A Phytochrome-Interacting Factor Gene CaPIF7a Positively Regulates the Defense Response against Phytophthora capsici Infection in Pepper (Capsicum annuum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Cloning and Sequence Analyses of CaPIF7a of Pepper
2.3. Subcellular Localization Assay
2.4. Quantitative Real-Time PCR (qRT-PCR)
2.5. Virus-Induced Gene Silencing (VIGS) of CaPIF7a in Pepper
2.6. Pathogens Growth and Inoculation Procedures
2.7. Determination of Resistance Indexes
2.8. DNA Affinity Purification Followed by Sequencing (DAP-Seq)
2.9. Yeast One-Hybrid (Y1H) Assay
2.10. Statistical Analysis
3. Results
3.1. Cloning and Sequence Analyses of Pepper CaPIF7a Gene and Its Encoded Protein
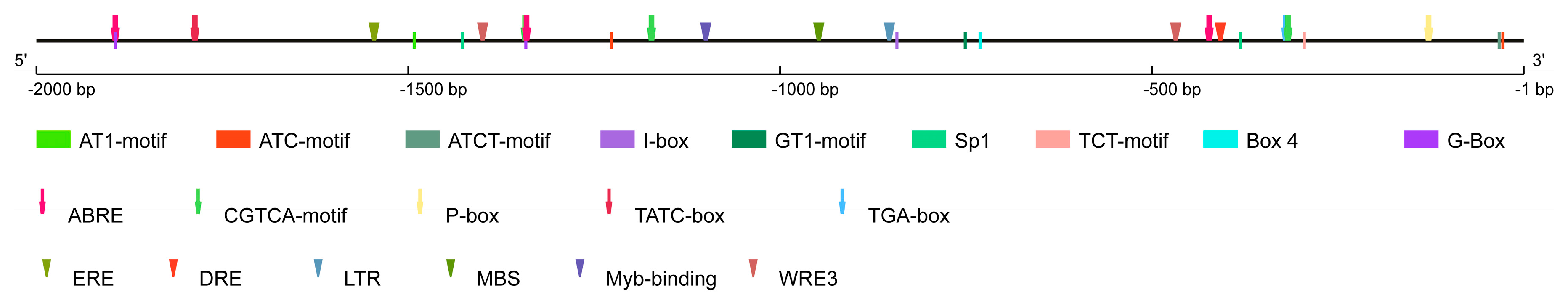
3.2. Promoter Analysis of the Pepper CaPIF7a Gene
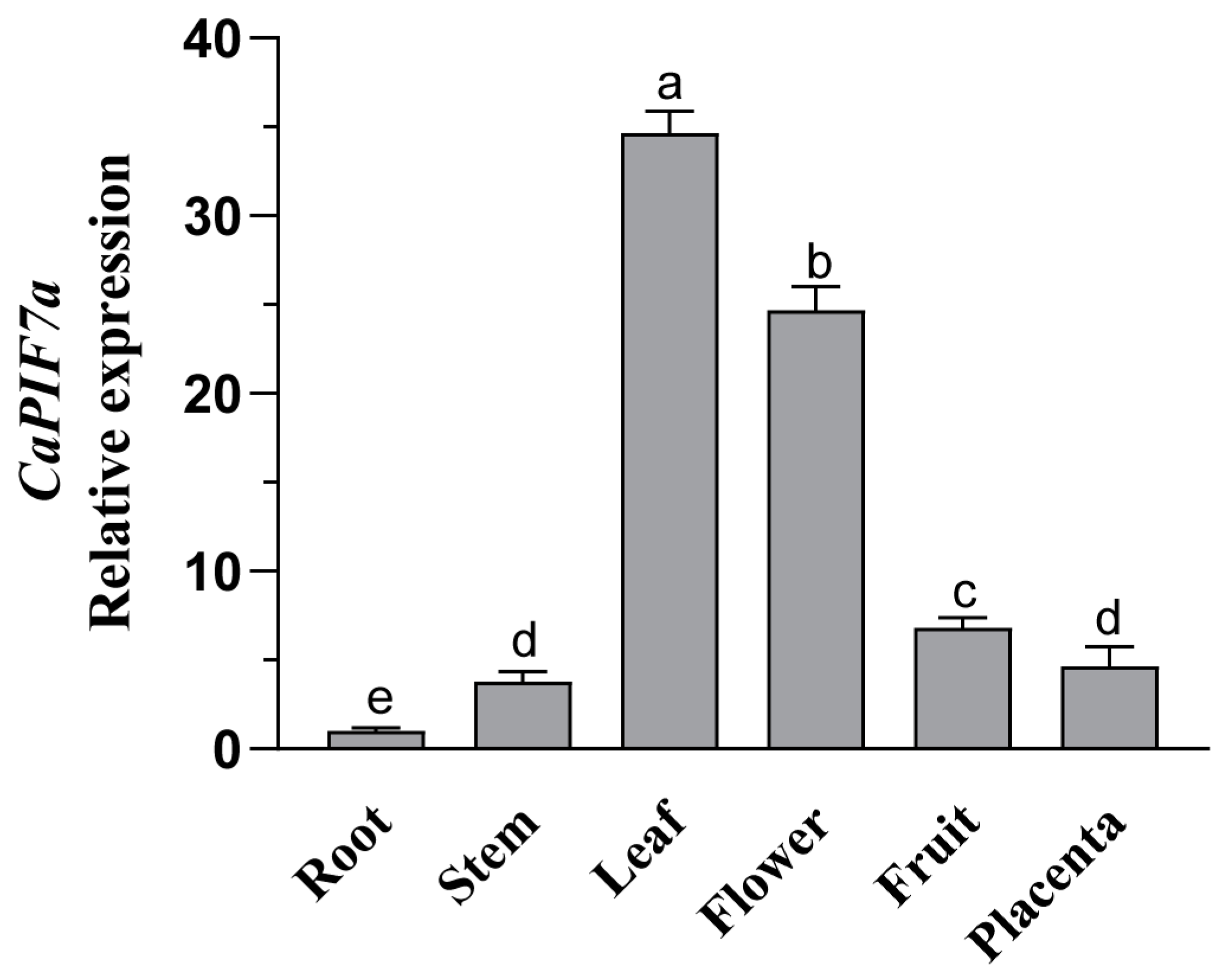
3.3. Expression Profiles of CaPIF7a in Different Tissues
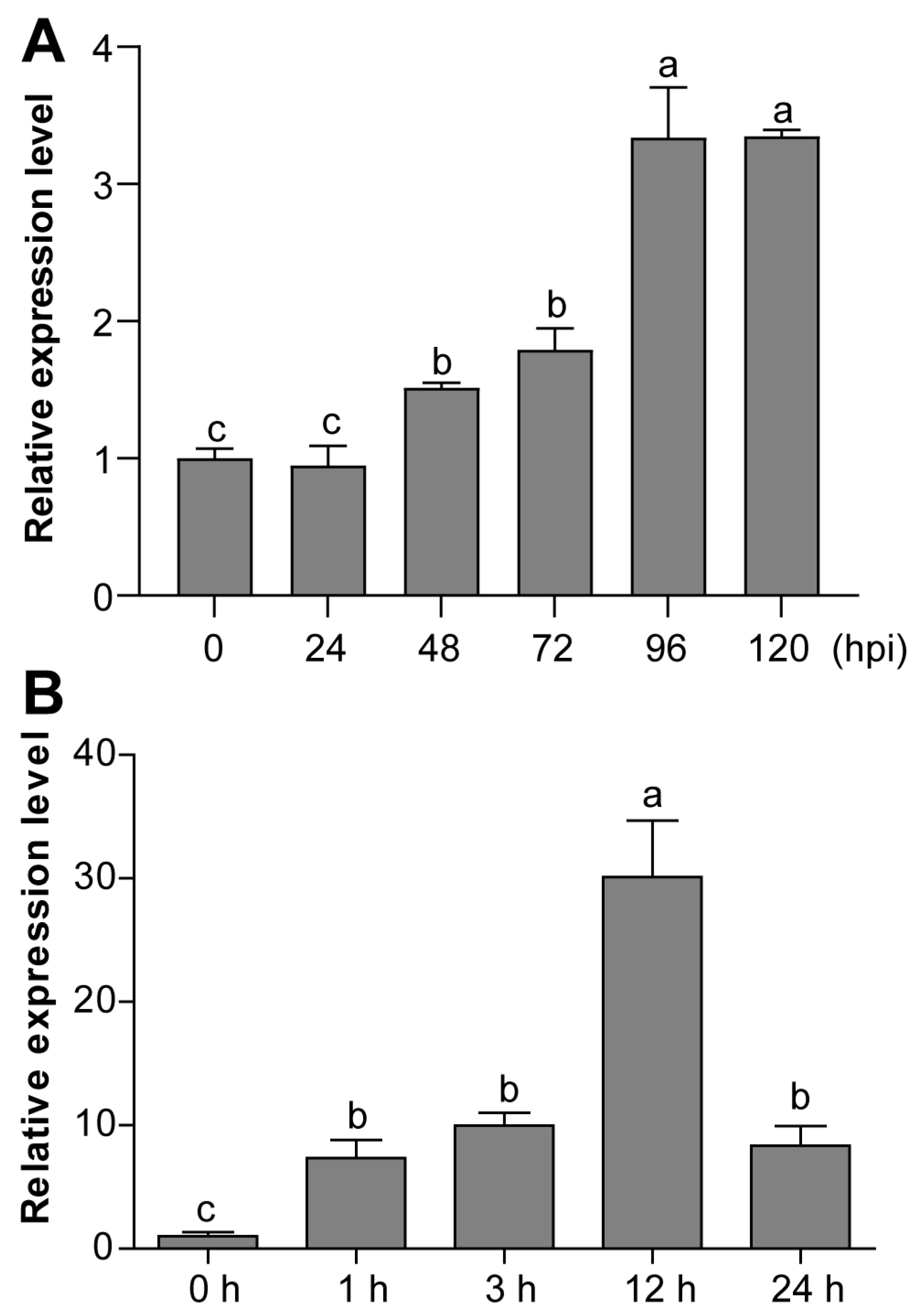
3.4. Expression Profiles of CaPIF7a under PCI and SA Treatment
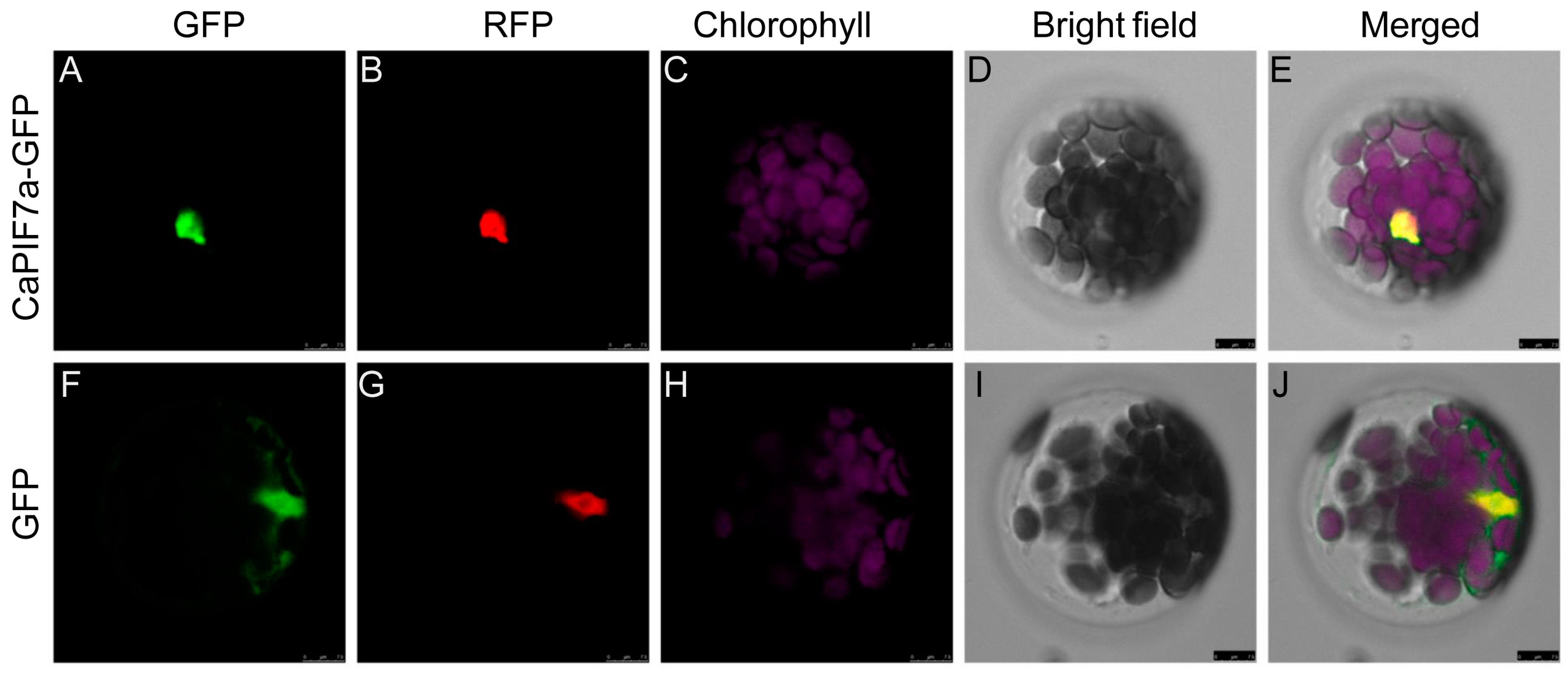
3.5. Subcellular Localization of CaPIF7a
3.6. VIGS-Silenced of CaPIF7a Increased Pepper Sensitivity to PCI
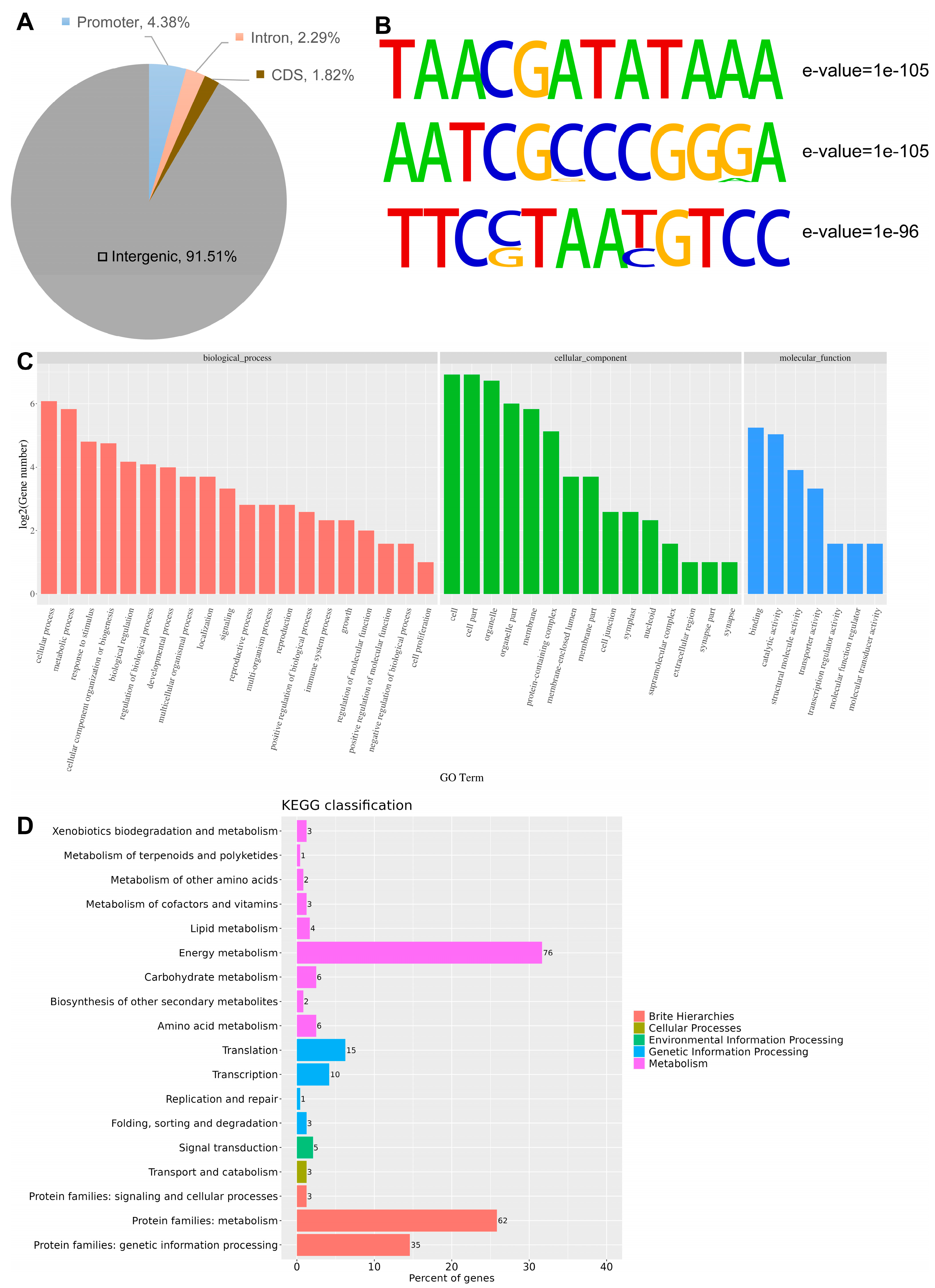
3.7. Genome-Wide Identification of CaPIF7a Binding Regions and Motifs by DAP-Seq
3.8. CaPIF7a Regulates the Expression of CaHY5
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ni, M.; Tepperman, J.M.; Quail, P.H. Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature 1999, 400, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Chu, L.; Lin, H.; Qi, Y.; Fang, Z.; Xu, D. PIFs- and COP1-HY5-mediated temperature signaling in higher plants. Stress Biol. 2022, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Abid, M.A.; Qanmber, G.; Askari, M.; Zhou, L.; Song, Y.; Liang, C.; Meng, Z.; Malik, W.; Wei, Y.; et al. Photomorphogenesis in plants: The central role of phytochrome interacting factors (PIFs). Environ. Exp. Bot. 2022, 194, 104704. [Google Scholar] [CrossRef]
- Leivar, P.; Monte, E.; Oka, Y.; Liu, T.; Carle, C.; Castillon, A.; Huq, E.; Quail, P.H. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr. Biol. CB 2008, 18, 1815–1823. [Google Scholar] [CrossRef]
- Leivar, P.; Monte, E. PIFs: Systems integrators in plant development. Plant Cell 2014, 26, 56–78. [Google Scholar] [CrossRef]
- Park, E.; Kim, Y.; Choi, G. Phytochrome B requires PIF degradation and sequestration to induce light responses across a wide range of light conditions. Plant Cell 2018, 30, 1277–1292. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, W.; Liang, W.; Ling, X.; Ma, J.; Yang, C.; Li, L. Phytochrome B inhibits the activity of phytochrome-interacting factor 7 involving phase separation. Cell Rep. 2023, 42, 113562. [Google Scholar] [CrossRef]
- Song, Y.; Yang, C.; Gao, S.; Zhang, W.; Li, L.; Kuai, B. Age-triggered and dark-induced leaf senescence require the bHLH transcription factors PIF3, 4, and 5. Mol. Plant 2014, 7, 1776–1787. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Jeong, J.; Kang, M.Y.; Kim, J.; Paek, N.C.; Choi, G. Phytochrome-interacting transcription factors PIF4 and PIF5 induce leaf senescence in Arabidopsis. Nat. Commun. 2014, 5, 4636. [Google Scholar] [CrossRef]
- Sun, J.; Lu, J.; Bai, M.; Chen, Y.; Wang, W.; Fan, C.; Liu, J.; Ning, G.; Wang, C. Phytochrome-interacting factors interact with transcription factor CONSTANS to suppress flowering in rose. Plant Physiol. 2021, 186, 1186–1201. [Google Scholar] [CrossRef]
- Liu, L.Y.; Jia, M.Z.; Wang, S.N.; Han, S.; Jiang, J. Identification and characterization of cotton PHYTOCHROME-INTERACTING FACTORS in temperature-dependent flowering. J. Exp. Bot. 2023, 74, 3765–3780. [Google Scholar] [CrossRef] [PubMed]
- Rosado, D.; Trench, B.; Bianchetti, R.; Zuccarelli, R.; Rodrigues Alves, F.R.; Purgatto, E.; Segal Floh, E.I.; Silveira Nogueira, F.T.; Freschi, L.; Rossi, M. Downregulation of PHYTOCHROME-INTERACTING FACTOR 4 influences plant development and fruit production. Plant Physiol. 2019, 181, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hou, Y.; Wang, Q.; Pan, X.; Sun, Y.; Zhu, X.; Li, H.; Guo, M.; Gao, Y. Phytochrome interacting factor ZmPIF6 simultaneously enhances chilling tolerance and grain size in rice. Plant Physiol. Biochem. PPB 2024, 214, 108954. [Google Scholar] [CrossRef] [PubMed]
- Zhuge, Y.; Sheng, H.; Zhang, M.; Fang, J.; Lu, S. Grape phytochrome-interacting factor VvPIF1 negatively regulates carotenoid biosynthesis by repressing VvPSY expression. Plant Sci. Int. J. Exp. Plant Biol. 2023, 331, 111693. [Google Scholar] [CrossRef]
- Michael, R.; Ranjan, A.; Gautam, S.; Trivedi, P.K. HY5 and PIF antagonistically regulate HMGR expression and sterol biosynthesis in Arabidopsis thaliana. Plant Sci. Int. J. Exp. Plant Biol. 2024, 346, 112168. [Google Scholar] [CrossRef]
- Nie, N.; Huo, J.; Sun, S.; Zuo, Z.; Chen, Y.; Liu, Q.; He, S.; Gao, S.; Zhang, H.; Zhao, N.; et al. Genome-wide characterization of the PIFs family in sweet potato and functional identification of IbPIF3.1 under drought and Fusarium wilt stresses. Int. J. Mol. Sci. 2023, 24, 4092. [Google Scholar] [CrossRef]
- Sng, B.J.R.; Vu, K.V.; Choi, I.K.Y.; Chin, H.J.; Jang, I.C. LONG HYPOCOTYL IN FAR-RED 1 mediates a trade-off between growth and defence under shade in Arabidopsis. J. Exp. Bot. 2023, 74, 3560–3578. [Google Scholar] [CrossRef]
- Xiang, S.; Wu, S.; Zhang, H.; Mou, M.; Chen, Y.; Li, D.; Wang, H.; Chen, L.; Yu, D. The PIFs redundantly control plant defense response against Botrytis cinerea in Arabidopsis. Plants 2020, 9, 1246. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, X.; Zhang, X.; Wang, W.; Cai, G.; Bi, G.; Chen, S.; Sun, C.; Zhou, J.M. PIF3 is phosphorylated by MAPK to modulate plant immunity. New Phytol. 2023, 240, 372–381. [Google Scholar] [CrossRef]
- Gangappa, S.N.; Berriri, S.; Kumar, S.V. PIF4 coordinates thermosensory growth and immunity in Arabidopsis. Curr. Biol. CB 2017, 27, 243–249. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Zhao, P.; Sun, Y.; Fang, R.X.; Ye, J. Near-infrared light and PIF4 promote plant antiviral defense by enhancing RNA interference. Plant Commun. 2024, 5, 100644. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, L.; Xing, Q.; Zhao, Y.; Qi, H. CmPIF8-CmERF27-CmACS10-mediated ethylene biosynthesis modulates red light-induced powdery mildew resistance in oriental melon. Plant Cell Environ. [CrossRef]
- Yang, Y.; Guang, Y.; Wang, F.; Chen, Y.; Yang, W.; Xiao, X.; Luo, S.; Zhou, Y. Characterization of phytochrome-interacting factor genes in pepper and functional analysis of CaPIF8 in cold and salt stress. Front. Plant Sci. 2021, 12, 746517. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Guang, Y.; Lin, J.; Zhou, Y.; Yu, T.; Ding, F.; Wang, Y.; Chen, J.; Zhou, Y.; et al. Red light induces salicylic acid accumulation by activating CaHY5 to enhance pepper resistance against Phytophthora capsici. Hortic. Res. 2023, 10, uhad213. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, R.C.; Huang, S.C.; Song, L.; Lewsey, M.G.; Bartlett, A.; Nery, J.R.; Galli, M.; Gallavotti, A.; Ecker, J.R. Cistrome and epicistrome features shape the regulatory DNA landscape. Cell 2016, 165, 1280–1292. [Google Scholar] [CrossRef]
- Mai, Y.; Sun, P.; Suo, Y.; Li, H.; Han, W.; Diao, S.; Wang, L.; Yuan, J.; Wang, Y.; Ye, L.; et al. Regulatory mechanism of MeGI on sexuality in Diospyros oleifera. Front. Plant Sci. 2023, 14, 1046235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, T.; Meyer, C.A.; Eeckhoute, J.; Johnson, D.S.; Bernstein, B.E.; Nusbaum, C.; Myers, R.M.; Brown, M.; Li, W.; et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008, 9, R137. [Google Scholar] [CrossRef]
- Ma, W.; Noble, W.S.; Bailey, T.L. Motif-based analysis of large nucleotide data sets using MEME-ChIP. Nat. Protoc. 2014, 9, 1428–1450. [Google Scholar] [CrossRef]
- Roeber, V.M.; Bajaj, I.; Rohde, M.; Schmülling, T.; Cortleven, A. Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 2021, 44, 645–664. [Google Scholar] [CrossRef]
- Pham, V.N.; Kathare, P.K.; Huq, E. Phytochromes and phytochrome interacting factors. Plant Physiol. 2018, 176, 1025–1038. [Google Scholar] [CrossRef]
- Zhang, K.; Zheng, T.; Zhu, X.; Jiu, S.; Liu, Z.; Guan, L.; Jia, H.; Fang, J. Genome-wide identification of PIFs in grapes (Vitis vinifera L.) and their transcriptional analysis under lighting/shading conditions. Genes 2018, 9, 451. [Google Scholar] [CrossRef]
- Zheng, P.F.; Wang, X.; Yang, Y.Y.; You, C.X.; Zhang, Z.L.; Hao, Y.J. Identification of phytochrome-interacting factor family members and functional analysis of MdPIF4 in Malus domestica. Int. J. Mol. Sci. 2020, 21, 7350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Feng, X.H.; Jin, J.H.; Khan, A.; Guo, W.L.; Du, X.H.; Gong, Z.H. CaSBP11 participates in the defense response of pepper to Phytophthora capsici through regulating the expression of defense-related genes. Int. J. Mol. Sci. 2020, 21, 9065. [Google Scholar] [CrossRef]
- Zhang, H.X.; Ali, M.; Feng, X.H.; Jin, J.H.; Huang, L.J.; Khan, A.; Lv, J.G.; Gao, S.Y.; Luo, D.X.; Gong, Z.H. A novel transcription factor CaSBP12 gene negatively regulates the defense response against Phytophthora capsici in pepper (Capsicum annuum L.). Int. J. Mol. Sci. 2019, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, X.; Dong, S.; Jiang, X.; Wang, L.; Yu, J.; Zhou, Y. Crosstalk of PIF4 and DELLA modulates CBF transcript and hormone homeostasis in cold response in tomato. Plant Biotechnol. J. 2020, 18, 1041–1055. [Google Scholar] [CrossRef]
- Oh, E.; Zhu, J.Y.; Wang, Z.Y. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat. Cell Biol. 2012, 14, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, L.; Chen, Y.; Xiao, N.; Zhang, D.; Zhang, M.; Wang, W.; Zhang, C.; Zhang, A.; Li, H.; et al. Phytochrome interacting factor regulates stomatal aperture by coordinating red light and abscisic acid. Plant Cell 2022, 34, 4293–4312. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Milmanda, G.L.; Crocco, C.D.; Reichelt, M.; Mazza, C.A.; Köllner, T.G.; Zhang, T.; Cargnel, M.D.; Lichy, M.Z.; Fiorucci, A.S.; Fankhauser, C.; et al. A light-dependent molecular link between competition cues and defence responses in plants. Nat. Plants 2020, 6, 223–230. [Google Scholar] [CrossRef]
- Lei, G.; Zhou, K.H.; Chen, X.J.; Huang, Y.Q.; Yuan, X.J.; Li, G.G.; Xie, Y.Y.; Fang, R. Transcriptome and metabolome analyses revealed the response mechanism of pepper roots to Phytophthora capsici infection. BMC Genom. 2023, 24, 626. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Xing, Q.; Zhao, Y.; Yu, B.; Ma, Y.; Wang, F.; Qi, H. PIF8-WRKY42-mediated salicylic acid synthesis modulates red light induced powdery mildew resistance in oriental melon. Plant Cell Environ. 2023, 46, 1726–1742. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, N.; Li, Y.; Zhou, X.; Bai, X.; Liu, L.; Ma, X.; Wang, S.; Li, X.; Gong, B.; et al. CaWRKY01-10 and CaWRKY08-4 confer pepper’s resistance to Phytophthora capsici infection by directly activating a cluster of defense-related genes. J. Agric. Food Chem. 2024, 72, 11682–11693. [Google Scholar] [CrossRef]
- Zribi, I.; Ghorbel, M.; Brini, F. Pathogenesis related proteins (PRs): From cellular mechanisms to plant defense. Curr. Protein Pept. Sci. 2021, 22, 396–412. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, T.; Wu, T.; Wang, R.; Wang, H.; Du, H.; Xu, X.; Xie, D.; Xu, X. The dynamic transcriptome of pepper (Capsicum annuum) whole roots reveals an important role for the phenylpropanoid biosynthesis pathway in root resistance to Phytophthora capsici. Gene 2020, 728, 144288. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Xia, Z.; Fan, R.; Tan, L.; Hu, L.; Wu, B.; Wu, H. De novo transcriptome sequencing of black pepper (Piper nigrum L.) and an analysis of genes involved in phenylpropanoid metabolism in response to Phytophthora capsici. BMC Genom. 2016, 17, 822. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wu, D.; Yu, T.; Liu, B.; Gao, X.; Han, H.; Chen, J.; Zhou, Y.; Yang, Y. A Phytochrome-Interacting Factor Gene CaPIF7a Positively Regulates the Defense Response against Phytophthora capsici Infection in Pepper (Capsicum annuum L.). Agronomy 2024, 14, 2035. https://doi.org/10.3390/agronomy14092035
Li Y, Wu D, Yu T, Liu B, Gao X, Han H, Chen J, Zhou Y, Yang Y. A Phytochrome-Interacting Factor Gene CaPIF7a Positively Regulates the Defense Response against Phytophthora capsici Infection in Pepper (Capsicum annuum L.). Agronomy. 2024; 14(9):2035. https://doi.org/10.3390/agronomy14092035
Chicago/Turabian StyleLi, Yu, Dan Wu, Ting Yu, Bing Liu, Xuchun Gao, Huibin Han, Jinyin Chen, Yong Zhou, and Youxin Yang. 2024. "A Phytochrome-Interacting Factor Gene CaPIF7a Positively Regulates the Defense Response against Phytophthora capsici Infection in Pepper (Capsicum annuum L.)" Agronomy 14, no. 9: 2035. https://doi.org/10.3390/agronomy14092035
APA StyleLi, Y., Wu, D., Yu, T., Liu, B., Gao, X., Han, H., Chen, J., Zhou, Y., & Yang, Y. (2024). A Phytochrome-Interacting Factor Gene CaPIF7a Positively Regulates the Defense Response against Phytophthora capsici Infection in Pepper (Capsicum annuum L.). Agronomy, 14(9), 2035. https://doi.org/10.3390/agronomy14092035





