Abstract
The steady increase in temperature due to global warming can significantly impact weed growth. This study investigates the response of the yellow foxtail (Setaria pumila (Poir.) Roem. & Schult.) grass weed and the Palmer amaranth (Amaranthus palmeri S. Watson) broadleaf weed to increasing temperature conditions, simulating future climate conditions. Temperature treatments included growing these weeds in three temperature-controlled growth chambers: control or ambient conditions (15.5/12.8 °C day/night), moderately elevated temperatures (17.2/14.4 °C), and elevated temperatures (18.8/16.1 °C). Monthly adjustments in the growth chambers simulated the natural temperature rise observed from April to June in the Southern USA, aligning with the respective treatments. Different plant parameters recorded included days to emergence, number of tillers/leaves, chlorophyll content, days to first inflorescence, biomass, and seed set. One-way ANOVA indicated a significant temperature impact on all the parameters assessed (p < 0.05), except for biomass (for both weeds) and days to first inflorescence and yield (for Palmer amaranth (p > 0.05)). The average days to emergence were the lowest under elevated temperatures (8 days for yellow foxtail and 11 days for Palmer amaranth) when compared to that for the control (10 days for yellow foxtail and 19 days for Palmer amaranth). By week 5, yellow foxtail exhibited notably greater tiller numbers under elevated temperatures compared to that of the control; a similar trend was noticed regarding the number of Palmer amaranth leaves. The average chlorophyll content was the highest under elevated temperature conditions up to week 6 and began decreasing after that for both weeds. The average yield of yellow foxtail under elevated, moderately elevated, and control temperatures was 7.55, 2.69, and 0.88 g, respectively. Even though not significant, the yield of Palmer amaranth was higher under elevated temperature conditions as compared to that under the ambient condition. The biomass of both yellow foxtail and Palmer amaranth were not significantly impacted by temperature (p > 0.05). Our research shows that as temperatures rise, weeds exhibit more vigorous growth and show higher photosynthetic efficiency, which has important implications for how we manage weeds in agriculture. These findings suggest that under warmer conditions, weeds could display more vigorous vegetative growth, thus significantly impacting crop yields. As we face ongoing global warming, it is crucial to consider how temperatures influences weed growth when designing strategies to manage weeds effectively.
1. Introduction
Global climate models predict that annual temperatures will rise by an average of 0.3 °C to 5.8 °C by the end of the 21st century [1]. The worldwide agricultural sector is expected to be greatly impacted by temperature increase and climate change. A month-by-month analysis of average temperatures in Oklahoma, USA, from 2002 to 2023 shows an increase from 19.20 °C in 2002 to 20.60 °C in 2023 during the peak summer month of May (Figure 1). Such a temperature increase holds true for the entire Southern United States. With the global population increase and a higher demand for food and fuel, the climate change scenario is expected to become more severe in the future. Rising temperatures linked to global warming have intensified in recent years, posing significant impacts on agricultural ecosystems and altering weed species dynamics, distribution, and competitiveness within crops [2]. Research indicates that elevated temperatures directly influence weed growth and development, fostering increased proliferation and competitive behaviors [3].
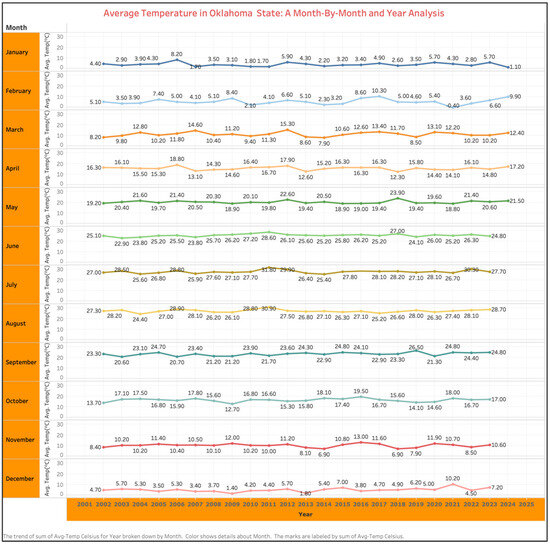
Figure 1.
Month-by-month analysis of average temperature in Oklahoma, USA, from 2002 to 2023.
Palmer amaranth, or pigweed (Amaranthus palmeri S. Watson), and yellow foxtail (Setaria pumila (Poir.) Roem. & Schult.) represent persistent weeds inflicting substantial costs on farmers through crop yield losses and heightened weed control expenditures [4]. Yellow foxtail, an annual grass weed originating from Eurasia, has markedly expanded its presence across the United States [5], becoming a predominant species that adversely impacts spring cereal grain, corn, alfalfa, and soybean production [6]. Palmer amaranth is a problematic annual broadleaf weed native to the Southwest United States, with few cases of the weed spreading northbound [7]. Because of its competitive nature and enormous seed production capacity, Palmer amaranth currently accounts for 91% of yield loss in corn and 79% in soybeans [8]. Both species are listed among the top ten most common and troublesome weeds among all broadleaf crops, fruits, and vegetables, with Palmer amaranth ranked at number one, and yellow foxtail ranked at number four [6]. Even though the current recorded optimal temperature for germination and development of yellow foxtail is approximately 21 °C to 30 °C and 32 °C to 36 °C in case of Palmer amaranth, the weeds can germinate in early spring by March/April, becoming competitive with both summer and winter crops [9,10]. The highly adaptive responses of these weeds to changing environmental conditions could significantly alter their growth patterns, potentially enhancing their competitiveness and impact on agricultural systems.
In order to understand the response of yellow foxtail and Palmer amaranth to increased temperature condition, the purpose of this study is to examine the temperature responses of these two weeds within a controlled growth chamber system at moderately elevated and highly elevated temperature conditions. Such information can be of use in predicting the potential growth and vegetative behavior of each species in the face of global warming. Results from the study would be relevant in the development of futuristic weed management plans, particularly in the Southern United States, but also globally, as the temperature increase is a global phenomenon. Past research has suggested that optimal growth of broadleaf weeds such as sicklepod (Senna obtusifolia (L.) H.S. Irwin & Barneby) respond positively to temperatures as high as 34 °C [11]. Similarly, rice weeds were shown exhibit have significant increases in weight, height, and leaf area at temperature treatments 3.4 °C above their typical range [3]. Positive correlations were also found in the predominant grassweed, itch grass (Rottboellia cochinchinensis (Lour.) Clayton), where a 3 °C rise in temperature increased overall biomass by 88% [12]. To predict how yellow foxtail and Palmer amaranth species may behave in crop systems during enhanced temperature conditions, preliminary research is needed to understand the specific developmental responses amongst various temperature parameters in these species outside of a crop–weed experiment. Thus, the objective of this research was to grow grass weeds (yellow foxtail) and broadleaf weeds (Palmer amaranth) under elevated temperature conditions to understand their morphological and physiological responses to elevated temperatures.
2. Materials and Methods
Yellow foxtail and Palmer amaranth seeds were sourced from Azlin Seed Services, Leland, MS, USA. The experiments were conducted in two runs using growth chambers at the controlled environmental research laboratory facility of Oklahoma State University. Seeds of yellow foxtail and Palmer amaranth were sown in 10 cm diameter pots containing SUNGRO 360 SOIL RSI potting mix (American Plant Products, Oklahoma City, OK, USA), amended with 4N-4.2P-11.6K controlled-release fertilizer (Scotts, Marysville, OH, USA). For each weed, five seeds were sown per pot, and the plants were later thinned to establish a single plant per pot. Each pot was placed in a single non-porous tray (28 × 54.6 × 6.3 cm3) and was irrigated with 500 mL of water supplied in the tray every other day during the first month. A total of 1000 mL of irrigation water of was supplied daily during the second and third month. The experiment was conducted in a completely randomized design, with three replications. Each replication consisted of two plants in separate pots (n = 6). Treatment consisted of exposing these plants to three different temperatures; this was achieved by setting the growth chambers to (i) normal/ambient temperature: GC-1; (ii) moderately elevated temperature: GC-2; and (iii) highly elevated temperature: GC-3. Since both yellow foxtail and Palmer amaranth are summer annuals that mostly germinate in early April and start producing seeds by June/July, the temperature of the ambient growth chamber was adjusted to mirror Oklahoma’s natural temperature rise from April to June. The state of Oklahoma was selected to fix the baseline temperature for the study because diverse summer and winter crops are grown in the state, and yellow foxtail and Palmer amaranth are the major weeds in a diverse crop production system. Full detail of the temperature maintained in all the growth chambers over the 90-day experimental period is provided in Table 1. Daytime temperatures were maintained from 8 a.m. to 4 p.m., and the remaining time was maintained at the nighttime temperature. During month two and three, the daytime temperatures of GC-2 and GC-3 were maintained at 1.7 °C and 3.4 °C higher, respectively, than that of GC-1 for the first month, and temperatures were increased accordingly, mimicking the increase in temperature expected in the future due to global warming [3]. Precautions were taken not to increase the temperature of the elevated growth chambers to a dangerous level in order to ensure that the plants survived the higher temperatures, enabling comparative analysis. The experiment was terminated at 90 days (3 months) after sowing to eliminate the impact of time on yield and biomass.

Table 1.
Temperature of three different growth chambers used in the study.
Different parameters measured in the study are as follows:
- (a)
- Seedling emergence was recorded by counting the number of seedlings emerging from the soil surface. Daily observation was performed every day for three weeks to record the seedling emergence.
- (b)
- Tiller and leaf number were recorded every week for five weeks after emergence. Recording was terminated at five weeks because the vegetative growth was highly vigorous after five weeks, making it overwhelming to count the tiller and leaf numbers.
- (c)
- Chlorophyll content recording was initiated at four weeks after emergence when the leaves of both weed types were significantly developed to provide enough surface area for observation. Chlorophyll content was recorded every week and terminated at the ninth week, as the plants in GC-3 had already completed the life cycle at this stage, and the leaves were yellowing. The chlorophyll content was recorded using a chlorophyll meter (Apogee MC-100 Chlorophyll Concentration Meter, Logan, UT, USA). The chlorophyll for each plant was measured by choosing three fully grown leaves at random and measuring the chlorophyll of each leaf individually using the chlorophyll meter. The average of the three readings was finalized as the chlorophyll content of the plant.
- (d)
- Yield was measured in grams by weighing the seeds produced by each plant on a weighing scale; the seeds were dried for one week at room temperature before measuring their weight.
- (e)
- Dry biomass was recorded by cutting the plants at the base of the stem, oven-drying them for three days at 26.6 °C, followed by weighing them, in grams, using the weighing scale.
All measured parameters were subjected to one-way analysis of variance at the 0.05 level of probability, followed by a least significant difference test to determine which specific treatments are significantly different from each other. The analysis was performed using R-4.4.1 statistical software (R core team 2021). The normality of the data was tested using a Shapiro–Wilk test before analysis.
3. Results
To understand the impact of elevated temperature on the growth and yield of grass and broadleaf weeds, yellow foxtail and Palmer amaranth plants were grown at ambient (GC-1), moderately elevated (GC-2), and highly elevated (GC-3) temperature conditions. The results of both the runs were pooled together for analysis, as there were no significant difference between the two runs (p < 0.05). The Shapiro–Wilk test indicated that the data met the assumptions of ANOVA.
3.1. Impact of Elevated Temperature on Seedling Emergence
Emergence is one of the fundamental processes in the life cycle of weeds, dictating their potential impact on crops. Temperature had a significant impact on weed seed emergence of both yellow foxtail and Palmer amaranth (p < 0.01). The mean emergence time for yellow foxtail under normal temperatures was 10 days. This rate experienced a modest decrease to 8.3 days under moderately elevated temperatures, and emergence occurred most rapidly at 8.2 days in GC-3, which were subjected to the highest temperature condition in the study (Figure 2).
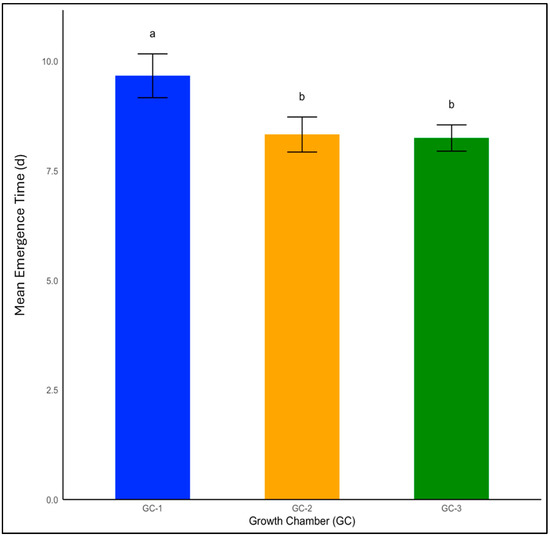
Figure 2.
Mean emergence time for yellow foxtail plants treated under different temperature conditions. Bars with the same letter are not significantly different from each other. GC-1 represents plants under control or ambient temperatures, GC-2 represents plants under moderately elevated temperatures, and GC-3 represents plants under highly elevated temperatures. The error bar represents the standard error of the mean in all the graphs.
In Palmer amaranth, which is a broadleaf weed, the mean emergence time was 19 days under the control or ambient temperature conditions of GC-1. This rate sharply decreased to 12 days under moderately elevated temperatures, and the number of days to emergence was the shortest in GC-3, with an average rate of 11 days (Figure 3). The LSD test indicated that GC-2 and GC-3 were not significantly different in terms of their impact on seed emergence for both yellow foxtail and Palmer amaranth.
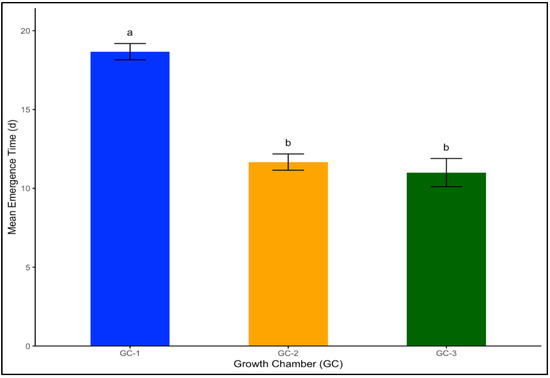
Figure 3.
Mean emergence time for Palmer amaranth plants treated under different temperature conditions. Bars with the same letter are not significantly different from each other. GC-1 represents plants under control or ambient temperatures, GC-2 represents plants under moderately elevated temperatures, and GC-3 represents plants under highly elevated temperatures.
3.2. Impact of Elevated Temperature on Vegetative Vigor
To understand the vegetative growth response of yellow foxtail and Palmer amaranth under thermal stress, the number of tillers and number of leaves were recorded, respectively, up to five weeks after emergence. In the case of yellow foxtail, GC-3 consistently displayed the highest number of tillers when compared to the results for GC-2 and GC-1 (Figure 4). At five weeks after emergence, the average number of tillers in GC-3 was approximately three times higher than the average number of tillers in GC-1. This ascending trend is a clear indicator of the grass weed’s adaptability to higher temperatures, with ANOVA results reinforcing a significant influence of temperature on tiller numbers (p = 0.003). The mean tiller number up to five weeks after emergence in GC-1 was 4, while in GC-2 and GC-3, the numbers were 7 and 11, respectively.

Figure 4.
Average tiller numbers of yellow foxtail up to five weeks after temperature treatment. GC-1 represents plants under control or ambient temperatures, GC-2 represents plants under moderately elevated temperatures, and GC-3 represents plants under highly elevated temperatures.
Palmer amaranth’s leaf production exhibited a similar positive impact of enhanced temperature conditions. GC-3 consistently displayed a higher number of leaves each week as compared to the results for GC-2 and GC-1 (p = 0.004). Figure 5 shows the leaf growth in Palmer amaranth over a five-week time period for each growth chamber. The mean number of leaves up until five weeks after emergence in GC-1, GC-2, and GC-3 was 9, 21, and 33, respectively. In the case of both yellow foxtail and Palmer amaranth, plants in GC-3 consistently showed the most vigorous vegetative growth as compared to that of plants in GC-2 and GC-1.
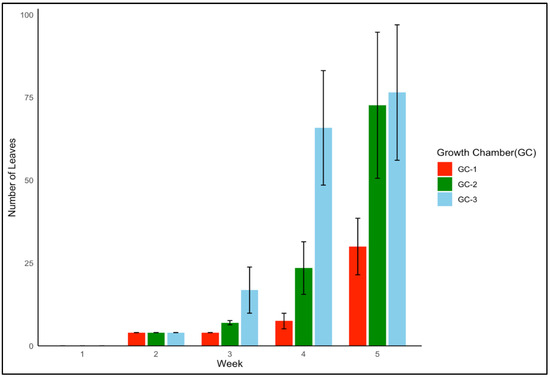
Figure 5.
Leaf growth trend of Palmer amaranth up until five weeks after emergence under different temperature treatments. GC-1 represents plants under control or ambient temperature, GC-2 represents plants under moderately elevated temperatures, and GC-3 represents plants under highly elevated temperatures.
3.3. Impact of Elevated Temperature on Chlorophyll Content
For yellow foxtail and Palmer amaranth, the chlorophyll content is a vital measure of potential photosynthetic activity and overall health. One-way ANOVA indicated that chlorophyll content was significantly associated with temperature treatments in both yellow foxtail and Palmer amaranth (p = 0.003 and p < 0.01). Yellow foxtail’s average chlorophyll content from week 4 to week 9 increased from an average of approximately 42.5 µmol/m2 under control conditions to about 44.5 µmol/m2 under moderately elevated temperatures, reaching up to 46 µmol/m2 under the most elevated thermal conditions. This gradational rise reflects the species’ resilience and suggests a possible optimization of photosynthetic processes in the face of temperature increase (Figure 6). To get a better idea of how chlorophyll content varied weekly in response to temperature, weekly average chlorophyll content was visualized from week 4 to week 9 (Figure 7). In the case of yellow foxtail, the weekly chlorophyll content was the highest in GC-3 until week 6, when it gradually started declining. This indicates that plants in GC-3 approached maturity earlier and started senescence or yellowing after week 6, leading to a decrease in chlorophyll content.
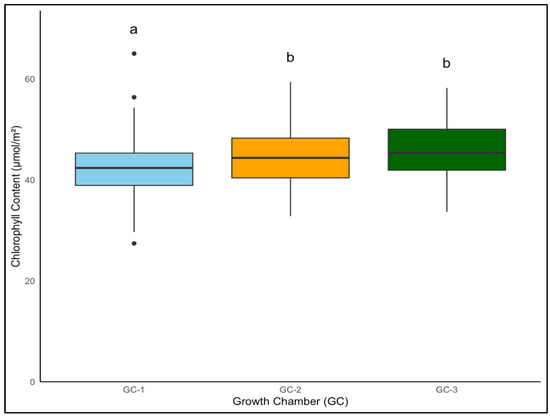
Figure 6.
Average chlorophyll content of yellow foxtail in µmol/m2 from week 4 to week 9 under different temperature treatments. Bars with the same letter are not significantly different from each other. GC-1 represents plants under control or ambient temperatures, GC-2 represents plants under moderately elevated temperatures, and GC-3 represents plants under highly elevated temperatures.
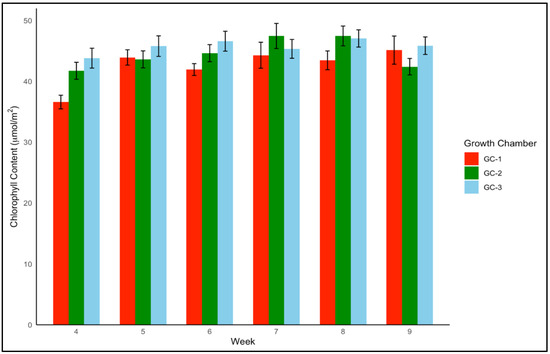
Figure 7.
Average chlorophyll content trend of yellow foxtail in µmol/m2 from week 4 to week 9 under different temperature treatments. GC-1 represents plants under control or ambient temperature, GC-2 represents plants under moderately elevated temperature, and GC-3 represents plants under highly elevated temperatures.
Similarly, Palmer amaranth’s chlorophyll content increased from a controlled average of 40.3 µmol/m2 to approximately 43.0 µmol/m2 under moderately elevated temperatures, with the most pronounced increase to roughly 48.0 µmol/m2 at the highest temperature elevation (Figure 8). Chlorophyll content in Palmer amaranth increased gradually up to 6 weeks in Palmer amaranth and started declining after 6 weeks, suggesting a similar life cycle response between yellow foxtail and Palmer amaranth under elevated conditions (Figure 9).
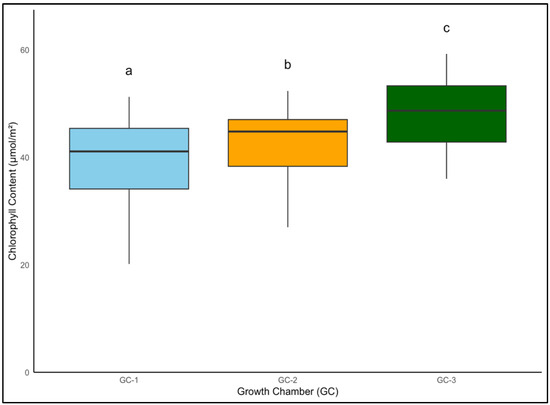
Figure 8.
Average chlorophyll content of Palmer amaranth in µmol/m2 from week 4 to week 9 under different temperature treatments. Bars with the same letter are not significantly different from each other. GC-1 represents plants under control or ambient temperatures, GC-2 represents plants under moderately elevated temperatures, and GC-3 represents plants under highly elevated temperatures.
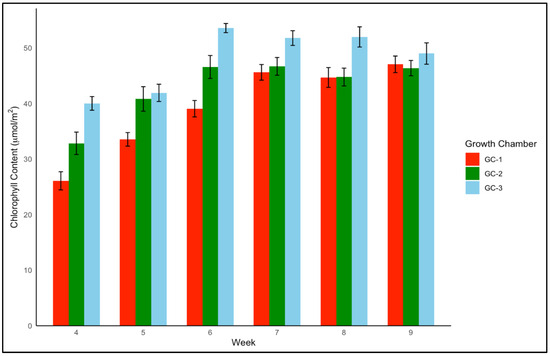
Figure 9.
Average chlorophyll content trend of Palmer amaranth in µmol/m2 from week 4 to week 9 under different temperature treatments. GC-1 represents plants under control or ambient temperatures, GC-2 represents plants under moderately elevated temperatures, and GC-3 represents plants under highly elevated temperatures.
3.4. Impact of Elevated Temperature on First Inflorescence
The timing of inflorescence is a pivotal event in the life cycle of a plant, particularly under the lens of temperature influences. Our research delved into the initial appearance of inflorescence in yellow foxtail and Palmer amaranth under varied temperature conditions. Temperature had a significant impact on the appearance of the first inflorescence in yellow foxtail (p < 0.01). Yellow foxtail’s days to the first inflorescence decreased markedly from an average of 53 days at normal temperature to 28 days and 26 days under moderately elevated and highly elevated temperature conditions, respectively (Figure 10). The advanced onset of flowering under elevated temperature conditions signals a significant shift in developmental timing with temperature increase.
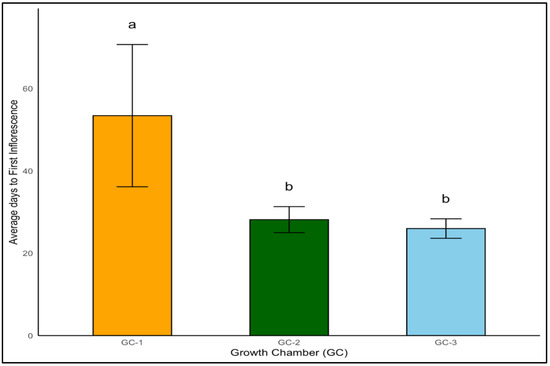
Figure 10.
Average days to first inflorescence of yellow foxtail plants exposed to different temperature conditions. Bars with the same letter are not significantly different from each other. GC-1 represents plants under control or ambient temperatures, GC-2 represents plants under moderately elevated temperatures, and GC-3 represents plants under highly elevated temperatures.
In case of Palmer amaranth, even though the effects of temperature were not significantly different regarding the onset of first inflorescence (p = 0.958), the average days to inflorescence was the lowest for GC-3 (45 days) and the highest for GC-1 (48 days) (Figure 11).
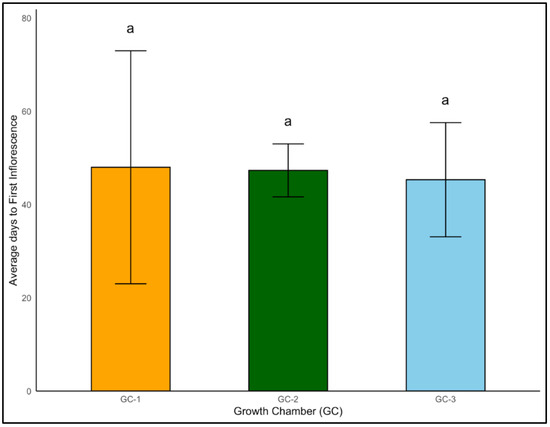
Figure 11.
Average days to first inflorescence of Palmer amaranth plants exposed to different temperature conditions. Bars with the same letter are not significantly different from each other. GC-1 represents plants under control or ambient temperatures, GC-2 represents plants under moderately elevated temperatures, and GC-3 represents plants under highly elevated temperatures.
3.5. Impact of Elevated Temperature on Biomass and Yield
For yellow foxtail, the average biomass decreased slightly from 62.3 g under control conditions to 52.5 g under highly elevated temperatures and 50.9 g under moderately elevated conditions (Figure 12). There was no significant impact of temperature on yellow foxtail’s biomass (p = 0.423); however, temperature impacted the yield significantly (p < 0.01). The yield increased notably from 0.88 g in GC-1 to 7.55 g in GC-3, and GC-2 exhibited an average yield of 2.69 g. This might be indicative of a possible compensatory growth and yield mechanism (Figure 12).
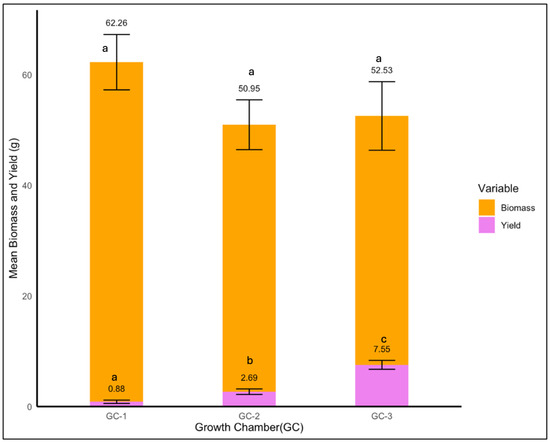
Figure 12.
Mean yield and biomass of yellow foxtail plants under control/ambient temperature conditions (GC-1), moderately elevated temperature conditions (GC-1), and highly elevated temperature conditions (GC-3). Bars with the same letter are not significantly different from each other.
In the case of Palmer amaranth, neither biomass nor yield were significantly different among various temperature conditions (p = 0.251 and p = 0.757, respectively). Even though the yield was not significantly impacted by temperature, plants in GC-3 and GC-2, which were exposed to higher temperatures, showed a yield of 15.3 and 14.7 g, respectively, and GC-1 displayed the lowest yield of 10.4 g (Figure 13).
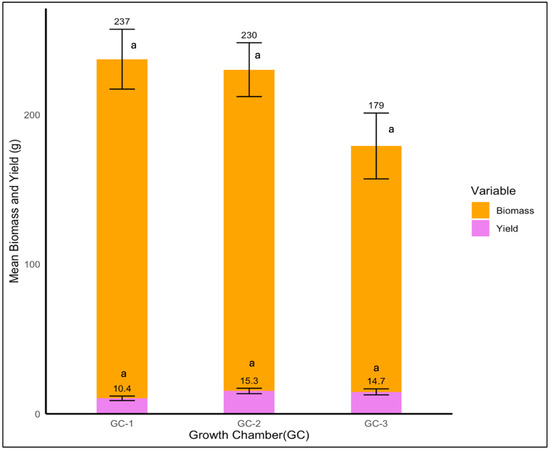
Figure 13.
Mean yield and biomass of Palmer amaranth plants under control/ambient temperature conditions (GC-1), moderately elevated temperature conditions (GC-1), and highly elevated temperature condition (GC-3). Bars with the same letter are not significantly different from each other.
In the case of biomass, Palmer amaranth plants showed the highest dry mass biomass of 237 g in GC-1, which was followed by 230 g in GC-2, and the lowest biomass of 179 g was observed in GC-3 (Figure 13).
4. Discussion
In recent years, climate change has caused significant impact on agricultural production system [13]. Various climate-driven extremes like drought, flooding, erratic rainfall, temperature rise, and increased disease and pest pressure have negatively impacted crop productivity [14]. Weeds are one of the major limitations to crop production systems, and left uncontrolled, they can cause up to a 40% yield loss [15]. In the face of climate change, it is essential to understand the impact of elevated temperatures on weeds to optimize climate-smart agricultural practices.
The effect of temperature on the seedling emergence of grass and broadleaf weeds indicated that under a higher temperature regime, weed seeds are likely to emerge earlier in the growing season. Many prior studies presenting results of higher germination under increased temperatures [3,11] support the findings of this study. Both yellow foxtail and Palmer amaranth are among the most problematic weeds for summer crop like soybean, cotton, and corn [16,17]. Future temperature increases may lead to earlier weed emergence, allowing them to compete with young crops during critical growth stages, potentially causing substantial yield losses without the initiation of timely weed management practices. Earlier emergence of these weeds would also provide growers with a very small window to control weeds after the sowing of summer crops. Thus, in the future, growers need to keep in mind the impact of increasing temperature while developing their weed management practices. The use of pre-emergent herbicides with high residual activity would be a good option for these summer crops, as the residual activity of herbicide can suppress weed emergence throughout the growing season [16].
Our study also suggested that both grass and broadleaf weeds will exhibit vigorous vegetative growth with increased temperatures. For example, the average number of tillers in yellow foxtail and the average number of leaves in Palmer amaranth five weeks after emergence were the highest under elevated temperature conditions. Our study results are supported by numerous studies performed by various researchers to assess the impact of increased temperature on several weeds, like hemp sesbania (Sesbania herbacea (Mill.) McVaugh), sicklepod, showy crotalaria (Crotalaria spectabilis Roth), and weedy rice (Oryza sativa L.) [3,18]. Since weeds are known for their competitive nature and adaptiveness, they can become even more aggressive in the future scenario involving climate change. Further, the negative impact of climate change would make crops less competitive, which would again provide advantages to the weeds. This paints a worrisome picture, with crops becoming less competitive and weeds becoming more competitive in the future. It is imperative that plant breeders and weed scientists work together to develop crops that would be resilient to weed interference in the future so that yield loss due to the aggressiveness of the weeds can be minimized under global warming conditions [19,20].
Chlorophyll content is a vital measurement of potential photosynthetic activity and overall health of the plant. Our study revealed that both yellow foxtail and Palmer amaranth are highly resilient to increased temperature conditions in terms of chlorophyll pigment synthesis. In fact, both the species showed the highest chlorophyll content up until 6 weeks after emergence in GC-3, which included the highest temperatures. In the GC-3 group, the chlorophyll content began gradually decreasing after week 6. The decrease in chlorophyll content after week 6 suggests that the weed species complete their life cycle faster under elevated temperature conditions. In the study, both of the weed species displayed a reduced chlorophyll content after week 6, which means that the plants were approaching senescence. These results were also supported by visual observation, as the plants in GC-3 (highly elevated temperature conditions) started yellowing, with active seed production after week 6. While studying the effect of elevated temperature on chlorophyll content, it is imperative to keep in mind that the temperatures used in our study were not high enough to damage the photosynthetic apparatus of plants in either the grass or broadleaf weeds, as the pigment synthesis process was not impacted by this temperature. With global warming, instead of a sudden temperature increase, there is gradual increase in temperatures. Our study suggests that, under such a scenario, the photosynthetic apertures of the weeds will be more efficient, thus resulting in a higher chlorophyll content and higher yields.
Inflorescence initiation is an important morphological characteristic, as it marks the transition from the vegetative to the reproductive stage, ultimately affecting the life cycle of the plants [21]. It is well recorded in the literature that inflorescence initiation is significantly associated with light, photoperiod, and temperature [22,23]. Our study showed that higher temperatures initiated earlier flowering in both yellow foxtail grass weed and Palmer amaranth broadleaf weed. In case of grassweed (yellow foxtail), the days to first inflorescence decreased significantly from 53 days to 26 days under highly elevated temperature conditions. In case of broadleaf Palmer amaranth, even though temperature did not significantly impact flowering initiation, plants exposed to higher temperatures required the lowest number of days to flower (GC-3—45 days; GC-1—48 days). Both yellow foxtail and Palmer amaranth are C4 plants, and they are more efficient than C3 plants. Studies have shown that C4 plants acclimatize to higher temperatures and can exhibit enhanced photosynthetic efficiency. Earlier flowering behavior under increased temperature condition suggests that both yellow foxtail and Palmer amaranth are highly efficient under high temperature conditions.
Although chlorophyll content, leaf number, and flowering showed a positive association with increased temperature in the current study, the yield of Palmer amaranth was not significantly impacted by temperature. It is possible that broadleaf weeds like Palmer amaranth might display high photosynthetic activity at higher temperatures, but this might not always be linked with higher yield. It can also be concluded from this study that the high temperatures used in the experiment were within the range tolerated by the plant’s photosynthetic apparatus, and that high temperatures do not negatively impact the plants’ functioning.
In the case of yellow foxtail, elevated temperature was associated with greater tiller number, higher chlorophyll content, and increased yield, suggesting a direct correlation between photosynthetic activity and yield in yellow foxtail, unlike the results for Palmer amaranth. In the future, it will be imperative to dive into the physiological studies of yellow foxtail and Palmer amaranth under elevated temperature conditions to understand the mechanisms impacting yield and biomass under elevated temperature conditions in these weeds.
In our study, during the experiment, termination biomass was the highest in GC-1, which was set at ambient temperatures, in case of both yellow foxtail and Palmer amaranth. This might be due to the fact that the plants in GC-1 were still actively growing when the experiment was terminated, while the plants exposed to higher temperatures had already completed their life cycle and had entered the senescence phase.
In the current study, we observed that most of the parameters measured did not show a significant difference between the moderately elevated and highly elevated temperature conditions. This might be because the temperature range for the highly elevated conditions was set close to that of the moderately elevated conditions. We intentionally avoided using very high temperatures to prevent plant mortality, which would have made the comparative analysis impossible.
Taking all the results into consideration, it is safe to say that higher temperatures shorten the life cycle of both grass and broadleaf weeds, enhancing their vegetative growth in the early life stages. Thus, it is important to be proactive in developing immediate weed management practices in the future, as weeds will likely be more aggressive, with earlier emergence and shorter life cycles, in the face of climate change.
5. Conclusions
Our study underscores the profound influence of rising temperatures on weed species like yellow foxtail and Palmer amaranth, highlighting their heightened growth and competitive vigor under elevated thermal conditions. These findings emphasize the pressing need for implementing adaptive weed management strategies to counteract the escalating challenges posed by climate change in agriculture. Effective solutions will necessitate integrated approaches that encompass cultural, biological, and chemical control methods. Future research endeavors should prioritize unraveling the mechanistic responses of weeds to temperature shifts and developing predictive models to inform robust management strategies. In the future, it is also important to conduct climate change impact experiments focusing on weed biology and ecology by using more in-depth physiological parameters, such as the growing degree days (GDD) component. GDD are a measure of heat accumulation in a species and are used to estimate the developmental phases of plants [24]. Collaborative interdisciplinary efforts will be pivotal in advancing our knowledge base and ensuring the resilience of agricultural systems amid global warming pressures. By fostering innovation and adaptation, we can effectively safeguard food security and promote sustainable agricultural practices in a progressively warming environment.
Author Contributions
Conceptualization, S.S. (Swati Shrestha) and A.O.; methodology, S.S. (Swati Shrestha) and A.O.; software, A.O.; validation, S.S. (Swati Shrestha), A.O., and S.S. (Sanju Shrestha); formal analysis, A.O. and S.S. (Swati Shrestha); investigation, A.O.; resources, A.O. and S.S. (Swati Shrestha); data curation, G.O.; writing—original draft preparation, A.O., G.O., and S.S. (Sanju Shrestha); writing—review and editing, A.O., G.O., T.-M.T., and A.D.; visualization, S.S. (Sanju Shrestha) and A.D.; supervision, S.S. (Swati Shrestha) and T.-M.T.; project administration, S.S. (Sanju Shrestha). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Oklahoma State University, funding number 3-326240.
Data Availability Statement
Data will be made available according to requests to the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- IPCC. Climate Change 2013: The Physical Science Basis; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Amare, T. Review on Impact of Climate Change on Weed and Their Management. Am. J. Biol. Environ. Sci. 2016, 2, 21–27. [Google Scholar] [CrossRef]
- Flint, E.P.; Patterson, D.T.; Mortensen, D.A.; Riechers, G.H.; Beyers, J.L. Temperature Effects on Growth and Leaf Production in Three Weed Species. Weed Sci. 1984, 32, 655–663. [Google Scholar] [CrossRef]
- Zimdahl, R.L. Weed-Crop Competition: A Review, 2nd ed.; Blackwell Publishing: Oxford, UK; Ames, IA, USA, 2004. [Google Scholar]
- Gressel, J. World Weeds: Natural Histories and Distributions; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Van Wychen, L. Survey of the Most Common and Troublesome Weeds in Broadleaf Crops, Fruits & Vegetables in the United States and Canada; Weed Science Society of America: Westminster, CO, USA, 2022. [Google Scholar]
- Yu, E.; O’Neill, J.L.; Jensen, S.B.; Thomason, I.J. Timeline of Palmer Amaranth (Amaranthus palmeri) Invasion and Eradication in Minnesota. Weed Technol. 2021, 35, 802–810. [Google Scholar] [CrossRef]
- USDA NRCS. Palmer Amaranth; USDA NRCS: Washington, DC, USA, 2017.
- Abbey, T.; Delvalle, T.; Landschoot, P. Lawn and Turfgrass Weeds: Yellow Foxtail and Green Foxtail; Penn State Extension: University Park, PA, USA, 2017. [Google Scholar]
- Keeley, P.E.; Carter, C.H.; Thullen, R.J. Influence of Planting Date on Growth of Palmer Amaranth (Amaranthus palmeri). Weed Sci. 1987, 35, 199–204. [Google Scholar] [CrossRef]
- Wright, S.R.; Coble, H.D.; Raper, C.D.; Rufty, T.W. Comparative Responses of Soybean (Glycine max), Sicklepod (Senna obtusifolia), and Palmer Amaranth (Amaranthus palmeri) to Root Zone and Aerial Temperatures. Weed Sci. 1999, 47, 167–174. [Google Scholar] [CrossRef]
- Patterson, D.T.; Westbrook, J.K.; Joyce, R.J.V.; Lingren, P.D.; Rogasik, J. Weeds, insects, and diseases. Clim. Chang. 1999, 43, 711–727. [Google Scholar] [CrossRef]
- Habib-ur-Rahman, M.; Khan, S.; Ali, R.; Hussain, S. Impact of Climate Change on Agricultural Production: Issues, Challenges, and Opportunities in Asia. Front. Plant Sci. 2022, 13, 925548. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Van Der Velde, M.; Lin, E.; Xiong, W.; Li, Y. The Impacts of Climate Change on Agricultural Production Systems in China. Clim. Chang. 2013, 120, 313–324. [Google Scholar] [CrossRef]
- Oerke, E.-C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Gibson, K.D.; Johnson, W.G.; Hillger, D.E. Farmer Perceptions of Problematic Corn and Soybean Weeds in Indiana. Weed Technol. 2005, 19, 1065–1070. [Google Scholar] [CrossRef]
- Pala, F.; Menan, H. Common weeds in cotton fields. In Cotton Production under Abiotic Stress; Karademir, E., Karademir, C., Eds.; Iksad Publishing House: Ankara, Turkey, 2021; pp. 270–271. [Google Scholar] [CrossRef]
- Piveta, L.B.; Salles, M.L.; Costa, C.F.; Rocha, M.T. Molecular and Physiological Responses of Rice and Weedy Rice to Heat and Drought Stress. Agriculture 2020, 11, 9. [Google Scholar] [CrossRef]
- Banga, S.S.; Kang, M.S. Developing Climate-Resilient Crops. J. Crop Improv. 2014, 28, 57–87. [Google Scholar] [CrossRef]
- Kole, C.; Liu, X.; Schafleitner, R.; Khan, M. Application of Genomics-Assisted Breeding for Generation of Climate-Resilient Crops: Progress and Prospects. Front. Plant Sci. 2015, 6, 563. [Google Scholar] [CrossRef] [PubMed]
- Kirchoff, B.K.; Claßen-Bockhoff, R. Inflorescences: Concepts, Function, Development and Evolution. Ann. Bot. 2013, 112, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Antoun, M.; Ouellet, F. Growth Temperature Affects Inflorescence Architecture in Arabidopsis thaliana. Botany 2013, 91, 642–651. [Google Scholar] [CrossRef]
- Oh, W. Effects of Temperature, Photoperiod and Light Intensity on Growth and Flowering in Eustoma grandiflorum. HortScience 2015, 33, 349–355. [Google Scholar] [CrossRef]
- Sallenave, M.; Pornaro, R.; Velasco-Cruz, C.; Macolino, S.; Leinauer, B. Base Temperatures Affect Accuracy of Growing Degree Days Models for Turfgrass Species. Agronomy 2021, 11, 1218. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).