. ISBN 978-981-10-5589-8. [Google Scholar]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gupta, V.K.; Dahms, T.E.; Silva, R.N.; Singh, H.B.; Upadhyay, R.S.; Gomes, E.V.; Tsui, C.K.M.; Nayak, S.C. Friends or foes? Emerging insights from fungal interactions with plants. FEMS Microbiol. Rev. 2016, 40, 182–207. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Investigating the mechanisms for the opposing pH relationships of fungal and bacterial growth in soil. Soil Biol. Biochem. 2010, 42, 926–934. [Google Scholar] [CrossRef]
- Schimel, J.P.; Balser, T.C.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- Miller, J.R.; Biswas, K.B.; Hazelbauer, G.L. A bacterial chemoreceptor with magnitudes of stimulus response. Nat. Chem. Biol. 2010, 6, 521–528. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loiko, N.; Islam, M.N. Plant–Soil Microbial Interaction: Differential Adaptations of Beneficial vs. Pathogenic Bacterial and Fungal Communities to Climate-Induced Drought. Agronomy 2024, 14, 1949. https://doi.org/10.3390/agronomy14091949
Loiko N, Islam MN. Plant–Soil Microbial Interaction: Differential Adaptations of Beneficial vs. Pathogenic Bacterial and Fungal Communities to Climate-Induced Drought. Agronomy. 2024; 14(9):1949. https://doi.org/10.3390/agronomy14091949
Chicago/Turabian StyleLoiko, Nataliya, and M. Nazrul Islam. 2024. "Plant–Soil Microbial Interaction: Differential Adaptations of Beneficial vs. Pathogenic Bacterial and Fungal Communities to Climate-Induced Drought" Agronomy 14, no. 9: 1949. https://doi.org/10.3390/agronomy14091949
APA StyleLoiko, N., & Islam, M. N. (2024). Plant–Soil Microbial Interaction: Differential Adaptations of Beneficial vs. Pathogenic Bacterial and Fungal Communities to Climate-Induced Drought. Agronomy, 14(9), 1949. https://doi.org/10.3390/agronomy14091949






