Occurrence of Polycyclic Aromatic Hydrocarbons (PAHs) in Pyrochar and Hydrochar during Thermal and Hydrothermal Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Char Samples
2.3. Extraction of PAHs from Chars
2.4. Instrumental Analysis
2.5. Statistical Analysis
3. Results
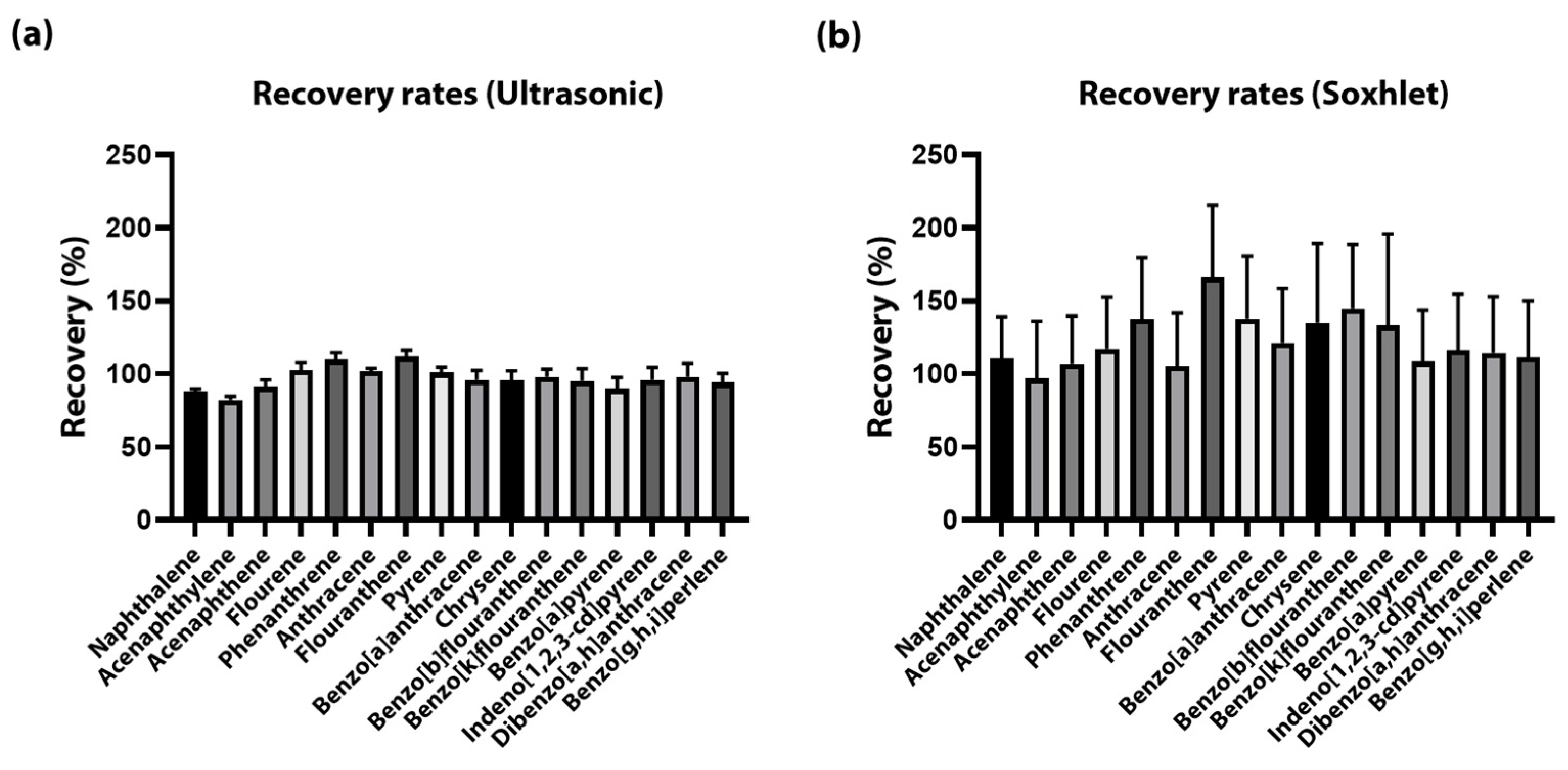
3.1. Validation of the Methods
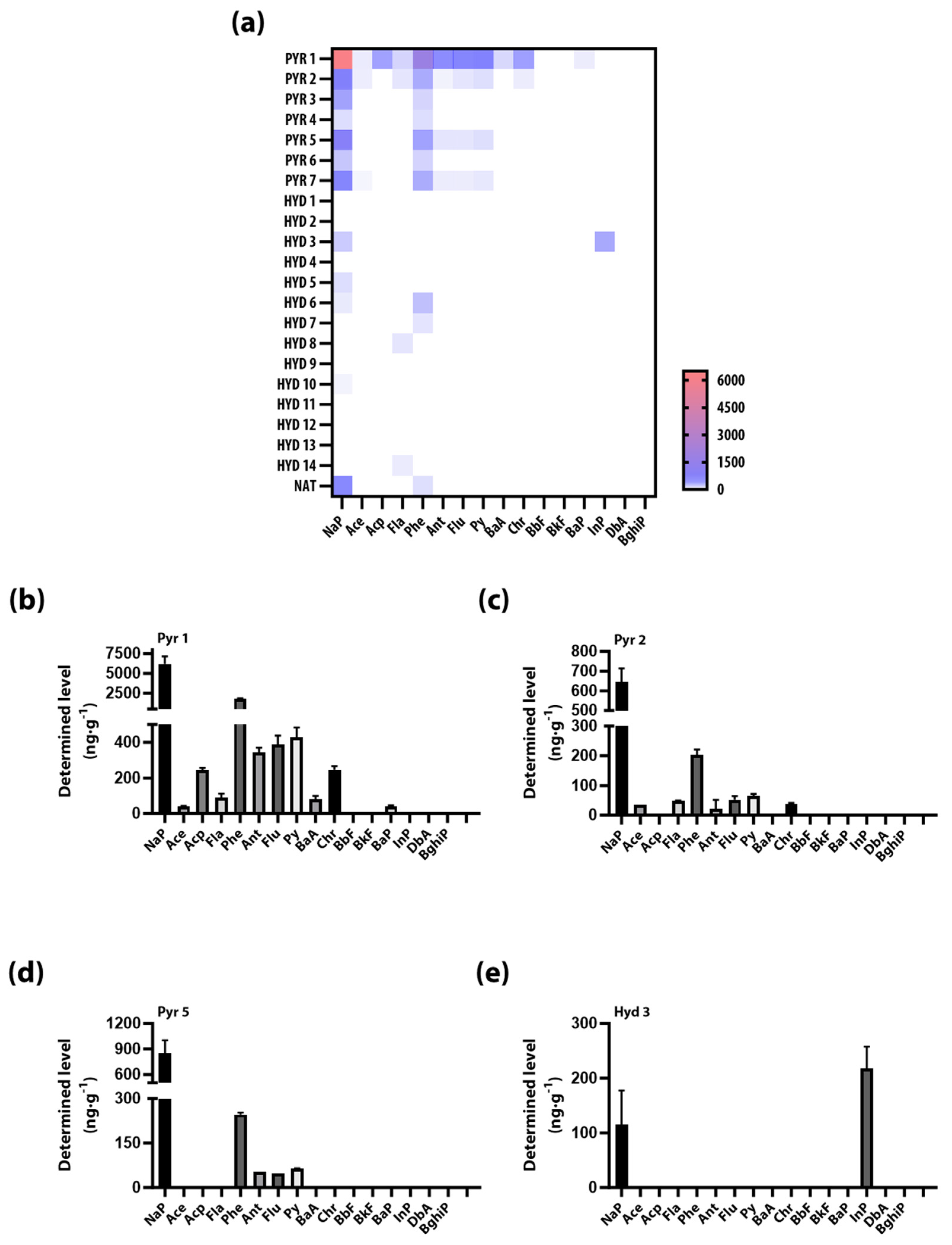
3.2. Determined Level of PAHs in Pyrochars and Hydrochars
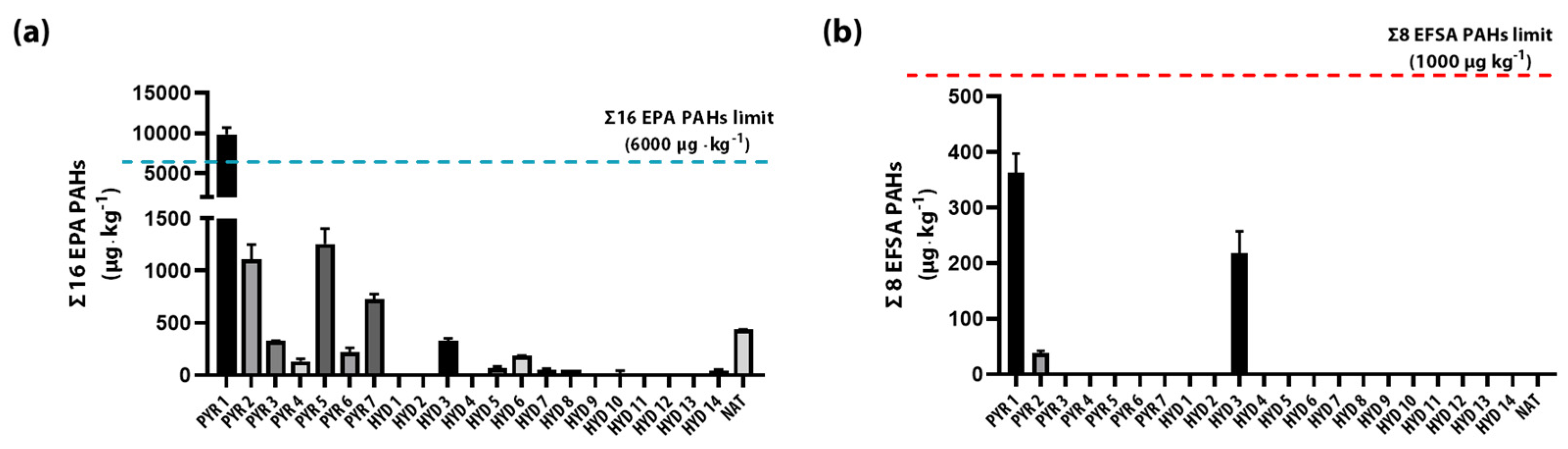
3.3. Evaluation of 16 EPA PAHs in Pyrochar and Hydrochar
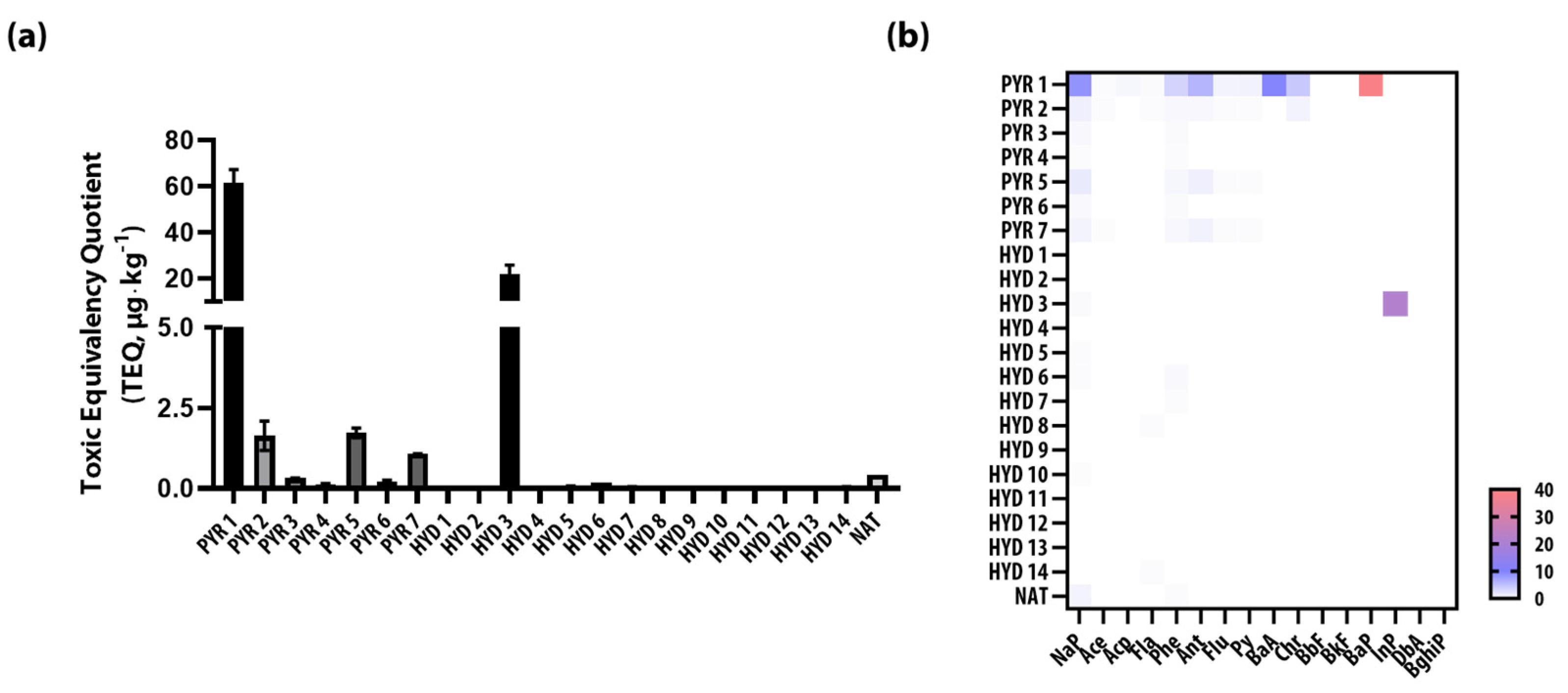
3.4. Toxic Equivalency of PAHs in Pyrochar and Hydrochar
4. Discussion
4.1. PAH Levels in Pyrochars
4.2. PAH Levels in Hydrochars
4.3. Comparison between PAH Levels in Pyrochars vs. Hydrochars
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ace | Acenaphthylene |
| Acp | Acenaphthene |
| Ant | Anthracene |
| BaA | Benzo(a)anthracene |
| BaP | Benzo(a)pyrene |
| BbF | Benzo(b)fluoranthene |
| BghiP | Benzo(g,h,i)perylene |
| BkF | Benzo(k)fluoranthene |
| Chr | Chrysene |
| DbA | Dibenzo(a,h)anthracene |
| EBC | European Biochar Certificate |
| EFSA | European Food Safety Authority |
| Flu | Fluoranthene |
| Fl | Fluorene |
| GHG | Greenhouse gas |
| HTC | Hydrothermal carbonization |
| HTH | Hydrothermal humification |
| HYD | Hydrochar |
| IBI | International Biochar Initiative |
| InP | Indeno(1,2,3-cd)pyrene |
| NaP | Naphthalene |
| PAH | Polycyclic aromatic hydrocarbons |
| Phe | Phenanthrene |
| Py | Pyrene |
| PYR | Pyrochar |
| TEF | Toxic equivalency factor |
| TEQ | Toxic equivalent (or toxic equivalency quotient) |
References
- Alkharabsheh, H.M.; Seleiman, M.F.; Battaglia, M.L.; Shami, A.; Jalal, R.S.; Alhammad, B.A.; Almutairi, K.F.; Al-Saif, A.M. Biochar and Its Broad Impacts in Soil Quality and Fertility, Nutrient Leaching and Crop Productivity: A Review. Agronomy 2021, 11, 993. Available online: https://www.mdpi.com/2073-4395/11/5/993 (accessed on 2 September 2024). [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef] [PubMed]
- Kanaly, R.A.; Harayama, S. Advances in the field of high-molecular-weight polycyclic aromatic hydrocarbon biodegradation by bacteria. Microb. Biotechnol. 2010, 3, 136–164. [Google Scholar] [CrossRef]
- Hussar, E.; Richards, S.; Lin, Z.Q.; Dixon, R.P.; Johnson, K.A. Human Health Risk Assessment of 16 Priority Polycyclic Aromatic Hydrocarbons in Soils of Chattanooga, Tennessee, USA. Water Air Soil Pollut. 2012, 223, 5535–5548. [Google Scholar] [CrossRef]
- Odinga, E.S.; Waigi, M.G.; Gudda, F.O.; Wang, J.; Yang, B.; Hu, X.; Li, S.; Gao, Y. Occurrence, formation, environmental fate and risks of environmentally persistent free radicals in biochars. Environ. Int. 2020, 134, 105172. [Google Scholar] [CrossRef]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (US EPA). Provisional Guidance for Quantitative Risk Assessment of Poly Aromatic Hydrocarbons (EPA 600/R-93/089). 1993. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=49732 (accessed on 2 September 2024).
- Wang, J.; Xia, K.; Waigi, M.G.; Gao, Y.; Odinga, E.S.; Ling, W.; Liu, J. Application of biochar to soils may result in plant contamination and human cancer risk due to exposure of polycyclic aromatic hydrocarbons. Environ. Int. 2018, 121, 169–177. [Google Scholar] [CrossRef]
- Brandt, M.; Einhenkel-Arle, D. Polycyclic Aromatic Hydrocarbons Harmful to the Environment! Toxic! Inevitable? German Environmental Agency. 2016. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/376/publikationen/polycyclic_aromatic_hydrocarbons_web.pdf (accessed on 2 September 2024).
- Zelinkova, Z.; Wenzl, T. The Occurrence of 16 EPA PAHs in Food—A Review. Polycycl. Aromat. Compd. 2015, 35, 248–284. [Google Scholar] [CrossRef]
- Certificate, E.B. Guidelines of the European Biochar Certificate—Version 10.3. 2023. Available online: https://www.european-biochar.org/media/doc/2/version_en_10_3.pdf (accessed on 2 September 2024).
- Initiative, I.B. Standardizaed Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil. 2013. Available online: https://www.biochar-international.org/wp-content/uploads/2018/04/IBI_Biochar_Standards_V1.1.pdf (accessed on 2 September 2024).
- Liu, Z.; Wang, Z.; Chen, H.; Cai, T.; Liu, Z. Hydrochar and pyrochar for sorption of pollutants in wastewater and exhaust gas: A critical review. Environ. Pollut. 2021, 268 Pt B, 115910. [Google Scholar] [CrossRef]
- Djandja, O.S.; Liew, R.K.; Liu, C.; Liang, J.; Yuan, H.; He, W.; Feng, Y.; Lougou, B.G.; Duan, P.G.; Lu, X.; et al. Catalytic hydrothermal carbonization of wet organic solid waste: A review. Sci. Total Environ. 2023, 873, 162119. [Google Scholar] [CrossRef]
- Li, B.; Guo, J.Z.; Liu, J.L.; Fang, L.; Lv, J.Q.; Lv, K. Removal of aqueous-phase lead ions by dithiocarbamate-modified hydrochar. Sci. Total Environ. 2020, 714, 136897. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kuramochi, H. Catalytic pyrolysis of biomass using fly ash leachate to increase carbon monoxide production and improve biochar properties to accelerate anaerobic digestion. Bioresour. Technol. 2023, 387, 129583. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhu, X.; Dutta, S.; Khanal, S.K.; Lee, K.T.; Masek, O.; Tsang, D.C.W. Catalytic co-hydrothermal carbonization of food waste digestate and yard waste for energy application and nutrient recovery. Bioresour. Technol. 2022, 344 Pt B, 126395. [Google Scholar] [CrossRef]
- Cavali, M.; Libardi Junior, N.; de Sena, J.D.; Woiciechowski, A.L.; Soccol, C.R.; Belli Filho, P.; Bayard, R.; Benbelkacem, H.; de Castilhos, A.B., Jr. A review on hydrothermal carbonization of potential biomass wastes, characterization and environmental applications of hydrochar, and biorefinery perspectives of the process. Sci. Total Environ. 2023, 857 Pt 3, 159627. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Cheng, K.; Antonietti, M. A hydrothermal process to turn waste biomass into artificial fulvic and humic acids for soil remediation. Sci. Total Environ. 2019, 686, 1140–1151. [Google Scholar] [CrossRef]
- Marzban, N.; Libra, J.A.; Rotter, V.S.; Herrmann, C.; Ro, K.S.; Filonenko, S.; Hoffmann, T.; Antonietti, M. Maximizing the value of liquid products and minimizing carbon loss in hydrothermal processing of biomass: An evolution from carbonization to humification. Biochar 2024, 6, 44. [Google Scholar] [CrossRef]
- Pavlovic, I.; Knez, Z.; Skerget, M. Hydrothermal reactions of agricultural and food processing wastes in sub- and supercritical water: A review of fundamentals, mechanisms, and state of research. J. Agric. Food Chem. 2013, 61, 8003–8025. [Google Scholar] [CrossRef] [PubMed]
- Gollakota, A.; Savage, P.E. Fast and Isothermal Hydrothermal Liquefaction of Polysaccharide Feedstocks. ACS Sustain. Chem. Eng. 2020, 8, 3762–3772. [Google Scholar] [CrossRef]
- Anna, Z.; Patryk, O. The conversion of sewage sludge into biochar reduces polycyclic aromatic hydrocarbon content and ecotoxicity but increases trace metal content. Biomass Bioenergy 2015, 75, 235–244. [Google Scholar] [CrossRef]
- Buss, W.; Hilber, I.; Graham, M.C.; Mašek, O. Composition of PAHs in Biochar and Implications for Biochar Production. ACS Sustain. Chem. Eng. 2022, 10, 6755–6765. [Google Scholar] [CrossRef]
- Keiluweit, M.; Kleber, M.; Sparrow, M.A.; Simoneit, B.R.; Prahl, F.G. Solvent-extractable polycyclic aromatic hydrocarbons in biochar: Influence of pyrolysis temperature and feedstock. Environ. Sci. Technol. 2012, 46, 9333–9341. [Google Scholar] [CrossRef]
- McGrath, T.E.; Wooten, J.B.; Geoffrey Chan, W.; Hajaligol, M.R. Formation of polycyclic aromatic hydrocarbons from tobacco: The link between low temperature residual solid (char) and PAH formation. Food Chem. Toxicol. 2007, 45, 1039–1050. [Google Scholar] [CrossRef]
- Dai, Q.; Jiang, X.; Jiang, Y.; Jin, Y.; Wang, F.; Chi, Y.; Yan, J.; Xu, A. Temperature Influence and Distribution in Three Phases of PAHs in Wet Sewage Sludge Pyrolysis Using Conventional and Microwave Heating. Energy Fuels 2014, 28, 3317–3325. [Google Scholar] [CrossRef]
- Hale, S.E.; Lehmann, J.; Rutherford, D.; Zimmerman, A.R.; Bachmann, R.T.; Shitumbanuma, V.; O’Toole, A.; Sundqvist, K.L.; Arp, H.P.; Cornelissen, G. Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ. Sci. Technol. 2012, 46, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Quilliam, R.S.; Rangecroft, S.; Emmett, B.A.; Deluca, T.H.; Jones, D.L. Is biochar a source or sink for polycyclic aromatic hydrocarbon (PAH) compounds in agricultural soils? GCB Bioenergy 2013, 5, 96–103. [Google Scholar] [CrossRef]
- Buss, W.; Graham, M.C.; MacKinnon, G.; Mašek, O. Strategies for producing biochars with minimum PAH contamination. J. Anal. Appl. Pyrolysis 2016, 119, 24–30. [Google Scholar] [CrossRef]
- Hilber, I.; Blum, F.; Leifeld, J.; Schmidt, H.-P.; Bucheli, T.D. Quantitative Determination of PAHs in Biochar: A Prerequisite To Ensure Its Quality and Safe Application. J. Agric. Food Chem. 2012, 60, 3042–3050. [Google Scholar] [CrossRef]
- Lang, Q.; Zhang, B.; Li, Y.; Liu, Z.; Jiao, W. Formation and toxicity of polycyclic aromatic hydrocarbons during CaO assisted hydrothermal carbonization of swine manure. Waste Manag. 2019, 100, 84–90. [Google Scholar] [CrossRef]
- Erdogan, E.; Atila, B.; Mumme, J.; Reza, M.T.; Toptas, A.; Elibol, M.; Yanik, J. Characterization of products from hydrothermal carbonization of orange pomace including anaerobic digestibility of process liquor. Bioresour. Technol. 2015, 196, 35–42. [Google Scholar] [CrossRef]
- Peng, N.; Li, Y.; Liu, T.; Lang, Q.; Gai, C.; Liu, Z. Polycyclic Aromatic Hydrocarbons and Toxic Heavy Metals in Municipal Solid Waste and Corresponding Hydrochars. Energy Fuels 2017, 31, 1665–1671. [Google Scholar] [CrossRef]
- Mohammad, I.A.-W.; Muhammad Imran, R.; Mahtab, A.; Munir, A.; Abid, H.; Adel, R.A.U. Pyrolytic and hydrothermal carbonization of date palm leaflets: Characteristics and ecotoxicological effects on seed germination of lettuce. Saudi J. Biol. Sci. 2019, 26, 665–672. [Google Scholar] [CrossRef]
- Heinrich, T.; Kaetzl, K.; Libra, J.A.; Hoffmann, T. Influence of Thermochemical Conversion Technologies on Biochar Characteristics from Extensive Grassland for Safe Soil Application. Energies 2023, 16, 1896. Available online: https://www.mdpi.com/1996-1073/16/4/1896 (accessed on 2 September 2024). [CrossRef]
- Fernandes, M.B.; Brooks, P. Characterization of carbonaceous combustion residues: II. Nonpolar organic compounds. Chemosphere 2003, 53, 447–458. [Google Scholar] [CrossRef]
- Kim, L.; Jeon, H.-J.; Kim, Y.-C.; Yang, S.-H.; Choi, H.; Kim, T.-O.; Lee, S.-E. Monitoring polycyclic aromatic hydrocarbon concentrations and distributions in rice paddy soils from Gyeonggi-do, Ulsan, and Pohang. Appl. Biol. Chem. 2019, 62, 18. [Google Scholar] [CrossRef]
- Wang, D.; Ma, J.; Li, H.; Zhang, X. Concentration and Potential Ecological Risk of PAHs in Different Layers of Soil in the Petroleum-Contaminated Areas of the Loess Plateau, China. Int. J. Environ. Res. Public Health 2018, 15, 1785. Available online: https://www.mdpi.com/1660-4601/15/8/1785 (accessed on 2 September 2024). [CrossRef] [PubMed]
- Fagernäs, L.; Kuoppala, E.; Simell, P. Poly cyclic Aromatic Hydrocarbons in Birch Wood Slow Pyrolysis Products. Energy Fuels 2012, 26, 6960–6970. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of Slow Pyrolysis Biochars: Effects of Feedstocks and Pyrolysis Temperature on Biochar Properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef]
- Canadian Council of Ministers of the Environment. Polycyclic Aromatic Hydrocarbons. Canadian Soil Quality Guide-Lines for Protection of Environmental and Human Health. In Canadian Soil Quality Guidelines; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada; Available online: http://ceqg-rcqe.ccme.ca/en/index.html (accessed on 18 August 2024).
- Kuśmierz, M.; Oleszczuk, P. Biochar production increases the polycyclic aromatic hydrocarbon content in surrounding soils and potential cancer risk. Environ. Sci. Pollut. Res. 2014, 21, 3646–3652. [Google Scholar] [CrossRef]
- Dieguez-Alonso, A.; Funke, A.; Anca-Couce, A.; Rombolà, A.G.; Ojeda, G.; Bachmann, J.; Behrendt, F. Towards Biochar and Hydrochar Engineering—Influence of Process Conditions on Surface Physical and Chemical Properties, Thermal Stability, Nutrient Availability, Toxicity and Wettability. Energies 2018, 11, 496. Available online: https://www.mdpi.com/1996-1073/11/3/496 (accessed on 2 September 2024). [CrossRef]
- Schimmelpfennig, S.; Glaser, B. One Step Forward toward Characterization: Some Important Material Properties to Distinguish Biochars. J. Environ. Qual. 2012, 41, 1001–1013. [Google Scholar] [CrossRef]
- Bargmann, I.; Rillig, M.C.; Buss, W.; Kruse, A.; Kuecke, M. Hydrochar and Biochar Effects on Germination of Spring Barley. J. Agron. Crop Sci. 2013, 199, 360–373. [Google Scholar] [CrossRef]
- Garlapalli, R.K.; Wirth, B.; Reza, M.T. Pyrolysis of hydrochar from digestate: Effect of hydrothermal carbonization and pyrolysis temperatures on pyrochar formation. Bioresour. Technol. 2016, 220, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Wiedner, K.; Naisse, C.; Rumpel, C.; Pozzi, A.; Wieczorek, P.; Glaser, B. Chemical modification of biomass residues during hydrothermal carbonization—What makes the difference, temperature or feedstock? Org. Geochem. 2013, 54, 91–100. [Google Scholar] [CrossRef]
- Li, Y.; Zhai, Y.; Zhu, Y.; Peng, C.; Wang, T.; Zeng, G.; Wu, D.; Zhao, X. Distribution and Conversion of Polycyclic Aromatic Hydrocarbons during the Hydrothermal Treatment of Sewage Sludge. Energy Fuels 2017, 31, 9542–9549. [Google Scholar] [CrossRef]
| Sample | Sample Type | Inputs: Feedstock/Water/Additive | Reactor | Temperature (°C) | Duration (h) | %Solids (%wt, db) | Stirring (rpm) | Initial pH * (-) | Final pH † (-) | SY (%wt, db) |
|---|---|---|---|---|---|---|---|---|---|---|
| PYR 1 | Pyrochar | Grass briquettes | farm-scale SPSC oven | n.m. | 4 | n.a. | n.a. | 5.6 | 9.6 | 36.3 |
| PYR 2 | Pyrochar | Grass bale (loose) | Farm-scale carbon Twister oven | 600 | 2 | n.a. | n.a. | 6.3 | 9.9 | 12.9 |
| PYR 3 | Pyrochar | Grass pellets | farm-scale Biomacon oven | 800 | n.m. | n.a. | n.a. | 5.9 | 10.8 | 20.5 |
| PYR 4 | Pyrochar | Grass pellets | lab-scale top-lit up-draft | n.m. | n.m. | n.a. | n.a. | 5.9 | 9.3 | 23.8 |
| PYR 5 | Pyrochar | Grass pellets | lab-scale muffle oven | 450 | 1.3 | n.a. | n.a. | 5.9 | 10.6 | 32.5 |
| PYR 6 | Pyrochar | Grass pellets | lab-scale muffle oven | 550 | 0.9 | n.a. | n.a. | 5.9 | 10.4 | 27.4 |
| PYR 7 | Pyrochar | Grass pellets | lab-scale muffle oven | 650 | 0.67 | n.a. | n.a. | 5.9 | 10.5 | 26.6 |
| HYD 1 | Hydrochar | Grass pellets | 18.75 L Parr reactor | 220 | 5 | 15 | 125 | 5.9 | 4.2 | 61 |
| HYD 2 | HTH-solids | Sugar beet pellets/unseparated cattle manure digestate from biogas plant/DI water/KOH | 18.75 L Parr reactor | 200 | 6 | 40 | 200 | 13 | 8.3 | 75.6 |
| HYD 3 | HTH-solids | Unseparated cattle manure digestate from biogas plant/KOH | 18.75 L Parr reactor | 240 | 4 | 12.5 | 200 | 13 | 10.6 | 23.9 |
| HYD 4 | HTH-solids | Grass pellets/unseparated cattle manure biogas digestate/KOH | 18.75 L Parr reactor | 220 | 6 | 29.5 | 200 | 13 | 8.1 | 53.6 |
| HYD 5 | Hydrochar | Separated cattle manure digestate from biogas plant/DI water | 18.75 L Parr reactor | 240 | 1 | 13 | 200 | 9.0 | 5.3 | 62.6 |
| HYD 6 | Hydrochar | Fresh skin from coffee berries/DI water/Mg(OH)2 | 1 L Parr reactor | 160 | 5 | 15 | 100 | 13 (9.5) | 7.5 | 98.8 |
| HYD 7 | Hydrochar | Fresh skin from coffee berries/DI water | 1 L Parr reactor | 160 | 5 | 20 | 200 | 7 (9.5) | 6.9 | 94.3 |
| HYD 8 | Hydrochar | Fresh skin from coffee berries/DI water | 1 L Parr reactor | 240 | 5 | 25 | 0 | 7 (9.5) | 7.5 | 61.6 |
| HYD 9 | Hydrochar | Dry husk from dried coffee berries/DI water | 1 L Parr reactor | 240 | 5 | 15 | 200 | 7 (6.5) | 4.7 | 50.5 |
| HYD 10 | Hydrochar | Dry husk from dried coffee berries/DI water | 1 L Parr reactor | 240 | 1 | 25 | 100 | 7 (6.5) | 4.5 | 61.7 |
| HYD 11 | Hydrochar | Brown macroalgae, Fucus vesiculosus/DI water | 1 L Parr reactor | 190 | 3 | 35 | 200 | n.m. | 5.1 | 68 |
| HYD 12 | Hydrochar | Brown macroalgae, Fucus vesiculosus/recirculated process water | 1 L Parr reactor | 190 | 3 | 35 | 200 | n.m. | 4.9 | 73.4 |
| HYD 13 | Hydrochar | Halophyte, Salicornia/DI water | 1 L Parr reactor | 190 | 3 | 35 | 200 | n.m. | 4.2 | 51.9 |
| HYD 14 | Hydrochar | Halophyte, Salicornia/recirculated process water | 1 L Parr reactor | 190 | 3 | 35 | 200 | n.m. | 4.2 | 97.2 |
| NAT | Soil/char from burned forest | 5 cm topsoil/forest fire (pine trees) | n.a. | Natural forest fire | n.a. | n.a. | n.a. | n.m. | n.m. | n.a. |
| Feedstock Type | MC (%wt) | Source |
|---|---|---|
| Grass briquettes | 14.4 | Extensive grassland (harvest 2021), Lower Oder Valley National Park, Germany |
| Grass bale (loose) | 9 | |
| Grass pellets | 6.7 | |
| Sugar beet pellets | 9.4 | Purchased animal feed in Germany |
| Unseparated digestate from biogas plant | 92.6 | Biogas plant (input from cowshed), Brandenburg, Germany |
| Separated digested from biogas plant | 72.3 | Biogas plant (input from cowshed), Brandenburg, Germany |
| Fresh skin from wet processing of coffee berries | 72.3–74.9 | Byproducts from coffee semi-wash process (wet processing), Vietnam |
| Dry husk from processing of dried coffee berries | 7.3–13.4 | Byproducts from coffee dry processing, Vietnam |
| Brown macroalgae, Fucus vesiculosus | 15.6 | Coast of North Sea, Germany |
| Halophyte, Salicornia | 14.5 | Greenhouse trials, Germany |
| 5 cm topsoil/forest fire (pine trees) | n.m. | Topsoil sample from floor of pine forest after forest fire, Brandenburg, Germany |
| PYR 1 | PYR 2 | PYR 3 | PYR 4 | PYR 5 | PYR 6 | PYR 7 | HYD 1 | HYD 2 | HYD 3 | HYD 4 | HYD 5 | HYD 6 | HYD 7 | HYD 8 | HYD 9 | HYD 10 | HYD 11 | HYD 12 | HYD 13 | HYD 14 | NAT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nap | 6191.06 ± 77.02 | 644.42 ± 48.80 | 234.28 ± 5.62 | 64.80 ± 11.48 | 847.33 ± 10.18 | 128.13 ± 41.05 | 389.24 ± 24.93 | 0 | 0 | 115.22 ± 44.14 | 0 | 65.78 ± 13.34 | 41.86 ± 4.84 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 370.87 ± 2.39 |
| Ace | 40.16 ± 2.32 | 35.61 ± 0.07 | 0 | 0 | 0 | 0 | 15.43 ± 15.43 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Acp | 243.27 ± 9.36 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fl | 90.63 ± 15.35 | 47.61 ± 1.40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 51.10 ± 0.03 | 0 | 0 | 0 | 0 | 0 | 40.59 ± 9.73 | 0 |
| Phe | 1769.75 ± 48.50 | 203.05 ± 13.01 | 93.25 ± 2.78 | 65.94 ± 7.41 | 246.12 ± 4.64 | 88.79 ± 9.43 | 204.82 ± 0.8 | 0 | 0 | 0 | 0 | 0 | 144.29 ± 3.95 | 55.39 ± 5.03 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 67.02 ± 0.05 |
| Ant | 342.92 ± 18.79 | 21.48 ± 21.48 | 0 | 0 | 52.34 ± 0.0 | 0 | 39.19 ± 3.46 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Flu | 389.37 ± 34.35 | 52.58 ± 8.49 | 0 | 0 | 47.81 ± 0.13 | 0 | 37.80 ± 0.52 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Py | 427.46 ± 39.67 | 64.68 ± 4.88 | 0 | 0 | 63.36 ± 1.31 | 0 | 43.39 ± 2.71 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BaA * | 79.38 ± 14.58 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chr * | 245.80 ± 14.65 | 37.84 ± 2.98 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BbF * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BkF * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BaP * | 38.44 ±5.33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| InP * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 217.53 ± 28.40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DbA * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BghiP * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Σ16 EPA PAHs | 9858.24 | 1107.28 | 327.53 | 130.74 | 1256.96 | 216.92 | 729.87 | 0 | 0 | 332.75 | 0 | 65.78 | 186.15 | 55.39 | 51.10 | 0 | 0 | 0 | 0 | 0 | 40.59 | 437.89 |
| Σ8 EFSA PAHs * | 363.62 | 37.84 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 217.53 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TEQ † | 61.42 | 1.64 | 0.33 | 0.13 | 1.73 | 0.22 | 1.08 | 0.00 | 0.00 | 21.87 | 0.00 | 0.07 | 0.19 | 0.06 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, H.-J.; Kim, D.; Scheufele, F.B.; Ro, K.S.; Libra, J.A.; Marzban, N.; Chen, H.; Ribeiro, C.; Jeong, C. Occurrence of Polycyclic Aromatic Hydrocarbons (PAHs) in Pyrochar and Hydrochar during Thermal and Hydrothermal Processes. Agronomy 2024, 14, 2040. https://doi.org/10.3390/agronomy14092040
Jeon H-J, Kim D, Scheufele FB, Ro KS, Libra JA, Marzban N, Chen H, Ribeiro C, Jeong C. Occurrence of Polycyclic Aromatic Hydrocarbons (PAHs) in Pyrochar and Hydrochar during Thermal and Hydrothermal Processes. Agronomy. 2024; 14(9):2040. https://doi.org/10.3390/agronomy14092040
Chicago/Turabian StyleJeon, Hwang-Ju, Donghyeon Kim, Fabiano B. Scheufele, Kyoung S. Ro, Judy A. Libra, Nader Marzban, Huan Chen, Caroline Ribeiro, and Changyoon Jeong. 2024. "Occurrence of Polycyclic Aromatic Hydrocarbons (PAHs) in Pyrochar and Hydrochar during Thermal and Hydrothermal Processes" Agronomy 14, no. 9: 2040. https://doi.org/10.3390/agronomy14092040






