Influence of Bacterial Fertilizers on the Structure of the Rhizospheric Fungal Community of Cereals South of Western Siberia
Abstract
1. Introduction
2. Materials and Methods
2.1. Conditions and Scheme of Experiments
2.2. Characteristics of Object Research
2.3. Taxonomic Analysis of the Mushroom Community
3. Results
3.1. Taxonomic Profile of the Fungal Component of the Microbiome
3.2. Study of the Population of Rhizoctonia solani Kuehn. in the Soil
3.3. In the Population of the Soil with Conidia, the Causative Agent of Ordinary Root Rot of Grain Crops Is Bipolaris sorokiniana Sacc. Shoem. (syn. Helminthosporium Pam., King et Bakke)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Glossary
| Soil fertility | a set of soil properties that ensure a harvest of crops |
| Soil microflora | a set of microorganisms present in the soil environment |
References
- Ratnikov, A.N.; Sviridenko, D.G.; Arysheva, S.P.; Semeshkina, P.S. The influence of the new organomineral complex “Gumiton” on the productivity and quality of grain crops on various types of soils. Agrar. Bull. Ural. 2020, 4, 29–37. [Google Scholar] [CrossRef]
- Alferov, A.A.; Chernova, L.S.; Zavalin, A.A.; Chebotar, V.K. The effectiveness of the use of endophytic biologics and nitrogen fertilizers. Bull. Russ. Agric. Sci. 2017, 5, 21–24. (In Russian) [Google Scholar]
- Hu, D.; Baskin, J.M.; Baskin, C.C.; Liu, R.; Yang, X.; Huang, Z. A seed mucilage-degrading fungus from the rhizosphere strengthens the plant-soil-microbe continuum and potentially regulates root nutrients of a cold desert shrub. Mol. Plant Microbe Interact. 2021, 34, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.A.; Atmapoojya, S.L. Experimental study on factors affecting the efficiency of microbially induced carbonate precipitation in soil. Mater. Today Proc. 2022, 60, 275–280. [Google Scholar] [CrossRef]
- Sun, X.; Sun, M.; Chao, Y.; Wang, H.; Pan, H.; Yang, Q.; Cui, X.; Lou, Y.; Zhuge, Y. Alleviation of lead toxicity and phytostimulation in perennial ryegrass by the Pb-resistant fungus Trichoderma asperellum SD-5. Funct. Plant Biol. 2021, 48, 333–341. [Google Scholar] [CrossRef]
- Vasileva, E.N.; Akhtemova, G.A.; Zhukov, V.A.; Tikhonovich, I.A. Endophytic microorganisms in fundamental research and agriculture. Ecol. Genet. 2019, 17, 19–32. [Google Scholar] [CrossRef]
- Alguacil, M.M.; Torrecillas, E.; García-Orenes, F.; Roldán, A. Changes in the composition and diversity of AMF communities mediated by management practices in a Mediterranean soil are related with increases in soil biological activity. Soil Biol. Biochem. 2014, 76, 34–44. [Google Scholar] [CrossRef]
- Shuliko, N.N.; Khamova, O.F.; Timokhin, A.Y.; Boiko, V.S.; Tukmacheva, E.V.; Krempa, A. Influence of long-term intensive use of irrigated meadow-chernozem soil on the biological activity and productivity of the arable layer. Sci. Rep. 2022, 12, 14672. Available online: https://www.nature.com/articles/s41598-022-18639-1 (accessed on 28 May 2024).
- Cheng, Q.; Ma, J.; Ren, R.; Zheng, L.J.; Guo, X.; Sun, X. Effects of Fertilization Management under WSPI on Soil Nitrogen Distribution and Nitrogen Absorption in Apple Orchard in Loess Plateau. Agronomy 2020, 10, 1386. [Google Scholar] [CrossRef]
- Khamova, O.F.; Shuliko, N.N.; Tukmacheva, E.V. The number of microorganisms of the barley rhizosphere with prolonged use of mineral fertilizers, straw and inoculation of seeds with associative diazotrophs. Omsk Sci. Bull. 2015, 1, 127–131. (In Russian) [Google Scholar]
- Voronkova, H.A. Ways of biologization of intensification processes in agriculture in Western Siberia. Achiev. Sci. Technol. Agroind. Complex 2008, 12, 28–30. (In Russian) [Google Scholar]
- Pershina, E.V.; Kutovaya, O.V.; Kogut, B.M.; Andronov, E.E. The Main Achievements and Prospects of Soil Metagenomics; Inform Navigator: St. Petersburg, Russia, 2004; 288p. (In Russian) [Google Scholar]
- Pershina, E.V.; Ivanova, E.A.; Nagieva, A.G.; Zhiengaliev, A.T.; Chirak, E.L.; Andronov, E.E.; Sergaliev, N.H. Comparative analysis of microbiomes of natural and anthropogenic disturbed soils of North-western Kazakhstan. Soil Sci. 2016, 6, 720–732. (In Russian) [Google Scholar]
- Zhuravleva, A.S.; Labutova, N.M.; Andronov, E.E. The effect of oil pollution on the microbiocenosis of soils adjacent to the oil storage facility. Ecol. Genet. 2017, 15, 60–68. (In Russian) [Google Scholar] [CrossRef]
- Andronov, E.E.; Petrova, S.N.; Pinaev, A.G.; Pershina, E.V.; Rakhimgalieva, S.Z.; Akhmedenov, K.M.; Gorobets, A.V.; Sergaliev, N.H. Studying the structure of the microbial community of soils of different degrees of salinity using t-RFLP and PCR with real-time detection. Soil Sci. 2012, 2, 173–183. (In Russian) [Google Scholar]
- Rosseev, V.M.; Belan, I.A.; Rosseeva, L.P. The use of the in vitro method in wheat breeding. Bull. Plant Prot. 2016, 3, 142–143. (In Russian) [Google Scholar]
- Pinaev, A.G.; Kichko, A.A.; Aksenova, T.S.; Safronova, V.I.; Kozhenkova, E.V.; Andronov, E.E. RIAM: A Universal Accessible Protocol for the Isolation of High Purity DNA from Various Soils and Other Humic Substances. Methods Protoc. 2022, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Mcmurdie, P.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Klaubauf, S.; Inselsbacher, E.; Zechmeister-Boltenstern, S.; Wanek, W.; Gottsberger, R.; Strauss, J.; Gorfer, M. Molecular diversity of fungal communities in agricultural soils from lower Austria. Fungal Divers. 2010, 44, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef] [PubMed]
- Lukhmenev, V.P. Phytopathology. In A Textbook for University Students Studying in the Specialty of Agronomy; Publishing house of the OGAU Center: Orenburg, Russia, 2012; 299p. (In Russian) [Google Scholar]
- Challacombe, J.; Hesse, C.N.; Bramer, L.M.; McCue, L.A.; Lipton, M.; Purvine, S.; Nicora, C.; Gallegos-Graves, V.; Porras-Alfaro, A.; Kuske, C.R. Genomes and secretomes of Ascomycota fungi reveal diverse functions in plant biomass decomposition and pathogenesis. BMC Genom. 2019, 20, 976. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Smith, M.E. Lineages of ectomycorrhizal fungi revisited: Foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biol. Rev. 2013, 27, 83–99. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, Q.; Adamowski, J.F.; Biswas, A.; Cao, J.; Liu, W.; Yanyan, Q.; Meng, Z. Conversion of grassland to abandoned land and afforested land alters soil bacterial and fungal communities on the Loess Plateau. Appl. Soil Ecol. 2023, 183, 104758. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Garrido-Jurado, I.; González-Mas, N.; Yousef-Yousef, M. Ecosystem services of entomopathogenic ascomycetes. J. Invertebr. Pathol. 2023, 201, 108015. [Google Scholar] [CrossRef]
- Karpov, S.A.; Mamkaeva, M.A.; Aleoshin, V.V.; Nassonova, E.; Lilje, O.; Gleason, F.H. Morphology, phylogeny and ecology of the aphelids (Aphelidea, Opisthokonta) and proposal for the new superphylum Opisthosporidia. Front. Microbiol. 2014, 5, 112. [Google Scholar] [CrossRef]
- Gromov, B.V. Parasites of algae from the group of “Monads” of the Tsenkovsky genera Aphelidium, Amoeboaphelidium and Pseudaphelidium as representatives of a new class. Zool. J. 2000, 79, 517–525. (In Russian) [Google Scholar]
- Bonfante, P.; Venice, F. Mucoromycota: Going to the roots of plant-interacting fungi. Fungal Biol. Rev. 2020, 34, 100–113. [Google Scholar] [CrossRef]
- Prasad, P.V.; Bheemanahalli, R.; Jagadish, S.K. Field crops and the fear of heat stress—Opportunities, challenges and future directions. Field Crops Res. 2017, 200, 114–121. [Google Scholar] [CrossRef]
- Vega, F.E.; Meyling, N.V.; Luangsa-ard, J.J.; Blackwell, M. Fungal Entomopathogens. In Insect Pathology; Elsevier: London, UK, 2012; pp. 171–220. [Google Scholar] [CrossRef]
- Khalikova, L.V.; Kavelenova, L.M. Soil microbiocenosis in the agricultural environment as a dynamic system: Primary results of assessment of changes. Samara Sci. Bull. 2023, 12, 91–97. [Google Scholar] [CrossRef]
- Chernov, T.I. Metagenomic Analysis of Prokaryotic Communities of Soil Profiles in the European Part of Russia. Ph.D. Thesis, Moscow State University, Moscow, Russia, 2016; 111p. (In Russian). [Google Scholar]
- Chirak, E.L.; Pershina, E.V.; Dolnik, A.S.; Kutovaya, O.V.; Vasilenko, E.S.; Kogut, B.M.; Merzlyakova, Y.V.; Andronov, E.E. Taxonomic structure of microbial communities in soils of various types according to high-performance sequencing of 16S-rRNA gene libraries. Agric. Biol. 2013, 3, 100–109. (In Russian) [Google Scholar]
- Woudenberg, J.H.C.; Groenewald, J.Z.; Binder, M.; Crous, P.W. Alternaria redefined. Stud. Mycol. 2013, 75, 171–212. [Google Scholar] [CrossRef] [PubMed]
- Ramires, F.A.; Masiello, M.; Somma, S.; Villani, A.; Susca, A.; Logrieco, A.F.; Luz, C.; Meca, G.; Moretti, A. Phylogeny and Mycotoxin Characterization of Alternaria Species Isolated from Wheat Grown in Tuscany, Italy. Toxins 2018, 10, 472. [Google Scholar] [CrossRef]
- López-López, A.M.; León-Félix, J.; Allende, R.; Lima, N.B.; Tovar-Pedraza, J.; García-Estrada, R. First Report of Setophoma terrestris Causing Corky and Pink. Root of Tomato in Sinaloa, Mexico. Plant Dis. 2020, 104, 1553. [Google Scholar] [CrossRef]
- Ikeda, K.; Kuwabara, K.; Urushibara, T.; Soyai, P.; Miki, S.; Shibata, S. Pink root rot of squash caused by Setophoma terrestris in Japan. J. Gen. Plant Pathol. 2012, 78, 372–375. [Google Scholar] [CrossRef]
- Waqar, S.; Bhat, A.A.; Khan, A.A. Endophytic fungi: Unravelling plant-endophyte interaction and the multifaceted role of fungal endophytes in stress amelioration. Plant Physiol. Biochem. 2024, 206, 108174. [Google Scholar] [CrossRef]
- Simeone, R.; Piarulli, L.; Nigro, D.; Signorile, M.; Blanco, E.; Mangini, G.; Blanco, A. Mapping Powdery Mildew (Blumeria graminis f. sp. tritici) Resistance in Wild and Cultivated Tetraploid Wheats. Int. J. Mol. Sci. 2020, 21, 7910. [Google Scholar] [CrossRef]
- Both, M.; Csukai, M.; Stumpf, M.P.H.; Spanu, P.D. Gene expression profiles of Blumeria graminis indicate dynamic changes to primary metabolism during development of an obligate biotrophic pathogen. Plant Cell 2005, 17, 2107–2122. [Google Scholar] [CrossRef]
- Villanueva, P.; Vásquez, G.; Gil-Durán, C.; Oliva, V.; Díaz, A.; Henríquez, M.; Álvarez, E.; Laich, F.; Chávez, R.; Vaca, I. Description of the First Four Species of the Genus Pseudogymnoascus From Antarctica. Front. Microbiol. 2021, 12, 713189. [Google Scholar] [CrossRef]
- Schroers, H.-J. A monograph of Bionectria (Ascomycota, Hypocreales, Bionectriaceae) and its Clonostachys anamorphs. Stud. Mycol. 2001, 46, 73–79. [Google Scholar]
- Bondar, P.N. Strains of Fungi of the Genus Trichoderma (Pers.: Fr.) as a Basis for the Creation of Plant Protection Products and the Production of Feed Additives: Abstract. Ph.D. Thesis, Moscow State University, Moscow, Russia, 2011; 21p. (In Russian). [Google Scholar]
- Zhang, X.; Wang, H.; Que, Y.; Yu, D.; Wang, H. The influence of rhizosphere soil fungal diversity and complex community structure on wheat root rot disease. PeerJ 2021, 9, 12601. [Google Scholar] [CrossRef] [PubMed]
- Kirtsideli, I.Y. Soil-Dwelling Microscopic Fungi in the Ecosystems of the Arctic and Antarctic: Abstract. Ph.D. Thesis, Russian Academy of Sciences, St. Petersburg, Russia, 2019; 58p. (In Russian). [Google Scholar]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus. Penicillium Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, M.; Saupe, S.J. The genome sequence of Podospora anserina, a classic model fungus. Genome Biol. 2008, 9, 223. [Google Scholar] [CrossRef]
- Gołębiowska, G.; Dyda, M.; Wajdzik, K. Quantitative Trait Loci and Candidate Genes Associated with Cold-Acclimation and Microdochium nivale Tolerance/Susceptibility in Winter Triticale (x Triticosecale). Plants 2021, 10, 2678. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Hao, G.; Yang, B.; Feng, Y.; Wang, Y.; Feng, L.; Zhao, J.; Song, Y.; Zhang, H.; et al. Role of the phenylalanine-hydroxylating system in aromatic substance degradation and lipid metabolism in the oleaginous fungus Mortierella alpine. Appl. Environ. Microbiol. 2013, 79, 3225–3233. [Google Scholar] [CrossRef]
- Dix, N.J.; Webster, J. Fungi of Extreme Environments. Fungal Ecology. Available online: https://www.sci-hub.ru/10.1007/978-94-011-0693-1?ysclid=lvmfebywmp214493048 (accessed on 30 April 2024).
- Borodin, S.G.; Kotlyarova, I.A.; Tereshchenko, G.A. Species composition of fungi of the genus Rhizopus Ehrenb on sunflower. Oilseed Crops 2012, 2, 151–152. (In Russian) [Google Scholar]
- Toropova, E.Y.; Porsev, I.N.; Maslennikov, A.A. Phytosanitary assessment of soil tillage methods in the Trans-Urals. Bull. Kurgan State Agric. Acad. 2013, 1, 27–31. (In Russian) [Google Scholar]
- Shuliko, N.N.; Timokhin, A.Y.; Khamova, O.F.; Boyko, V.S.; Tukmacheva, E.V.; Korchagina, I.A.; Weinbender, A.A. Biological and agrochemical properties of meadow-chernozem soil of the Omsk Irtysh region in connection with the productivity of forage crops when using mineral fertilizers. Agric. Biol. 2024, 59, 156–173. (In Russian) [Google Scholar] [CrossRef]
- Bozhko, A.A.; Popolzukhina, N.A.; Khamova, O.F.; Popolzukhin, P.V.; Seituarova, A.D.; Shuliko, N.N.; Parshutkin, Y.Y. Biological activity of the soil of the rhizosphere of oats (Hordeum vulgare L.) during seed inoculation with associative diazotrophs. Probl. Agrochem. Ecol. 2019, 2, 60–64. (In Russian) [Google Scholar] [CrossRef]
- Kursakova, V.S. The effectiveness of the use of root diazotrophic preparations in spring wheat crops with minimal tillage. Bull. Altai. State Agrar. Univ. 2018, 10, 5–12. (In Russian) [Google Scholar]
- Zverev, A.O.; Kurchak, O.N.; Orlova, O.V.; Onishchuk, O.P.; Kichko, A.A.; Eregin, A.V.; Naliukhin, A.N.; Pinaev, A.G.; Andronov, E.E. Dynamic of the Soil Microbiota in Short-Term Crop Rotation. Life 2023, 13, 400. [Google Scholar] [CrossRef]
- Abakumov, E.V.; Gladkov, G.V.; Kimeklis, A.K.; Andronov, E.E. The Microbiomes of Various Types of Abandoned Fallow Soils of South Taiga (Novgorod Region, Russian North-West). Agronomy 2023, 13, 2592. [Google Scholar] [CrossRef]
- Korobova, L.N.; Riksen, V.S.; Lomova, T.G. Microbiological parameters as an indicator of the agrogenic transformation of medium-sized salt shale by forage crop rotations. Vestn. NGAU 2023, 2, 51–59. (In Russian) [Google Scholar] [CrossRef]
- Yakimenko, V.N.; Malyuga, A.A. Dependence of the agroecological state of the soil on the potassium balance in the agrocenosis. Bull. Tomsk. State Univ. Biol. 2014, 1, 26–41. (In Russian) [Google Scholar]
- Hannibal, F.B.; Gagkaeva, T.Y.; Gomzhina, M.M.; Poluektova, E.V.; Gultyaeva, E.I. Wheat-associated micromycetes and their significance as pathogens in Russia. Bull. Plant Prot. 2022, 105, 164–180. (In Russian) [Google Scholar]
- Hannibal, F.B.; Poluektova, E.V.; Lukyanets, Y.V.; Gagkaeva, T.Y.; Gomzhina, M.M. Barley-associated micromycetes and their significance as pathogens in Russia. Bull. Plant Prot. 2023, 106, 172–186. (In Russian) [Google Scholar] [CrossRef]


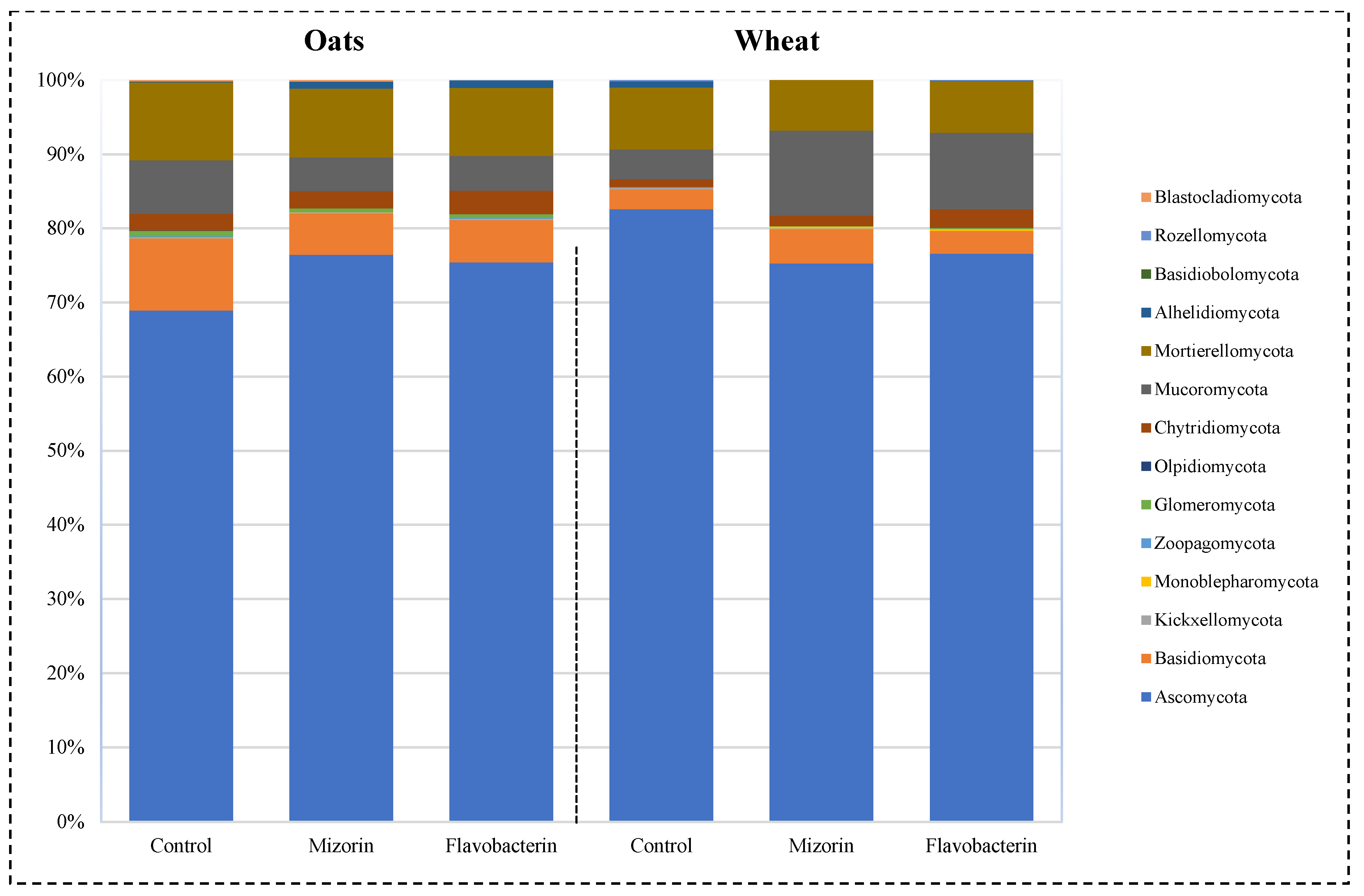


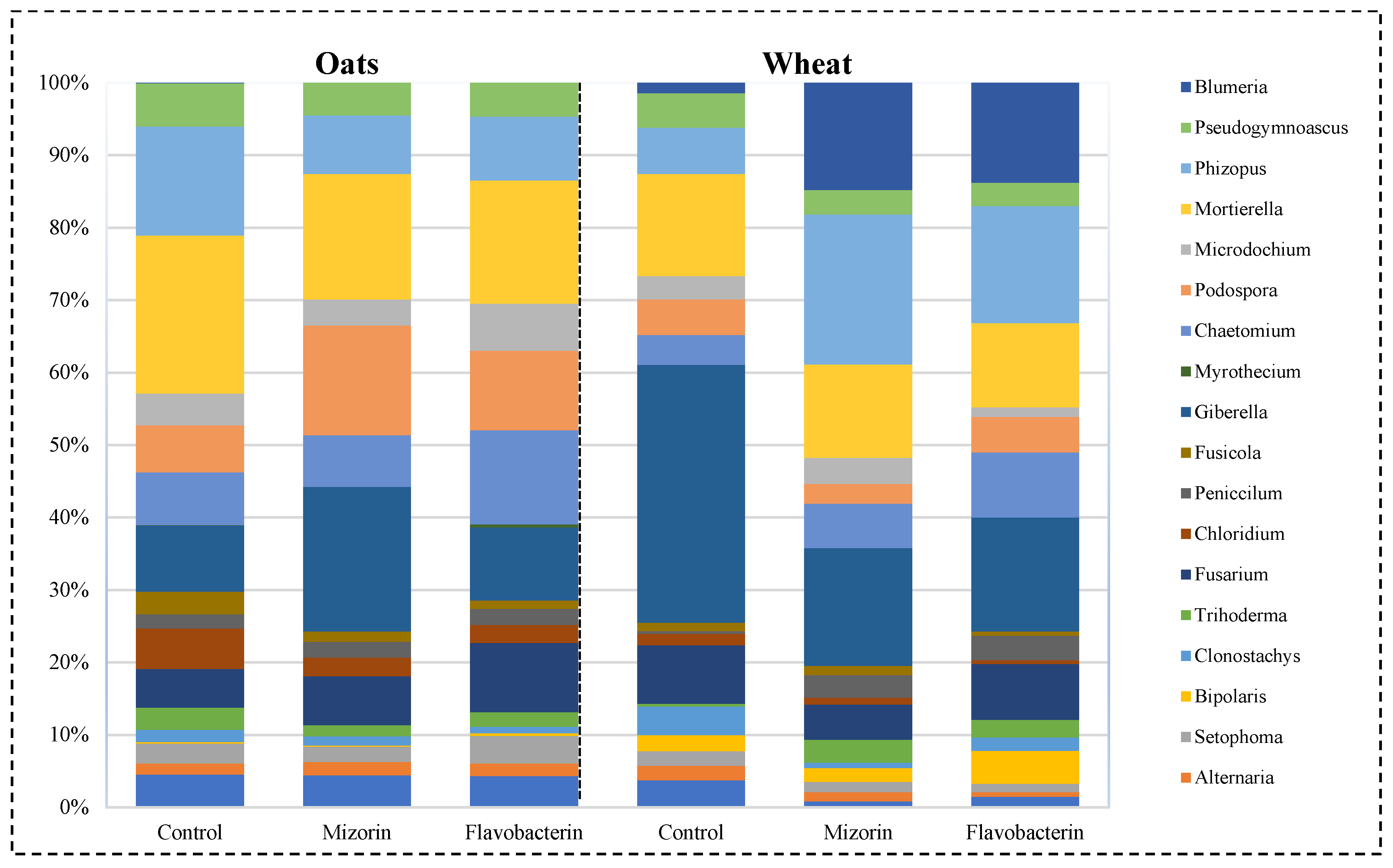
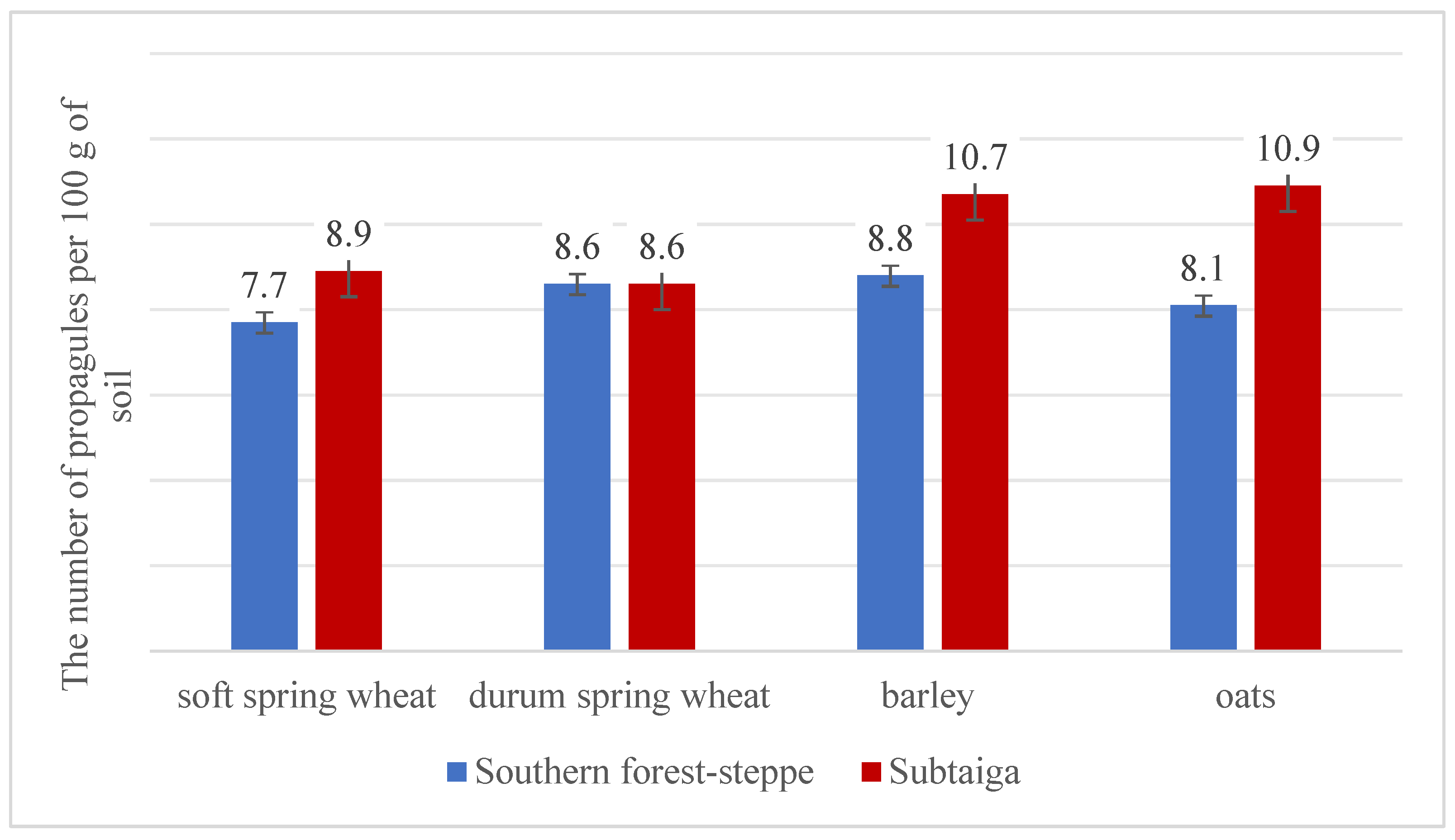
| Options | Southern Forest-Steppe (Meadow-Chernozem Soil) | The Average for the Zone | Subtaiga (Grey Forest Soil) | The Average for the Zone | |||||
|---|---|---|---|---|---|---|---|---|---|
| Tillering | Earing | Filling of Grain | Tillering | Earing | Filling of Grain | ||||
| Soft spring wheat Omsk 42 | Control | 12.2 | 8.6 | 7.9 | 9.6 | 15.6 | 9.4 | 9.7 | 11.6 |
| Mizorin | 17.5 | 13.4 | 7.2 | 12.7 | 14.6 | 8.9 | 9.4 | 11.0 | |
| Flavobacterin | 16.4 | 11.9 | 6.9 | 11.7 | 14.9 | 8.9 | 9.6 | 11.0 | |
| Average | 15.4 | 11.3 | 7.3 | 11.3 | 15.0 | 9.1 | 9.6 | 11.2 | |
| Soft spring wheat Tarskaya 12 | Control | 18.0 | 11.4 | 10.1 | 13.2 | 16.6 | 10.6 | 7.9 | 11.7 |
| Mizorin | 16.6 | 14.6 | 11.0 | 14.1 | 23.0 | 9.0 | 8.4 | 13.5 | |
| Flavobacterin | 15.0 | 14.2 | 9.7 | 13.0 | 23.0 | 9.1 | 6.8 | 13.0 | |
| Average | 16.5 | 13.4 | 10.3 | 13.4 | 20.9 | 9.6 | 7.7 | 12.7 | |
| Soft spring wheat Omsk 44 | Control | 18.8 | 14.7 | 12.9 | 15.5 | 16.5 | 13.7 | 10.1 | 13.4 |
| Mizorin | 16.2 | 14.4 | 11.0 | 13.9 | 15.3 | 11.3 | 10.0 | 12.2 | |
| Flavobacterin | 16.2 | 13.9 | 10.3 | 13.5 | 14.1 | 10.8 | 9.8 | 11.6 | |
| Average | 17.1 | 14.3 | 11.4 | 14.3 | 15.3 | 11.9 | 10.0 | 12.4 | |
| Durum spring wheat Omsk coral | Control | 18.8 | 12.7 | 9.0 | 13.5 | 13.6 | 11.9 | 9.9 | 11.8 |
| Mizorin | 18.0 | 11.4 | 10.1 | 13.2 | 14.6 | 12.6 | 10.6 | 12.6 | |
| Flavobacterin | 18.6 | 13.7 | 12.3 | 14.9 | 18.5 | 14.0 | 13.3 | 15.3 | |
| Average | 18.5 | 12.6 | 10.5 | 13.8 | 15.6 | 12.8 | 11.3 | 13.2 | |
| Barley Omsk 101 | Control | 15.9 | 12.1 | 9.0 | 12.3 | 12.2 | 9.5 | 8.6 | 10.1 |
| Mizorin | 13.0 | 11.4 | 10.3 | 11.6 | 12.5 | 9.9 | 8.7 | 10.4 | |
| Flavobacterin | 11.5 | 10.7 | 9.4 | 10.5 | 11.9 | 8.9 | 8.9 | 9.9 | |
| Average | 13.5 | 11.4 | 9.6 | 11.5 | 12.2 | 9.4 | 8.7 | 10.1 | |
| Oats Siberian Hercules | Control | 10.0 | 6.9 | 6.5 | 7.8 | 10,3 | 8.4 | 10.2 | 9.6 |
| Mizorin | 11.3 | 10.7 | 8.0 | 10.0 | 10.7 | 9.4 | 9.8 | 10.0 | |
| Flavobacterin | 13.8 | 10.4 | 8.8 | 11.0 | 10.0 | 9.8 | 9.4 | 9.7 | |
| Average | 11.7 | 9.3 | 7.8 | 9.6 | 10.3 | 9.2 | 9.8 | 9.8 | |
| Average by experience | 15.4 | 12.1 | 9.5 | 12.3 | 14.9 | 10.3 | 9.5 | 11.6 | |
| Options | The Total Number of Conidia, pcs./g | The Number of Living Conidia, pcs./g | Proportion of Degraded Conidia, % | |||
|---|---|---|---|---|---|---|
| Meadow- Chernozem | Grey Forest Soil | Meadow- Chernozem | Grey Forest Soil | Meadow- Chernozem | Grey Forest Soil | |
| Soft spring wheat | 10 | 20 | 1 | 3 | 90.0 | 85.0 |
| Durum spring wheat | 25 | 15 | 2 | 2 | 92.0 | 86.7 |
| Barley | 5 | 25 | 1 | 2 | 80.0 | 92.0 |
| Oats | 15 | 20 | 1 | 2 | 93.3 | 90.0 |
| Average | 13.8 | 20.0 | 1.3 | 2.3 | 88.8 | 88.4 |
| Options | The Total Number of Conidia, pcs./g | The Number of Living Conidia, pcs./g | Proportion of Degraded Conidia, % | ||||
|---|---|---|---|---|---|---|---|
| Meadow- Chernozem | Grey Forest Soil | Meadow- Chernozem | Grey Forest Soil | Meadow- Chernozem | Grey Forest Soil | ||
| Soft spring wheat Omsk 42 | Control | 31.7 ± 3.3 | 21.7 ± 5.2 | 3.3 ± 0.7 | 2.7 ± 0.5 | 89.7 | 87.3 |
| Mizorin | 18.3 ± 4.4 | 18.3 ± 1.3 | 1.7 ± 0.3 | 1.7 ± 0.3 | 90.7 | 91.1 | |
| Flavobacterin | 18.3 ± 1.7 | 18.3 ± 4.7 | 2.0 ± 0.8 | 2.0 ± 0.8 | 89.4 | 90.0 | |
| Soft spring wheat Tarskaya 12 | Control | 20.0 ± 2.9 | 18.3 ± 2.6 | 2.7 ± 0.5 | 2.0 ± 0.3 | 86.9 | 88.5 |
| Mizorin | 25.0 ± 2.9 | 16.7 ± 1.3 | 3.0 ± 0.5 | 1.0 ± 0.3 | 88.2 | 93.9 | |
| Flavobacterin | 25.0 ± 2.9 | 18.3 ± 1.3 | 3.0 ± 0.5 | 1.3 ± 0.3 | 88.2 | 92.8 | |
| Soft spring wheat Omsk 44 | Control | 21.7 ± 3.3 | 16.7 ± 1.3 | 2.7 ± 0.5 | 2.3 ± 0.3 | 87.6 | 85.6 |
| Mizorin | 21.7 ± 4.4 | 21.7 ± 3.4 | 2.7 ± 0.5 | 2.0 ± 0.3 | 87.8 | 90.0 | |
| Flavobacterin | 21.7 ± 1.7 | 18.3 ± 1.3 | 3.0 ± 0.3 | 2.7 ± 0.3 | 86.0 | 85.6 | |
| Durum spring wheat Omsk coral | Control | 38.3 ± 1.7 | 21.7 ± 1.3 | 4.7 ± 0.3 | 2.7 ± 0.7 | 87.9 | 87.7 |
| Mizorin | 33.3 ± 1.7 | 23.3 ± 2.6 | 4.3 ± 0.3 | 2.3 ± 0.7 | 87.0 | 90.6 | |
| Flavobacterin | 28.3 ± 1.7 | 21.7 ± 1.3 | 4.0 ± 0.3 | 2.0 ± 0.5 | 85.8 | 90.7 | |
| Barley Omsk 101 | Control | 30.0 ± 2.9 | 23.3 ± 1.3 | 4.0 ± 0.5 | 2.7 ± 0.3 | 86.8 | 88.7 |
| Mizorin | 33.3 ± 3.3 | 30.0 ± 1.3 | 3.7 ± 0,5 | 3.0 ± 0.5 | 89.2 | 90.0 | |
| Flavobacterin | 23.3 ± 1.7 | 21.7 ± 3.4 | 3.3 ± 0.7 | 2.3 ± 0.3 | 85.7 | 87.8 | |
| Oats Siberian Hercules | Control | 31.7 ± 1.7 | 23.3 ± 2.6 | 4.0 ± 0.3 | 2.7 ± 0.5 | 87.3 | 88.9 |
| Mizorin | 31.7 ± 3.3 | 20.0 ± 2.2 | 4.0 ± 0.5 | 2.0 ± 0.5 | 87.4 | 90.4 | |
| Flavobacterin | 28.3 ± 4.4 | 18.3 ± 3.4 | 3.7 ± 0.5 | 2.0 ± 0.5 | 86.9 | 89.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuliko, N.N.; Selitskaya, O.V.; Tukmacheva, E.V.; Kiselyova, A.A.; Korchagina, I.A.; Kubasova, E.V.; Timokhin, A.Y. Influence of Bacterial Fertilizers on the Structure of the Rhizospheric Fungal Community of Cereals South of Western Siberia. Agronomy 2024, 14, 1989. https://doi.org/10.3390/agronomy14091989
Shuliko NN, Selitskaya OV, Tukmacheva EV, Kiselyova AA, Korchagina IA, Kubasova EV, Timokhin AY. Influence of Bacterial Fertilizers on the Structure of the Rhizospheric Fungal Community of Cereals South of Western Siberia. Agronomy. 2024; 14(9):1989. https://doi.org/10.3390/agronomy14091989
Chicago/Turabian StyleShuliko, Natalia Nikolaevna, Olga Valentinovna Selitskaya, Elena Vasilyevna Tukmacheva, Alina Andreevna Kiselyova, Irina Anatolyevna Korchagina, Ekaterina Vladimirovna Kubasova, and Artem Yuryevich Timokhin. 2024. "Influence of Bacterial Fertilizers on the Structure of the Rhizospheric Fungal Community of Cereals South of Western Siberia" Agronomy 14, no. 9: 1989. https://doi.org/10.3390/agronomy14091989
APA StyleShuliko, N. N., Selitskaya, O. V., Tukmacheva, E. V., Kiselyova, A. A., Korchagina, I. A., Kubasova, E. V., & Timokhin, A. Y. (2024). Influence of Bacterial Fertilizers on the Structure of the Rhizospheric Fungal Community of Cereals South of Western Siberia. Agronomy, 14(9), 1989. https://doi.org/10.3390/agronomy14091989






