Assessing Heat Stress Tolerance of Wheat Genotypes through Integrated Molecular and Physio-Biochemical Analyses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genomic DNA Extraction and SSR Markers
2.2. Phenotyping, and Data Recording
2.2.1. Experimental Design
2.2.2. Agro-Physio-Biochemical Traits
2.2.3. Quality Traits
2.3. Statistical Analysis
3. Results
3.1. Genotypic Analysis Based on SSR Markers
3.2. Exploring the Relationship between SSR Markers and Agro-Physio-Biochemical Traits
3.3. Phenotypic Analysis for Assessing Terminal Heat Tolerance in Wheat Genotyp
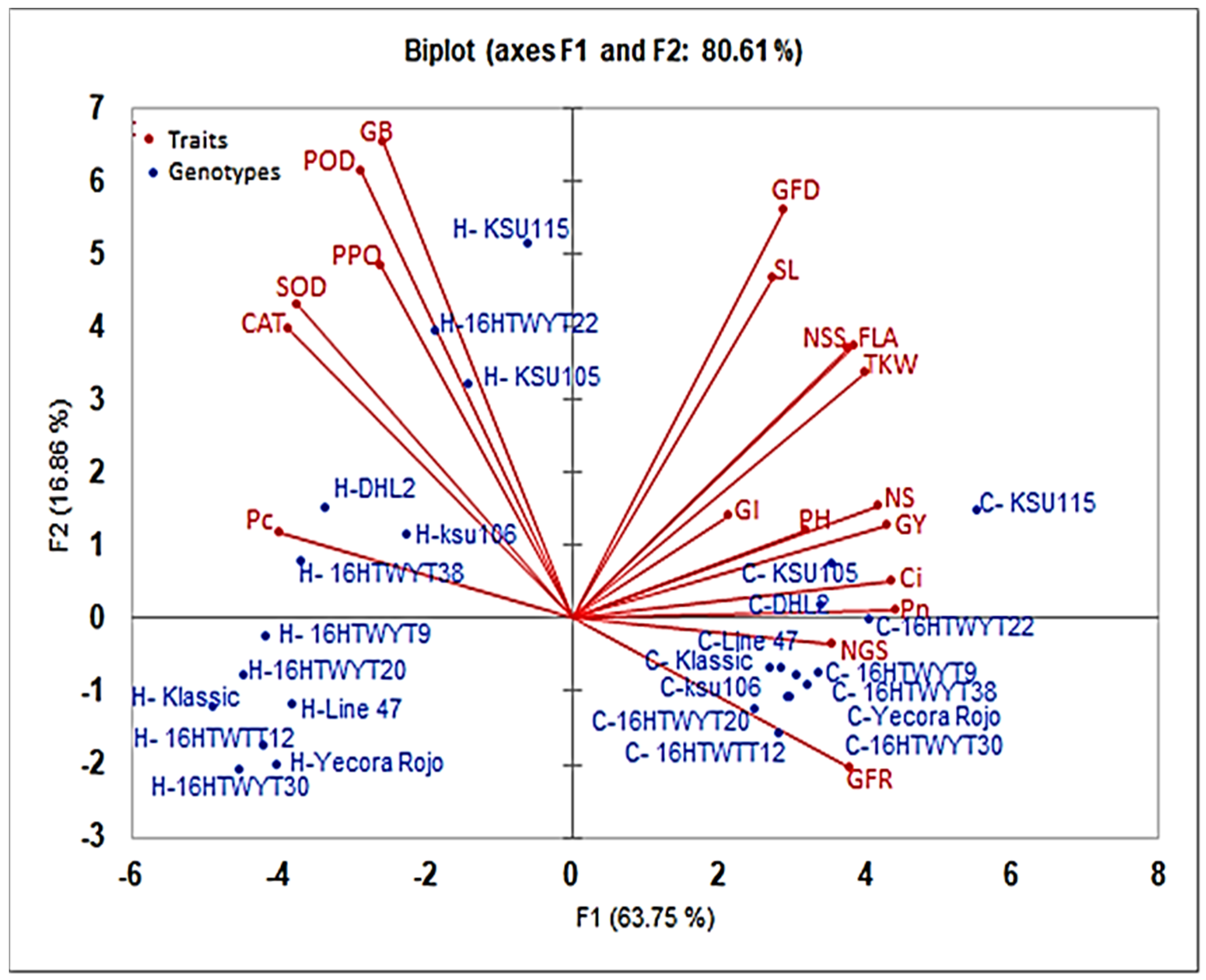
3.4. Principal Component Analysis of Agro-Physio-Biochemical Traits under Control and Heat Stress
3.5. SMLR and PC Analysis for the Preformance of Yield Traits
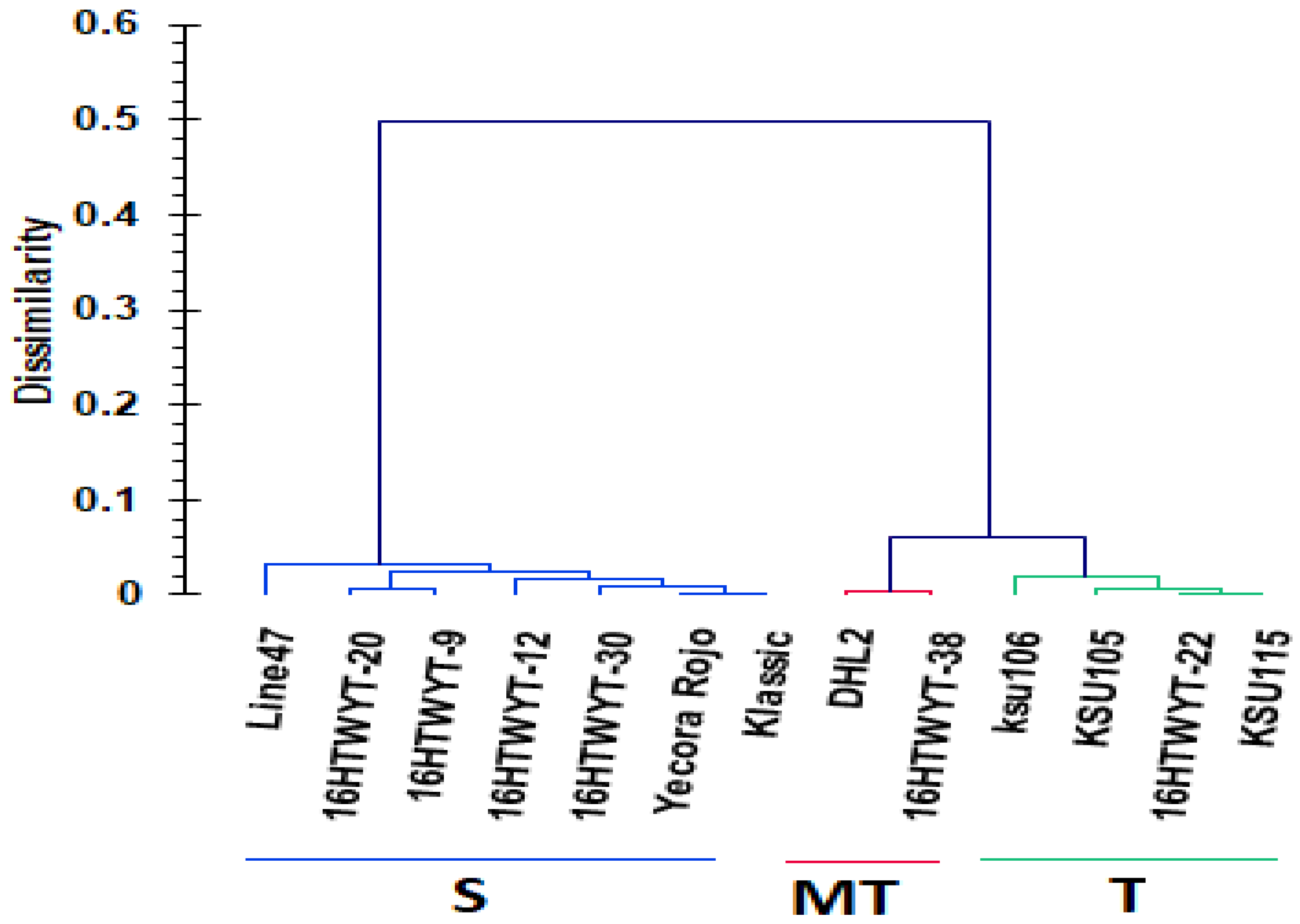
3.6. Hierarchical Clustering and Linear Discriminant Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haque, M.I.; Khan, M.R. Impact of climate change on food security in Saudi Arabia: A roadmap to agriculture-water sustainability. J. Agribus. Dev. Emerg. Econ. 2022, 12, 1–18. [Google Scholar] [CrossRef]
- IPCC. Climate Change: Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; 151p. [Google Scholar]
- Liu, B.; Asseng, S.; Wang, A.; Wang, S.; Tang, L.; Cao, W.; Zhu, Y.; Liu, L. Modelling the effects of post-heading heat stress on biomass growth of winter wheat. Agricultural 2017, 247, 476–490. [Google Scholar] [CrossRef]
- Oberle, B.; Bringezu, S.; Hatfield-Dodds, S.; Hellweg, S.; Schandl, H.; Clement, J. Global Resources Outlook: 2019; International Resource Panel, United Nations Envio: Paris, France, 2019. [Google Scholar]
- Akter, N.; Rafiqul Islam, M. Heat stress effects and management in wheat. A review. Agron. Sustain. Dev. 2017, 37, 1–17. [Google Scholar] [CrossRef]
- Zhang, C.X.; Feng, B.H.; Chen, T.T.; Fu, W.M.; Li, H.B.; Li, G.Y.; Jin, Q.Y.; Tao, L.X.; Fu, G.F. Heat stress-reduced kernel weight in rice at anthesis is associated with impaired source-sink relationship and sugars allocation. Environ. Exp. Bot. 2018, 155, 718–733. [Google Scholar] [CrossRef]
- Saini, H.; Aspinall, D. Abnormal sporogenesis in wheat (Triticum aestivum L.) induced by short periods of high temperature. Ann. Bot. 1982, 49, 835–846. [Google Scholar] [CrossRef]
- Giorno, F.; Wolters-Arts, M.; Mariani, C.; Rieu, I. Ensuring reproduction at high temperatures: The heat stress response during anther and pollen development. Plants 2013, 2, 489–506. [Google Scholar] [CrossRef]
- Ortiz, R.; Sayre, K.D.; Govaerts, B.; Gupta, R.; Subbarao, G.; Ban, T.; Reynolds, M. Climate change: Can wheat beat the heat? Agric. Ecosyst. Environ. 2008, 126, 46–58. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Chakraborty, U.; Pradhan, D. High temperature-induced oxidative stress in, role of antioxidants and amelioration of stress by chemical pre-treatments. J. Plant Interact. 2011, 6, 43–52. [Google Scholar] [CrossRef]
- Ashraf, M. Thermotolerance in plants: Potential physio-biochemical and molecular markers for crop improvement. Environ Exp. Bot. 2021, 186, 104454. [Google Scholar] [CrossRef]
- Edreira, J.I.R.; Otegui, M.E. Heat stress in temperate and tropical maize hybrids: Differences in crop growth, biomass partitioning and reserves use. Field Crop. Res. 2012, 130, 87–98. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Almeselmani, M.; Deshmukh, P.S.; Sairam, R.K.; Kushwaha, S.R.; Singh, T.P. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 2006, 171, 382–388. [Google Scholar] [CrossRef]
- Babu, R.N.; Devaraj, V.R. High temperature and salt stress response in French bean (Phaseolus vulgaris). Aust. J. Crop Sci. 2008, 2, 40–48. [Google Scholar]
- Xu, S.; Li, J.L.; Zhang, X.Q.; Wei, H.; Cui, L.J. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ. Exp. Bot. 2006, 56, 274–285. [Google Scholar] [CrossRef]
- Gong, M.; Chen, S.N.; Song, Y.Q.; Li, Z.G. Effect of calcium and calmodulin on intrinsic heat tolerance in relation to antioxidant systems in maize seedlings. Aust. J. Plant Physiol. 1997, 24, 371–379. [Google Scholar] [CrossRef]
- Dat, J.F.; Foyer, C.H.; Scott, I.M. Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol. 1998, 118, 1455–1461. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Almeselmani, M.; Deshmukh, P.; Sairam, R. High temperature stress tolerance in wheat genotypes: Role of antioxidant defence enzymes. Acta Agron. Hung. 2009, 57, 1–14. [Google Scholar] [CrossRef]
- Sarafraz-Ardakani, M.R.; Khavari-Nejad, R.A.; Moradi, F.; Najafi, F. Abscisic acid and cytokinin-induced osmotic and antioxidant regulation in two drought-tolerant and drought-sensitive cultivars of wheat during grain filling under water deficit in field conditions. Not. Sci. Biol. 2014, 6, 354–362. [Google Scholar] [CrossRef]
- Gupta, N.K.; Agarwal, S.; Agarwal, V.P.; Nathawat, N.S.; Gupta, S.; Singh, G. Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiol. Plant. 2013, 35, 1837–1842. [Google Scholar] [CrossRef]
- Anjali Anand, A.A.; Shantha Nagarajan, S.N.; Pathak, P. Effect of high temperature on hydrogen peroxide scavenging enzymes during reproductive phase in aromatic rice cultivars. Indian J. Plant Physiol. 2006, 11, 427–431. [Google Scholar]
- Sharma, P.; Mehta, G.; Shefali Muthusamy, S.K.; Singh, S.K.; Singh, G.P. Development and validation of heat-responsive candidate gene and miRNA gene based SSR markers to analysis genetic diversity in wheat for heat tolerance breeding. Mol. Biol. Rep. 2021, 48, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Senn, M.E.; Grozeff, G.E.G.; Alegre, M.L.; Barrile, F.; De Tullio, M.C.; Bartoli, C.G. Effect of mitochondrial ascorbic acid synthesis on photosynthesis. Plant Physiol. Bioch 2016, 104, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S. Physiological and Molecular Basis of Heat Tolerance with Emphasis on Oxidative Stress Metabolism in Wheat. Ph.D. Thesis, HNB Garhwal University, Srinagar, India, 2005. [Google Scholar]
- Balla, K.; Bencze, S.; Janda, T.; Veisz, O. Analysis of heat stress tolerance in winter wheat. Acta Agron. Hung. 2009, 57, 437–444. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Alotaibi, M.; Refay, Y.; Ghazy, A.; Zakri, A.; Al-Doss, A.J.A. Selection criteria for high-yielding and early-flowering bread wheat hybrids under heat stress. PLoS ONE 2020, 15, e0236351. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.S.; Lopes, M.S.; Collins, N.C.; Reynolds, M.P. Modelling and genetic dissection of staygreen under heat stress. Theor. Appl. Genet. 2016, 129, 2055–2074. [Google Scholar] [CrossRef]
- Pinto, R.S.; Reynolds, M.P.; Mathews, K.L.; McIntyre, C.L.; Olivares-Villegas, J.J.; Chapman, S.C. Heat and drought adaptive QTL in a wheat population designed to minimize confounding agronomic effects. Theor. Appl. Genet. 2010, 121, 1001–1021. [Google Scholar] [CrossRef]
- Barakat, M.N.; Saleh, M.; Al-Doss, A.A.; Moustafa, K.A.; Elshafei, A.A.; Al-Qurainy, F.H. Identification of New Ssr Markers Linked to Leaf Chlorophyll Content, Flag Leaf Senescence and Cell Membrane Stability Traits in Wheat under Water Stressed Condition. Acta Biol. Hung. 2015, 66, 93–102. [Google Scholar] [CrossRef]
- Liu, K.Y.; Xu, H.; Liu, G.; Guan, P.F.; Zhou, X.Y.; Peng, H.R.; Yao, Y.; Ni, Z.; Sun, Q.; Du, J. QTL mapping of flag leaf-related traits in wheat. Theor. Appl. Genet. 2018, 131, 839–849. [Google Scholar] [CrossRef]
- Rehman, H.U.; Tariq, A.; Ashra, F.I.; Ahmed, M.; Muscolo, A.; Basra, S.M.A.; Reynolds, M. Evaluation of Physiological and Morphological Traits for Improving Spring Wheat Adaptation to Terminal Heat Stress. Plants 2021, 10, 455. [Google Scholar] [CrossRef]
- Mondal, S.; Singh, R.P.; Crossa, J.; Huerta-Espino, J.; Sharma, I.; Chatrath, R.; Joshi, A.K. Earliness in wheat: A key to adaptation under terminal and continual high temperature stress in South Asia. Field Crop. Res. 2013, 151, 19–26. [Google Scholar] [CrossRef]
- Lamba, K.; Kumar, M.; Singh, V.; Chaudhary, L.; Sharma, R.; Yashveer, S.; Dalal, M.S. Heat stress tolerance indices for identification of the heat tolerant wheat genotypes. Sci. Rep. 2023, 13, 10842. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Jamil, M.; Napar, A.A.; Rahman, R.; Bano, A.; Afzal, F.; Gul Kazi, A.; Mujeeb Kazi, A. Heat stress in wheat and interdisciplinary approaches for yield maximization. In Plant-Environment Interaction: Responses Approaches to Mitigate Stress; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 161–183. [Google Scholar]
- Al-Ashkar, I.; Romdhane, W.B.; El-Said, R.A.; Ghazy, A.; Attia, K.; Al-Doss, A. Agro-Physiologic Responses and Stress-Related Gene Expression of Four Doubled Haploid Wheat Lines under Salinity Stress Conditions. Biology 2021, 10, 56. [Google Scholar] [CrossRef]
- Kushanov, F.N.; Turaev, O.S.; Ernazarova, D.K.; Gapparov, B.M.; Oripova, B.B.; Kudratova, M.K.; Rafieva, F.; Khalilov, K.; Erjigitov, D.; Khidivov, M.; et al. Genetic Diversity, QTL Mapping, and Marker-Assisted Selection Technology in Cotton (Gossypium spp.). Front. Plant Sci. 2021, 12, 779386. [Google Scholar] [CrossRef]
- Cao, L.-Y.; Zhao, J.-G.; Zhan, X.-D.; Li, D.-L.; He, L.-B.; Cheng, S.-H. Mapping QTLs for heat tolerance and correlation between heat tolerance and photosynthetic rate in rice. Chin. J. Rice Sci. 2003, 17, 223. [Google Scholar]
- Al-Doss, A.; Barakat, M.; Moustafa, K.; Motawei, M.; Alamri, M.; Elshafei, A.; Sallam, M.S.; Al-Ashkar, I.M. QTL analysis for grain failing rate and related traits in doubled haploid population of wheat under heat stress conditions. In Proceedings of the Conference Plant and Animal Genomics, San Diego, CA, USA, 10–15 January 2016; Volume 24. [Google Scholar]
- Barakat, M.N.; Al-Doss, A.A.; Elshafei, A.A.; Moustafa, K.A. Bulked segregant analysis to detect quantitative trait loci (QTL) related to heat tolerance at grain filling rate in wheat using simple sequence repeat (SSR) markers. Afr. J. Biotechnol. 2012, 11, 12436–12442. [Google Scholar]
- Ward, B.P.; Brown-Guedira, G.; Kolb, F.L.; Van Sanford, D.A.; Tyagi, P.; Sneller, C.H.; Griffey, C.A. Genome-wide association studies for yield-related traits in soft red winter wheat grown in Virginia. PLoS ONE 2019, 14, e0208217. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Ali, A.; Gul, A.; Ghafoor, A.; Napar, A.A.; Ibrahim, A.M.H.; Naveed, N.H.; Yasin, N.A.; Kazi, A.M. Genome-wide association studies of seven agronomic traits under two sowing conditions in bread wheat. BMC Plant Biol. 2019, 19, 149. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.; Skaggs, R.; Sneed, R. Stress day index models to predict corn and soybean relative yield under high water table conditions. Trans. ASAE 1991, 34, 1997–2005. [Google Scholar] [CrossRef]
- Kpoghomou, B.; Sapra, V.; Beyl, C.; Science, C. Screening for drought tolerance: Soybean germination and its relationship to seedling responses. J. Agron. 1990, 164, 153–159. [Google Scholar] [CrossRef]
- Urrea-Gómez, R.; Ceballos, H.; Pandey, S.; BahíaFilho, A.F.; León, L.A. A greenhouse screening technique for acid soil tolerance in maize. Am. Soc. Agron. 1996, 88, 806–812. [Google Scholar] [CrossRef]
- Richard, C.; Munyinda, K.; Kinkese, T.; Osiru, D.S. Genotypic variation in seedling tolerance to aluminum toxicity in historical maize inbred lines of Zambia. Agronomy 2015, 5, 200–219. [Google Scholar] [CrossRef]
- Ainsworth, C.; Beynon, J.; Buchanan-Wollaston, V. Techniques in Plant Biology: The Practical Manual; Wye College, University of London: London, UK, 1996. [Google Scholar]
- Al-Faifi, S.A.; Migdadi, H.M.; Algamdi, S.S.; Khan, M.A.; Ammar, M.H.; Al-Obeed, R.S.; Al-Thamra, M.; EL-Harty, E. Development, characterization and use of genomic SSR markers for assessment of genetic diversity in some Saudi date palm (Phoenix dactylifera L.) cultivars. Electron. J. Biotechnol. 2016, 21, 18–25. [Google Scholar] [CrossRef]
- Khierallah, H.; Bader, S.; Baum, M.; Hamwieh, A. Assessment of genetic diversity for some Iraqi date palms (Phoenix dactylifera L.) using amplified fragment length polymorphisms (AFLP) markers. Afr. J. Biotechnol. 2011, 10, 9570–9576. [Google Scholar] [CrossRef]
- Hasan, N.; Choudhary, S.; Naaz, N.; Sharma, N.; Laskar, R.A. Recent advancements in molecular marker-assisted selection and applications in plant breeding programmes. J. Genet. Eng. Biotechnol. 2021, 19, 128. [Google Scholar] [CrossRef]
- Powell, W.; Machray, G.C.; Provan, J. Polymorphism revealed by simple sequence repeats. Trends Plant Sci. 1996, 1, 215–222. [Google Scholar] [CrossRef]
- Chen, X.; Min, D.; Yasir, T.A.; Hu, Y.G. Genetic diversity, population structure and linkage disequilibrium in elite Chinese winter wheat investigated with SSR markers. PLoS ONE 2012, 7, e44510. [Google Scholar] [CrossRef]
- Singh, A.K.; Chaurasia, S.; Kumar, S.; Singh, R.; Kumari, J.; Yadav, M.C.; Singh, N.; Gaba, S.; Jacob, S.R. Identification, analysis and development of salt responsive candidate gene based SSR markers in wheat. BMC Plant Biol. 2018, 18, 249. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, W.; Ogbonnaya, F.C.; Jighly, A.; Sanchez-Garcia, M.; Sohail, Q.; Rajaram, S.; Baum, M. Genome-Wide Association Mapping of Yield and Grain Quality Traits in Winter Wheat Genotypes. PLoS ONE 2015, 10, e0141339. [Google Scholar] [CrossRef]
- Semenov, M.A.; Halford, N.G. Identifying target traits and molecular mechanisms for wheat breeding under a changing climate. J. Exp. Bot. 2009, 60, 2791–2804. [Google Scholar] [CrossRef]
- Beecher, F.W.; Mason, E.; Monda, L.S.; Awika, J.; Hays, D.; Ibrahim, A. Identification of quantitative trait loci (QTLs) associated with maintenance of wheat (Triticum aestivum Desf.) quality characteristics under heat stress conditions. Euphytica 2012, 188, 361–368. [Google Scholar] [CrossRef]
- Najeb Barakat, M.; Al-Doss, A.; Elshafei, A.A.; Moustafa, K.A. Identification of new microsatellite marker linked to the grain filling rate as indicator for heat tolerance genes in F2 wheat population. Aust. J. Crop Sci. 2011, 5, 104–110. [Google Scholar]
- Li, M.; Feng, J.; Zhou, H.; Najeeb, U.; Li, J.; Song, Y.; Zhu, Y. Overcoming Reproductive Compromise Under Heat Stress in Wheat: Physiological and Genetic Regulation, and Breeding Strategy. Front. Plant Sci. 2022, 13, 881813. [Google Scholar] [CrossRef]
- Sun, L.; Wen, J.; Peng, H.; Yao, Y.; Hu, Z.; Ni, Z.; Sun, Q.; Xin, M. The genetic and molecular basis for improving heat stress tolerance in wheat. Abiotech 2022, 3, 25–39. [Google Scholar] [CrossRef]
- Jha, U.C.; Bohra, A.; Singh, N.P. Heat stress in crop plants: Its nature, impacts and integrated breeding strategies to improve heat tolerance. Plant Breed. 2014, 133, 679–701. [Google Scholar] [CrossRef]
- More, K.; Achyut, B.; Jadhav, B.; Malge, H. Screening of Wheat (Triticum aestivum L.) genotypes for Heat Tolerance Trait by physio-molecular Markers. Biol. Forum Int. J. 2022, 14, 63–68. [Google Scholar]
- Acuña-Galindo, M.A.; Mason, R.E.; Subramanian, N.K.; Hays, D.B. Meta-analysis of wheat QTL regions associated with adaptation to drought and heat stress. Crop Sci. 2015, 55, 477–492. [Google Scholar] [CrossRef]
- Kumari, M.; Pudake, R.N.; Sing, V.; Breeding, P. Identification of microsatellite markers associated with staygreen trait in wheat RILs. Indian J. Genet. 2012, 72, 415–420. [Google Scholar]
- Paliwal, R.; Röder, M.S.; Kumar, U.; Srivastava, J.; Joshi, A.K.; Genetics, A. QTL mapping of terminal heat tolerance in hexaploid wheat (Triticum aestivum L.). J. Theor. 2012, 125, 561–575. [Google Scholar]
- Huang, S.S.; Sun, L.Q.; Hu, X.; Wang, Y.H.; Zhang, Y.J.; Nevo, E.; Peng, J.; Sun, D. Associations of canopy leaf traits with SNP markers in durum wheat (Triticum turgidum L. durum (Desf.)). PLoS ONE 2018, 13, e0206226. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, K.; Fritz, A.K.; Paulsen, G.M.; Bai, G.; Pandravada, S.; Gill, B.S. Modeling and mapping QTL for senescence-related traits in winter wheat under high temperature. Mol. Breed. 2010, 26, 163–175. [Google Scholar] [CrossRef]
- Breseghello, F.; Sorrells, M.E. Association mapping of kernel size and milling quality in wheat (Triticum aestivum L.) cultivars. Genetics 2006, 172, 1165–1177. [Google Scholar] [CrossRef]
- Ma, J.F.; Liu, Y.; Zhang, P.P.; Chen, T.; Tian, T.; Wang, P.; Che, Z.; Shahinnia, F.; Yang, D. Identification of quantitative trait loci (QTL) and meta-QTL analysis for kernel size-related traits in wheat (Triticum aestivum L). BMC Plant Biol. 2022, 22, 607. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, X.; Song, X.; Yan, J.; Song, T.; Dai, J.; Rocheford, T.; Li, J.S. Mapping quantitative trait loci for oil, starch, and protein concentrations in grain with high-oil maize by SSR markers. Euphytica 2008, 162, 335–344. [Google Scholar] [CrossRef]
- Kumari, M.; Pudake, R.; Singh, V.; Joshi, A.K. Association of staygreen trait with canopy temperature depression and yield traits under terminal heat stress in wheat (Triticum aestivum L.). Euphytica. 2013, 190, 87–97. [Google Scholar] [CrossRef]
- Talukder, S.K.; Babar, M.A.; Vijayalakshmi, K.; Poland, J.; Prasad, P.V.V.; Bowden, R.; Fritz, A. Mapping QTL for the traits associated with heat tolerance in wheat (Triticum aestivum L.). BMC Genom. 2014, 15, 97. [Google Scholar]
- Hussain, W.; Baenziger, P.; Belamkar, V.; Guttieri, M.J.; Venegas, J.P.; Easterly, A.; Sallam, A.; Poland, J. Genotyping-by-Sequencing Derived High-Density Linkage Map and its Application to QTL Mapping of Flag Leaf Traits in Bread Wheat. Sci. Rep. 2017, 7, 16394. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.H.; Pan, Y.; Luo, L.H.; Deng, H.B.; Zhang, G.L.; Tang, W.B.; Chen LiYun, C.L. Quantitative Trait Loci Associated with Pollen Fertility under High Temperature Stress at Flowering Stage in Rice (Oryza sativa). Rice Sci. 2011, 18, 204–209. [Google Scholar] [CrossRef]
- Shirdelmoghanloo, H.; Taylor, J.D.; Lohraseb, I.; Rabie, H.; Brien, C.; Timmins, A.; Martin, P.; Mather, D.E.; Emebiri, L.; Collins, N.C. A QTL on the short arm of wheat (Triticum aestivum L.) chromosome 3B affects the stability of grain weight in plants exposed to a brief heat shock early in grain filling. BMC Plant Biol. 2016, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Kilasi, N.L.; Singh, J.; Vallejos, C.E.; Ye, C.; Jagadish, S.K.; Kusolwa, P.; Rathinasabapathi, B. Heat stress tolerance in rice (Oryza sativa L.): Identification of quantitative trait loci and candidate genes for seedling growth under heat stress. Front. Plant Sci. 2018, 9, 1578. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Yang, W.T.; Kim, D.H.; Kim, K.M. Identification of a novel gene, osbht, in response to high temperature tolerance at booting stage in rice. J. Mol. Sci. 2020, 21, 5862. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Alderfasi, A.; El-Hendawy, S.; Al-Suhaibani, N.; El-Kafafi, S.; Seleiman, M.F. Detecting Salt Tolerance in Doubled Haploid Wheat Lines. Agronomy 2019, 9, 211. [Google Scholar] [CrossRef]
- Sallam, M.; Ghazy, A.; Al-Doss, A.; Al-Ashkar, I. Combining Genetic and Phenotypic Analyses for Detecting Bread Wheat Genotypes of Drought Tolerance through Multivariate Analysis Techniques. Life 2024, 14, 183. [Google Scholar] [CrossRef]
- Grzesiak, S.; Hordyńska, N.; Szczyrek, P.; Grzesiak, M.T.; Noga, A.; Szechyńska-Hebda, M. Variation among wheat (Triticum easativum L.) genotypes in response to the drought stress: I–selection approaches. J. Plant Interact. 2019, 14, 30–44. [Google Scholar] [CrossRef]
- El-Hendawy, S.; Al-Suhaibani, N.; Al-Ashkar, I.; Alotaibi, M.; Tahir, M.U.; Solieman, T.; Hassan, W.M. Combining Genetic Analysis and Multivariate Modeling to Evaluate Spectral Reflectance Indices as Indirect Selection Tools in Wheat Breeding under Water Deficit Stress Conditions. Remote Sens. 2020, 12, 1480. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Sallam, M.; Al-Suhaibani, N.; Ibrahim, A.; Alsadon, A.; Al-Doss, A. Multiple Stresses of Wheat in the Detection of Traits and Genotypes of High-Performance and Stability for a Complex Interplay of Environment and Genotypes. Agronomy 2022, 12, 2252. [Google Scholar] [CrossRef]
- Abdolshahi, R.; Nazari, M.; Safarian, A.; Sadathossini, T.S.; Salarpour, M.; Amiri, H. Integrated selection criteria for drought tolerance in wheat (Triticum easativum L.) breeding programs using discriminant analysis. Field Crop. Res. 2015, 174, 20–29. [Google Scholar] [CrossRef]
- Chakraborty, K.; Monda, L.S.; Ray, S.; Samal, P.; Pradhan, B.; Chattopadhyay, K.; Kar, K.M.; Swain, P.; Sarkar, R. Tissue Tolerance Coupled With Ionic Discrimination Can Potentially Minimize the Energy Cost of Salinity Tolerance in Rice. Front. Plant Sci. 2020, 11, 265. [Google Scholar] [CrossRef]
- Saghai-Maroof, M.A.; Soliman, K.M.; Jorgensen, R.A.; Allard, R.W. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. USA 1984, 81, 8014–8018. [Google Scholar] [CrossRef]
- Lorenz, T.C. Polymerase chain reaction: Basic protocol plus troubleshooting and optimization strategies. J. Vis. Exp. 2012, 63, e3998. [Google Scholar]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Chance, B.; Maehly, A.C. Preparation and assays of enzymes. Methods Enzymol. 1955, 2, 773–775. [Google Scholar]
- Duckworth, H.W.; Coleman, J.E. Physicochemical and kinetic properties of mushroom tyrosinase. J. Biol. Chem. 1970, 245, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y. Generation of Superoxide Radical during Autoxidation of Hydroxylamine and an Assay for Superoxide-Dismutase. Arch. Biochem. Biophys. 1978, 186, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Pütter, J. Peroxidases. In Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1974; pp. 685–690. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. J. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists; Approved Methods Committee. Approved Methods of the American Association of Cereal Chemists; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- Donkor, E.F.; Adjei, R.R.; Amadu, B.; Boateng, A.S. Genetic variability, heritability and association among yield components and proximate composition of neglected and underutilized Bambara ground nut (Vigna subterranea (L) Verdc) accessions for varietal development in Ghana. Heliyon 2022, 8, e09691. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Liu, B.; Asseng, S.; Müller, C.; Ewert, F.; Elliott, J.; Lobell, D.B.; Martre, P.; Ruane, A.C.; Wallach, D.; Jones, J.W.; et al. Similar estimates of temperature impacts on global wheat yield by three independent methods. Nat. Clim. Chang. 2016, 6, 1130–1136. [Google Scholar] [CrossRef]
- De Costa, W. A review of the possible impacts of climate change on forests in the humid tropics. Natl. Sci. Found. 2011, 39, 281–302. [Google Scholar] [CrossRef]
- Asseng, S.; Foster, I.; Turner, N.C. The impact of temperature variability on wheat yields. Glob. Chang. Biol. 2011, 17, 997–1012. [Google Scholar] [CrossRef]
- Bennett, D.; Izanloo, A.; Reynolds, M.; Kuchel, H.; Langridge, P.; Schnurbusch, T. Genetic dissection of grain yield and physical grain quality in bread wheat (Triticum aestivum L.) under water-limited environments. Theor. Appl. Genet. 2012, 125, 255–271. [Google Scholar] [CrossRef]
- Yu, Q.; Li, L.; Luo, Q.; Eamus, D.; Xu, S.; Chen, C.; Wang, E.; Liu, J.; Nielsen, D.C. Year patterns of climate impact on wheat yields. Int. J. Climatol. 2014, 34, 518–528. [Google Scholar] [CrossRef]
- Lo, M.-H.; Wey, H.-W.; Im, E.-S.; Tang, L.; Anderson, R.G.; Wu, R.-J.; Chien, R.; Wei, J.; AghaKouchak, A.; Wada, Y. Intense agricultural irrigation induced contrasting precipitation changes in Saudi Arabia. Environ. Res. Lett. 2021, 16, 064049. [Google Scholar] [CrossRef]
- Rahman, M.M.; Akter, R.; Abdul Bari, J.B.; Hasan, M.A.; Rahman, M.S.; Abu Shoaib, S.; Shatnawi, Z.N.; Alshayeb, A.F.; Shalabi, F.I.; Rahman, A.; et al. Analysis of climate change impacts on the food system security of Saudi Arabia. Sustainability 2022, 14, 14482. [Google Scholar] [CrossRef]
- Motawei, M.; Al-Doss, A.; Moustafa, K. Environment. Genetic diversity among selected wheat lines differing in heat tolerance using molecular markers. J. Food Agric. 2007, 5, 180. [Google Scholar]
- Sinha, D.; Maurya, A.K.; Abdi, G.; Majeed, M.; Agarwal, R.; Mukherjee, R.; Ganguly, S.; Aziz, R.; Bhatia, M.; Majgaonkar, A.; et al. Integrated genomic selection for accelerating breeding programs of climate-smart cereals. Genes 2023, 14, 1484. [Google Scholar] [CrossRef]
- Sadat, S.; Saeid, K.A.; Bihamta, M.R.; Torabi, S.; Salekdeh, S.G.H.; Ayeneh, G.A.L. Marker assisted selection for heat tolerance in bread wheat. World Appl. Sci. J. 2013, 21, 1181–1189. [Google Scholar]
- Dhillon, G.S.; Das, N.; Kaur, S.; Srivastava, P.; Bains, N.S.; Chhuneja, P. Marker assisted mobilization of heat tolerance QTLs from Triticum durum-Aegilops speltoides introgression lines to hexaploid wheat. Indian J. Genet. 2021, 81, 186–198. [Google Scholar]
- Khan, A.; Ahmad, M.; Ahmed, M.; Gill, K.S.; Akram, Z. Association analysis for agronomic traits in wheat under terminal heat stress. Saudi J. Biol. Sci. 2021, 28, 7404–7415. [Google Scholar] [CrossRef]
- Gahtyari, N.C.; Jaiswal, J.P.; Sharma, D.; Talha, M.; Kumar, N.; Singh, N.K. Genetic analysis and marker association of physiological traits under rainfed and heat stress conditions in spring wheat (Triticum aestivum L.). Genetika 2022, 54, 1049–1068. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Al-Doss, A.; Ullah, N. Accelerating Crop Improvement through Speed Breeding. In Climate-Resilient Agriculture, Vol 1: Crop Responses and Agroecological Perspectives; Hasanuzzaman, M., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 821–847. [Google Scholar]
- De Leon, T.B.; Linscombe, S.; Gregorio, G.; Subudhi, P.K. Genetic variation in Southern USA rice genotypes for seedling salinity tolerance. Front. Plant Sci. 2015, 6, 374. [Google Scholar] [CrossRef]
- Junaid, M.B.; El-Hendawy, S.; Al-Ashkar, I.; Al-Suhaibani, N.; Alotaibi, M. Integrating Agro-Morpho-Physiological Traits and SSR Markers for Detecting the Salt Tolerance of Advanced Spring Wheat Lines under Field Conditions. Agriculture 2023, 13, 2135. [Google Scholar] [CrossRef]
- Sodini, S.M.; Kemper, K.E.; Wray, N.R.; Trzaskowski, M. Comparison of Genotypic and Phenotypic Correlations: Cheverud’s Conjecture in Humans. Genetics 2018, 209, 941–948. [Google Scholar] [CrossRef]
- Hill, C.B.; Li, C. Genetic improvement of heat stress tolerance in cereal crops. Agronomy 2022, 12, 1205. [Google Scholar] [CrossRef]
- Mason, R.E.; Mondal, S.; Beecher, F.W.; Pacheco, A.; Jampala, B.; Ibrahim, A.M.H.; Hays, D.B. QTL associated with heat susceptibility index in wheat (Triticum aestivum L.) under short-term reproductive stage heat stress. Euphytica 2010, 174, 423–436. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Brisson, L.F.; Zelitch, I.; Havir, E.A. Manipulation of catalase levels produces altered photosynthesis in transgenic tobacco plants. J. Plant Physiol. 1998, 116, 259–269. [Google Scholar] [CrossRef]
- Feng, B.H.; Zhang, C.X.; Chen, T.T.; Zhang, X.F.; Tao, L.X.; Fu, G.F. Salicylic acid reverses pollen abortion of rice caused by heat stress. BMC Plant Biol. 2018, 18, 245. [Google Scholar] [CrossRef]
- Van Breusegem, F.; Dat, J.F. Reactive oxygen species in plant cell death. J. Plant Physiol. 2006, 141, 384–390. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Alderfasi, A.; Ben Romdhane, W.; Seleiman, M.; El-Said, R.; Al-Doss, A. Morphological and genetic diversity within salt tolerance detection in eighteen wheat genotypes. Plants 2020, 9, 287. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Sallam, M.; Almutairi, K.F.; Shady, M.; Ibrahim, A.; Alghamdi, S.S. Detection of high-performance wheat genotypes and genetic stability to determine complex interplay between genotypes and environments. Agronomy 2023, 13, 585. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Al-Suhaibani, N.; Abdella, K.; Sallam, M.; Alotaibi, M.; Seleiman, M.F. Combining genetic and multidimensional analyses to identify interpretive traits related to water shortage tolerance as an indirect selection tool for detecting genotypes of drought tolerance in wheat breeding. Plants 2021, 10, 931. [Google Scholar] [CrossRef]
- Khan, M.M.H.; Rafii, M.Y.; Ramlee, S.S.; Jusoh, M.; Al Mamun, M. Hereditary analysis and genotype× environment interaction effects on growth and yield components of Bambara groundnut (Vigna subterranea (L.) Verdc.) over multi-environments. Sci. Rep. 2022, 12, 15658. [Google Scholar] [CrossRef]
- Boakye-Peprah, B.; Ofori, K.; Asante, I.; Parkes, E. Genetic variability of three cassava traits across three locations in Ghana. Afr. J. Plant Sci. 2013, 7, 265–267. [Google Scholar] [CrossRef]
- Falconer, D.S. Introduction to Quantitative Genetics; Pearson Education India: Bengaluru, India, 1996. [Google Scholar]
- Obala, J.; Saxena, R.K.; Singh, V.K.; Vechalapu, S.; Das, R.; Rathore, A.; Sameer-Kumar, C.V.; Saxena, K.; Tongoona, P.; Sibiya, J.; et al. Genetic variation and relationships of total seed protein content with some agronomic traits in pigeon pea (‘Cajanus cajan’ (L.) Millsp.). Aust. J. Crop Sci. 2018, 12, 1859–1865. [Google Scholar] [CrossRef]
- Burton, G. Qualitative inheritance in grasses. In Proceedings of the 6th International Grassland Congress, State College, PA, USA, 17–23 August 1952; Volume 1, pp. 17–23. [Google Scholar]
- Al-Ashkar, I.; Sallam, M.; Ghazy, A.; Ibrahim, A.; Alotaibi, M.; Ullah, N.; AL-Doss, A. Agro-physiological indices and multidimensional analyses for detecting heat tolerance in wheat genotypes. Agronomy 2023, 13, 154. [Google Scholar] [CrossRef]
- Hosseini, S.J.; Sarvestani, Z.T.; Pirdashti, H. Responses of some rice genotypes to drought stress. Int. J. Agric. 2012, 2, 475–482. [Google Scholar]
- Khan, A.S.; Ashfaq, M.; Asad, M.A. A correlation and path coefficient analysis for some yield components in bread wheat. Asian J. Plant Sci. 2003, 2, 582–884. [Google Scholar]
- Ayer, D.; Sharma, A.; Ojha, B.; Paudel, A.; Dhakal, K. Correlation and path coefficient analysis in advanced wheat genotypes. J. Agric. 2017, 15, 1–12. [Google Scholar] [CrossRef]
- Novo, P.; Science, s. Cultivar and year effect on grain filling of winter barley. J. Plant Breed. 2001, 45, 45–58. [Google Scholar]
- Gunasekaran, K.; Sivakami, R.; Sabariappan, R.; Ponnaiah, G.; Nachimuthu, V.V.; Pandian, B.A. Assessment of genetic variability, correlation and path coefficient analysis for morphological and quality traits in rice (Oryza sativa L.). J. Agric. Sci. Dig.-Res. J. 2017, 37, 251–256. [Google Scholar] [CrossRef]
- Nitish, D.; Baranwal, D.; Kuma, S. Genetic evaluation of spring wheat (Triticum aestivum L.) genotypes for yield and spot blotch resistance in Eastern Gangetic Plains of India. J. Afr. J. Biotechnol. 2014, 13, 1867–1875. [Google Scholar] [CrossRef]
- Singh, D.; Singh, M.; Sharma, K. Correlation and path-coefficient analysis among flag leaf-area, yield and yield attributes in wheat (Triticum aestivum L.). J. Cereal Res. Commun. 1979, 7, 145–152. [Google Scholar]
- Bojarian, M.; Asadi-Gharneh, H.A.; Golabadi, M. Factor analysis, stepwise regression and path coefficient analyses of yield, yield-associated traits, and fruit quality in tomato. Int. J. Veg. Sci. 2019, 25, 542–553. [Google Scholar] [CrossRef]
- Del Moral, L.G.; Rharrabti, Y.; Villegas, D.; Royo, C. Evaluation of grain yield and its components in durum wheat under Mediterranean conditions: An ontogenic approach. J. Agron. J. 2003, 95, 266–274. [Google Scholar] [CrossRef]
- Arshad, M.; Ali, N.; Ghafoor, A. Character correlation and path coefficient in soybean Glycine max (L.) Merrill. J. Pak. J. Bot. 2006, 38, 121. [Google Scholar]
- Dewey, D.R.; Lu, K. A correlation and path-coefficient analysis of components of crested wheatgrass seed production. Agron. J. 1959, 51, 515–518. [Google Scholar] [CrossRef]
- Milligan, S.B.; Gravois, K.A.; Bischoff, K.P.; Martin, F.A. Crop Effects on Genetic-Relationships among Sugarcane Traits. Crop Sci. 1990, 30, 927–931. [Google Scholar] [CrossRef]
- Nasri, R.; Kashani, A.; Paknejad, F.; Vazan, S.; Barary, M.; Sciences, A.L. Correlation, path analysis and stepwise regression in yield and yield component in wheat (Triticum aestivum L.) under the temperate climate of Ilam province, Iran. Indian J. Fundam. 2014, 4, 188–198. [Google Scholar]
- Rameez Iftikhar, R.; IhsanKhaliq, I.K.; Muhammad Kashif, M.K.; Ahmad, M.; Smiullah, S. Study of morphological traits affecting grain yield in wheat (Triticum aestivum L.) under field stress condition. Middle-East. J. Sci. Res. 2012, 11, 19–23. [Google Scholar]
- Marenco, R.A.; Antezana-Vera, S.A.; Nascimento, H.C.S. Relationship between specific leaf area, leaf thickness, leaf water content and SPAD-502 readings in six Amazonian tree species. Photosynthetica 2009, 47, 184–190. [Google Scholar] [CrossRef]
- Yuan, S.; Li, Y.; Peng, S.B. Leaf Lateral Asymmetry in Morphological and Physiological Traits of Rice Plant. PLoS ONE 2015, 10, e0129832. [Google Scholar] [CrossRef]
- Gladun, I.V.; Karpov, E.A. Distribution of Assimilates from the Flag Leaf of Rice during the Reproductive Period of Development. Russ Plant Physiol. 1993, 40, 215–218. [Google Scholar]
- Briggs, K.G.; Aytenfisu, A. Relationships between morphological characters above the flag leaf node and grain yield in spring wheats. J. Crop Sci. 1980, 20, 350–354. [Google Scholar] [CrossRef]
- Murata, Y. Studies on the photosynthesis of rice plants and its culture significance. Agric. Sci. 1961, 9, 1–169. [Google Scholar]
- Cook, M.G.; Evans, L.T. Some Physiological-Aspects of the Domestication and Improvement of Rice (Oryza Spp.). Field Crop. Res. 1983, 6, 219–238. [Google Scholar] [CrossRef]
- Blanco, F.F.; Folegatti, M.V. Estimation of leaf area for greenhouse cucumber by linear measurements under salinity and grafting. Sci. Agric. 2005, 62, 305–309. [Google Scholar] [CrossRef]
- Fowler, C.; Rasmusson, D.C. Leaf area relationships and inheritance in barley. Crop Sci. 1969, 9, 729–731. [Google Scholar] [CrossRef]
- Jia, H.; Wan, H.; Yang, S.; Zhang, Z.; Kong, Z.; Xue, S.; Zhang, L.; Ma, Z. Genetic dissection of yield-related traits in a recombinant inbred line population created using a key breeding parent in China’s wheat breeding. J. Theor. 2013, 126, 2123–2139. [Google Scholar] [CrossRef]
- Wu, Q.H.; Chen, Y.X.; Fu, L.; Zhou, S.H.; Chen, J.J.; Zhao, X.J.; Zhang, D.; Zhang, D.; Ouyang, S.; Wang, Z.; et al. QTL mapping of flag leaf traits in common wheat using an integrated high-density SSR and SNP genetic linkage map. Euphytica 2016, 208, 337–351. [Google Scholar] [CrossRef]
- Liu, C.G.; Zhou, X.Q.; Chen, D.G.; Li, L.J.; Li, J.C.; Chen, Y.D. Natural Variation of Leaf Thickness and Its Association to Yield Traits in Rice. J. Integr. Agric. 2014, 13, 316–325. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, B.; He, Y. Improving Grain Yield via Promotion of Kernel Weight in High Yielding Winter Wheat Genotypes. Biology 2021, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Hussain Wani, S.; Brajendra Singh, N.; Haribhushan, A.; Iqbal Mir, J. Compatible solute engineering in plants for abiotic stress tolerance-role of glycine betaine. Curr. Genom. 2013, 14, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Mohi-Ud-Din, M.; Hossain, M.A.; Rohman, M.M.; Uddin, M.N.; Haque, M.S.; Dessoky, E.S.; Alqurashi, M.; Aloufi, S. Assessment of genetic diversity of bread wheat genotypes for drought tolerance using canopy reflectance-based phenotyping and SSR marker-based genotyping. Sustainability 2022, 14, 9818. [Google Scholar] [CrossRef]
- El-Rawy, M.A.; Hassan, M.I. Assessment of genetic diversity in durum and bread wheat genotypes based on drought tolerance and SSR markers. Plant Breed. Biotechnol. 2021, 9, 89–103. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Z.Q.; Yang, G.P.; Xu, C.G.; Liu, K.D.; Saghai Maroof, M.A. Molecular marker heterozygosity and hybrid performance in indica and japonica rice. Theor. Appl. Genet. 1996, 93, 1218–1224. [Google Scholar] [CrossRef]
- Boppenmaier, J.; Melchinger, A.E.; Brunklaus-Jung, E.; Geiger, H.H.; Herrmann, R.G. Genetic Diversity for RFLPs in European Maize Inbreds: I. Relation to Performance of Flint × Dent Crosses for Forage Traits. Crop Sci. 1992, 32, 895–902. [Google Scholar] [CrossRef]
- Jinxiong, S.; Guangyuan, L.; Tingdong, F.; Guangsheng, Y. Relationships between genetic diversity and hybrid performance in Oilseed rape (Brassica napus). Zuo Wu Xue Bao 2002, 28, 622–627. [Google Scholar]
- Martin, J.; Talbert, L.; Lanning, S.; Blake, N. Hybrid performance in wheat as related to parental diversity. Crop Sci. 1995, 35, 104–108. [Google Scholar] [CrossRef]

| Traits | Treatments | Markers | R2 Par. | R2 Com. | p-Value * |
|---|---|---|---|---|---|
| GY | Control | wmc527 | 0.790 | 0.790 | <0.0001 |
| Heat | wmc326 | 0.606 | 0.606 | 0.002 | |
| gwm337 | 0.180 | 0.786 | 0.016 | ||
| Relative change | wmc326 | 0.671 | 0.671 | 0.001 | |
| gwm337 | 0.133 | 0.805 | 0.026 | ||
| TKW | Control | wmc154 | 0.472 | 0.472 | 0.010 |
| Heat | wmc326 | 0.764 | 0.764 | <0.0001 | |
| Relative change | wmc326 | 0.672 | 0.672 | 0.001 | |
| FLA | Heat | wmc326 | 0.699 | 0.699 | 0.0001 |
| Relative change | wmc326 | 0.866 | 0.866 | <0.0001 | |
| wmc65 | 0.049 | 0.915 | 0.037 | ||
| PH | Control | wmc65 | 0.328 | 0.328 | 0.041 |
| Heat | wmc326 | 0.359 | 0.359 | <0.0001 | |
| wmc54 | 0.318 | 0.677 | <0.0001 | ||
| wmc65 | 0.205 | 0.882 | <0.0001 | ||
| gwm369 | 0.083 | 0.965 | 0.002 | ||
| Relative change | gwm369 | 0.603 | 0.603 | 0.002 | |
| GFD | Control | wmc65 | 0.736 | 0.736 | 0.000 |
| Heat | wmc326 | 0.873 | 0.873 | <0.0001 | |
| wmc65 | 0.048 | 0.921 | <0.0001 | ||
| wmc54 | 0.052 | 0.972 | 0.000 | ||
| wmc74 | 0.017 | 0.989 | 0.005 | ||
| gwm337 | 0.005 | 0.994 | 0.047 | ||
| Relative change | wmc326 | 0.746 | 0.746 | <0.0001 | |
| gwm337 | 0.125 | 0.871 | 0.001 | ||
| wmc527 | 0.062 | 0.933 | 0.006 | ||
| wmc54 | 0.032 | 0.965 | 0.028 | ||
| Ci | Control | wmc326 | 0.462 | 0.462 | 0.011 |
| Heat | wmc326 | 0.764 | 0.764 | <0.0001 | |
| Relative change | wmc326 | 0.656 | 0.656 | 0.001 |
| Source | DF | PPO | CAT | POD | SOD | GB | Pn | Ci | FLA | GFD | GFR |
| H | 1 | 0.35 ** | 103.99 ** | 215.662 ** | 4.54 ** | 33.28 ** | 1067.31 ** | 171,829.81 ** | 3924.31 ** | 284.62 ** | 308.07 ** |
| Error A | 2 | 0.002 | 0.005 | 0.668 | 0.004 | 0.003 | 0.537 | 24.453 | 6.888 | 0.205 | 0.059 |
| G | 12 | 0.023 ** | 0.470 ** | 5.706 ** | 0.031 ** | 1.568 ** | 6.901 ** | 1821.177 ** | 277.864 ** | 69.78 ** | 6.026 ** |
| H*G | 12 | 0.009 ** | 0.172 ** | 4.637 ** | 0.018 ** | 0.611 ** | 2.964 ** | 501.600 ** | 61.524 ** | 26.10 ** | 2.500 ** |
| Error B | 48 | 0.001 | 0.012 | 0.13 | 0.004 | 0.021 | 0.595 | 77.837 | 9.595 | 2.896 | 0.327 |
| Genetic Parameters | |||||||||||
| σ2G | 0.002 | 0.05 | 0.178 | 0.002 | 0.16 | 0.656 | 219.929 | 36.057 | 7.281 | 0.587 | |
| σ2e | 0.001 | 0.002 | 0.022 | 0.001 | 0.003 | 0.099 | 12.973 | 1.599 | 0.483 | 0.054 | |
| σ2Ph | 0.004 | 0.078 | 0.951 | 0.005 | 0.261 | 1.15 | 303.529 | 46.311 | 11.631 | 1.004 | |
| h2 % | 59.396 | 63.425 | 18.72 | 42.134 | 61.043 | 57.052 | 72.457 | 77.858 | 62.601 | 72.63 | |
| G.C.V. % | 14.666 | 12.67 | 12.815 | 11.203 | 15.933 | 8.656 | 7.161 | 14.884 | 6.6 | 6.07 | |
| Ph.C.V. % | 19.03 | 15.909 | 29.619 | 17.259 | 20.392 | 11.46 | 8.413 | 16.868 | 8.342 | 7.123 | |
| GA | 0.076 | 0.366 | 0.376 | 0.063 | 0.643 | 1.26 | 26.005 | 10.915 | 4.398 | 9.342 | |
| GG % | 23.284 | 20.785 | 11.422 | 14.981 | 25.643 | 13.469 | 12.557 | 27.055 | 10.757 | 10.65 | |
| Source | DF | PH | SL | NG | NS | NSS | TKW | GY | PC | GI | |
| Rep | 2 | 1.423 | 2.013 | 19.828 | 217.782 | 1.59 | 1.34 | 0.027 | 0.745 | 28.057 | |
| H | 1 | 2820.013 ** | 29.538 ** | 4078.154 ** | 233,317.385 ** | 108.513 ** | 997.064 ** | 92.966 ** | 430.755 ** | 1829.522 ** | |
| Error A | 2 | 17.321 | 0.346 | 41.734 | 160.731 | 0.359 | 0.237 | 0.013 | 0.779 | 52.474 | |
| G | 12 | 233.902 ** | 11.959 ** | 207.238 ** | 5597.440 ** | 7.504 ** | 48.428 ** | 1.629 ** | 7.021 ** | 731.008 ** | |
| H*G | 12 | 64.013 ** | 0.733 ns | 76.916 * | 2051.051 ** | 2.957 ** | 20.011 ** | 0.728 ** | 3.685 ** | 179.671 ** | |
| Error B | 48 | 8.191 | 0.471 | 33.133 | 87.701 | 0.808 | 1.2 | 0.015 | 0.723 | 17.454 | |
| Genetic Parameters | |||||||||||
| σ2G | 28.315 | 1.871 | 21.72 | 591.065 | 0.758 | 4.736 | 0.15 | 0.556 | 91.889 | ||
| σ2e | 1.365 | 0.079 | 5.522 | 14.617 | 0.135 | 0.2 | 0.002 | 0.121 | 2.909 | ||
| σ2Ph | 38.984 | 1.993 | 34.54 | 932.907 | 1.251 | 8.071 | 0.271 | 1.17 | 121.835 | ||
| h2 % | 72.633 | 93.872 | 62.885 | 63.357 | 60.592 | 58.679 | 55.29 | 47.517 | 75.421 | ||
| G.C.V. % | 6.071 | 14.302 | 12.52 | 5.04 | 5.485 | 5.175 | 5.918 | 5.471 | 12.42 | ||
| Ph.C.V. % | 7.123 | 14.762 | 15.788 | 6.332 | 7.046 | 6.756 | 7.958 | 7.936 | 14.301 | ||
| GA | 9.342 | 2.73 | 7.613 | 39.864 | 1.396 | 3.434 | 0.593 | 1.059 | 17.149 | ||
| GG % | 10.658 | 28.545 | 20.452 | 8.264 | 8.795 | 8.167 | 9.064 | 7.768 | 22.22 | ||
| PCA1 | PCA2 | PCA3 | |
|---|---|---|---|
| Eigenvalue | 12.113 | 3.204 | 1.217 |
| Variability (%) | 63.752 | 16.861 | 6.404 |
| Cumulative % | 63.752 | 80.613 | 87.018 |
| Eigenvectors: | |||
| POD | 0.412 | 0.495 | 0.000 |
| SOD | 0.691 | 0.244 | 0.000 |
| PPO | 0.337 | 0.307 | 0.212 |
| CAT | 0.745 | 0.208 | 0.007 |
| GB | 0.331 | 0.561 | 0.012 |
| Pn | 0.974 | 0.000 | 0.001 |
| Ci | 0.951 | 0.003 | 0.001 |
| FLA | 0.739 | 0.184 | 0.013 |
| GFD | 0.416 | 0.415 | 0.016 |
| GFR | 0.713 | 0.054 | 0.003 |
| PH | 0.507 | 0.019 | 0.289 |
| SL | 0.374 | 0.286 | 0.006 |
| NS | 0.875 | 0.031 | 0.004 |
| NSS | 0.700 | 0.180 | 0.001 |
| NGS | 0.623 | 0.002 | 0.061 |
| TKW | 0.793 | 0.149 | 0.003 |
| GY | 0.918 | 0.021 | 0.000 |
| Pc | 0.785 | 0.018 | 0.015 |
| GI | 0.231 | 0.026 | 0.572 |
| Stepwise Regression | Path Coefficient | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dependent | Source | Partitioning the Correlation | R2 | ||||||
| Regression Coefficient | p-Value | R2 Par. | R2 Com. | Direct Effect | Indirect Effect | Correlation Value | Direct Effect | ||
| GY | Intercept | 0.011 | |||||||
| TKW | 0.585 | 0.004 | 0.868 | 0.868 | 0.494 | 0.437 | 0.931 | 0.244 | |
| PH | 0.530 | 0.005 | 0.066 | 0.934 | 0.343 | 0.474 | 0.817 | 0.118 | |
| Ci | 0.316 | 0.047 | 0.025 | 0.958 | 0.259 | 0.581 | 0.840 | 0.067 | |
| Total direct effect | 0.429 | ||||||||
| Total indirect effect | 0.529 | ||||||||
| Total R2 | 0.958 | 0.958 | |||||||
| Residual | 0.205 | 0.205 | |||||||
| TKW | Intercept | 0.010 | |||||||
| FLA | 0.481 | <0.001 | 0.780 | 0.780 | |||||
| Total direct effect | |||||||||
| Total indirect effect | |||||||||
| Total R2 | 0.780 | 0.780 | |||||||
| Residua | |||||||||
| FLA | Intercept | −0.117 | |||||||
| GFD | 0.794 | 0.011 | 0.699 | 0.699 | 0.511 | 0.325 | 0.836 | 0.261 | |
| Ci | 0.951 | 0.012 | 0.146 | 0.845 | 0.502 | 0.331 | 0.833 | 0.252 | |
| Total direct effect | 0.513 | ||||||||
| Total indirect effect | 0.332 | ||||||||
| Total R2 | 0.845 | 0.845 | |||||||
| Residual | 0.394 | 0.394 | |||||||
| Genotypes | Classification | Cross-Validation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Prior | Posterior | Membership Probabilities | Posterior | Membership Probabilities | |||||
| Pr(MT) | Pr(S) | Pr(T) | MT | S | T | ||||
| 16HTWYT-30 | S | S | 0.000 | 1.000 | 0.000 | S | 0.000 | 1.000 | 0.000 |
| DHL2 | MT | MT | 1.000 | 0.000 | 0.000 | MT | 1.000 | 0.000 | 0.000 |
| 16HTWYT-20 | S | S | 0.000 | 1.000 | 0.000 | S | 0.000 | 1.000 | 0.000 |
| 16HTWYT-38 | MT | MT | 1.000 | 0.000 | 0.000 | MT | 1.000 | 0.000 | 0.000 |
| 16HTWYT-9 | S | S | 0.000 | 1.000 | 0.000 | S | 0.000 | 1.000 | 0.000 |
| KSU105 | T | T | 0.000 | 0.000 | 1.000 | T | 0.000 | 0.000 | 1.000 |
| 16HTWYT-12 | S | S | 0.000 | 1.000 | 0.000 | S | 0.000 | 1.000 | 0.000 |
| Yecora Rojo | S | S | 0.000 | 1.000 | 0.000 | S | 0.000 | 1.000 | 0.000 |
| 16HTWYT-22 | T | T | 0.000 | 0.000 | 1.000 | T | 0.000 | 0.000 | 1.000 |
| KSU115 | T | T | 0.000 | 0.000 | 1.000 | T | 0.000 | 0.000 | 1.000 |
| Klassic | S | S | 0.000 | 1.000 | 0.000 | S | 0.000 | 1.000 | 0.000 |
| Line 47 | MT | MT | 1.000 | 0.000 | 0.000 | MT | 1.000 | 0.000 | 0.000 |
| KSU106 | T | T | 0.000 | 0.000 | 1.000 | T | 0.000 | 0.000 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sallam, M.; Al-Ashkar, I.; Al-Doss, A.; Al-Gaadi, K.A.; Zeyada, A.M.; Ghazy, A. Assessing Heat Stress Tolerance of Wheat Genotypes through Integrated Molecular and Physio-Biochemical Analyses. Agronomy 2024, 14, 1999. https://doi.org/10.3390/agronomy14091999
Sallam M, Al-Ashkar I, Al-Doss A, Al-Gaadi KA, Zeyada AM, Ghazy A. Assessing Heat Stress Tolerance of Wheat Genotypes through Integrated Molecular and Physio-Biochemical Analyses. Agronomy. 2024; 14(9):1999. https://doi.org/10.3390/agronomy14091999
Chicago/Turabian StyleSallam, Mohammed, Ibrahim Al-Ashkar, Abdullah Al-Doss, Khalid A. Al-Gaadi, Ahmed M. Zeyada, and Abdelhalim Ghazy. 2024. "Assessing Heat Stress Tolerance of Wheat Genotypes through Integrated Molecular and Physio-Biochemical Analyses" Agronomy 14, no. 9: 1999. https://doi.org/10.3390/agronomy14091999






