Mitigation of Salinity Stress on Vetiver Grass (Vetiveria zizanioides) through Application of Micrococcus yunnanensis and Indole-3-Acetic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Analysis of Soil
2.2. Preparation of Bacterial Inoculum
2.3. Plant Growth and Treatments
2.4. Statistics
3. Results
3.1. The Effect of Salinity Levels, IAA Bacterial Inoculatiand on on Growth Characteristics and Yield of Vetiver
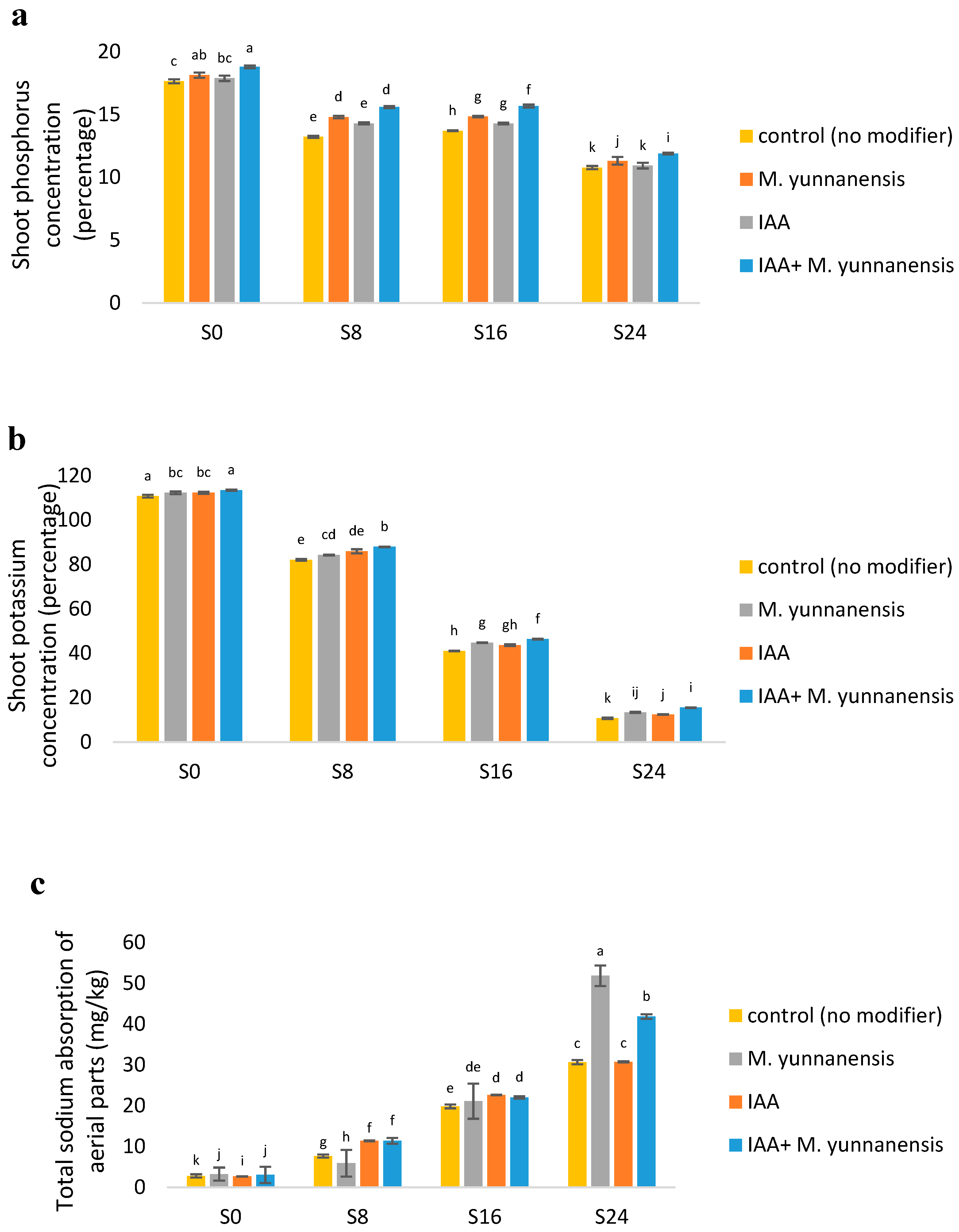
3.2. Effect of Salinity Levels and IAA and M. yunnanensis on the Chemical Composition of Vetiver
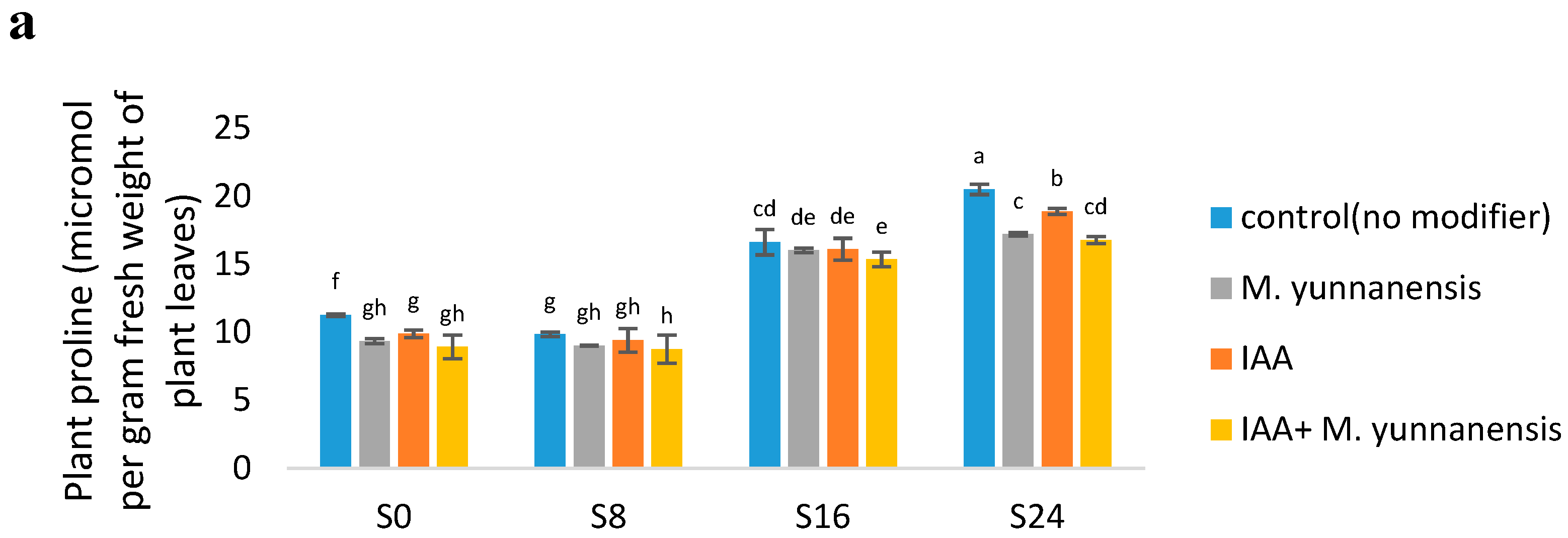
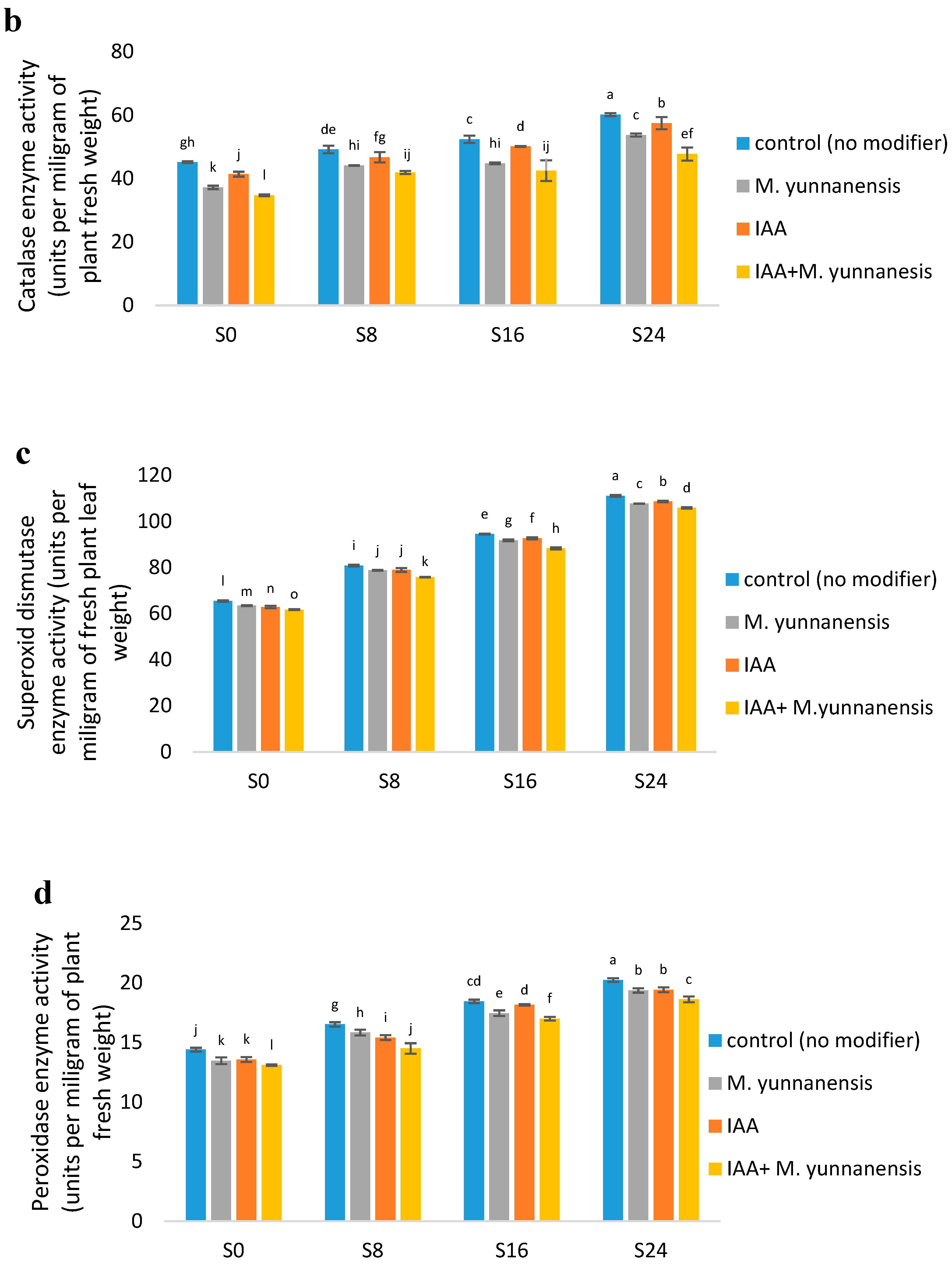
3.3. Effect of Salinity Levels, IAA and M. yunnanensis on Physiological Properties of Vetiver
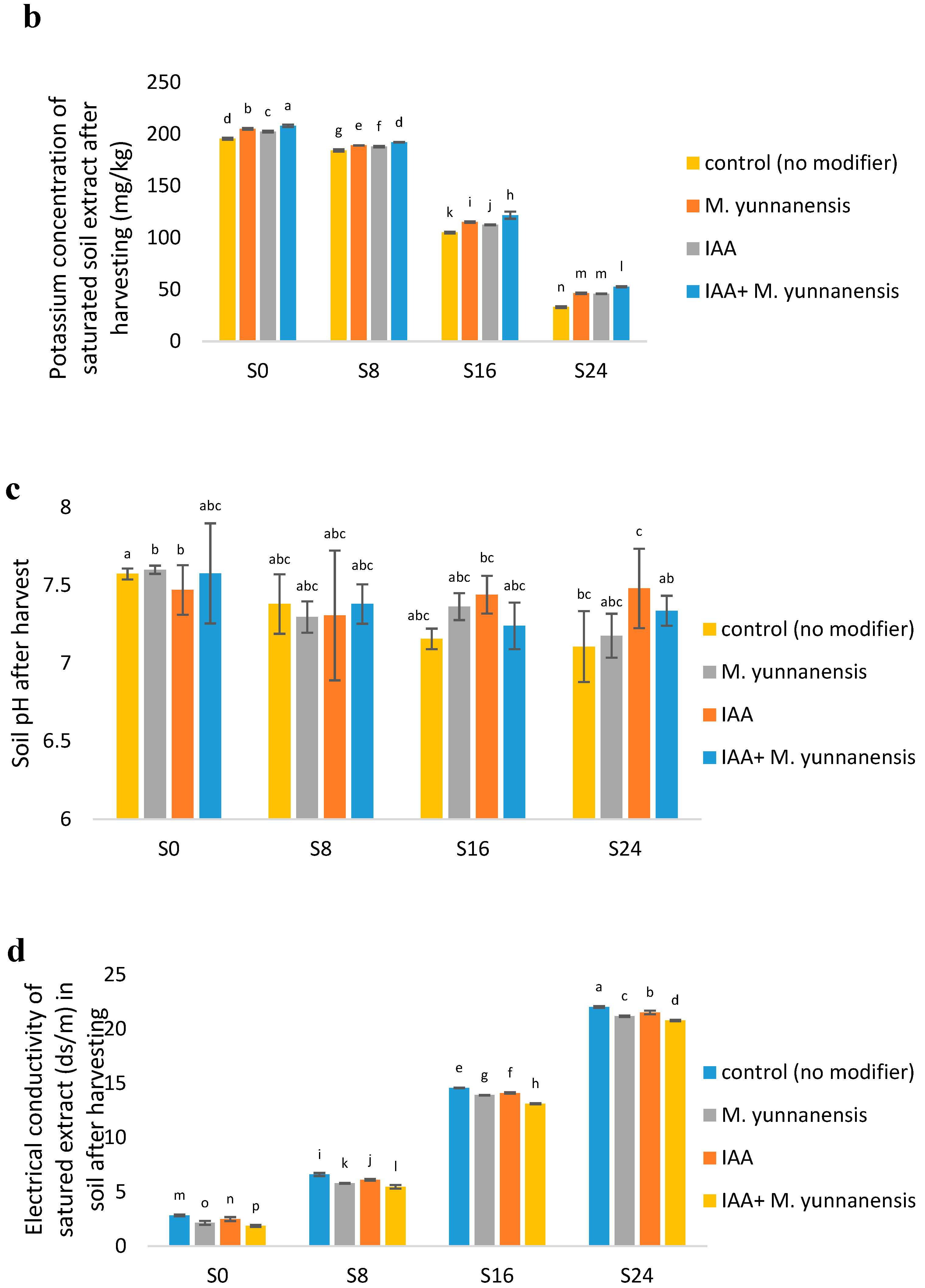
3.4. Chemical and Biological Properties of Soil after Vetiver Harvest
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| S | M | S × M | |
|---|---|---|---|
| PH (cm) | ns | ** | * |
| FW (g) | ** | ** | * |
| DW (g) | ** | ** | ** |
| RDW (g) | ns | ** | ns |
| ShP (%) | ** | ** | ** |
| ShK (%) | ** | ** | ** |
| ShNa (mg/kg) | ** | ns | ns |
| Pr (µmol/g) | ** | ** | ** |
| CAT (U/mg) | ** | ** | * |
| DIS (U/mg) | ** | ** | ** |
| POD (U/mg) | ** | ** | ** |
| SP (mg/kg) | ** | ns | ns |
| SK (mg/kg) | ** | ** | ** |
| SpH | ** | ns | ns |
| EC (ds/m) | ** | ** | ** |
| MR (mg CO2-C/Kg·h) | ** | ** | ** |
| MB (mgC/kg) | ** | ** | ** |
References
- Allahverdi, A.; Škvára, F. Acidic corrosion of hydrated cement based materials. Ceram. Silikáty 2000, 44, 152–160. [Google Scholar] [CrossRef]
- Gong, X.; Chao, L.; Zhou, M.; Hong, M.; Luo, L.; Wang, L.; Ying, W.; Cai, J.; Gong, S.; Hong, F. Oxidative damages of maize seedlings caused by exposure to a combination of potassium deficiency and salt stress. Plant Soil 2011, 340, 443–452. [Google Scholar] [CrossRef]
- Dietz, K.J.; Sauter, A.; Wichert, K.; Messdaghi, D.; Hartung, W. Extracellular β-glucosidase activity in barley involved in the hydrolysis of ABA glucose conjugate in leaves. J. Exp. Bot. 2000, 51, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Mousavi, S.S.; Karami, A.; Saharkhiz, M.J.; Etemadi, M.; Ravanbakhsh, M. Microbial amelioration of salinity stress in endangered accessions of Iranian licorice (Glycyrrhiza glabra L.). BMC Plant Biol. 2022, 22, 322. [Google Scholar] [CrossRef]
- Fathizad, H.; Ardakani, M.A.H.; Sodaiezadeh, H.; Kerry, R.; Taghizadeh-Mehrjardi, R. Investigation of the spatial and temporal variation of soil salinity using random forests in the central desert of Iran. Geoderma 2020, 365, 114233. [Google Scholar] [CrossRef]
- Hassani, A.; Azapagic, A.; Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef]
- Alghamdi, A.G.; Aly, A.A.; Ibrahim, H.M. Effect of climate change on the quality of soil, groundwater, and pomegranate fruit production in Al-Baha Region, Saudi Arabia: A modeling study using SALTMED. Sustainability 2022, 14, 13275. [Google Scholar] [CrossRef]
- Alqasemi, A.S.; Ibrahim, M.; Fadhil Al-Quraishi, A.M.; Saibi, H.; Al-Fugara, A.k.; Kaplan, G. Detection and modeling of soil salinity variations in arid lands using remote sensing data. Open Geosci. 2021, 13, 443–453. [Google Scholar] [CrossRef]
- Abbasipour Bahrani, H.; Ghazvini, H.; Amiri, B.; Bazrafshan, F.; Nikkhah, H. Responses of barley (Hordeum vulgare L.) genotypes to salinity stress under controlled and field conditions. Gesunde Pflanz. 2023, 75, 499–513. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.; AlKahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Saleem, M.; Hussain, S. Perspectives of bacterial ACC deaminase in phytoremediation. TRENDS Biotechnol. 2007, 25, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo-and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant growth-promoting bacteria as bioinoculants: Attributes and challenges for sustainable crop improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- Zhao, G.-Z.; Li, J.; Qin, S.; Zhang, Y.-Q.; Zhu, W.-Y.; Jiang, C.-L.; Xu, L.-H.; Li, W.-J. Micrococcus yunnanensis sp. nov., a novel actinobacterium isolated from surface-sterilized Polyspora axillaris roots. Int. J. Syst. Evol. Microbiol. 2009, 59, 2383–2387. [Google Scholar] [CrossRef]
- Borzoo, S.; Mohsenzadeh, S.; Moradshahi, A.; Kahrizi, D.; Zamani, H.; Zarei, M. Characterization of physiological responses and fatty acid compositions of Camelina sativa genotypes under water deficit stress and symbiosis with Micrococcus yunnanensis. Symbiosis 2021, 83, 79–90. [Google Scholar] [CrossRef]
- Majhi, K.; Let, M.; Bandopadhyay, R. Efficacious use of Micrococcus yunnanensis GKSM13 for the growth of rice seedlings under copper stress with elucidation into genomic traits. Curr. Plant Biol. 2024, 37, 100318. [Google Scholar] [CrossRef]
- de Araújo, V.L.V.P.; Junior, M.A.L.; de Souza Júnior, V.S.; de Araújo Filho, J.C.; Fracetto, F.J.C.; Andreote, F.D.; de Araujo Pereira, A.P.; Júnior, J.P.M.; do Rêgo Barros, F.M.; Fracetto, G.G.M. Bacteria from tropical semiarid temporary ponds promote maize growth under hydric stress. Microbiol. Res. 2020, 240, 126564. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, Z.; Mohsenzadeh, S.; Rowshan, V.; Moradshahi, A. Evaluation of the growth, essential oil composition and antioxidant activity of Dracocephalum moldavica under water deficit stress and symbiosis with Claroideoglomus etunicatum and Micrococcus yunnanensis. Sci. Hortic. 2019, 256, 108652. [Google Scholar] [CrossRef]
- Khan, M.; Asaf, S.; Khan, A.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.M.; Lee, I.J. Plant growth-promoting endophytic bacteria augment growth and salinity tolerance in rice plants. Plant Biol. 2020, 22, 850–862. [Google Scholar] [CrossRef]
- Afrangan, F.; Kazemeini, S.A.; Alinia, M.; Mastinu, A. Glomus versiforme and Micrococcus yunnanensis reduce the negative effects of salinity stress by regulating the redox state and ion homeostasis in Brassica napus L. crops. Biologia 2023, 78, 3049–3061. [Google Scholar] [CrossRef]
- Borzoo, S.; Mohsenzadeh, S.; Moradshahi, A.; Kahrizi, D.; Zamani, H.; Zarei, M. Effects of water deficit stress and symbiosis with Micrococcus yunnanensis at the reproductive stage on yield and seed composition of Camelina sativa. Authorea Prepr. 2020. [Google Scholar] [CrossRef]
- Riva, V.; Mapelli, F.; Dragonetti, G.; Elfahl, M.; Vergani, L.; Crepaldi, P.; La Maddalena, N.; Borin, S. Bacterial inoculants mitigating water scarcity in tomato: The importance of long-term in vivo experiments. Front. Microbiol. 2021, 12, 675552. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.; Valipour, M.; Nawaz, F.; Raheel, M.; Abbas, H.T.; Sajid, M.; Manan, A.; Kanwal, S.; Mahmoud, E.A.; Casini, R. Evaluation of silicon supplementation for drought stress under water-deficit conditions: An application of sustainable agriculture. Agronomy 2023, 13, 599. [Google Scholar] [CrossRef]
- Abdar, N.; Zarei, M.; Ronaghi, A.M. Mitigation effects of Rhizophagus intraradices and Micrococcus yunnanensis on boron toxicity in maize (Zea may L.) plant. J. Plant Nutr. 2023, 46, 3312–3324. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Kaczyński, P.; Pietkun, M.; Łozowicka, B. Evaluation of titanium and silicon role in mitigation of fungicides toxicity in wheat expressed at the level of biochemical and antioxidant profile. Chemosphere 2022, 308, 136284. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Łuniewski, S.; Kaczyński, P.; Łozowicka, B. The influence of humic acids and nitrophenols on metabolic compounds and pesticide behavior in wheat under biotic stress. Agronomy 2023, 13, 1378. [Google Scholar] [CrossRef]
- Maffei, M. The Genus Vetiveria; Taylor and Francis: London, UK, 2002. [Google Scholar]
- Bertea, C.M.; Camusso, W. Anatomy, biochemistry and physiology. In Vetiveria; CRC Press: Boca Raton, FL, USA, 2002; pp. 25–49. [Google Scholar]
- Lal, R.; Gupta, P.; Gupta, V.; Sarkar, S.; Singh, S. Genetic variability and character associations in vetiver (Vetiveria zizanioides L. Nash). Ind. Crops Prod. 2013, 49, 273–277. [Google Scholar] [CrossRef]
- Ahmadi Beni, M.; Niknahad Gharmakher, H.; Sadat Azimi, M.; Maramaei, M.G. Investigation of forage quality of Vetiveria zizanioides in semi-steppe region of Maravehtappeh, Golestan province, Iran. J. Rangel. Sci. 2014, 4, 287–297. [Google Scholar]
- Adams, R.; Dafforn, M. Lessons in diversity: DNA sampling of the pantropical vetiver grass uncovers genetic uniformity in erosion-control germplasm. Diversity 1997, 13, 27–28. [Google Scholar]
- Huang, X.-D.; El-Alawi, Y.; Penrose, D.M.; Glick, B.R.; Greenberg, B.M. Responses of three grass species to creosote during phytoremediation. Environ. Pollut. 2004, 130, 453–463. [Google Scholar] [CrossRef]
- Siripin, S. Microbiology associated with the vetiver plant. In Proceedings of the Second International Conference on Vetiver (ICV-2), Phetchaburi, Thailand, 18–22 January 2020; Office of the Royal Development Projects Board: Bangkok, Thailand, 2020; pp. 374–376. [Google Scholar]
- Alghanem, S.; Abu-Elsaoud, A.M.; Soliman, M.H.; ALHaithloul, H.A.S.; Zia-ur-Rehman, M.; Usman, M.; Abdel-Azeem, A.M. Effect of endophytic fungi in combating salinity and drought stress in date palm: A case study in Saudi Arabia. Pak. J. Agric. Sci. 2023, 60, 497–506. [Google Scholar]
- Bayomy, H.M.; Alamri, E.S.; Alharbi, B.M.; Foudah, S.H.; Genaidy, E.A.; Atteya, A.K. Response of Moringa oleifera trees to salinity stress conditions in Tabuk region, Kingdom of Saudi Arabia. Saudi J. Biol. Sci. 2023, 30, 103810. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef]
- Peng, M.; Jiang, Z.; Zhou, F.; Wang, Z. From salty to thriving: Plant growth promoting bacteria as nature’s allies in overcoming salinity stress in plants. Front. Microbiol. 2023, 14, 1169809. [Google Scholar] [CrossRef]
- Shilev, S. Plant-growth-promoting bacteria mitigating soil salinity stress in plants. Appl. Sci. 2020, 10, 7326. [Google Scholar] [CrossRef]
- Kandeler, E. Chapter 7: Physiological and Biochemical Methods for Studying Soil Biota and Their Functions en Paul EA (Eds) Soil Microbiology, Ecology and Biochemistry; Academic Press: Boston, MA, USA, 2015. [Google Scholar]
- Kumar, V.; Shriram, V.; Nikam, T.; Jawali, N.; Shitole, M. Sodium chloride-induced changes in mineral nutrients and proline accumulation in indica rice cultivars differing in salt tolerance. J. Plant Nutr. 2008, 31, 1999–2017. [Google Scholar] [CrossRef]
- Cruz, R.E.D.; Husain, T. Effect of vesicular arbuscular mycorrhiza (VAM) fungi inoculation on coppicing ability and drought resistance of Senna spectabilis. Pak. J. Bot. 2008, 40, 2217–2224. [Google Scholar]
- Sundara, B.; Natarajan, V.; Hari, K. Influence of phosphorus solubilising bacteria on soil available P status and sugarcane development on a tropical vertisol. In Proceedings of the XXIV Congress on International Society of Sugar Cane Technologists, Brisbane, Australia, 17–21 September 2001. [Google Scholar]
- Gutiérrez-Mañero, F.J.; Ramos-Solano, B.; Probanza, A.N.; Mehouachi, J.; Tadeo, F.R.; Talon, M. The plant-growth-promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins. Physiol. Plant. 2001, 111, 206–211. [Google Scholar] [CrossRef]
- Ghorbani Javid, M.; Sorooshzadeh, A.; Modarres Sanavy, S.A.M.; Allahdadi, I.; Moradi, F. Effects of the exogenous application of auxin and cytokinin on carbohydrate accumulation in grains of rice under salt stress. Plant Growth Regul. 2011, 65, 305–313. [Google Scholar] [CrossRef]
- Zahir, Z.; Asghar, H.; Arshad, M. Cytokinin and its precursors for improving growth and yield of rice. Soil Biol. Biochem. 2001, 33, 405–408. [Google Scholar] [CrossRef]
- Maleki, T.; Attaeian, B.; Mohammadparast, B.; Akhzari, D. Effects of sodium chloride stress on growth and physiological characteristics of plants Chrysopogon zizanioides in the greenhouse. J. Plant Ecosyst. Conserv. 2017, 5, 119–137. [Google Scholar]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ response mechanisms to salinity stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Levitt, J. Responses of Plants to Environmental Stress, Volume 1: Chilling, Freezing, and High Temperature Stresses; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Yu, X.; Ai, C.; Xin, L.; Zhou, G. The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur. J. Soil Biol. 2011, 47, 138–145. [Google Scholar] [CrossRef]
- Zabihi, H.; Savabeghi, A.; Khavazi, K.; Ganjeali, A. Effect of application of Pseudomonas fluorescents on yield and yield components of wheat under different soil salinity levels. Water Soil 2009, 23, 199–208. [Google Scholar] [CrossRef]
- Khalid, S.; Parvaiz, M.; Nawaz, K.; Hussain, H.; Arshad, A.; Shawaka, S.; Sarfaraz, Z.N.; Waheed, T. Effect of Indole Acetic Acid (IAA) on morphological, biochemical and chemical attributes of Two varieties of maize (Zea mays L.) under salt stress. World Appl. Sci. J. 2013, 26, 1150–1159. [Google Scholar]
- Ma, C.; Yuan, S.; Xie, B.; Li, Q.; Wang, Q.; Shao, M. IAA plays an important role in alkaline stress tolerance by modulating root development and ROS detoxifying systems in rice plants. Int. J. Mol. Sci. 2022, 23, 14817. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Dikilitas, M.; Tuna, A.L. Alleviation of salt stress-induced adverse effects on maize plants by exogenous application of indoleacetic acid (IAA) and inorganic nutrients-A field trial. Aust. J. Crop Sci. 2013, 7, 249–254. [Google Scholar]
- Tilak, K.; Ranganayaki, N.; Pal, K.; De, R.; Saxena, A.; Nautiyal, C.S.; Mittal, S.; Tripathi, A.; Johri, B. Diversity of plant growth and soil health supporting bacteria. Curr. Sci. 2005, 89, 136–150. [Google Scholar]
- Earnapalli, V. Screening of antagonistic microorganisms for biological control of early blight of tomato caused by Alternaria solani. Master Thesis, University of Agricultural Sciences, Dharwad, India, 2005. [Google Scholar]
- Young, L.-S.; Hameed, A.; Peng, S.-Y.; Shan, Y.-H.; Wu, S.-P. Endophytic establishment of the soil isolate Burkholderia sp. CC-Al74 enhances growth and P-utilization rate in maize (Zea mays L.). Appl. Soil Ecol. 2013, 66, 40–47. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Arasu, V.S.; Kathiresan, K. Effect of Trichoderma on soil phosphate solubilization and growth improvement of Avicennia marina. Aquat. Bot. 2013, 104, 101–105. [Google Scholar] [CrossRef]
- Flowers, T.; Hajibagheri, M. Salinity tolerance in Hordeum vulgare: Ion concentrations in root cells of cultivars differing in salt tolerance. Plant Soil 2001, 231, 1–9. [Google Scholar] [CrossRef]
- Shady, M.; Ibrahim, I.; Afify, A. Mobilisation of elements and their effects on certain plant growth characteristics as influenced by some silicate bacteria. Egypt. J. Bot. 1984, 27, 17–30. [Google Scholar]
- Greenway, H.; Munns, R. Mechanisms of salt tolerance in nonhalophytes. Annu. Rev. Plant Physiol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Sadeghi, H.; Emam, Y. Effect of different sodium chloride levels on morphological characteristics, chemical composition and yield components of two bread wheat cultivars. BIABAN 2005, 10, 267–278. [Google Scholar]
- Ashraf, M.; McNeilly, T. Salinity tolerance in Brassica oilseeds. Crit. Rev. Plant Sci. 2004, 23, 157–174. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Khedr, A.H.A.; Abbas, M.A.; Wahid, A.A.A.; Quick, W.P.; Abogadallah, G.M. Proline induces the expression of salt-stress-responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt-stress. J. Exp. Bot. 2003, 54, 2553–2562. [Google Scholar] [CrossRef]
- Gohari, G.; Panahirad, S.; Sepehri, N.; Akbari, A.; Zahedi, S.M.; Jafari, H.; Dadpour, M.R.; Fotopoulos, V. Enhanced tolerance to salinity stress in grapevine plants through application of carbon quantum dots functionalized by proline. Environ. Sci. Pollut. Res. 2021, 28, 42877–42890. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.-J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Z.; Yao, L.; Yue, H.; Li, H.; Li, C. Effect of IAA produced by Klebsiella oxytoca Rs-5 on cotton growth under salt stress. J. Gen. Appl. Microbiol. 2013, 59, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.R.; Chaitanya, K.; Sundar, D. Water stress-mediated changes in antioxidant enzyme activities of mulberry (Morus alba L.). J. Sericultural Sci. Jpn. 2000, 69, 169–175. [Google Scholar]
- Upadhyay, S.K.; Singh, J.S.; Saxena, A.K.; Singh, D.P. Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol. 2012, 14, 605–611. [Google Scholar] [CrossRef]
- Baniaghil, N.; Arzanesh, M.; Ghorbanli, M.; Shahbazi, M. The effect of plant growth promoting rhizobacteria on growth parameters, antioxidant enzymes and microelements of canola under salt stress. J. Appl. Environ. Biol. Sci 2013, 3, 17–27. [Google Scholar]
- Omar, M.; Osman, M.; Kasim, W.; Abd El-Daim, I. Improvement of salt tolerance mechanisms of barley cultivated under salt stress using Azospirillum brasilense. In Salinity and Water Stress: Improving Crop Efficiency; Springer: Dordrecht, The Netherlands, 2009; pp. 133–147. [Google Scholar]
- Sairam, R.K.; Rao, K.V.; Srivastava, G. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Gossett, D.R.; Millhollon, E.P.; Lucas, M.C. Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Sci. 1994, 34, 706–714. [Google Scholar] [CrossRef]
- Arora, A.; Sairam, R.; Srivastava, G. Oxidative stress and antioxidative system in plants. Curr. Sci. 2002, 82, 1227–1238. [Google Scholar]
- Jingnan, L.; Liping, T.; Tongwei, S.; Jie, L.; Lijun, W.; Zengliang, Y. Impact of ion implantation on licorice (Glycyrrhiza uralensis Fisch) growth and antioxidant activity under drought stress. Plasma Sci. Technol. 2007, 9, 301. [Google Scholar]
- Ramírez, I.; Estay, D.; Stange, C.; Cardemil, L. Superoxide dismutase is a critical enzyme to alleviate oxidative stress in Aloe vera (L.) Burm. plants subjected to water deficit. Plant Ecol. Divers. 2012, 5, 183–195. [Google Scholar] [CrossRef]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Ashraf, M.; Ahmad, S. Influence of sodium chloride on ion accumulation, yield components and fibre characteristics in salt-tolerant and salt-sensitive lines of cotton (Gossypium hirsutum L.). Field Crops Res. 2000, 66, 115–127. [Google Scholar] [CrossRef]
- Tuna, A.L.; Kaya, C.; Dikilitas, M.; Higgs, D. The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ. Exp. Bot. 2008, 62, 1–9. [Google Scholar] [CrossRef]
- Han, H.; Lee, K. Physiological responses of soybean-inoculation of Bradyrhizobium japonicum with PGPR in saline soil conditions. Res. J. Agric. Biol. Sci. 2005, 1, 216–221. [Google Scholar]
- Chandra, D.; Srivastava, R.; Sharma, A. Influence of IAA and ACC deaminase producing fluorescent pseudomonads in alleviating drought stress in wheat (Triticum aestivum). Agric. Res. 2018, 7, 290–299. [Google Scholar] [CrossRef]
- Al-Turki, A.; Murali, M.; Omar, A.F.; Rehan, M.; Sayyed, R. Recent advances in PGPR-mediated resilience toward interactive effects of drought and salt stress in plants. Front. Microbiol. 2023, 14, 1214845. [Google Scholar] [CrossRef]
- Meddich, A. Biostimulants for resilient agriculture—Improving plant tolerance to abiotic stress: A concise review. Gesunde Pflanz. 2023, 75, 709–727. [Google Scholar] [CrossRef]
- Ben-Laouane, R.; Baslam, M.; Ait-El-Mokhtar, M.; Anli, M.; Boutasknit, A.; Ait-Rahou, Y.; Toubali, S.; Mitsui, T.; Oufdou, K.; Wahbi, S. Potential of native arbuscular mycorrhizal fungi, rhizobia, and/or green compost as alfalfa (Medicago sativa) enhancers under salinity. Microorganisms 2020, 8, 1695. [Google Scholar] [CrossRef]
- Baltazar-Bernal, O.; Spinoso-Castillo, J.L.; Mancilla-Álvarez, E.; Bello-Bello, J.J. arbuscular mycorrhizal fungi induce tolerance to salinity stress in Taro plantlets (Colocasia esculenta L. Schott) during acclimatization. Plants 2022, 11, 1780. [Google Scholar] [CrossRef] [PubMed]
- Alinia, M.; Kazemeini, S.A.; Sepehri, M.; Dadkhodaie, A. Simultaneous application of rhizobium strain and melatonin improves the photosynthetic capacity and induces antioxidant defense system in common bean (Phaseolus vulgaris L.) under salinity stress. J. Plant Growth Regul. 2022, 41, 1367–1381. [Google Scholar] [CrossRef]
- Ouhaddou, R.; Ech-Chatir, L.; Anli, M.; Ben-Laouane, R.; Boutasknit, A.; Meddich, A. Secondary metabolites, osmolytes and antioxidant activity as the main attributes enhanced by biostimulants for growth and resilience of lettuce to drought stress. Gesunde Pflanz. 2023, 75, 1737–1753. [Google Scholar] [CrossRef]
- Khoshru, B.; Mitra, D.; Khoshmanzar, E.; Myo, E.M.; Uniyal, N.; Mahakur, B.; Mohapatra, P.K.D.; Panneerselvam, P.; Boutaj, H.; Alizadeh, M. Current scenario and future prospects of plant growth-promoting rhizobacteria: An economic valuable resource for the agriculture revival under stressful conditions. J. Plant Nutr. 2020, 43, 3062–3092. [Google Scholar]
- Ikram, M.; Ali, N.; Jan, G.; Jan, F.G.; Pervez, R.; Romman, M.; Zainab, R.; Yasmin, H.; Khan, N. Isolation of endophytic fungi from halophytic plants and their identification and screening for auxin production and other plant growth promoting traits. J. Plant Growth Regul. 2023, 42, 4707–4723. [Google Scholar] [CrossRef]
- Vimal, S.R.; Gupta, J.; Singh, J.S. Effect of salt tolerant Bacillus sp. and Pseudomonas sp. on wheat (Triticum aestivum L.) growth under soil salinity: A comparative study. Microbiol. Res. 2018, 9, 7462. [Google Scholar] [CrossRef]
- Rietz, D.; Haynes, R. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol. Biochem. 2003, 35, 845–854. [Google Scholar] [CrossRef]
- Pathak, H.; Rao, D. Carbon and nitrogen mineralization from added organic matter in saline and alkali soils. Soil Biol. Biochem. 1998, 30, 695–702. [Google Scholar] [CrossRef]
- Yan, N.; Marschner, P. Response of soil respiration and microbial biomass to changing EC in saline soils. Soil Biol. Biochem. 2013, 65, 322–328. [Google Scholar] [CrossRef]
- Jafari, S.; Chorom, M.; Enayatizamir, N.; Motamedi, H. Evaluating the effects of Bacillus Subtilis and Corynebacterium glutamicum on soil microbial indexes in different levels of salinity. Agric. Eng. 2013, 35, 55–70. [Google Scholar]
- Chandarana, K.A.; Pramanik, R.S.; Amaresan, N. Interaction between ciliate and plant growth promoting bacteria influences the root structure of rice plants, soil PLFAs and respiration properties. Rhizosphere 2022, 21, 100466. [Google Scholar] [CrossRef]
- Weldmichael, T.; Márton, D.; Simon, B.; Michéli, E.; Reda, G.; Adiyah, F.; Cserháti, M. Bacterial community characterization and microbial respiration of selected arable soils of ethiopia. Eurasian Soil Sci. 2021, 54, 1921–1934. [Google Scholar] [CrossRef]
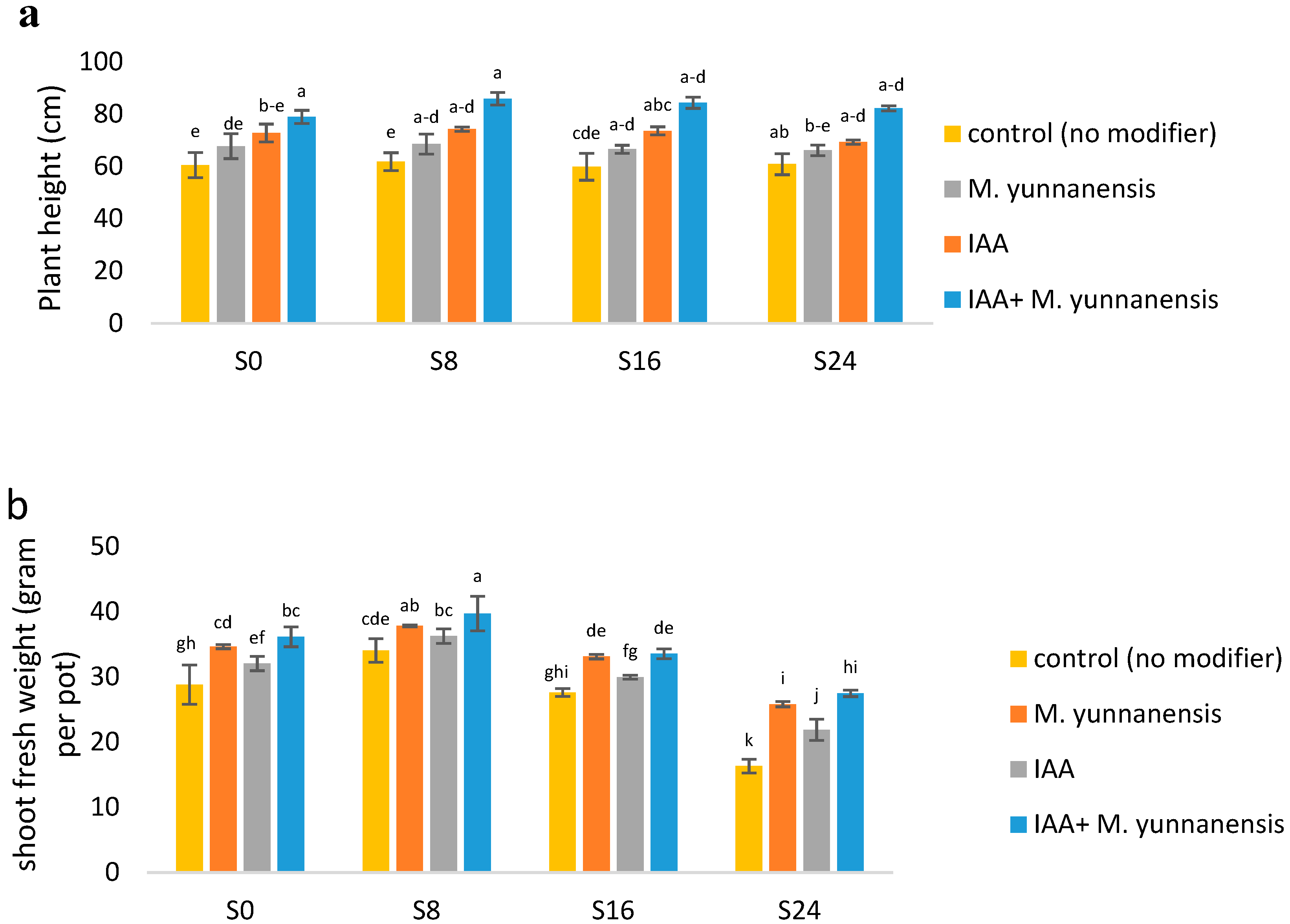
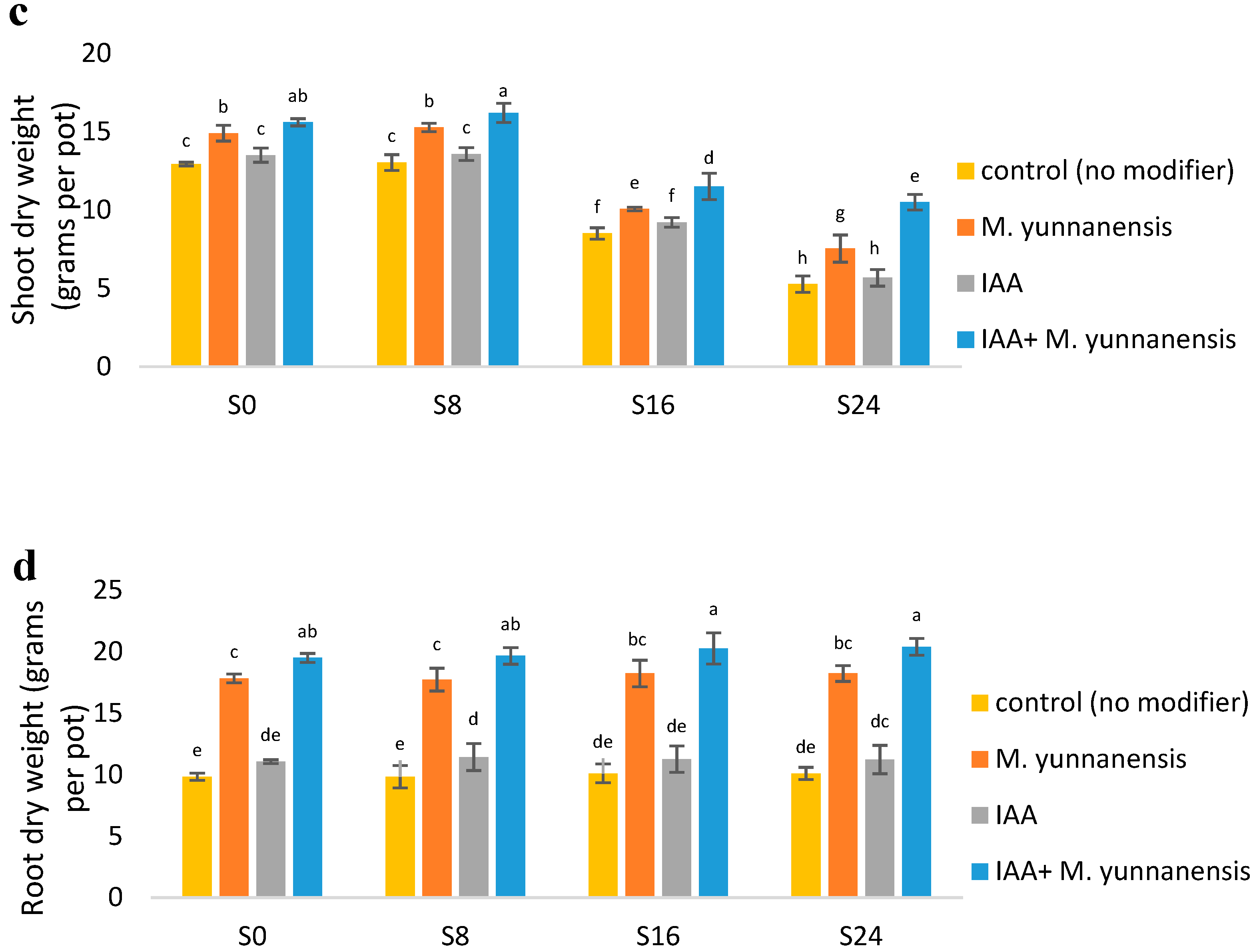
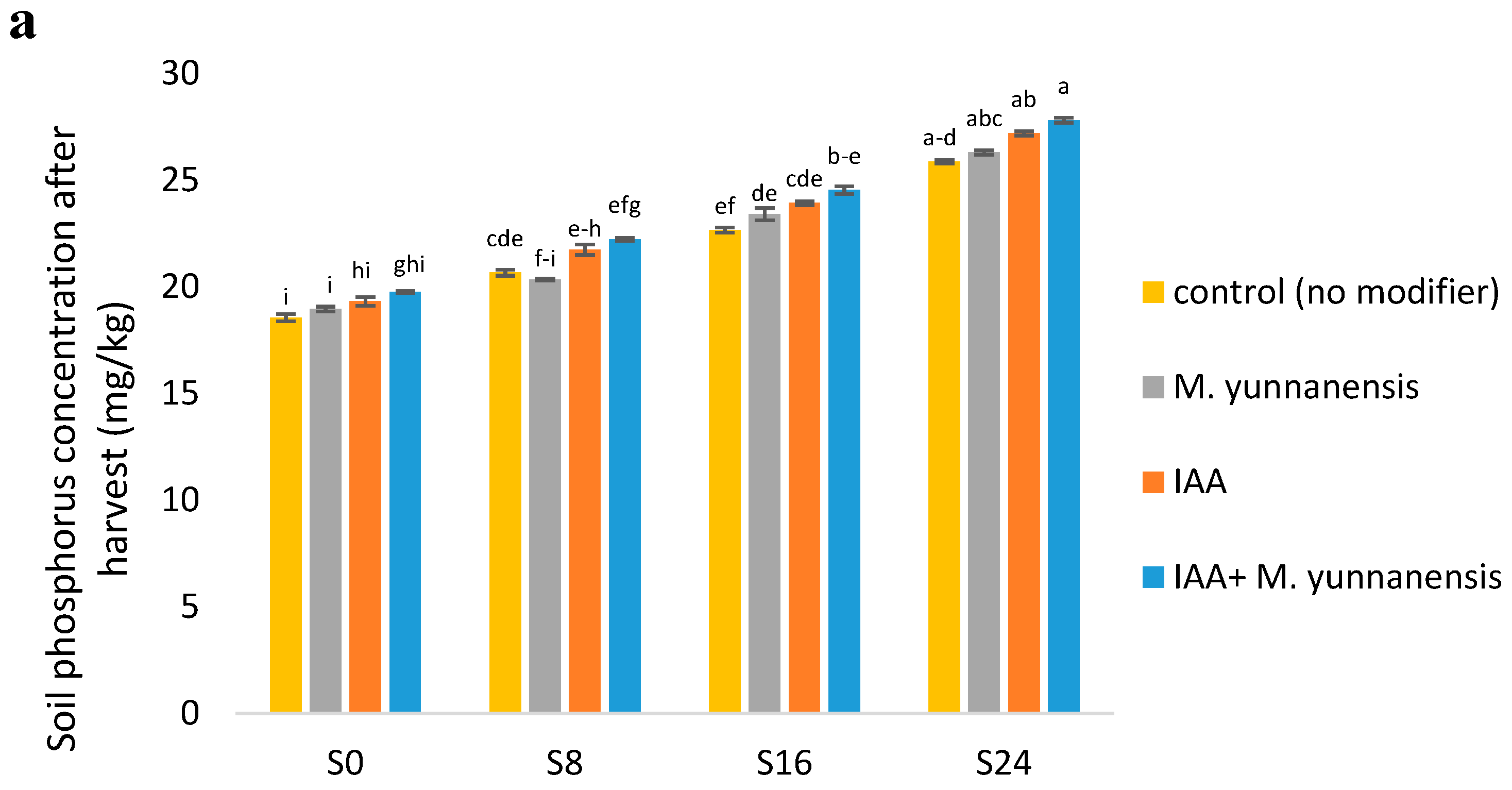



| Feature | The Amount |
|---|---|
| Sand | 57.72% |
| Silt | 12.56% |
| Clay | 29.72 |
| Texture class | Sandy clay loam |
| pH-saturated dough | 7.6 |
| Electrical conductivity of the saturated extract | 2.15 dS/m |
| Cation exchange capacity | 18 cmol+/kg |
| Organic matter | 1.3% |
| Total N | 0.07% |
| P extractable by NaHCO3 | 15 mg/kg |
| K extractable by C2H7NO2 | 420 mg/kg |
| Extractable Cu with DTPA | 5.1 mg/kg |
| Extractable Mn with DTPA | 0.6 mg/kg |
| Extractable Zn with DTPA | 3 mg/kg |
| Extractable Cd with DTPA | 1.3 mg/kg |
| Microbial respiration | 5.2 mg CO2-C kg−1·h−1 |
| Soil Microbial biomass carbon | 15.15 mg of C/kg of soil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosallanejad, N.; Zarei, M.; Ghasemi-Fasaei, R.; Shahriari, A.G.; Mohkami, A.; Majláth, I.; Vetukuri, R.R. Mitigation of Salinity Stress on Vetiver Grass (Vetiveria zizanioides) through Application of Micrococcus yunnanensis and Indole-3-Acetic Acid. Agronomy 2024, 14, 1952. https://doi.org/10.3390/agronomy14091952
Mosallanejad N, Zarei M, Ghasemi-Fasaei R, Shahriari AG, Mohkami A, Majláth I, Vetukuri RR. Mitigation of Salinity Stress on Vetiver Grass (Vetiveria zizanioides) through Application of Micrococcus yunnanensis and Indole-3-Acetic Acid. Agronomy. 2024; 14(9):1952. https://doi.org/10.3390/agronomy14091952
Chicago/Turabian StyleMosallanejad, Negar, Mehdi Zarei, Reza Ghasemi-Fasaei, Amir Ghaffar Shahriari, Afsaneh Mohkami, Imre Majláth, and Ramesh R. Vetukuri. 2024. "Mitigation of Salinity Stress on Vetiver Grass (Vetiveria zizanioides) through Application of Micrococcus yunnanensis and Indole-3-Acetic Acid" Agronomy 14, no. 9: 1952. https://doi.org/10.3390/agronomy14091952







