Utilization of Diversified Cover Crops as Green Manure-Enhanced Soil Organic Carbon, Nutrient Transformation, Microbial Activity, and Maize Growth
Abstract
:1. Introduction
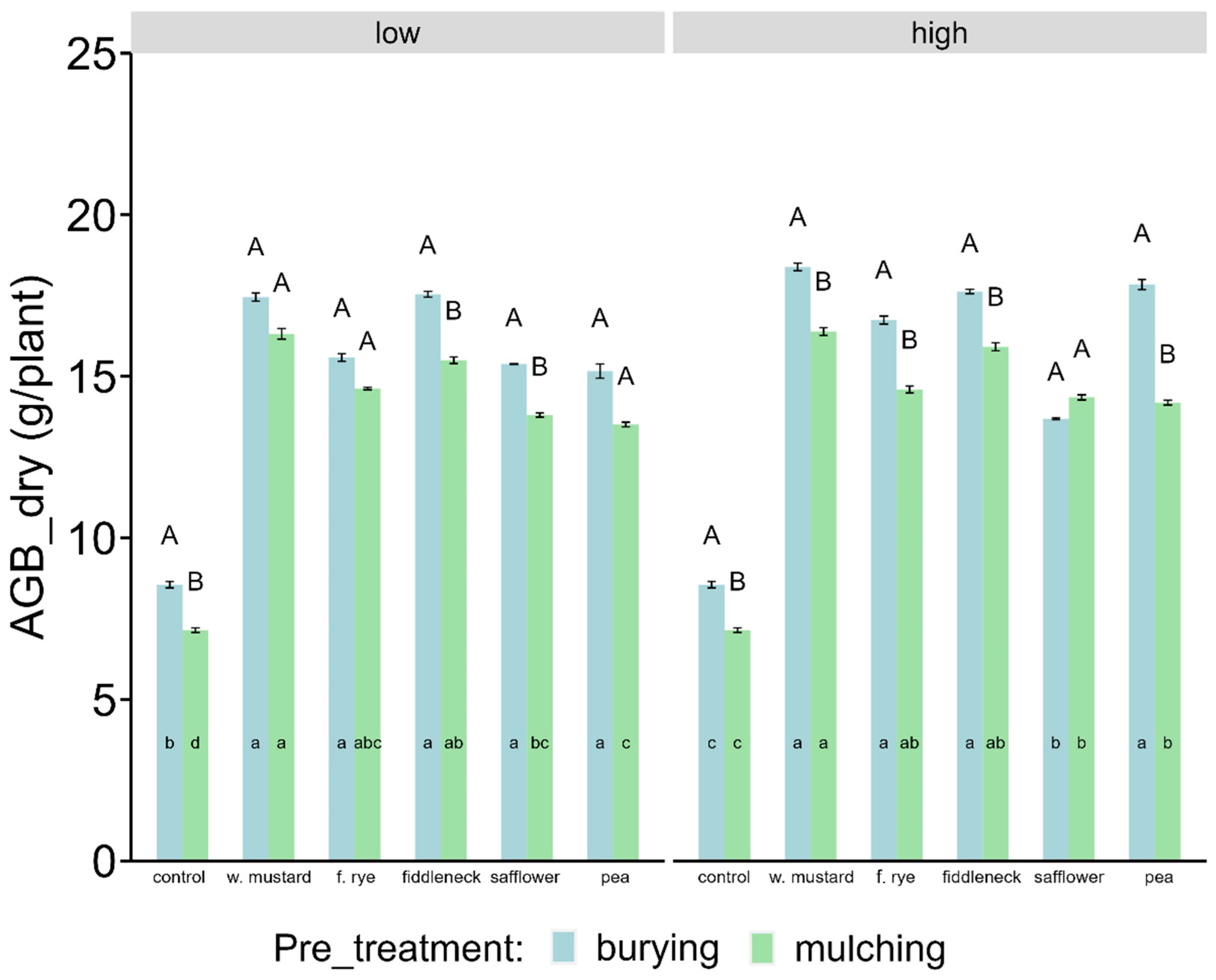
- Green manuring limited to the incorporation of solely aboveground biomass (without roots) of cover crops increases the dry maize biomass compared to untreated soil, regardless of the pre-treatment (both after burying or mulching).
- The type of cover crop and its nutrient characteristics determine the yield of dry maize biomass. For example, N-rich biomass may derive a higher yield than others.
- The pre-treatment of green-manured cover crop biomass determines the differences (between burying and mulching) in the soil organic carbon mineralization.
2. Materials and Methods
2.1. Soil Collection and Properties
2.2. Preparation, Procurement, and Composition of Green Manure Biomass
2.3. Plant Growth Experiment and Treatment Description
2.4. Soil Analyses
2.5. Data Processing and Statistical Analyses
3. Results
3.1. Nutritional and Biomass Characteristics of Green Manure
3.2. Dry Aboveground Biomass of Maize
3.3. Soil pH(CaCl2)
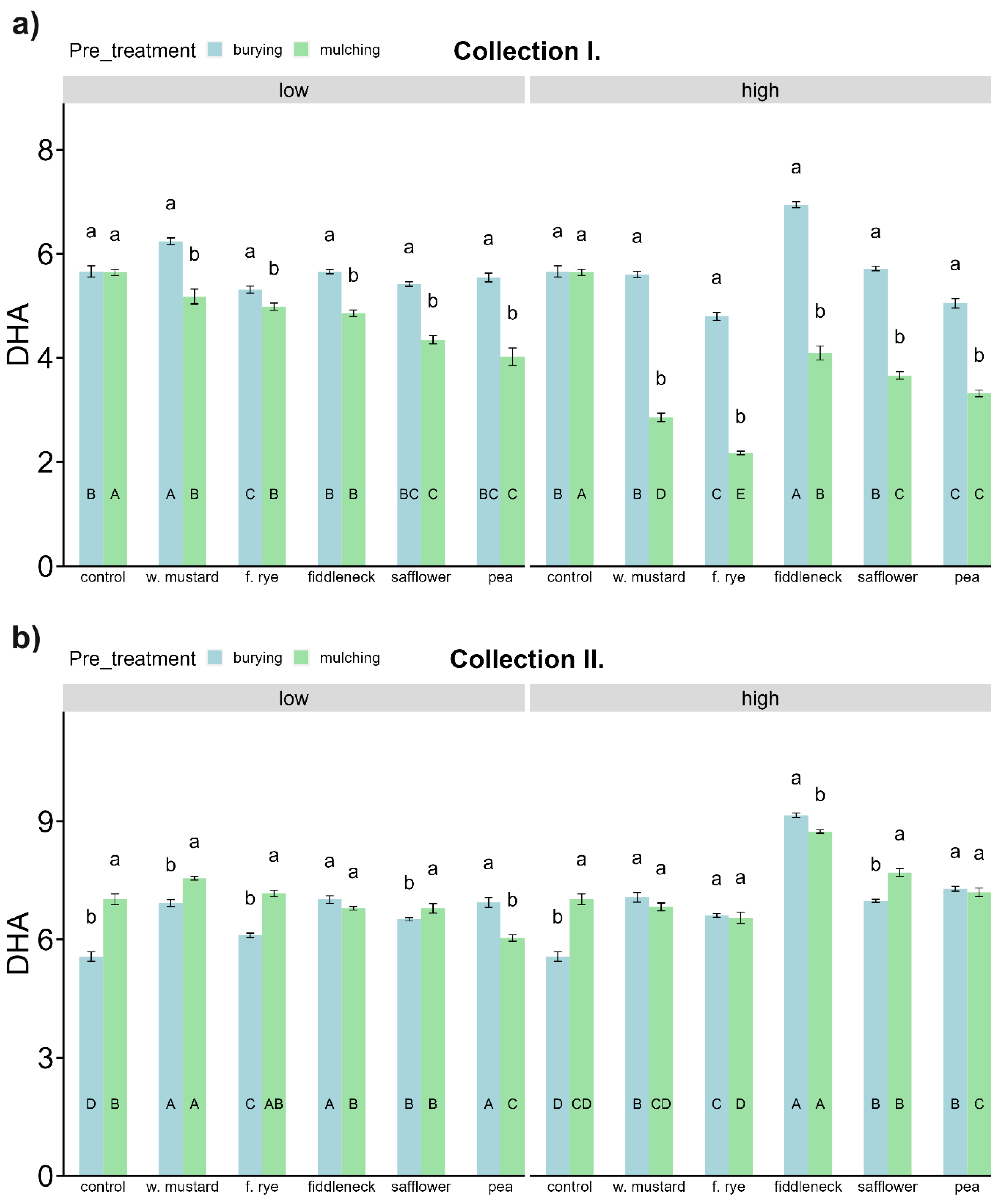
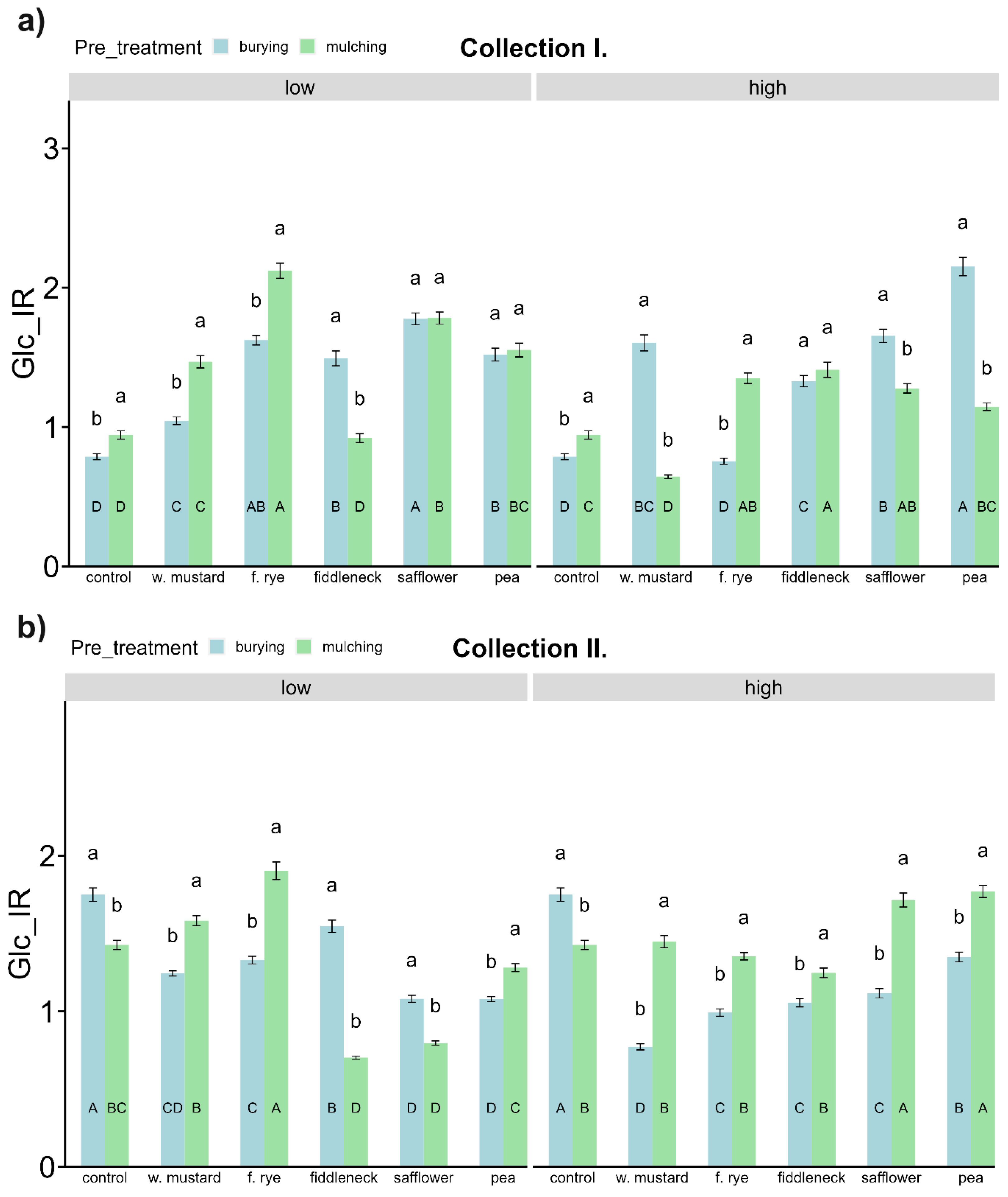
3.4. Soil Carbon (C) and C Mineralizing Microbial Activities
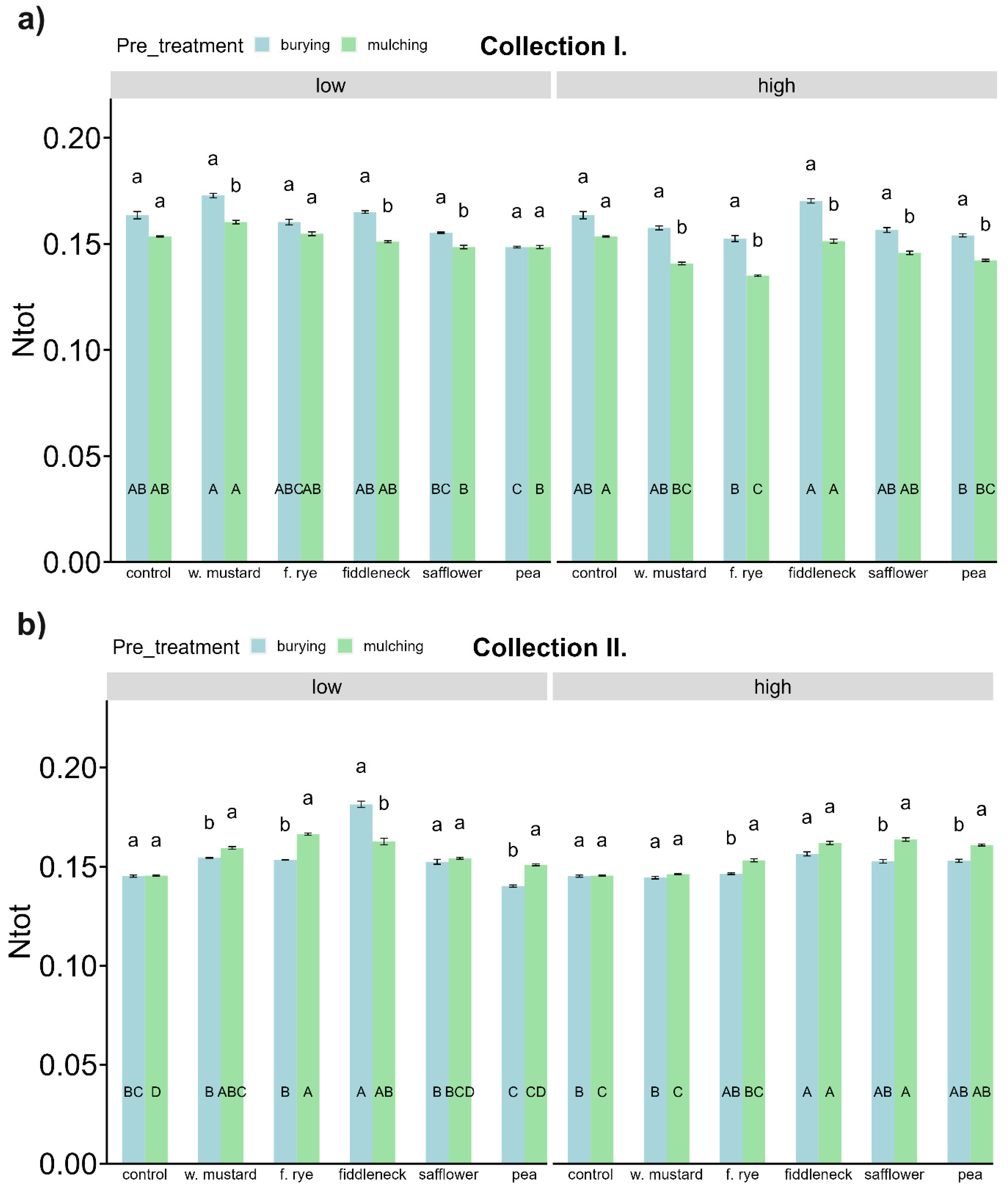
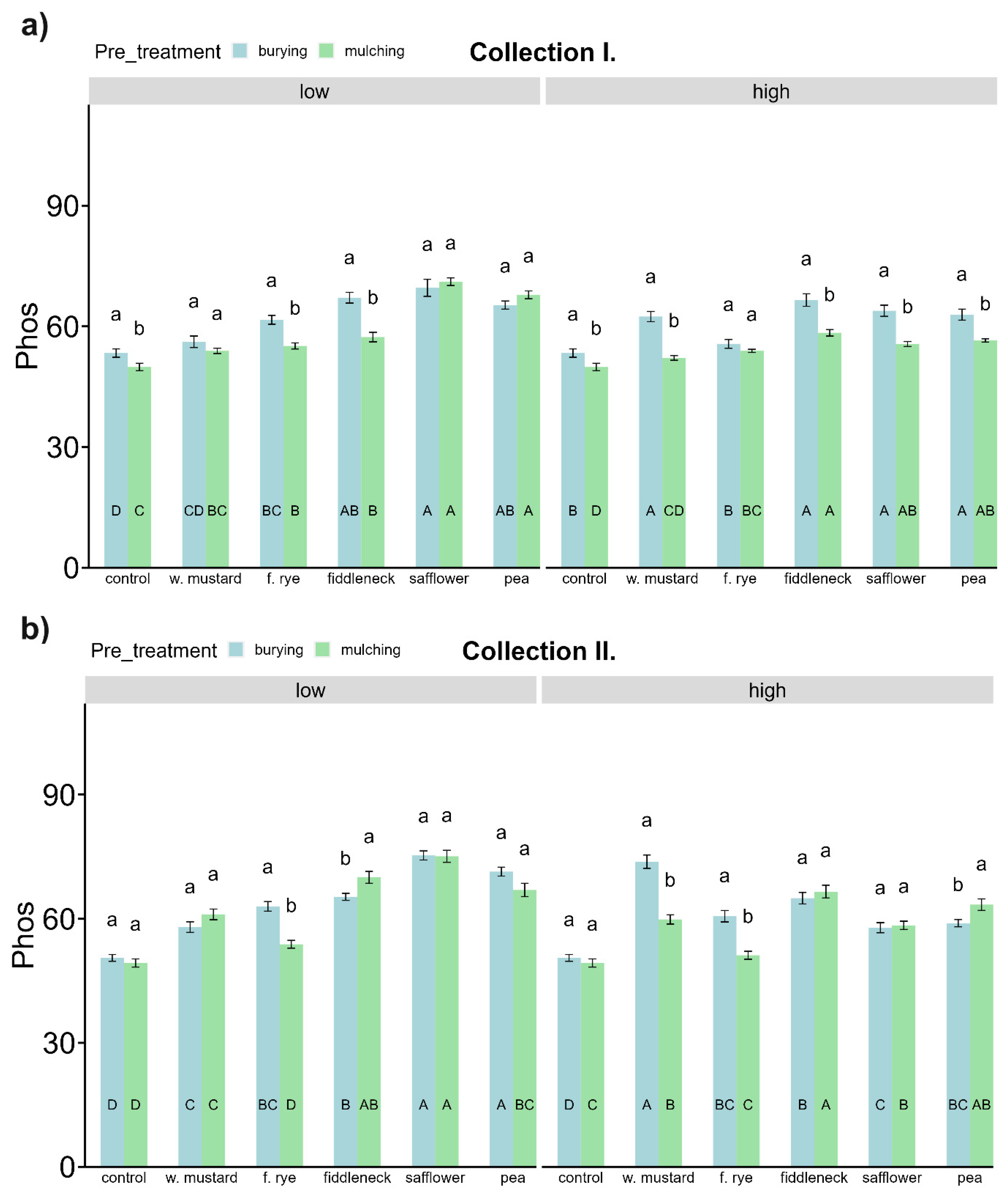
3.5. Soil Nitrogen (N) and N, P Mineralizing Microbial Activities
3.6. Statistical Evaluation of the Green Manure Treatment on Soil and Plant Parameters
4. Discussion
4.1. Impact on Soil Prior to Sowing
4.2. Impact on Soil after the Harvest
4.2.1. Influence of Cover Crops on above Ground Biomass Production
4.2.2. Influence of Green Manure on Soil Microbial Characteristics
4.2.3. Soil pH (CaCl2)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schnitkey, G.D.; Sellars, S.C.; Gentry, L.F. Cover crops, farm economics, and policy. Appl. Econ. Perspect. Policy 2023, 46, 595–608. [Google Scholar] [CrossRef]
- Boulet, A.K.; Alarcão, C.; Ferreira, C.; Kalantari, Z.; Veiga, A.; Campos, L.; Ferreira, A.; Hessel, R. Agro-ecological services delivered by legume cover crops grown in succession with grain corn crops in the Mediterranean region. Open Agric. 2021, 6, 609–626. [Google Scholar] [CrossRef]
- Thapa, R.; Tully, K.L.; Reberg-Horton, C.; Cabrera, M.; Davis, B.W.; Fleisher, D.; Gaskin, J.; Hitchcock, R.; Poncet, A.; Schomberg, H.H. Cover crop residue decomposition in no-till cropping systems: Insights from multi-state on-farm litter bag studies. Agric. Ecosyst. Environ. 2022, 326, 107823. [Google Scholar] [CrossRef]
- Antosh, E.; Liebig, M.A.; Archer, D.W.; Luciano, R. Cover crop interseeding effects on aboveground biomass and corn grain yield in western North Dakota. Crop Forage Turfgrass Manag. 2022, 8, e20148. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Ncube, B.; Mulidzi, R.; Lewu, F.B. Management impact and benefit of cover crops on soil quality: A review. Soil Tillage Res. 2020, 204, 104717. [Google Scholar] [CrossRef]
- dos Santos Cordeiro, C.F.; Echer, F.R.; Araujo, F.F. Cover Crops Impact Crops Yields by Improving Microbiological Activity and Fertility in Sandy Soil. J. Soil Sci. Plant Nutr. 2021, 21, 1968–1977. [Google Scholar] [CrossRef]
- Saleem, M.; Pervaiz, Z.H.; Contreras, J.; Lindenberger, J.H.; Hupp, B.M.; Chen, D.; Zhang, Q.; Wang, C.; Iqbal, J.; Twigg, P. Cover crop diversity improves multiple soil properties via altering root architectural traits. Rhizosphere 2020, 16, 100248. [Google Scholar] [CrossRef]
- Arruda, B.; Herrera, W.F.B.; Rojas-García, J.C.; Turner, C.; Pavinato, P.S. Cover crop species and mycorrhizal colonization on soil phosphorus dynamics. Rhizosphere 2021, 19, 100396. [Google Scholar] [CrossRef]
- Feng, H.; Sekaran, U.; Wang, T.; Kumar, S. On-farm assessment of cover cropping effects on soil C and N pools, enzyme activities, and microbial community structure. J. Agric. Sci. 2021, 159, 216–226. [Google Scholar] [CrossRef]
- Couëdel, A.; Alletto, L.; Justes, É. Crucifer-legume cover crop mixtures provide effective sulphate catch crop and sulphur green manure services. Plant Soil 2018, 426, 61–76. [Google Scholar] [CrossRef]
- Bogužas, V.; Mikučionienė, R.; Šlepetienė, A.; Sinkevičienė, A.; Feiza, V.; Steponavičienė, V.; Adamavičienė, A. Long-term effect of tillage systems, straw and green manure combinations on soil organic matter. Zemdirbyste-Agriculture 2015, 102, 243–250. [Google Scholar] [CrossRef]
- Liu, A.; Ku, Y.S.; Contador, C.A.; Lam, H.M. The Impacts of Domestication and Agricultural Practices on Legume Nutrient Acquisition through Symbiosis With Rhizobia and Arbuscular Mycorrhizal Fungi. Front. Genet. 2020, 11, 583954. [Google Scholar] [CrossRef]
- Ferreira, L.L.; Dalbosco, L.E.; Carvalho, I.R.; Carnevale, A.B.; Moura, N.B.; Lautenchleger, F. Multivariate and canonical models applied to corn: Benefits of green manure with Vigna unguiculate. Holos 2020, 7, 1–15. [Google Scholar] [CrossRef]
- Li, S.; Liang, H.; Wang, Y.; Zhang, Z.; Zhang, L.; Zhou, G.; Gao, S.; Cao, W. Responses of functional genes involved in nitrogen cycling to green manuring in different paddy soils in south China. Plant Soil 2022, 478, 519–532. [Google Scholar] [CrossRef]
- Vásquez-Castro, D.C.; Rodrigues, R.R.; Meli, P.; Brancalion, P.H.S.; Silva, R.R.; Couto, H.T.Z.d. Preliminary results of using green manure species as a cost-effective option for forest restoration. Sci. For. 2020, 48, 7. [Google Scholar] [CrossRef]
- Kolota, E.; Adamczewska-Sowinska, K. Living mulches in vegetable crops production: Perspectives and limitations (a reviev). Acta Sci. Pol. Hortorum Cultus 2013, 12, 127–142. [Google Scholar]
- Garcia-Franco, N.; Albaladejo, J.; Almagro, M.; Martínez-Mena, M. Beneficial effects of reduced tillage and green manure on soil aggregation and stabilization of organic carbon in a Mediterranean agroecosystem. Soil Tillage Res. 2015, 153, 66–75. [Google Scholar] [CrossRef]
- Su, G.; Zhao, R.; Wang, Y.; Yang, Y.; Wu, X.; Wang, J.; Ge, J. Green Manure Return Strategies to Improve Soil Properties and Spring Maize Productivity under Nitrogen Reduction in the North China Plain. Agronomy 2022, 12, 2734. [Google Scholar] [CrossRef]
- Li, P.; Jia, L.; Chen, Q.; Zhang, H.; Deng, J.; Lu, J.; Xu, L.; Li, H.; Hu, F.; Jiao, J. Adaptive evaluation for agricultural sustainability of different fertilizer management options for a green manure-maize rotation system: Impacts on crop yield, soil biochemical properties and organic carbon fractions. Sci. Total Environ. 2024, 908, 168170. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Q.; Jiang, Y.; Yu, J.; Li, J.; Liu, W.; Wu, Z. Improving soil pH, nutrient concentrations, and enzyme activities by green manure returning in young and mature rubber plantation on Hainan Island, China. Plant Soil 2023, 495, 341–358. [Google Scholar] [CrossRef]
- Ablimit, R.; Li, W.; Zhang, J.; Gao, H.; Zhao, Y.; Cheng, M.; Meng, X.; An, L.; Chen, Y. Altering microbial community for improving soil properties and agricultural sustainability during a 10-year maize-green manure intercropping in Northwest China. J. Environ. Manag. 2022, 321, 115859. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, N. Green Manures Alter Taxonomic and Functional Characteristics of Soil Bacterial Communities. Microb. Ecol. 2022, 85, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.X.; Desaeger, J.A. Volatile compounds as potential bio-fumigants against plant-parasitic nematodes—A mini review. J. Nematol. 2021, 53, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhao, Q.; Zhang, X.; Ning, X.; Liang, H.; Cao, W. Winter Green Manure Decreases Subsoil Nitrate Accumulation and Increases N Use Efficiencies of Maize Production in North China Plain. Plants 2023, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.; Hartung, J.; Perkons, U.; Möller, K.; Zikeli, S. Plant and soil N of different winter cover crops as green manure for subsequent organic white cabbage. Nutr. Cycl. Agroecosyst. 2023, 127, 285–298. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, J.; Cao, W.; Gao, Y.; Lu, Y.; Liao, Y. Long-term green manuring to substitute partial chemical fertilizer simultaneously improving crop productivity and soil quality in a double-rice cropping system. Eur. J. Agron. 2023, 142, 126641. [Google Scholar] [CrossRef]
- Kumar, S.; Meena, R.N.; Singh, R.K.; Srivastava, V.K.; Kumar, V.; Hemalatha, K. Studies on tillage and green manuring for enhancing productivity of wheat (Triticum aestivum). Indian J. Agric. Sci. 2022, 92, 353–356. [Google Scholar] [CrossRef]
- Luo, Y.; Xiao, H.B.; Wu, X.; Wu, X.; Wu, Y.P.; Jiang, Y.B.; Hu, R.G. Mitigation of nutrient runoff loss using reduced nitrogen application and green manure planting in citrus orchard in Hubei, China. J. Soils Sediments 2023, 23, 582–595. [Google Scholar] [CrossRef]
- Regina, K.; Singh, P.; Känkänen, H. Impacts of green manure on crop yield, nitrogen leaching and nitrous oxide emissions in sandy and clay soil lysimeters. Agric. Food Sci. 2021, 30, 53–62. [Google Scholar] [CrossRef]
- Elfstrand, S.; Båth, B.; Mårtensson, A. Influence of various forms of green manure amendment on soil microbial community composition, enzyme activity and nutrient levels in leek. Appl. Soil Ecol. 2007, 36, 70–82. [Google Scholar] [CrossRef]
- Piotrowska-Dlugosz, A.; Wilczewski, E. Influence of field pea (Pisum sativum L.) as catch crop cultivated for green manure on soil phosphorus and P-cycling enzyme activity. Arch. Agron. Soil Sci. 2020, 66, 1570–1582. [Google Scholar] [CrossRef]
- Xu, J.; Si, L.L.; Zhang, X.; Cao, K.; Wang, J.H. Various green manure-fertilizer combinations affect the soil microbial community and function in immature red soil. Front. Microbiol. 2023, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Glab, T.; Kulig, B. Effect of mulch and tillage system on soil porosity under wheat (Triticum aestivum). Soil Tillage Res. 2008, 99, 169–178. [Google Scholar] [CrossRef]
- Frøseth, R.B.; Bakken, A.K.; Bleken, M.A.; Riley, H.; Pommeresche, R.; Thorup-Kristensen, K.; Hansen, S. Effects of green manure herbage management and its digestate from biogas production on barley yield, N recovery, soil structure and earthworm populations. Eur. J. Agron. 2014, 52, 90–102. [Google Scholar] [CrossRef]
- Dahlin, A.S.; Stenberg, M.; Marstorp, H. Mulch N recycling in green manure leys under Scandinavian conditions. Nutr. Cycl. Agroecosyst. 2011, 91, 119–129. [Google Scholar] [CrossRef]
- Sharma, A.R.; Ghosh, A. Effect of green manuring with Sesbania aculeata and nitrogen fertilization on the performance of direct-seeded flood-prone lowland rice. Nutr. Cycl. Agroecosyst. 2000, 57, 141–153. [Google Scholar] [CrossRef]
- Ranaivoson, L.; Falconnier, G.N.; Affholder, F.; Leroux, L.; Autfray, P.; Muller, B.; Auzoux, S.; Ripoche, A. Can green manure contribute to sustainable intensification of rainfed rice production in Madagascar? Field Crops Res. 2022, 289, 14. [Google Scholar] [CrossRef]
- Liu, J.X.; Peng, S.J.; Faivre-Vuillin, B.; Xu, Z.H.; Zhang, D.Q.; Zhou, G.Y. Erigeron annuus (L.) Pers., as a green manure for ameliorating soil exposed to acid rain in Southern China. J. Soils Sediments 2008, 8, 452–460. [Google Scholar] [CrossRef]
- Xie, Z.J.; Shah, F.R.; Zhou, C.H. Combining Rice Straw Biochar with Leguminous Cover Crop as Green Manure and Mineral Fertilizer Enhances Soil Microbial Biomass and Rice Yield in South China. Front. Plant Sci. 2022, 13, 778738. [Google Scholar] [CrossRef]
- Gautam, R.; Shriwastav, C.P.; Lamichhane, S.; Baral, B.R. The Residual Effect of Pre-Rice Green Manuring on a Succeeding Wheat Crop (Triticum aestivum L.) in the Rice-Wheat Cropping System in Banke, Nepal. Int. J. Agron. 2021, 2021, 4142743. [Google Scholar] [CrossRef]
- Balota, E.L.; Chaves, J.C. Enzymatic activity and mineralization of carbon and nitrogen in soil cultivated with coffee and green manures. Rev. Bras. Cienc. Solo 2010, 34, 1573–1583. [Google Scholar] [CrossRef]
- Nazir, S.; Zaman, Q.U.; Abbasi, A.; Komal, N.; Riaz, U.; Ashraf, K.; Ahmad, N.; Agarwal, S.; Nasir, R.; Chen, Y.L. Bioresource Nutrient Recycling in the Rice-Wheat Cropping System: Cornerstone of Organic Agriculture. Plants 2021, 10, 2323. [Google Scholar] [CrossRef] [PubMed]
- Liaudanskiene, I.; Zukaitis, T.; Velykis, A.; Satkus, A.; Parasotas, I. The impact of tillage practices on the distribution of humified organic carbon in a clay loam. Zemdirbyste-Agriculture 2021, 108, 11–18. [Google Scholar] [CrossRef]
- Wilczewski, E.; Sadkiewicz, J.; Piotrowska-Dlugosz, A.; Galezewski, L. Change of Plant Nutrients in Soil and Spring Barley Depending on the Field Pea Management as a Catch Crop. Agriculture 2021, 11, 394. [Google Scholar] [CrossRef]
- Gimsing, A.L.; Kirkegaard, J.A. Glucosinolate and isothiocyanate concentration in soil following incorporation of Brassica biofumigants. Soil Biol. Biochem. 2006, 38, 2255–2264. [Google Scholar] [CrossRef]
- Ates, E.; Tekeli, A.S.; Boynukara, B. Performance of fodder pea (Pisum arvense L.)-fiddleneck (Phacelia tanacetifolia Benth.) mixture under different nitrogen doses. Rom. Agric. Res. 2014, 31, 213–218. [Google Scholar]
- Wysokinski, A.; Lozak, I. The Dynamic of Nitrogen Uptake from Different Sources by Pea (Pisum sativum L.). Agriculture 2021, 11, 81. [Google Scholar] [CrossRef]
- ISO_13878; Soil Quality—Determination of Total Nitrogen Content by Dry Combustion (Elemental Analysis). International Organization for Standardization: Geneva, Switzerland, 1998.
- ISO_10694; Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elemental Analysis). International Organization for Standardization: Geneva, Switzerland, 1995.
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 2008, 15, 1409–1416. [Google Scholar] [CrossRef]
- ISO_13906; Animal Feeding Stuffs—Determination of Acid Detergent Fibre (ADF) and Acid Detergent Lignin (ADL) Contents. International Organization for Standardization: Geneva, Switzerland, 2008.
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Tambone, F.; Genevini, P.; D’Imporzano, G.; Adani, F. Assessing amendment properties of digestate by studying the organic matter composition and the degree of biological stability during the anaerobic digestion of the organic fraction of MSW. Bioresour. Technol. 2009, 100, 3140–3142. [Google Scholar] [CrossRef]
- Marrubini, G.; Papetti, A.; Genorini, E.; Ulrici, A. Determination of the Sugar Content in Commercial Plant Milks by Near Infrared Spectroscopy and Luff-Schoorl Total Glucose Titration. Food Anal. Methods 2017, 10, 1556–1567. [Google Scholar] [CrossRef]
- Kintl, A.; Hammerschmiedt, T.; Vítěz, T.; Brtnický, M.; Vejražka, K.; Huňady, I.; Látal, O.; Elbl, J. Possibility of using tannins to control greenhouse gas production during digestate storage. Waste Manag. 2023, 156, 75–83. [Google Scholar] [CrossRef] [PubMed]
- ISO_6865; Animal Feeding Stuffs—Determination of Crude Fibre Content—Method with Intermediate Filtration. International Organization for Standardization: Geneva, Switzerland, 2000.
- Priego-Capote, F.; De Castro, M.L. Analytical uses of ultrasound I. Sample preparation. Trends Analyt. Chem. 2004, 23, 644–653. [Google Scholar] [CrossRef]
- Dhaliwal, G.S.; Gupta, N.; Kukal, S.S.; Meetpal, S. Standardization of Automated Vario EL III CHNS Analyzer for Total Carbon and Nitrogen Determination in Plants. Commun. Soil Sci. Plant Anal. 2014, 45, 1316–1324. [Google Scholar] [CrossRef]
- ISO_11261; Soil Quality—Determination of Total Nitrogen: Modified Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 1995.
- ISO_6869; Animal Feeding Stuffs—Determination of the Contents of Calcium, Copper, Iron, Magnesium, Manganese, Potassium, Sodium and Zinc—Method Using Atomic Absorption Spectrometry. International Organization for Standardization: Geneva, Switzerland, 2000.
- ISO_10390; Soil Quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2005.
- Doi, R.; Ranamukhaarachchi, S.L. Soil dehydrogenase in a land degradation-rehabilitation gradient: Observations from a savanna site with a wet/dry seasonal cycle. Rev. Biol. Trop. 2009, 57, 223–234. [Google Scholar] [CrossRef]
- ISO_20130; Soil Quality—Measurement of Enzyme Activity Patterns in Soil Samples Using Colorimetric Substrates in Micro-Well Plates. International Organization for Standardization: Geneva, Switzerland, 2018.
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl. Environ. Microbiol. 2003, 69, 3593–3599. [Google Scholar] [CrossRef]
- R_Core_Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 23 August 2024).
- Wickham, H.; Pedersen, T.; Seidel, D. Scale Functions for Visualization. 2023. Available online: https://scales.r-lib.org (accessed on 23 August 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Wickham, H., Navarro, D., Pedersen, T.L., Eds.; Taylor & Francis: Abingdon, UK, 2016. [Google Scholar]
- Auguie, B.; Antonov, A. gridExtra: Miscellaneous Functions for "Grid" Graphics, R Package Version 2.3; 2017. Available online: https://CRAN.R-project.org/package=gridExtra (accessed on 23 August 2024).
- Arnold, J. ggthemes: Extra Themes, Scales and Geoms for ‘ggplot2’. 2023. Available online: https://cran.r-project.org/web/packages/ggthemes/index.html (accessed on 23 August 2024).
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1984; p. 718. [Google Scholar]
- Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research, version 1.3.7; 2023. Available online: https://cran.r-project.org/web/packages/agricolae/index.html (accessed on 23 August 2024).
- Faraway, R.J.J. Linear Models with R; Chapman & Hall/CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses, version 1.0.7; 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 23 August 2024).
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences, 5th ed.; Houghton Mifflin: Boston, MA, USA, 2003. [Google Scholar]
- Peterson, B.; Carl, P. PerformanceAnalytics: Econometric Tools for Performance and Risk Analysis (R Package Version 2.0. 4) [Computer Software]. 2020. Available online: https://rdrr.io/cran/PerformanceAnalytics/ (accessed on 23 August 2024).
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix, Version 0.92; 2021. Available online: https://rdrr.io/cran/corrplot/ (accessed on 23 August 2024).
- Pekár, S.; Brabec, M. Modern Analysis of Biological Data. Generalized Linear Models in R; Masaryk University Press: Brno, Czech Republic, 2016. [Google Scholar]
- Yue, K.; Peng, C.; Yang, W.; Peng, Y.; Zhang, C.; Huang, C.; Wu, F. Degradation of lignin and cellulose during foliar litter decomposition in an alpine forest river. Ecosphere 2016, 7, e01523. [Google Scholar] [CrossRef]
- Palucha, N.; Fojt, J.; Holátko, J.; Hammerschmiedt, T.; Kintl, A.; Brtnický, M.; Řezáčová, V.; De Winterb, K.; Uitterhaegen, E.; Kučerík, J. Does poly-3-hydroxybutyrate biodegradation affect the quality of soil organic matter? Chemosphere 2024, 352, 141300. [Google Scholar] [CrossRef]
- Eckel, F.; Sinzinger, K.; Van Opdenbosch, D.; Schieder, D.; Sieber, V.; Zollfrank, C. Influence of microbial biomass content on biodegradation and mechanical properties of poly(3-hydroxybutyrate) composites. Biodegradation 2024, 35, 209–224. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Xia, Q.; Rufty, T.; Shi, W. Soil microbial diversity and composition: Links to soil texture and associated properties. Soil Biol. Biochem. 2020, 149, 107953. [Google Scholar] [CrossRef]
- Fontaine, S.; Henault, C.; Aamor, A.; Bdioui, N.; Bloor, J.M.G.; Maire, V.; Mary, B.; Revaillot, S.; Maron, P.A. Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol. Biochem. 2011, 43, 86–96. [Google Scholar] [CrossRef]
- Chowdhury, S.; Farrell, M.; Bolan, N. Priming of soil organic carbon by malic acid addition is differentially affected by nutrient availability. Soil Biol. Biochem. 2014, 77, 158–169. [Google Scholar] [CrossRef]
- Sauvadet, M.; Lashermes, G.; Alavoine, G.; Recous, S.; Chauvat, M.; Maron, P.-A.; Bertrand, I. High carbon use efficiency and low priming effect promote soil C stabilization under reduced tillage. Soil Biol. Biochem. 2018, 123, 64–73. [Google Scholar] [CrossRef]
- Razanamalala, K.; Fanomezana, R.; Razafimbelo, T.; Chevallier, T.; Trap, J.; Blanchart, E.; Bernard, L. The priming effect generated by stoichiometric decomposition and nutrient mining in cultivated tropical soils: Actors and drivers. Appl. Soil Ecol. 2018, 126, 21–33. [Google Scholar] [CrossRef]
- Guenet, B.; Neill, C.; Bardoux, G.; Abbadie, L. Is there a linear relationship between priming effect intensity and the amount of organic matter input? Appl. Soil Ecol. 2010, 46, 436–442. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, J.; Li, Y.; Xu, X.; He, L.; Wang, J.; Ren, C.; Guo, Y. Microbial functional genes driving the positive priming effect in forest soils along an elevation gradient. Soil Biol. Biochem. 2022, 165, 108498. [Google Scholar] [CrossRef]
- Bernard, L.; Basile-Doelsch, I.; Derrien, D.; Fanin, N.; Fontaine, S.; Guenet, B.; Karimi, B.; Marsden, C.; Maron, P.-A. Advancing the mechanistic understanding of the priming effect on soil organic matter mineralisation. Funct. Ecol. 2022, 36, 1355–1377. [Google Scholar] [CrossRef]
- Xu, M.; Zeng, Q.; Liu, Y.; Liu, C.; Zhang, Q.; Mei, K.; Yuan, X.; Zhang, X.; Chen, Y. Keystone Soil Microbial Modules Associated with Priming Effect under Nitrogen- and Glucose-Addition Treatments. Forests 2023, 14, 1207. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Carrillo, Y.; Pendall, E.; Morgan, J.A. Rhizosphere priming: A nutrient perspective. Front. Microbiol. 2013, 4, 216. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L. A theoretical model of litter decay and microbial interaction. Ecol. Monogr. 2006, 76, 151–174. [Google Scholar] [CrossRef]
- Craine, J.M.; Morrow, C.; Fierer, N. Microbial nitrogen limitation increases decomposition. Ecology 2007, 88, 2105–2113. [Google Scholar] [CrossRef]
- Tian, Q.; Yang, X.; Wang, X.; Liao, C.; Li, Q.; Wang, M.; Wu, Y.; Liu, F. Microbial community mediated response of organic carbon mineralization to labile carbon and nitrogen addition in topsoil and subsoil. Biogeochemistry 2016, 128, 125–139. [Google Scholar] [CrossRef]
- Wild, B.; Schnecker, J.; Alves, R.J.E.; Barsukov, P.; Bárta, J.; Čapek, P.; Gentsch, N.; Gittel, A.; Guggenberger, G.; Lashchinskiy, N.; et al. Input of easily available organic C and N stimulates microbial decomposition of soil organic matter in arctic permafrost soil. Soil Biol. Biochem. 2014, 75, 143–151. [Google Scholar] [CrossRef]
- Koranda, M.; Kaiser, C.; Fuchslueger, L.; Kitzler, B.; Sessitsch, A.; Zechmeister-Boltenstern, S.; Richter, A. Fungal and bacterial utilization of organic substrates depends on substrate complexity and N availability. FEMS Microbiol. Ecol. 2014, 87, 142–152. [Google Scholar] [CrossRef]
- Murphy, C.J.; Baggs, E.M.; Morley, N.; Wall, D.P.; Paterson, E. Rhizosphere priming can promote mobilisation of N-rich compounds from soil organic matter. Soil Biol. Biochem. 2015, 81, 236–243. [Google Scholar] [CrossRef]
- Tipping, E.; Somerville, C.J.; Luster, J. The C:N:P:S stoichiometry of soil organic matter. Biogeochemistry 2016, 130, 117–131. [Google Scholar] [CrossRef]
- Fang, X.-M.; Zhang, X.-L.; Chen, F.-S.; Zong, Y.-Y.; Bu, W.-S.; Wan, S.-Z.; Luo, Y.; Wang, H. Phosphorus addition alters the response of soil organic carbon decomposition to nitrogen deposition in a subtropical forest. Soil Biol. Biochem. 2019, 133, 119–128. [Google Scholar] [CrossRef]
- Lenzi, A.; Antichi, D.; Bigongiali, F.; Mazzoncini, M.; Migliorini, P.; Tesi, R. Effect of different cover crops on organic tomato production. Renew. Agric. Food Syst. 2009, 24, 92–101. [Google Scholar] [CrossRef]
- Marinari, S.; Mancinelli, R.; Brunetti, P.; Campiglia, E. Soil quality, microbial functions and tomato yield under cover crop mulching in the Mediterranean environment. Soil Tillage Res. 2015, 145, 20–28. [Google Scholar] [CrossRef]
- Wayne Reeves, D.; Follett, R.; Delgado, J. Winter Cover Crops. In Encyclopedia of Soil Science, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar] [CrossRef]
- Zhang, D.B.; Yao, Z.Y.; Chen, J.; Yao, P.W.; Zhao, N.; He, W.X.; Li, Y.Y.; Zhang, S.Q.; Zhai, B.N.; Wang, Z.H.; et al. Improving soil aggregation, aggregate-associated C and N, and enzyme activities by green manure crops in the Loess Plateau of China. Eur. J. Soil Sci. 2019, 70, 1267–1279. [Google Scholar] [CrossRef]
- Caban, J.R.; Kuppusamy, S.; Kim, J.H.; Yoon, Y.E.; Kim, S.Y.; Lee, Y.B. Green manure amendment enhances microbial activity and diversity in antibiotic-contaminated soil. Appl. Soil Ecol. 2018, 129, 72–76. [Google Scholar] [CrossRef]
- Janusauskaite, D.; Arlauskienė, A.; Maikštėnienė, S. Soil mineral nitrogen and microbial parameters as influenced by catch crops and straw management. Zemdirbyste-Agriculture 2013, 100, 9–18. [Google Scholar] [CrossRef]
- Ansari, M.A.; Babu, S.; Choudhary, J.; Ravisankar, N.; Panwar, A.S. Soil quality restoration and yield stabilization in acidic soils of northeastern Himalayas: Five years impact of green manuring and crop residue management. Front. Environ. Sci. 2022, 10, 940349. [Google Scholar] [CrossRef]
- Scavo, A.; Fontanazza, S.; Restuccia, A.; Pesce, G.R.; Abbate, C.; Mauromicale, G. The role of cover crops in improving soil fertility and plant nutritional status in temperate climates. A review. Agron. Sustain. Dev. 2022, 42, 93. [Google Scholar] [CrossRef]
- Piotrowska-Dlugosz, A.; Wilczewski, E. Influences of Catch Crop and Its Incorporation Time on Soil Carbon and Carbon-Related Enzymes. Pedosphere 2015, 25, 569–579. [Google Scholar] [CrossRef]
- Solangi, F.; Bai, J.S.; Gao, S.J.; Yang, L.; Zhou, G.P.; Cao, W.D. Improved Accumulation Capabilities of Phosphorus and Potassium in Green Manures and Its Relationship with Soil Properties and Enzymatic Activities. Agronomy 2019, 9, 708. [Google Scholar] [CrossRef]
- Dick, R.P. Soil Enzyme Activities as Indicators of Soil Quality. In Defining Soil Quality for a Sustainable Environment; Wiley: Hoboken, NJ, USA, 1994; pp. 107–124. [Google Scholar]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Ding, T.; Yan, Z.; Zhang, W.; Duan, T. Green Manure Crops Affected Soil Chemical Properties and Fungal Diversity and Community of Apple Orchard in the Loess Plateau of China. J. Soil Sci. Plant Nutr. 2021, 21, 1089–1102. [Google Scholar] [CrossRef]
- Gao, H.; Tian, G.; Khashi, U.R.M.; Wu, F. Cover Crop Species Composition Alters the Soil Bacterial Community in a Continuous Pepper Cropping System. Front. Microbiol. 2021, 12, 789034. [Google Scholar] [CrossRef] [PubMed]
- Domagała-Świątkiewicz, I.; Siwek, P. Effect of field pea (Pisum sativum subsp. arvense (L.) Asch.) and pea-oat (Avena sativa L.) biculture cover crops on high tunnel vegetable under organic production system. Org. Agric. 2022, 12, 91–106. [Google Scholar] [CrossRef]
- Céspedes, C.; Espinoza, S.; Maass, V. Nitrogen transfer from legume green manure in a crop rotation to an onion crop using 15N natural abundance technique. Chil. J. Agric. Res. 2022, 82, 44–51. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]




| Depth | Ctot [%] | Ntot [%] | C:N | P [mg·kg −1] | K [mg·kg −1] | Mg [mg·kg −1] | Ca [mg·kg −1] |
|---|---|---|---|---|---|---|---|
| Mean | 1.56 | 0.18 | 8.7 | 118.00 | 298.93 | 234.23 | 2954.33 |
| SD | 0.15 | 0.02 | 1.1 | 1.73 | 25.92 | 3.58 | 9.81 |
| Green Manure Type | Biomass Dose | Treatment | Abbreviation | Collection | |
|---|---|---|---|---|---|
| Before | After | ||||
| Control (no plant)—C | - | buried | CB | CB_I | CB_II |
| - | mulched | CM | CM_I | CM_II | |
| White mustard (Sinapis alba L.)—WM | low | buried | WMLB | WMLB_I | WMLB_II |
| mulched | WMLM | WMLM_I | WMLM_II | ||
| high | buried | WMHB | WMHB_I | WMHB_II | |
| mulched | WMHM | WMHM_I | WMHM_II | ||
| Forest rye (Secale cereale L. var. multicaule Metzg. ex Alef.)—R | low | buried | RLB | RLB_I | RLB_II |
| mulched | RLM | RLM_I | RLM_II | ||
| high | buried | RHB | RHB_I | RHB_II | |
| mulched | RHM | RHM_I | RHM_II | ||
| Fiddleneck (Phacelia tanacetifolia Benth.)—F | low | buried | FLB | FLB_I | FLB_II |
| mulched | FLM | FLM_I | FLM_II | ||
| high | buried | FHB | FHB_I | FHB_II | |
| mulched | FHM | FHM_I | FHM_II | ||
| Safflower (Carthamus tinctorius L.)—S | low | buried | SLB | SLB_I | SLB_II |
| mulched | SLM | SLM_I | SLM_II | ||
| high | buried | SHB | SHB_I | SHB_II | |
| mulched | SHM | SHM_I | SHM_II | ||
| Pea (Pisum sativum L.)—P | low | buried | PLB | PLB_I | PLB_II |
| mulched | PLM | PLM_I | PLM_II | ||
| high | buried | PHB | PHB_I | PHB_II | |
| mulched | PHM | PHM_I | PHM_II | ||
| Green Manure | Repetition | Dry Mass | Lignin | Cellulose | Hemicellulose | Sugars | Lipids | Ash |
| White mustard | mean | 93.14 | 6.95 | 19.89 | 2.30 | 12.53 | 3.17 | 13.74 |
| SE | 0.03 | 0.16 | 0.08 | 0.29 | 0.29 | 0.03 | 0.16 | |
| HSD | a | a | a | b | c | b | b | |
| Perennial forest rye | mean | 93.18 | 2.46 | 13.90 | 1.40 | 12.57 | 2.21 | 14.56 |
| SE | 0.04 | 0.03 | 0.11 | 0.22 | 0.03 | 0.05 | 0.35 | |
| HSD | a | e | b | b | c | c | ab | |
| Fiddleneck | mean | 92.83 | 5.41 | 9.73 | 2.22 | 14.12 | 1.97 | 15.75 |
| SE | 0.03 | 0.10 | 0.14 | 0.03 | 0.03 | 0.06 | 0.39 | |
| HSD | b | c | d | b | b | c | a | |
| Safflower | mean | 92.73 | 4.42 | 11.52 | 1.38 | 10.68 | 1.97 | 13.12 |
| SE | 0.05 | 0.04 | 0.24 | 0.17 | 0.02 | 0.03 | 0.34 | |
| HSD | b | d | c | b | d | c | b | |
| Pea | mean | 93.11 | 6.21 | 14.62 | 22.31 | 30.13 | 3.46 | 8.32 |
| SE | 0.06 | 0.11 | 0.00 | 0.07 | 10.06 | 0.07 | 0.10 | |
| HSD | a | b | b | a | a | a | c | |
| Green Manure | Repetition | N_AGB | P_AGB | K_AGB | C_AGB | Ca_AGB | Mg_AGB | |
| White mustard | mean | 3.41 | 0.38 | 3.80 | 46.53 | 1.74 | 0.22 | |
| SE | 0.09 | 0.01 | 0.09 | 1.29 | 0.04 | 0.00 | ||
| HSD | bc | b | b | a | b | b | ||
| Perennial forest rye | mean | 3.91 | 0.44 | 3.80 | 45.43 | 1.69 | 0.21 | |
| SE | 0.10 | 0.01 | 0.08 | 1.25 | 0.03 | 0.01 | ||
| HSD | a | a | b | a | b | b | ||
| Fiddleneck | mean | 2.32 | 0.30 | 4.60 | 45.66 | 2.27 | 0.21 | |
| SE | 0.06 | 0.01 | 0.11 | 1.23 | 0.05 | 0.01 | ||
| HSD | d | c | a | a | a | b | ||
| Safflower | mean | 3.47 | 0.23 | 3.35 | 47.23 | 2.08 | 0.28 | |
| SE | 0.08 | 0.00 | 0.08 | 1.04 | 0.05 | 0.01 | ||
| HSD | ab | d | c | a | a | a | ||
| Pea | mean | 2.97 | 0.23 | 2.62 | 49.46 | 0.77 | 0.22 | |
| SE | 0.08 | 0.00 | 0.06 | 0.95 | 0.02 | 0.00 | ||
| HSD | c | d | d | a | c | b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucerik, J.; Brtnicky, M.; Mustafa, A.; Hammerschmiedt, T.; Kintl, A.; Sobotkova, J.; Alamri, S.; Baltazar, T.; Latal, O.; Naveed, M.; et al. Utilization of Diversified Cover Crops as Green Manure-Enhanced Soil Organic Carbon, Nutrient Transformation, Microbial Activity, and Maize Growth. Agronomy 2024, 14, 2001. https://doi.org/10.3390/agronomy14092001
Kucerik J, Brtnicky M, Mustafa A, Hammerschmiedt T, Kintl A, Sobotkova J, Alamri S, Baltazar T, Latal O, Naveed M, et al. Utilization of Diversified Cover Crops as Green Manure-Enhanced Soil Organic Carbon, Nutrient Transformation, Microbial Activity, and Maize Growth. Agronomy. 2024; 14(9):2001. https://doi.org/10.3390/agronomy14092001
Chicago/Turabian StyleKucerik, Jiri, Martin Brtnicky, Adnan Mustafa, Tereza Hammerschmiedt, Antonin Kintl, Julie Sobotkova, Saud Alamri, Tivadar Baltazar, Oldrich Latal, Muhammad Naveed, and et al. 2024. "Utilization of Diversified Cover Crops as Green Manure-Enhanced Soil Organic Carbon, Nutrient Transformation, Microbial Activity, and Maize Growth" Agronomy 14, no. 9: 2001. https://doi.org/10.3390/agronomy14092001






