Abstract
Phaseolus vulgaris L. is a legume of high nutraceutical value, widely cultivated and consumed. However, common bean production faces challenges such as water stress that severely affects its growth and yield. This study evaluated the morphological and physiological response of four native P. vulgaris accessions subjected to different irrigation treatments under greenhouse conditions. A completely randomized design with factorial arrangement was used, evaluating three irrigation frequencies (100%, 50%, 25%) in combination with four accessions (PER1003541, PER1003542, PER1003543, PER1003544). The results showed that with the 25% irrigation treatment, PER1003544 showed a 54.62% decrease in leaf area, while PER1003542 and PER1003543 experienced reductions of 56.56% and 59.24%, respectively. In addition, accession PER1003544 reported a smaller reduction in the number of flowers and pods, with decreases of 40.21% and 29.9%, in contrast to PER1003543, which showed decreases of 60.66% and 52.63%, respectively. Accessions PER1003541 and PER1003544 also recorded the lowest reductions in dry biomass, with 31.85% and 35.41%, respectively. Regarding yield, PER1003544 and PER1003541 experienced reductions of 59.01% and 69.79%, respectively, unlike PER1003543, which showed a 90% decrease. In relation to stomatal density, PER1003541 recorded a reduction of 28.28%, while PER1003544 had a decrease of 37.10%, and PER1003543 experienced a reduction of 47.05%; chlorophyll content showed a similar trend. Finally, PER1003544 maintained a relatively stable stomatal index, with a reduction of 29.01%, compared to PER1003543, which reduced by 60.99%. In conclusion, accession PER1003544 stands out as a promising variety for breeding programs focused on water stress tolerance, contributing to food security and agricultural sustainability in areas affected by limited water availability. However, PER1003541 would be a suitable additional option, offering farmers flexibility in their crop selection according to the specific conditions of their environment.
1. Introduction
The common bean (Phaseolus vulgaris L.) is a legume of high nutraceutical value, widely cultivated and consumed worldwide. Its nutritional composition is remarkable, as it includes carbohydrates (50–60%), proteins (20–25%), minerals, B-complex vitamins, calories, and polyphenols, which contribute significantly to the strengthening of the immune system and a balanced diet [1,2,3]. In addition to its nutritional benefits, the common bean plays a crucial role in agricultural sustainability, fixing between 30 and 251 kg ha−1 of biological nitrogen through symbiosis with bacteria of the genus Rhizobium, which improves soil fertility and increases the productivity of associated crops [4,5,6].
However, common bean production faces numerous challenges, with water stress being one of the most significant [7]. Water stress, including drought and waterlogging, severely affects plant growth and yield [8]. Mladenov et al. [9] highlighted that water stress is one of the main limiting factors for bean production in many regions. To address this problem, several studies have investigated the response of different bean accessions to water stress conditions to identify more resistant varieties and improve crop management practices. Huerta-Lara et al. [10] conducted an investigation in which they evaluated the impact of water stress under rainfall conditions on four black bean accessions. Their results indicated that the accessions showed significant variations in their resistance to water stress, which is crucial for developing more resistant varieties. Similarly, Arruda et al. [11] investigated four common bean genotypes and found that water deficiency is a critical factor in reducing grain yield, highlighting the need to develop more tolerant genotypes.
Beans are highly vulnerable to water and heat stress, factors that often coincide during the most critical phenological stages of the plant. These stages, which include the onset of flowering, the beginning of pod growth, and bean filling in rainfed areas, are critical for yield formation. This type of abiotic stress causes a decrease in both yield and yield quality [12,13]. Recent research has consistently shown that beans are susceptible to abiotic stress conditions, such as water stress and waterlogging, resulting in significant reductions in yield and other physiological parameters. Geleta et al. [14] reported a yield decrease of up to 40.46% under waterlogging conditions and 71.91% under moisture deficit in five common bean varieties in a controlled environment, also observing more significant reductions in leaf fresh and dry weight and leaf area under waterlogging conditions, while shoot and root dry weight were more affected by water deficit. Similarly, Campos et al. [8] documented yield reductions of 36.81% and 63.56% in cultivars subjected to irrigations of 50% and 30% of crop evapotranspiration, respectively, although they found no significant differences in 100-seed weight and pod length.
In the Amazon region, the common bean is of considerable economic and social importance. Local research, such as that of Lápiz-Culqui et al. [15], has evaluated the physiological and morphological response of five bean accessions subjected to different levels of water deficit. The results showed that parameters such as leaf area, root length, and chlorophyll content are significantly affected by water stress, depending on the tolerance level of each genotype.
Climate change has exacerbated problems related to global water distribution, mainly affecting crops such as common beans, which depend on irrigation [16]. In this context, irrigation is fundamental for water security in agriculture, being crucial for a rational use of water to ensure productivity and reduce water and energy costs [17]. Therefore, it is essential to identify and conserve genetic resources that provide water stress tolerance mechanisms. Genetic conservation and the development of breeding programs are key strategies to ensure common bean sustainability and productivity under limited water availability.
In this context, the present research aims to evaluate the morphological and physiological response of four P. vulgaris accessions subjected to different irrigation treatments under greenhouse conditions. This study also studied the association between the traits studied to improve crop productivity and sustainability in contexts of limited water availability.
2. Materials and Methods
2.1. Area of Research
This research was carried out at the EEA-Amazonas of the National Institute for Agrarian Innovation (INIA) located at Fundo San Juan, longitude 77°52′21″ and latitude 06°13′45″ in the district of Chachapoyas, Chachapoyas province, Amazonas region, at an altitude of 2335 m.a.s.l. (Figure 1).
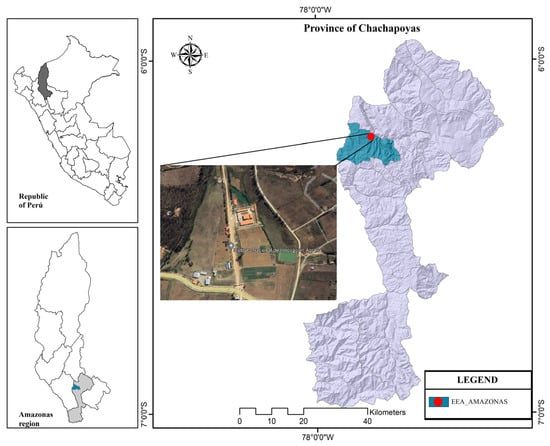
Figure 1.
Geographical location of the experimental area.
2.2. Seed Selection and Substrate Preparation
The seeds of the four accessions (Figure 2) originate from the provinces of Luya and Chachapoyas in the Amazon region. The seeds were selected based on their distinctiveness, homogeneity, and agro-morphological stability, which put them in high demand in local markets [18,19].

Figure 2.
P. vulgaris accessions: PER1003541(A), PER1003542 (B), PER1003543 (C), and PER1003544 (D).
The substrate was prepared using agricultural soil and pine needles in a 2:1 ratio and then disinfected by solarization. To determine its chemical characteristics, a representative sample was taken to the Soil and Water Research Laboratory, an accredited laboratory of the Universidad Nacional Toribio Rodríguez de Mendoza de Amazonas.
The results showed that the macronutrient levels were 17.54 ppm phosphorus (P), 350.97 ppm potassium (K), 3.20% carbon (C), and 0.28% nitrogen (N). Cation exchange capacity (CEC) was 10.40 meq/100 g, with exchangeable cation concentrations of 4.35 meq/100 g calcium (Ca2⁺), 1. 52 meq/100 g magnesium (Mg2⁺), 0.64 meq/100 g potassium (K⁺), 0.26 meq/100 g sodium (Na⁺) and 0.08 meq/100 g aluminum plus hydrogen (Al3⁺ + H⁺). Soil pH was 4.96, electrical conductivity (EC) was 0.41 dS/m, and organic matter (OM) content was recorded at 5.52%.
2.3. Experimental Design
A Completely Randomized Design (CRD) was used, with a factorial arrangement (4 × 3) having as factors the three irrigation treatments (A) and four bean accessions (B), totaling twelve interactions (Table 1). Each interaction had ten replications for a total of 120 experimental units. Each experimental unit was represented by a bag.

Table 1.
Factors and description of interactions.
2.4. Sowing of P. vulgaris Accessions and Establishment of Irrigation Frequency
Three bean seeds were sown per bag to ensure germination. After approximately 20 days, the seedlings were removed from each bag, leaving one plant per bag, taking into account the most vigorous and healthy plant. Irrigation was maintained at 100% of field capacity during this period (1 month) to allow vegetative development.
To determine the field capacity of the substrate, 10 × 5 kg bags were saturated with spring water using a known volume of water. Water evaporation was controlled by hermetically covering the top of the bags. After 48 h, the volume of water drained was measured. The difference between the volume applied and the volume drained made it possible to calculate the amount of water needed to reach 100%, 50%, and 25% of the field capacity of the substrate. This irrigation was carried out every seven days when the plants reached the permanent wilting point [20].
2.5. Installation of the Greenhouse Experiment
The experiment was installed on 29 September 2023. One hundred twenty bean plants were sown in 20 × 35 polyethylene bags in 5 kg of substrate, composed of agricultural soil and pine needles in a 2:1 proportion, in a greenhouse covered with anti-aphid mesh and a roof with an anti-UV film. Sowing was manual. The first seedlings germinated ten days after sowing, reaching 100% germination after 18 days; this was influenced by seed dormancy.
2.6. Evaluation of Morphological and Physiological Characteristics
2.6.1. Morphological Characteristics
Plant height: Plant height was measured from the base of the stem to the highest point of the plant. For each interaction, measurements were made on five plants using a tape measure. Data were collected at the end of the growing season.
Flower cluster/plant: These variables were evaluated at the flowering stage by counting the number of flower clusters generated on each of the five plants.
Pods/plant: The number of pods per plant was determined at the end of the crop cycle. The number of pods developed on each of the five selected plants per interaction was counted.
Seeds/pod: The number of seeds per pod was assessed at harvest time. Five pods were randomly selected from each of the five plants per interaction, and the number of seeds in each pod was counted.
Root length: Root length was measured at the end of the experiment. The plants were carefully dug up, and the roots were washed to remove any soil residue. Using a tape measure, the length of the main root was measured from the base of the stem to the furthest tip. These measurements were made on five plants per interaction.
Foliar area: Five plants were evaluated weekly for each interaction from the beginning of the experiment. Two leaves from each third (middle, apical, and basal) were selected and digitized in JPG format. Subsequently, the dimension was calculated using ImageJ v.1.48 software, and this value was multiplied by the total number of leaves to determine the total leaf area.
Fresh and dry biomass (foliage and root): Initially, whole plants were weighed to determine fresh biomass. The samples were then dried in an oven at 70 °C until a constant weight was reached, from which the dry biomass was determined.
Yield/plant: Yield was quantified by the total weight of seeds collected throughout the production cycle in each experimental unit. For this purpose, five plants were evaluated for each interaction.
2.6.2. Physiological Characteristics
Chlorophyll content (SPAD): Five plants were evaluated every two weeks for each treatment; evaluations were performed in the early morning hours (9–10 a.m.) using a chlorophyllometer (SPAD-502, Konica Minolta, Tokyo, Japan).
Stomata index: Observations were made during the flowering period of the accessions with the support of a stereoscope; the number of epidermal cells and the number of stomata were observed.
Stomatic density (mm2): A leaf was taken and placed under the stereoscope, and the number of stomata was counted in an area equivalent to the diameter of the observed field.
To determine the stomatal index and density, a uniform layer of glaze was applied to the lower surface of the leaf. After a few minutes, the enamel was carefully removed and fixed on a slide for subsequent examination under a microscope (Leica MC170 HD, Leica, Wetzlar, Germany) equipped with a digital camera. In each field of view, with an area of 4.5 mm2 (40× magnification), the number of stomata (NE) and epidermal cells (EC) were counted. The stomatal index (SI) was calculated using the formula of Xu et al. [21]: SI = [(NE/(EC + NE)] * 100, and stomatal density was expressed in stomata per mm2.
2.7. Data Analysis
The first step was calculating descriptive statistics for each variable according to the treatments. Subsequently, the normal distribution of the data was contrasted using the Shapiro–Wilk test. Data that did not follow a normal distribution were tested using the Kruskal–Wallis test with the subsequent Dunn’s test. All tests were performed with 95% confidence in the statistical program InfoStat professional version 2018. In addition, since the data did not show a normal distribution, a Spearman correlation analysis was applied to evaluate the relationship between morphological and physiological variables using the R programming language (version 4.3.3).
3. Results
3.1. Morphological Characteristics
Table 2 presents the morphological characteristics of four P. vulgaris accessions under different irrigation treatments. The results show significant differences between accessions and irrigation treatments.

Table 2.
Descriptive statistics of morphological characteristics of four P. vulgaris accessions (mean ± standard deviation).
Regarding plant height, accession PER1003544 (B4) reached the greatest height (45.03 cm), significantly exceeding the other accessions, especially under full irrigation (A1), where it reached 62.74 cm. However, reducing irrigation to 25% (A3) resulted in a remarkable 50.71% decrease in B4 height, showing some sensitivity to drought water stress. Despite this reduction, B4 remained the accession with the greatest height under the most severe stress conditions.
The number of flowers per plant also decreased significantly with reduced irrigation in all accessions. Accession B4 showed the highest number of flowers (15.20 flowers/plant), maintaining higher flower production even under 50% (A2) and 25% (A3) irrigation conditions, with reductions of 24.74% and 40.21%, respectively, compared to full irrigation. This contrasts with accession PER1003543 (B3), which showed the lowest number of flowers under all irrigation treatments. However, flower reduction with 25% irrigation treatment was around 60% for all accessions.
The number of pods per plant was significantly higher in B4 at 100% irrigation (19.40 pods/plant) and decreased by 10.31% and 29.9% under 50% and 25% irrigation, respectively. B3, in comparison, showed a more drastic decrease of 52.63% when irrigation was reduced to 25%, highlighting the superiority of B4 under stress conditions.
Accession 4 had the highest number of seeds per pod, followed closely by accession 1. Under 25% irrigation conditions, accessions 1 and 4 showed the least reduction, with 33.33%. Accession 3 experienced the greatest reduction, with 58.82%.
Regarding root length, B4 presented the longest roots (87.02 cm), suggesting a better adaptation to water stress. Although reducing irrigation to 25% decreased root length by 43.36%, B4 maintained significantly longer roots than other accessions. On the other hand, B2 experienced the least reduction, 38.05%, under 25% irrigation conditions.
Finally, leaf area, a critical indicator of photosynthetic capacity, significantly decreased with decreasing irrigation in all accessions. B4, with a leaf area of 189.58 cm2 under full irrigation, suffered a reduction of 54.62% when irrigation was reduced to 25%, in contrast to B3, which reduced 59.24%.
Figure 3 shows the fresh biomass produced by the different P. vulgaris accessions under the different irrigation treatments. It was observed that the 100% irrigation treatment generated the highest values of fresh biomass in all accessions, with a progressive decrease as irrigation was reduced. Accessions PER1003541 (B1) and PER1003544 (B4) maintained higher relative fresh biomass under severe water stress conditions (25% irrigation), showing reductions of 31.42% and 35.4%, respectively, compared to their yield under full irrigation, while other accessions, such as PER1003543 (B3), experienced a much steeper decrease with 66.97%.
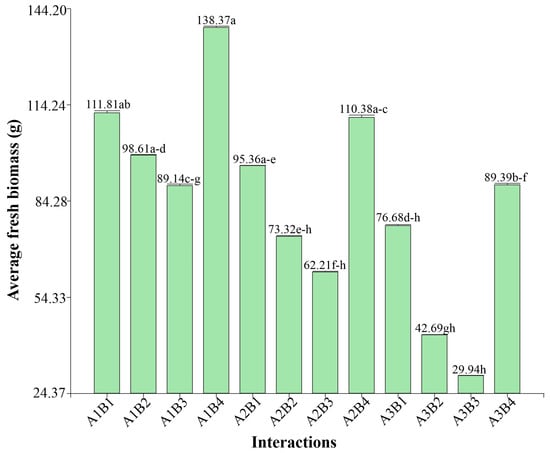
Figure 3.
Fresh biomass by interactions, different letters between bars indicate significant differences (Kruskal–Wallis test, p ≤ 0.05).
Figure 4 shows the dry biomass for each combination of accession and irrigation treatment. As with fresh biomass, dry biomass was significantly higher under fully irrigated conditions, decreasing as water availability decreased. Accessions B1 and B4 stood out for their ability to maintain relatively high dry biomass even with reduced irrigation, showing decreases of 31.85% and 35.41%, respectively, under the 25% irrigation treatment, in contrast to accession B3, which showed a much steeper reduction of 66.73%.
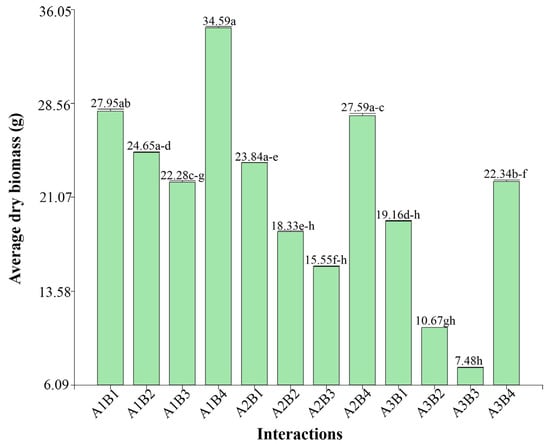
Figure 4.
Dry biomass by interactions, different letters between bars indicate significant differences (Kruskal–Wallis test, p ≤ 0.05).
Figure 5 presents the yield per plant of the accessions under different irrigation levels. The analysis reveals that reduced irrigation significantly affected yield in all accessions, although the magnitude of this decrease varied significantly. Accessions B4 and B1 showed greater resilience, with reductions of 59.01% and 69.79%, respectively, when irrigation was reduced to 25%. In contrast, other accessions, such as B3, experienced more than 90% reductions, underlining a lower tolerance to drought water stress.
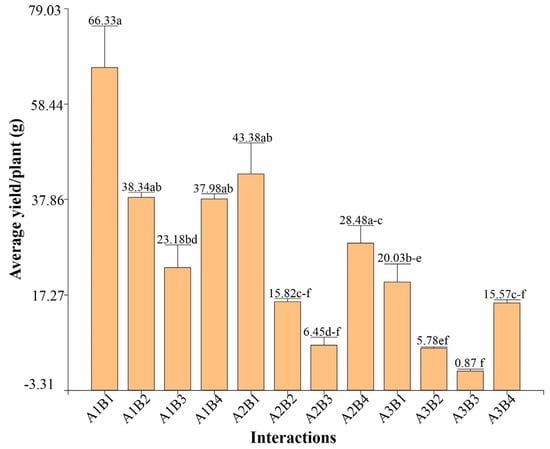
Figure 5.
Yield by interactions, different letters between bars indicate significant differences (Kruskal–Wallis test, p ≤ 0.05).
3.2. Physiological Characteristics
Table 3 details the physiological characteristics of the accessions under different irrigation regimes. Chlorophyll content was significantly higher in accessions B1 and B4, reaching 43.71 and 51.90 SPAD, respectively, suggesting higher photosynthetic efficiency. Despite the reduction of irrigation to 25%, chlorophyll content in B1 decreased by 28.28% and in B4 by 37.10%, remaining above other accessions such as B3, which showed a reduction of 47.05%.

Table 3.
Descriptive statistics of physiological characteristics of P. vulgaris accessions (mean ± standard deviation).
The stomatal index, an indicator of the plant’s ability to regulate its water balance, was also higher in B4 (46.04). Although under 25% irrigation conditions, this value decreased by 29.01%, A4 maintained its advantage over accessions such as B3, which showed a reduction of 60.99% with the same irrigation treatment.
Stomatic density followed a similar pattern, with B4 registering the highest density (10.13 mm2). Reducing irrigation to 25% caused a 40.09% decrease in the stomatal density of B4, although this value continued to be higher than that of other accessions, such as B3, which experienced a 66.92% decrease.
3.3. Correlation of Morphological and Physiological Variables
Figure 6 presents a Spearman correlation analysis between several morphological and physiological characteristics in P. vulgaris under different irrigation treatments. Among the most outstanding observations, a strong positive correlation between root length and leaf area (r = 0.95) and plant height (r = 0.91) can be noted. Likewise, a strong association was observed between yield and root length (r = 0.9), fresh biomass (r = 0.9), and dry biomass (r = 0.9). Chlorophyll content also significantly correlated with dry biomass (r = 0.94). In addition, a significant positive correlation was identified between stomatal density and stomatal index (r = 0.94). A correlation was also observed between plant height and dry biomass (r = 0.92) and yield (r = 0.86).
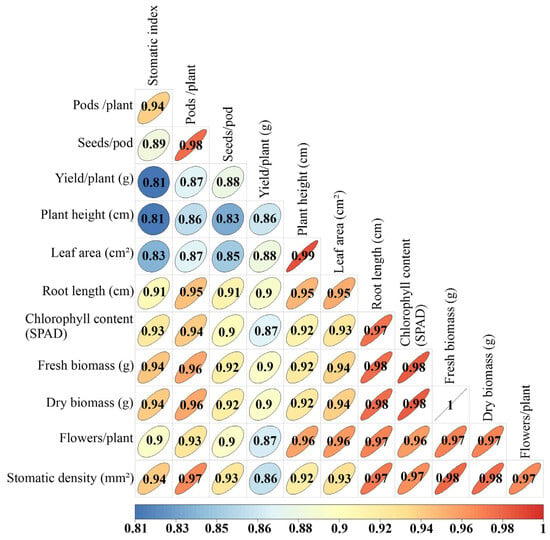
Figure 6.
Spearman’s correlation of morphological and physiological variables of P. vulgaris accessions.
4. Discussion
Plant height is a key indicator of overall health and vigor; our results showed highly significant correlations of this variable with dry biomass and yield, which is consistent with other research indicating that plant height is an indicator that can be used to predict advanced traits such as biomass and yield of a crop [22]. Accession B4 (PER1003544), which reached its greatest height with full irrigation, experienced a significant reduction of 50.71% when 25% irrigation was applied. Although this decrease is considerable, it is less pronounced than other accessions, suggesting that B4, despite the water deficit, maintains relatively robust growth even under adverse conditions. This capacity could be linked to a more extensive and efficient root system, which allows it to access and use available water resources more effectively [23]. Several authors agree that the correlation between plant growth and water availability is clear, as cell enlargement is more sensitive to water deficits than cell division, which inhibits growth due to reduced cell wall extensibility and turgor [24,25].
The reduction in the number of flowers per plant under limited irrigation highlights the sensitivity of reproductive processes to water availability. Despite this, accession B4 (PER1003544) showed the highest flower production at all irrigation levels, suggesting the presence of drought tolerance mechanisms that favor reproductive success even under water stress conditions [26]. This finding is consistent with previous studies indicating that certain bean varieties can maintain reproductive output despite decreased water availability [27,28], possibly due to increased water use efficiency and osmotic adjustment, key factors to ensure survival and reproductive success in dry environments [29].
The number of pods per plant was significantly influenced by irrigation treatments in all accessions evaluated. Particularly, accession 4 (PER1003544) presented a 10.31% decrease in pod number when 50% irrigation was applied. This behavior is congruent with the findings of Guerrero-Domínguez et al. [30], who reported an 11.11% reduction in the number of pods per plant in the black bean variety Triunfo 70 under a similar irrigation regime. The consistency among these studies reinforces the notion of Sosiawan et al. [31], who point out that, during the pod formation phase, water stress affects not only pod quantity but also pod filling, which could have direct implications on seed weight and quality. This suggests that irrigation strategies should be optimized to ensure adequate pod formation and development, especially in accessions that, like PER1003544, show a relative tolerance to water deficit.
Like the number of pods, the number of seeds per pod is a crucial factor that directly impacts final yield, and both are closely interrelated. For this reason, the number of seeds per pod is commonly used as a selection criterion in the genetic improvement processes of P. vulgaris [32]. In this study, it was observed that under conditions of reduced irrigation to 25%, accessions 1 (PER1003541) and 4 (PER1003544) showed a more moderate decrease in the number of seeds per pod, with a reduction of 33.33%. This relative resistance to drought water stress highlights the ability of these accessions to maintain a more stable yield even under adverse conditions, in contrast to accession 3 (PER1003543), which experienced the greatest decrease in the number of seeds per pod (58.82%). These results agree with the findings of Rai et al. [33], who reported reductions of up to 30% in the number of seeds per pod in the dry bean variety cv. Othello under arid field conditions and 25% irrigation. This suggests that, although water stress inevitably affects seed formation, some accessions possess mechanisms to mitigate this impact.
The greater root length observed in accession PER1003544 (B4) under all irrigation conditions, especially under 25% irrigation, suggests a superior water exploration and absorption capacity compared to the other accessions. This characteristic is essential to face drought conditions since it allows the plant to access deeper water sources, which can be crucial in water deficit situations [34]. In addition, the extensive root length in B4 could be related to greater water use efficiency, as longer and deeper roots not only allow water absorption in deeper soil layers but may also facilitate nutrient uptake, which contributes to more sustained and robust growth under drought water stress conditions [35].
It is important to highlight the correlations between root length and leaf area, as greater leaf area is generally associated with higher photosynthetic potential, which could contribute to maintaining biomass production even under limited irrigation conditions [36]. Therefore, accession B4 shows a structural adaptation through a more developed root system and maintains a functional balance between water uptake and maintenance of photosynthetic activity, strengthening its resistance to water stress.
Leaf Area
The highest values of leaf area were reached with full irrigation doses. However, it is interesting to note that B4(PER1003544), even under reduced irrigation conditions, shows a leaf area (187.65 ± 0.35 cm2) comparable to full irrigation treatments, which underlines the adaptability of accession 4 under water stress conditions. This behavior is consistent with research suggesting that certain bean varieties can adapt their morphology to optimize water use while maintaining sufficient leaf area for photosynthesis and growth [37]. The ability of some accessions, such as accession 4, to maintain considerable leaf area under reduced irrigation suggests their potential for cultivation in water-scarce regions. Selection of accessions with favorable and adaptable morphological characteristics could improve the resilience of agricultural systems in the face of climate change and water scarcity. Furthermore, these findings can guide irrigation management strategies, optimizing water use without significantly compromising plant growth and development [38].
The results reflect how water availability directly influences fresh biomass production in the different P. vulgaris accessions. In particular, accessions PER1003541 (B1) and PER1003544 (B4) showed a remarkable ability to maintain relatively high biomass levels even under severe water stress conditions. This behavior suggests the presence of intrinsic adaptations that allow them to optimize water and nutrient use, directly impacting fresh biomass production [39]. Such adaptations could include morphological modifications, such as increased efficiency in the root system, or physiological responses that facilitate the conservation and efficient use of available water and nutrients [23,29]. Previous research, such as that of Teshome et al. [40], also showed that there are bean varieties that can produce optimal fresh biomass levels under reduced irrigation conditions and be candidates for areas affected by water scarcity or for programs aimed at conserving water sources. Identifying accessions such as B1 and B4 opens opportunities for developing more resilient cultivars that can sustain satisfactory yields under adverse conditions, thus contributing to food security.
Like fresh biomass, dry biomass was also reduced in treatments with lower irrigation rates. However, it is propitious to note that accession 4 (PER1003544), with 50% reduced irrigation, had a dry biomass comparable to the fully irrigated treatments, indicating the ability of accession 4 to maintain a robust yield under water stress conditions. This is consistent with research results that have reported the resilience of certain bean varieties to reduced water supply while maintaining adequate dry biomass production [41]. The ability of these accessions to maintain dry biomass under limited irrigation conditions suggests efficient adaptation to water stress, possibly through mechanisms such as stomatal closure or increased water use efficiency [42].
Although reduced irrigation negatively impacted yield in all P. vulgaris accessions, accessions 1 (PER1003541) and 4 (PER1003544) stood out for their ability to maintain considerable yield even under water stress conditions with only 50% irrigation. This finding is consistent with previous research showing that certain bean varieties can sustain acceptable yield levels under moderate water stress [38,43].
This ability to maintain yield under suboptimal conditions suggests the presence of physiological and biochemical mechanisms that allow more efficient use of water at the cellular level, an osmotic adjustment that helps maintain cell turgor, or even a reconfiguration of growth patterns that favor the allocation of resources toward seed and fruit production rather than vegetative growth [44]. In addition, the maintenance of yield under water stress conditions in these accessions could be related to a higher efficiency in photosynthesis and a better capacity to maintain stomatal aperture under conditions of low water availability, which optimizes carbon assimilation [45].
Evidence suggests that adequate irrigation is critical for chlorophyll synthesis, a key component for photosynthesis and, ultimately, for plant productivity. Several studies have shown that water stress can significantly reduce plant photosynthetic capacity by decreasing chlorophyll content [15,46,47]. In this context, accessions PER1003544(B4) and PER1003541(B1) stood out for their higher chlorophyll content, indicating higher photosynthetic efficiency, even under limited irrigation. The lower reduction in chlorophyll content observed in these accessions under 25% irrigation conditions suggests the existence of adaptive mechanisms that allow them to maintain their photosynthetic activity [48,49]. On the other hand, accession PER1003543 (B3) recorded the greatest decrease in chlorophyll content under reduced irrigation, which correlates with its lower dry biomass production and final yield, thus highlighting the importance of chlorophyll content as an indicator of tolerance to water stress.
The variation in stomatal index observed among the accessions studied reflects the genetic diversity in the water regulation capacity of P. vulgaris. Accession PER1003544 (B4), which maintained a significantly elevated stomatal index even under reduced irrigation conditions, shows a remarkable ability to adapt to water stress. The lower decrease in this index in B4, compared to other accessions such as B3, suggests a potential adaptive advantage in terms of water use efficiency and gas exchange [50]. On the other hand, PER1003543 (B3) presented the lowest stomatal index, which could restrict the ability of plants to regulate their temperature and absorb CO2, negatively affecting their growth [51].
The reduction in stomatal density observed in all accessions under limited irrigation conditions is consistent with the general tendency of plants to minimize water loss during periods of water stress. This adjustment in stomatal density and the variation among accessions underscores the genetic diversity present in P. vulgaris regarding adaptation to water stress [41,52]. It is particularly notable that accession B4, despite the significant 40.09% reduction in stomatal density under 25% irrigation, maintained higher values compared to other accessions. This could suggest that B4 possesses an optimal balance between water use efficiency and gas exchange capacity, allowing it to maintain higher photosynthetic activity under stress conditions.
On the other hand, the greater decrease in stomatal density observed in B3 could indicate a different strategy aimed at greater water conservation, although at the possible cost of a lower photosynthetic capacity. This diversity in stomatal responses suggests that accessions may employ different strategies to cope with water stress, which is crucial for breeding programs seeking to develop more resilient cultivars [48,53]. Previous studies have highlighted the importance of stomatal traits as key indicators of drought resistance in legume crops [43,54].
5. Conclusions
Accession PER1003544 (B4) showed superior adaptability to water stress, maintaining height, flower number, and yield under reduced irrigation, compared to other accessions such as PER1003543 (B3). B4 also showed higher chlorophyll content, stomatal index, and stomatal density, highlighting its adaptability. These results are fundamental to improving and managing the cultivation of P. vulgaris in regions with variable water availability and provide valuable information for breeding programs and agronomic strategies aimed at increasing crop resistance and productivity under water scarcity conditions, thus increasing food security and production stability under climate variability scenarios.
Future research could focus on identifying the physiological and molecular mechanisms that confer to B4 its adaptability to reduced irrigation conditions. It would also be valuable to study the interaction of this accession with factors such as temperature and salinity to develop more resilient breeding strategies. Finally, comparative genomic studies between accessions that show tolerance to water stress and those that do not would allow the identification of genetic markers associated with resilience, thus ensuring a higher success rate in P. vulgaris breeding programs.
Author Contributions
A.S.H.: conceptualization, validation, resources, and writing—original draft preparation; J.J.T.-A.: conceptualization, formal analysis, and writing—original draft preparation; L.G.: conceptualization, supervision, and writing—original draft preparation; J.V.-G.: validation, supervision, and writing—original draft preparation; E.F.: methodology, data curation, and writing—review and editing; N.C.V.-V.: investigation, data curation, and writing—review and editing; M.O.-C.: investigation, formal analysis, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the technical and financial support of CUI project N° 2480490 “Mejoramiento de los Servicios de Investigación en la Caracterización de los Recursos Genéticos de la Agrobiodiversidad en 17 Departamentos del Perú (PROAGROBÍO)”, executed by the Instituto Nacional de Innovación Agraria (INIA) and to CUI Project N° 2590588 “Mejoramiento del Servicio de Promoción de la Ciencia, Tecnología e Innovación para el Centro de Investigación en Granos y Semillas de la UNTRM”—CEIGRAS. In addition, they thank the Vice Rectorate of Research of the National University Toribio Rodríguez de Mendoza of Amazonas.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Blair, M.W.; Li, H.; Nekkalapudi, L.; Becerra, V.; Paredes, M. Nutritional Traits of Beans (Phaseolus vulgaris): Nutraceutical Characterization and Genomics. In Compendium of Crop Genome Designing for Nutraceuticals; Kole, C., Ed.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Jannat, S.; Shah, A.H.; Sabir, S.M. Nutraceutical characterization of common bean (Phaseolus vulgaris L.) germplasm from Pakistan. Int. Food Res. J. 2019, 26, 1835–1843. [Google Scholar]
- Ramírez-Jiménez, A.K.; Reynoso-Camacho, R.; Tejero, M.E.; León-Galván, F. Potential role of bioactive compounds of Phaseolus vulgaris L. on lipid-lowering mechanisms. Food Res. Int. 2015, 76, 92–104. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Al-Amri, S.M.; Elsadek, A.W. Enhancing Rhizobium–Legume Symbiosis and Reducing Nitrogen Fertilizer Use Are Potential Options for Mitigating Climate Change. Agriculture 2023, 13, 2092. [Google Scholar] [CrossRef]
- Lirio-Paredes, J.; Ogata-Gutiérrez, K.; Zúñiga-Dávila, D. Effects of Rhizobia Isolated from Coffee Fields in the High Jungle Peruvian Region, Tested on Phaseolus vulgaris L. var. Red Kidney. Microorganisms 2022, 10, 823. [Google Scholar] [CrossRef]
- Goyal, R.K.; Mattoo, A.K.; Schmidt, M.A. Rhizobial–Host Interactions and Symbiotic Nitrogen Fixation in Legume Crops Toward Agriculture Sustainability. Front. Microbiol. 2021, 12, 669404. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.J.; Creamer, B. Major constraints and trends for common bean production and commercialization; establishing priorities for future research. Agron. Colomb. 2014, 32, 423–431. [Google Scholar] [CrossRef]
- Campos, K.; Schwember, A.R.; Machado, D.; Ozores-Hampton, M.; Gil, P.M. Physiological and Yield Responses of Green-Shelled Beans (Phaseolus vulgaris L.) Grown under Restricted Irrigation. Agronomy 2021, 11, 562. [Google Scholar] [CrossRef]
- Mladenov, P.; Aziz, S.; Topalova, E.; Renaut, J.; Planchon, S.; Raina, A.; Tomlekova, N. Physiological Responses of Common Bean Genotypes to Drought Stress. Agronomy 2023, 13, 1022. [Google Scholar] [CrossRef]
- Huerta-Lara, M.; Reyes-López, D.; Bautista-Calles, J.; Hernández-Zepeda, J.S.; Parraguirre-Lezama, J.F.C.; Romero-Arenas, O. Survival and yield of bean varieties with horizontal resistance to water stress in the Sierra Nororiental de Puebla. Nova Sci. 2021, 13, 1–21. [Google Scholar] [CrossRef]
- Arruda, I.M.; Moda-Cirino, V.; Koltun, A.; dos Santos, O.J.A.P.; Moreira, R.S.; Moreira, A.F.P.; Gonçalves, L.S.A. Physiological, biochemical and morphoagronomic characterization of drought-tolerant and drought-sensitive bean genotypes under water stress. Physiol. Mol. Biol. Plants 2018, 24, 1059–1067. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research Progress and Perspective on Drought Stress in Legumes: A Review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef]
- Farooq, M.; Nadeem, F.; Gogoi, N.; Ullah, A.; Alghamdi, S.S.; Nayyar, H.; Siddique, K.H.M. Heat stress in grain legumes during reproductive and grain-filling phases. Crop Pasture Sci. 2017, 68, 985–1005. [Google Scholar] [CrossRef]
- Geleta, R.J.; Roro, A.G.; Terfa, M.T. Phenotypic and yield responses of common bean (Phaseolus vulgaris L.) varieties to different soil moisture levels. BMC Plant Biol. 2024, 24, 242. [Google Scholar] [CrossRef]
- Lapiz-Culqui, Y.K.; Neri, J.C.; Vilca-Valqui, N.C.; Meléndez-Mori, J.B.; Huaman-Huaman, E.; Oliva, M. Efecto del estrés hídrico sobre el comportamiento morfo-fisiológico de cinco genotipos de frijol común (Phaseolus vulgaris L.). Rev. Científica Pakamuros 2021, 9, 73–86. [Google Scholar] [CrossRef]
- Martínez-Barradas, V.; Inostroza-Blancheteau, C.; Tighe-Neira, R.; Romero-Romero, J.L.; Schwember, A.R.; Arce-Johnson, P. Drought Tolerance Evaluation of ‘Zorzal’, the Most Cultivated Common Bean in Chile, a Country Facing Desertification. Agric. Res. 2024, 13, 41–52. [Google Scholar] [CrossRef]
- Santos, R.M.; Sanchéz-Román, R.M.; Grassi Filho, H.; Silva, V.M.; Pereira, A.d.J. Coeficiente de produtividade—Ky do feijão carioca (Phaseolus vulgaris L. TAA DAMA.) Para o Município de Botucatu-sp. Irriga 2022, 27, 785–794. [Google Scholar] [CrossRef]
- Vásquez, J.; Vilca-Valqui, N.C.; Malqui, R.; Fernpandez, E.; Duarez, E.; Ayala, R. Caracterización agromorfológica de accesiones de Phaseolus spp., en la región Amazonas, Perú. Bioagro 2024, 36, 129–142. [Google Scholar] [CrossRef]
- Vásquez-García, J.; Vilca-Valqui, N.C.; Malqui-Ramos, R.F. Catálogo de Frijol en Regiones Andinas del Banco de Germoplasma del INIA, Primera ed.; Instituto Nacional de Innovación Agraria-INIA: Lima, Perú, 2023. [Google Scholar]
- Vanderlinden, K.; Giráldez, J.V. Field Water Capacity. In Encyclopedia of Agrophysics; Gliński, J., Horabik, J., Lipiec, J., Eds.; Encyclopedia of Earth Sciences Series; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Zhou, G.S.; Shimizu, H. Effects of Soil Drought with Nocturnal Warming on Leaf Stomatal Traits and Mesophyll Cell Ultrastructure of a Perennial Grass. Crop Sci. 2009, 49, 1843–1851. [Google Scholar] [CrossRef]
- Wang, N.; Li, Q.; Liu, X.; Yi, S.; Zhao, M.; Sun, X.; Song, H.; Peng, X.; Fan, P.; Gao, Q.; et al. Plant Size Plays an Important Role in Plant Responses to Low Water Availability and Defoliation in Two Woody Leguminosae Species. Front. Plant Sci. 2021, 12, 643143. [Google Scholar] [CrossRef]
- Kou, X.; Han, W.; Kang, J. Responses of root system architecture to water stress at multiple levels: A meta-analysis of trials under controlled conditions. Front. Plant Sci. 2022, 13, 1085409. [Google Scholar] [CrossRef]
- Humplík, J.F.; Bergougnoux, V.; Van Volkenburgh, E. To stimulate or inhibit? That is the question for the function of abscisic acid. Trends Plant Sci. 2017, 22, 830–841. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Refay, Y.; Hafez, E.M. Integrative Effects of Rice-Straw Biochar and Silicon on Oil and Seed Quality, Yield and Physiological Traits of Helianthus annuus L. Grown under Water Deficit Stress. Agronomy 2019, 9, 637. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Otoro, G.G.; Hatiye, S.D. Field evaluation of haricot bean (Phaseolus vulgaris L.) response to supplemental irrigation in Arba Minch area: Southern Ethiopia. Irrig. Drain. 2024, 73, 470–485. [Google Scholar] [CrossRef]
- Sofi, P.A.; Djanaguiraman, M.; Siddique, K.H.M.; Prasad, P.V.V. Reproductive fitness in common bean (Phaseolus vulgaris L.) under drought stress is associated with root length and volume. Ind. J. Plant Physiol. 2018, 23, 796–809. [Google Scholar] [CrossRef]
- López-Galiano, M.J.; García-Robles, I.; González-Hernández, A.I.; Camañes, G.; Vicedo, B.; Real, M.D.; Rausell, C. Expression of miR159 Is Altered in Tomato Plants Undergoing Drought Stress. Plants 2019, 8, 201. [Google Scholar] [CrossRef]
- Guerrero-Domínguez, L.; González-Pérez, B.L.; Jerez-Mompie, E.I.; Morales-Guevara, D.; Amico-Rodríguez, J.D.; Cruz-Suárez, A.S. Behavior of Common Bean Plants (Phaseolus vulgaris L.) Subjected to Two Irrigation Systems. Rev. Cienc. Técnicas Agropecu. 2023, 32, e06. [Google Scholar]
- Sosiawan, H.; Adi, S.H.; Yusuf, W.A. Water-saving irrigation management for mung bean in acid soil. IOP Conf. Ser. Earth Environ. Sci. 2021, 648, 012144. [Google Scholar] [CrossRef]
- Contreras-Rojas, M.; Guerra Guzmán, D.G.; Salazar Mercado, S.A.; Salazar-Villareal, F.A. Path analysis of yield and yield components in snap bean (Phaseolus vulgaris L.) genotypes. Euphytica 2024, 220, 36. [Google Scholar] [CrossRef]
- Rai, A.; Sharma, V.; Heitholt, J. Dry Bean [Phaseolus vulgaris L.] Growth and Yield Response to Variable Irrigation in the Arid to Semi-Arid Climate. Sustainability 2020, 12, 3851. [Google Scholar] [CrossRef]
- Kalra, A.; Goel, S.; Elias, A.A. Understanding role of roots in plant response to drought: Way forward to climate-resilient crops. Plant Genome 2023, 17, e20395. [Google Scholar] [CrossRef] [PubMed]
- Paez-Garcia, A.; Motes, C.M.; Scheible, R.; Chen, R.; Blancaflor, E.B.; Monteros, M.J. Root Traits and Phenotyping Strategies for Plant Improvement. Plants 2015, 4, 334–355. [Google Scholar] [CrossRef] [PubMed]
- Baslam, M.; Mitsui, T.; Hodges, M.; Priesack, E.; Herritt, M.T.; Aranjuelo, I.; Sanz-Sáez, A. Photosynthesis in a Changing Global Climate: Scaling Up and Scaling Down in Crops. Front. Plant Sci. 2020, 11, 515969. [Google Scholar] [CrossRef] [PubMed]
- Porch, T.G.; Beaver, J.S.; Debouck, D.G.; Jackson, S.A.; Kelly, J.D.; Dempewolf, H. Use of Wild Relatives and Closely Related Species to Adapt Common Bean to Climate Change. Agronomy 2013, 3, 433–461. [Google Scholar] [CrossRef]
- Beebe, S.; Rao, I.; Blair, M.; Acosta, J. Phenotyping common beans for adaptation to drought. Front. Physiol. 2013, 4, 28034. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, R.M.; Simister, R.; Roberts, L.A.; Cambler, A.B.; Corke, F.M.; Han, J.; Ward, R.J.; Buckeridge, M.S.; Gomez, L.D.; Bosch, M. Nutrient and drought stress: Implications for phenology and biomass quality in miscanthus. Ann. Bot. 2019, 124, 553–566. [Google Scholar] [CrossRef]
- Teshome, F.T.; Bayabil, H.K.; Schaffer, B.; Ampatzidis, Y.; Hoogenboom, G.; Singh, A. Exploring deficit irrigation as a water conservation strategy: Insights from field experiments and model simulation. Agric. Water Manag. 2023, 289, 108490. [Google Scholar] [CrossRef]
- Polania, J.A.; Salazar-Chavarría, V.; Gonzalez-Lemes, I.; Acosta-Maspons, A.; Chater, C.C.C.; Covarrubias, A.A. Contrasting Phaseolus Crop Water Use Patterns and Stomatal Dynamics in Response to Terminal Drought. Front. Plant Sci. 2022, 13, 894657. [Google Scholar] [CrossRef]
- Ullah, H.; Santiago-Arenas, R.; Ferdous, Z.; Attia, A.; Datta, A. Improving water use efficiency, nitrogen use efficiency, and radiation use efficiency in field crops under drought stress: A review. Adv. Agron. 2019, 156, 109–157. [Google Scholar] [CrossRef]
- Alemu, M.M.; Gedebo, A.; Roro, A.G.; Geletu, T.T. Effect of Moisture Stress on Physiological and Yield Responses of Common Bean Varieties at Lath House Condition, Hawassa University, Southern Ethiopia. Int. J. Agron. 2023, 1, 2626225. [Google Scholar] [CrossRef]
- Lanna, A.C.; Taeko, S.; Rios, T.G.; Pereira, R.; Figueiredo, M.A. Physiological characterization of common bean (Phaseolus vulgaris L.) genotypes, waterstress induced with contrasting response towards drought. Aust. J. Crop Sci. 2016, 10, 1–6. [Google Scholar]
- Suárez, J.C.; Urban, M.O.; Contreras, A.T.; Noriega, J.E.; Deva, C.; Beebe, S.E.; Polanía, J.A.; Casanoves, F.; Rao, I.M. Water Use, Leaf Cooling and Carbon Assimilation Efficiency of Heat Resistant Common Beans Evaluated in Western Amazonia. Front. Plant Sci. 2021, 12, 644010. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, C.; Pan, D.; Zhang, Y.; Luo, B.; Ji, J. Effects of drought stress on photosynthesis and chlorophyll fluorescence images of soybean (Glycine max) seedlings. Int. J. Agric. Biol. Eng. 2018, 11, 196–201. [Google Scholar] [CrossRef]
- Sharifi, P.; Mohammadkhani, N. Effects of Drought Stress on Photosynthesis Factors in Wheat Genotypes during Anthesis. Cereal Res. Commun. 2016, 44, 229–239. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef]
- Mutari, B.; Sibiya, J.; Matova, P.M.; Gasura, E.; Simango, K. Drought stress impact on agronomic, shoot, physiological, canning and nutritional quality traits of navy beans (Phaseolus vulgaris L.) under field conditions in Zimbabwe. Field Crops Res. 2023, 292, 108826. [Google Scholar] [CrossRef]
- Lv, X.; Li, Y.; Chen, R.; Rui, M.; Wang, Y. Stomatal Responses of Two Drought-Tolerant Barley Varieties with Different ROS Regulation Strategies under Drought Conditions. Antioxidants 2023, 12, 790. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Zhou, M.; Shabala, S. How Does Stomatal Density and Residual Transpiration Contribute to Osmotic Stress Tolerance? Plants 2023, 12, 494. [Google Scholar] [CrossRef]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of Stomatal Density and Morphology on Water-Use Efficiency in a Changing World. Front. Plant Sci. 2019, 10, 427588. [Google Scholar] [CrossRef]
- Alomari-Mheidat, M.; Martín-Palomo, M.J.; Castro-Valdecantos, P.; Medina-Zurita, N.; Moriana, A.; Corell, M. Effect of Water Stress on the Yield of Indeterminate-Growth Green Bean Cultivars (Phaseolus vulgaris L.) during the Autumn Cycle in Southern Spain. Agriculture 2023, 13, 46. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).