Potential Impact of Drought and Rewatering on Plant Physiology and Fruit Quality in Long-Shelf-Life Tomatoes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Open-Field Experiment
2.2. Measurements in Field
2.3. Physiological Measurements
2.4. Laboratory Analyses
2.4.1. Total Solids (TS) and Total Soluble Solids (SS)
2.4.2. Reducing Sugars (RS)
2.4.3. Vitamin C (AscA)
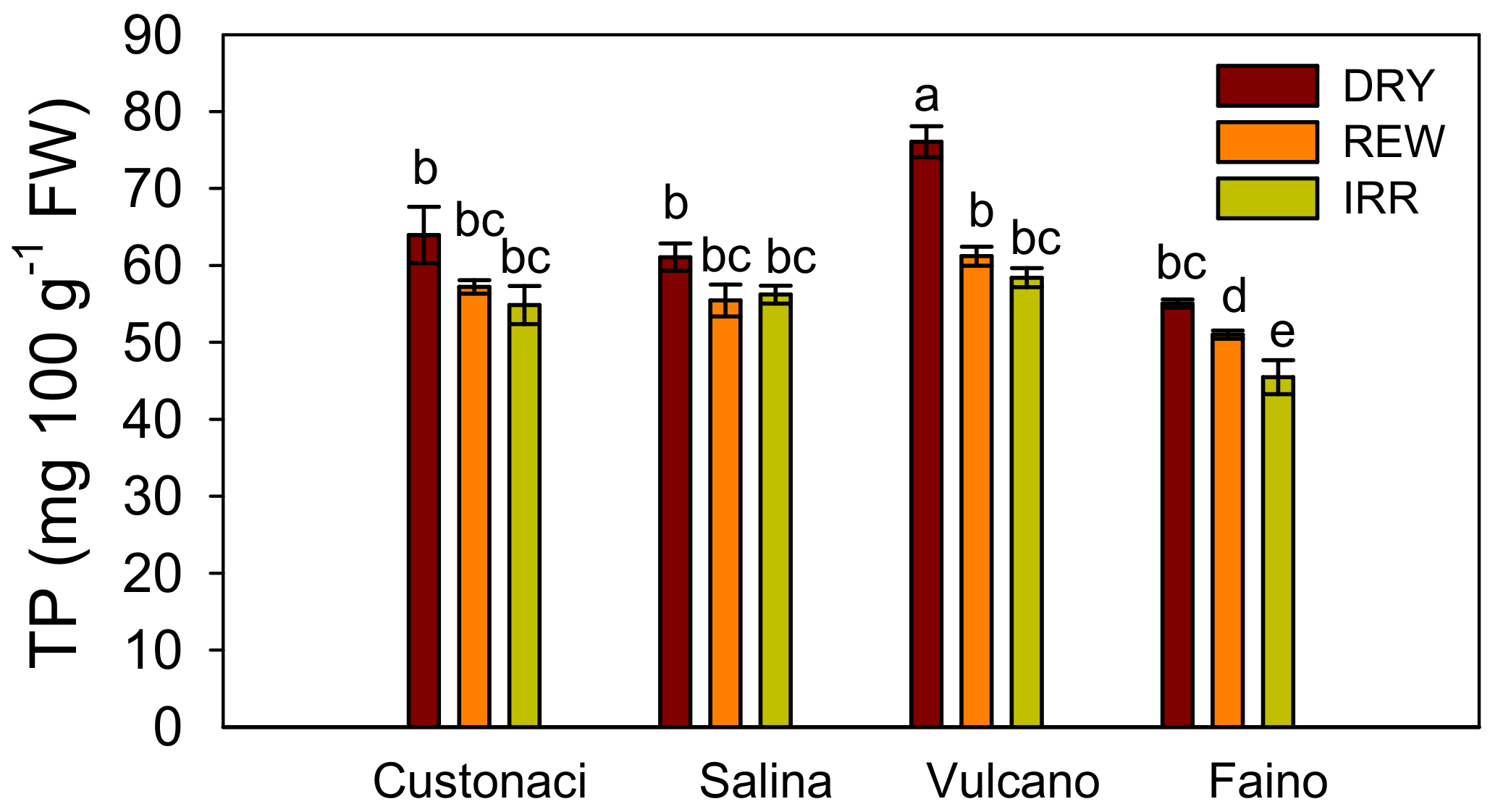
2.4.4. Total Phenols (TP)
2.5. Statistical Analyses
3. Results
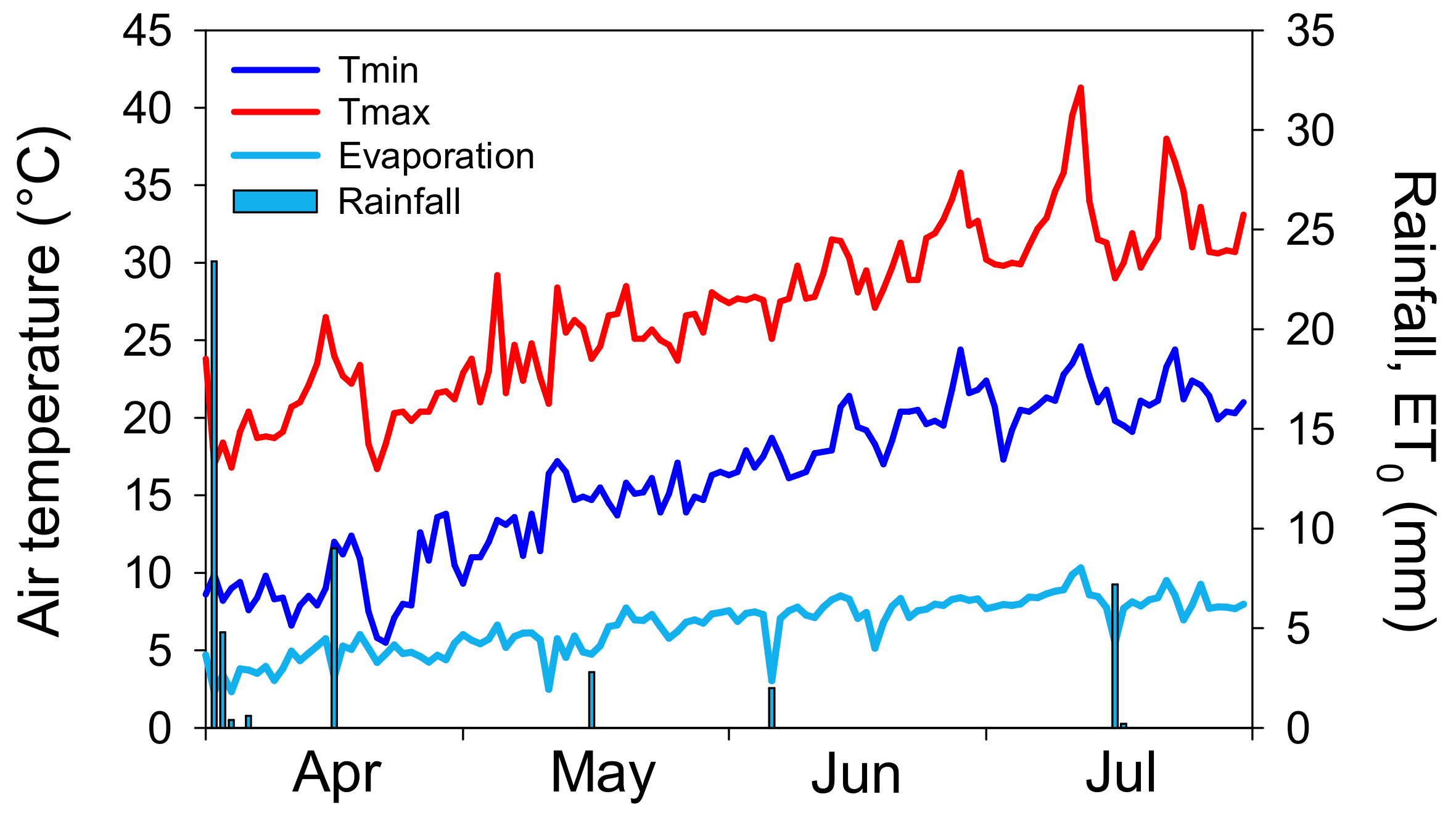
3.1. Weather Conditions during the Field Experiment
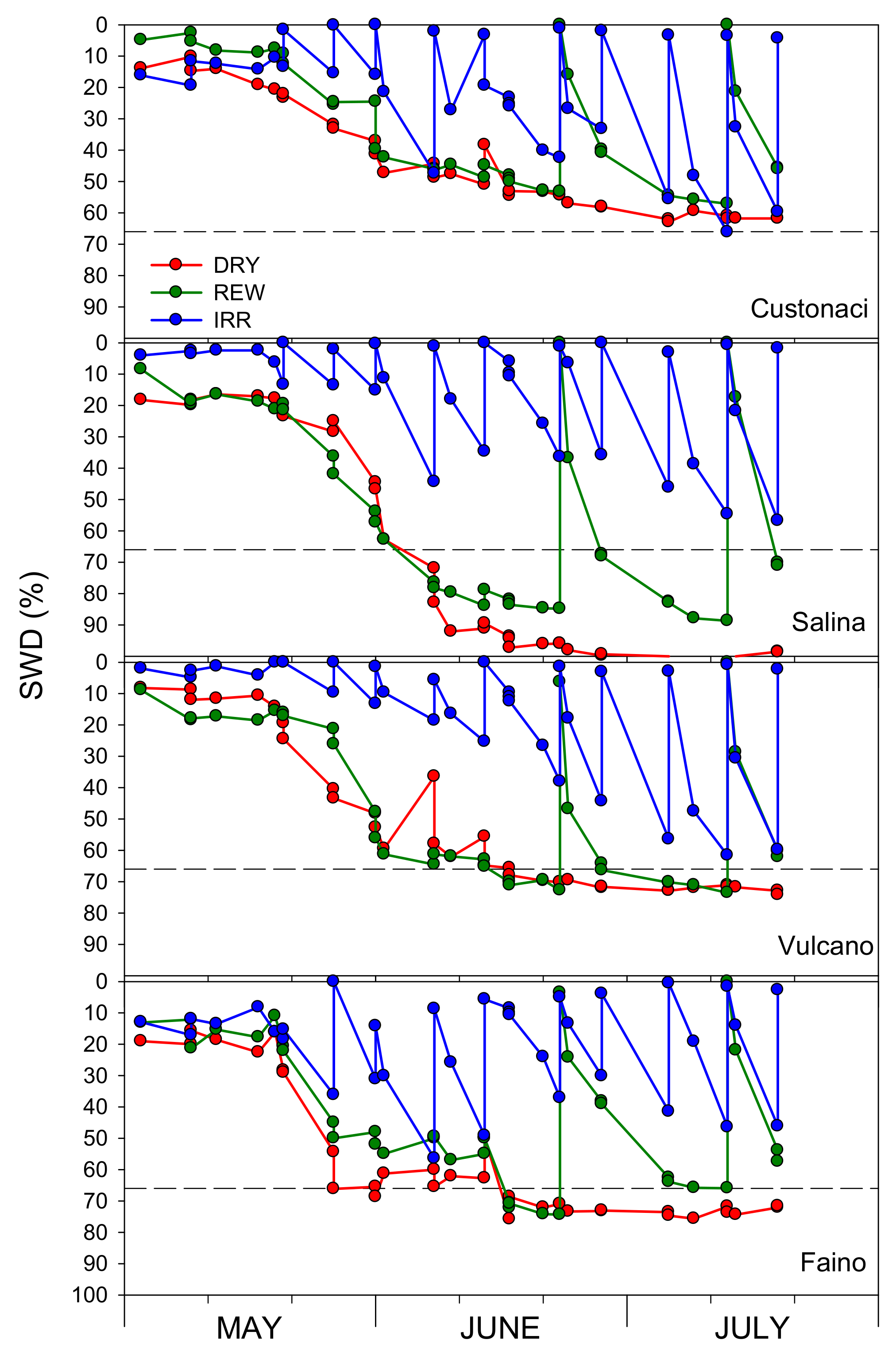
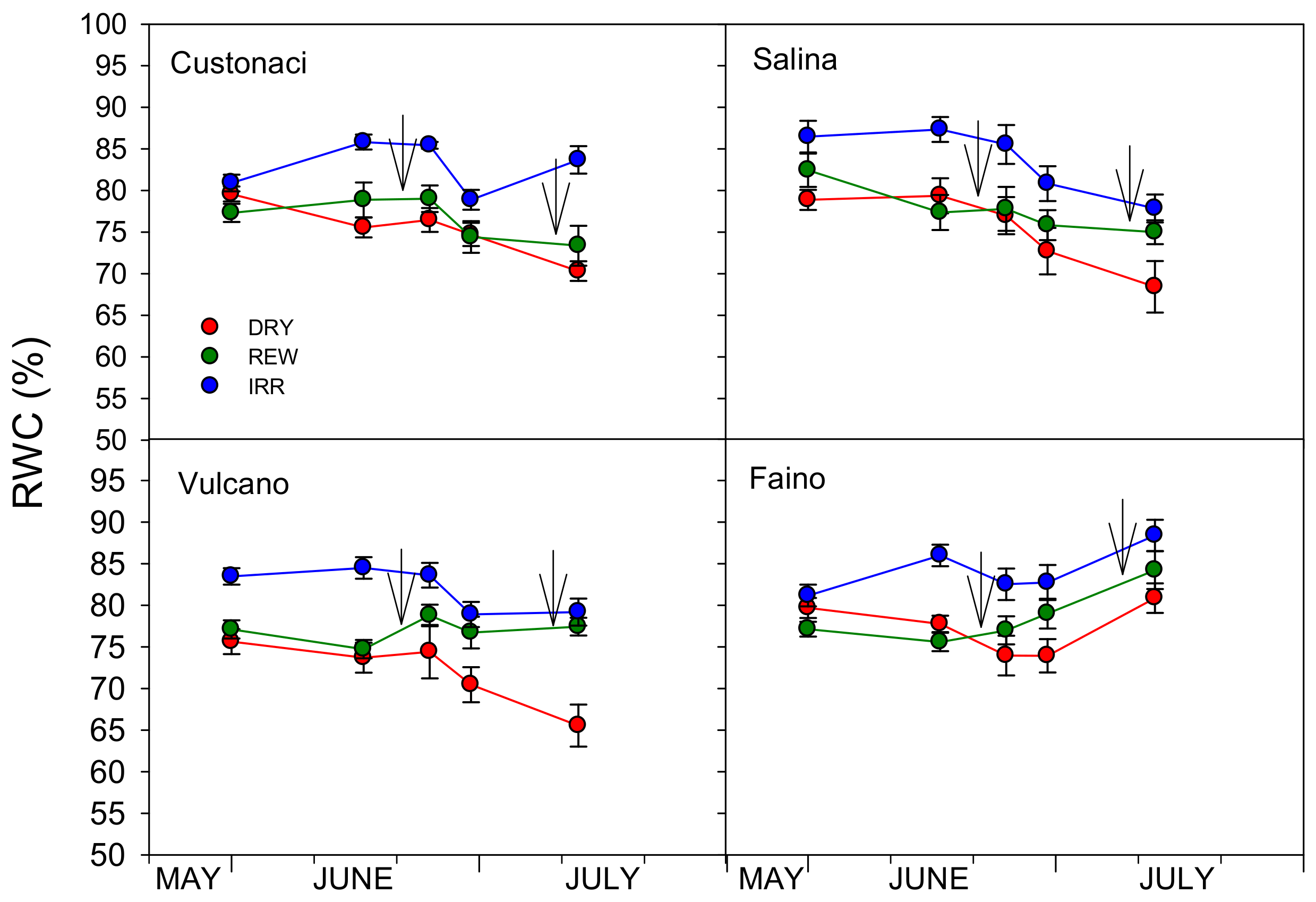
3.2. Course of Soil Water Content
3.3. Physiological Traits
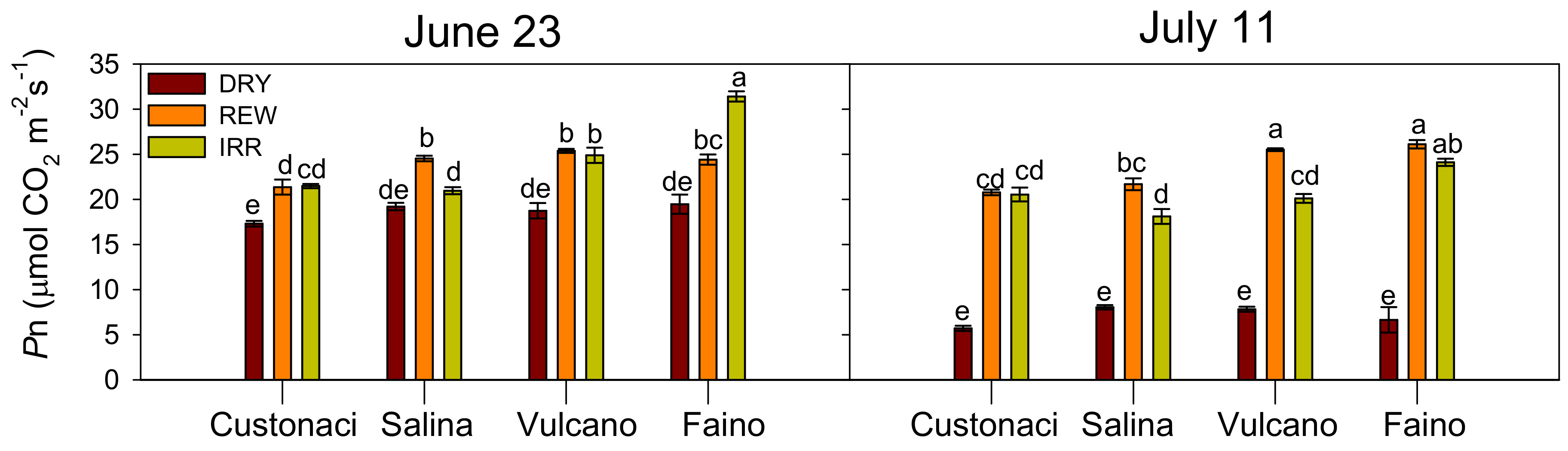
3.3.1. Net Photosynthetic Assimilation Rate (Pn)
3.3.2. Leaf Transpiration (E)
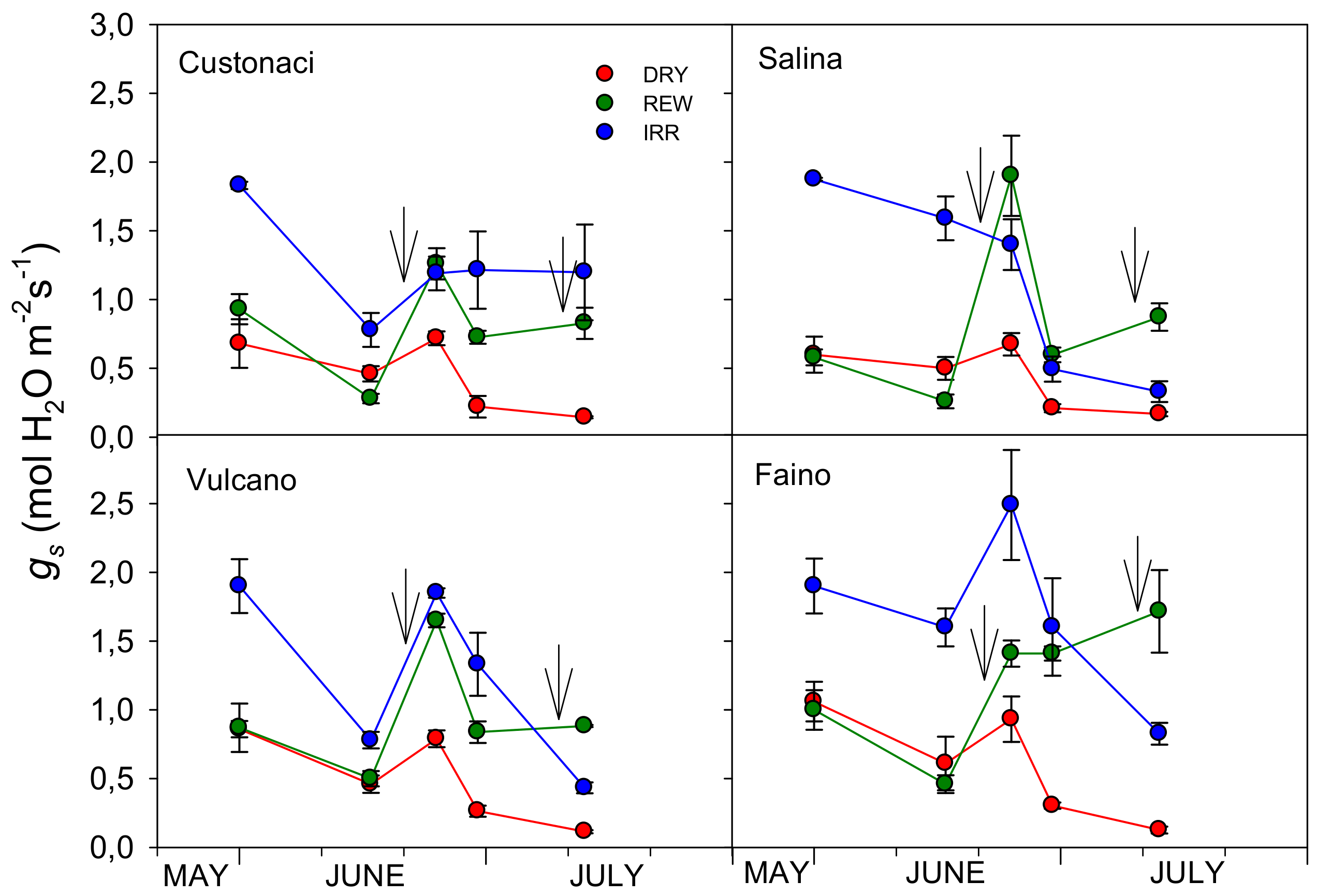
3.3.3. Stomatal Conductance (gs)
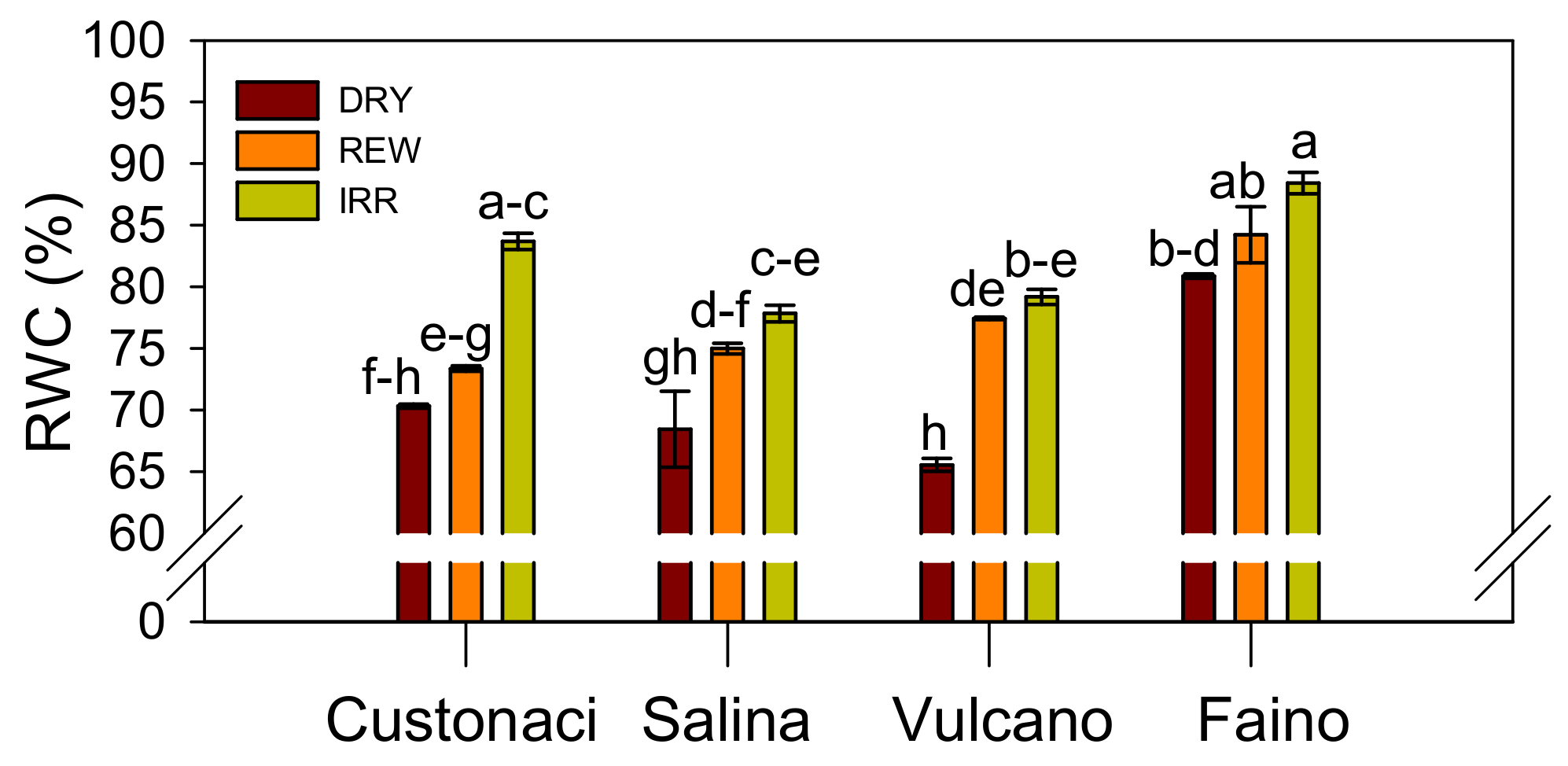
3.3.4. Leaf Relative Water Content (RWC)
3.3.5. Instantaneous Water Use Efficiency (WUEi)
3.3.6. Leaf Intercellular CO2 (Ci)
3.3.7. Stress Degree (Ta-Tl)
3.4. Fruit Production
3.5. Quality Traits
3.6. Pearson’s Correlation among Physiological and Quality Traits
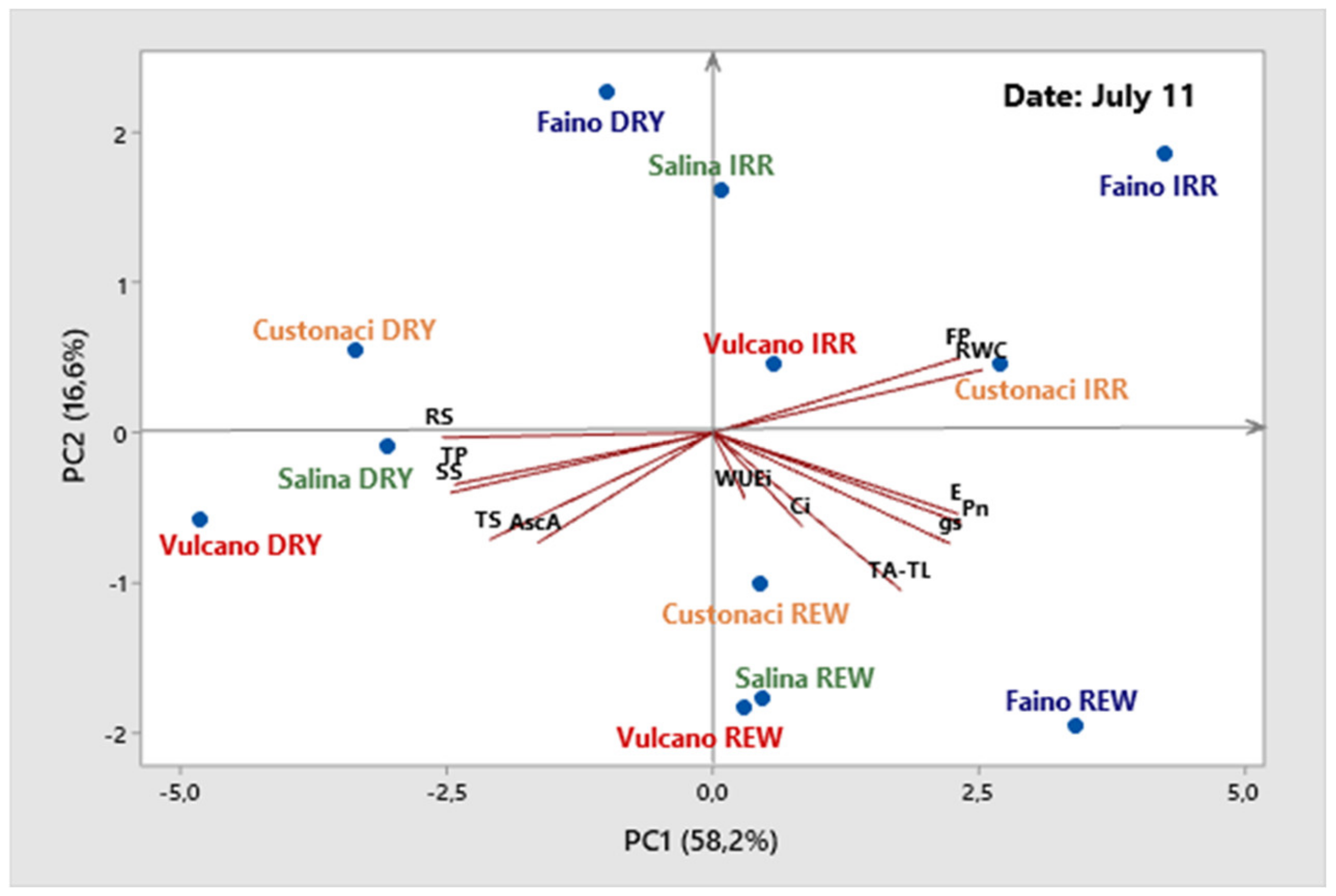
3.7. Principal Component Analysis of (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pope, J.O.; Brown, K.; Fung, F.; Hanlon, H.M.; Neal, R.; Palin, E.J.; Reid, A. Investigation of future climate change over the British Isles using weather patterns. Clim. Dyn. 2022, 58, 2405–2419. [Google Scholar] [CrossRef]
- Bibi, F.; Rahman, A. An overview of climate change impacts on agriculture and their mitigation strategies. Agriculture 2023, 13, 1508. [Google Scholar] [CrossRef]
- Straffelini, E.; Tarolli, P. Climate change-induced aridity is affecting agriculture in Northeast Italy. Agric. Syst. 2023, 208, 103647. [Google Scholar] [CrossRef]
- UN General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development. A/RES/70/1, 21 October 2015. Available online: https://www.refworld.org/legal/resolution/unga/2015/en/111816 (accessed on 3 July 2024).
- Kyoi, S.; Mori, K.; Matsushita, K. Solution of trade-offs between food production, water use, and climate change mitigation in global agriculture. Sustain. Dev. 2024, 32, 2314–2324. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Panda, S.K.; Dutta, B.K. Variation of physiological and antioxidative responses in tea cultivars subjected to elevated water stress followed by rehydration recovery. Acta Physiol. Plant. 2008, 30, 457–468. [Google Scholar] [CrossRef]
- Hao, S.; Cao, H.; Wang, H.; Pan, X. The physiological responses of tomato to water stress and re-water in different growth periods. Sci. Hortic. 2019, 249, 143–154. [Google Scholar] [CrossRef]
- Pereira, L.S.; Oweis, T.; Zairi, A. Irrigation management under water scarcity. Agric. Water Manag. 2002, 57, 175–206. [Google Scholar] [CrossRef]
- Patanè, C.; Saita, A. Biomass, fruit yield, water productivity and quality response of processing tomato to plant density and deficit irrigation under a semi-arid Mediterranean climate. Crop Pasture Sci. 2015, 66, 224–234. [Google Scholar] [CrossRef]
- Xu, J.; Wan, W.; Zhu, X.; Zhao, Y.; Chai, Y.; Guan, S.; Diao, M. Effect of regulated deficit irrigation on the growth, yield, and irrigation water productivity of processing tomatoes under drip irrigation and mulching. Agronomy 2023, 13, 2862. [Google Scholar] [CrossRef]
- Giorio, P.; Guida, G.; Mistretta, C.; Sellami, M.H.; Oliva, M.; Punzo, P.; Iovieno, P.; Arena, C.; De Maio, A.; Grillo, S.; et al. Physiological, biochemical and molecular responses to water stress and rehydration in Mediterranean adapted tomato landraces. Plant Biol. 2018, 20, 995–1004. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G.; Shimizu, H. Plant responses to drought and rewatering. Plant Signal. Behav. 2010, 5, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Patanè, C.; Malvuccio, A.; Saita, A.; Rizzarelli, P.; Siracusa, L.; Rizzo, V.; Muratore, G. Nutritional changes during storage in fresh-cut long storage tomato as affected by biocompostable polylactide and cellulose based packaging. LWT 2019, 101, 618–624. [Google Scholar] [CrossRef]
- Siracusa, L.; Avola, G.; Patanè, C.; Riggi, E.; Ruberto, G. Re-evaluation of traditional Mediterranean foods. The local landraces of ‘Cipolla di Giarratana’ (Allium cepa L.) and long-storage tomato (Lycopersicon esculentum L.): Quality traits and polyphenol content. J. Sci. Food Agric. 2013, 93, 3512–3519. [Google Scholar] [CrossRef] [PubMed]
- Conesa, M.A.; Fullana-Pericàs, M.; Granell, A.; Galmés, J. Mediterranean long shelf-life landraces: An untapped genetic resource for tomato improvement. Front. Plant Sci. 2020, 10, 1651. [Google Scholar] [CrossRef] [PubMed]
- Patanè, C.; Siah, S.; Pellegrino, A.; Cosentino, S.L.; Siracusa, L. Fruit yield, polyphenols, and carotenoids in long shelf-life tomatoes in response to drought stress and rewatering. Agronomy 2021, 11, 1943. [Google Scholar] [CrossRef]
- Patanè, C.; Tringali, S.; Sortino, O. Effects of deficit irrigation on biomass, yield, water productivity and fruit quality of processing tomato under semi-arid Mediterranean climate conditions. Sci. Hortic. 2011, 129, 590–596. [Google Scholar] [CrossRef]
- Patanè, C.; Cosentino, S.L.; Romano, D.; Toscano, S. Relative water content, proline, and antioxidant enzymes in leaves of long shelf-life tomatoes under drought stress and rewatering. Plants 2022, 11, 3045. [Google Scholar] [CrossRef]
- Yan, M.; Lian, H.; Zhang, C.; Chen, Y.; Cai, H.; Zhang, S. The role of root size and root efficiency in grain production, and water-and nitrogen-use efficiency in wheat. J. Sci. Food Agric. 2023, 103, 7083–7094. [Google Scholar] [CrossRef]
- Idso, S.B.; Jackson, R.D.; Pinter, P.J.; Reginato, R.J.; Hatfield, J.L. Normalizing the stress-degree-day parameter for environmental variability. Agric. Meteorol. 1981, 24, 45–55. [Google Scholar] [CrossRef]
- Bailey, M.J.; Biely, P.; Poutanen, K. Interlaboratory testing of methods for assay of xylanase activity. J. Biotech. 1992, 23, 257–270. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Patanè, C.; Scordia, D.; Testa, G.; Cosentino, S.L. Physiological screening for drought tolerance in Mediterranean long-storage tomato. Plant Sci. 2016, 249, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Guida, G.; Houssemeddine Sellami, M.H.; Mistretta, C.; Oliva, M.; Buonomo, R.; De Mascellis, R.; Patanè, C.; Rouphael, Y.; Albrizio, R.; Giorio, P. Agronomical, physiological and fruit quality responses of two Italian long-storage tomato landraces under rain-fed and full irrigation conditions. Agric. Water Manag. 2017, 180, 126–135. [Google Scholar] [CrossRef]
- Patanè, C.; Cosentino, S.L. Effects of soil water deficit on yield and quality of processing tomato under a Mediterranean climate. Agric. Water Manag. 2010, 97, 131–138. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance water-use efficiency, and yield potential-are theycompatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, J.K.; Yang, Y.L.; He, W.L.; Zhang, L.X. Changes in the pattern of antioxidant enzymes in wheat exposed to water deficit and rewatering. Acta Physiol. Plant. 2004, 26, 345–352. [Google Scholar] [CrossRef]
- Galić, V.; Mlinarić, S.; Marelja, M.; Zdunić, Z.; Brkić, A.; Mazur, M.; Begović, L.; Šimić, D. contrasting water withholding responses of young maize plants reveal link between lipid peroxidation and osmotic regulation corroborated by genetic analysis. Front Plant Sci. 2022, 6, 804630. [Google Scholar] [CrossRef]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of exogenous proline to abiotic stresses tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Abbas, S.; Hassan, M.U.; Saeed, F.; Haider, S.; Sharif, R.; Anand, A.; Corpas, F.J.; Jin, W.; et al. Assessment of proline function in higher plants under extreme temperatures. Plant Biol. 2023, 25, 379–395. [Google Scholar]
- Galmés, J.; Conesa, M.A.; Ochogavía, J.M.; Perdomo, J.A.; Francis, D.M.; Ribas-carbó, M.; Savé, R.; Flexas, L.; Medrano, H.; Cifre, J. Physiological and morphological adaptations in relation to water use efficiency in Mediterranean accessions of Solanum lycopersicum. Plant Cell Environ. 2011, 34, 245–260. [Google Scholar] [CrossRef]
- Li, G.; Wan, S.; Zhou, J.; Yang, Z.; Qin, P. Leaf chlorophyll fluorescence, hyperspectral reflectance, pigments content, malondialdehyde and proline accumulation responses of castor bean (Ricinus communis L.) seedlings to salt stress levels. Ind. Crop Prod. 2010, 31, 13–19. [Google Scholar] [CrossRef]
- Sreeman, S.; Prasad, B.T.K.; Nataraj, K.N.; Shankar, A.G.; Ganesh, P.; Udayakumar, M. Ratio of intercellular CO2 concentration to stomatal conductance is a reflection of mesophyll efficiency. Current Sci. 1996, 70, 672–675. [Google Scholar]
- Yuan, X.K.; Yang, Z.Q.; Li, Y.X.; Liu, Q.; Han, W. Effects of different levels of water stress on leaf photosynthetic characteristics and antioxidant enzyme activities of greenhouse tomato. Photosynthetica 2016, 54, 28–39. [Google Scholar] [CrossRef]
- Patanè, C.; Pellegrino, A.; Saita, A.; Siracusa, L.; Ruberto, G.; Barbagallo, R. Mediterranean long storage tomato as a source of novel products for the agrifood industry: Nutritional and technological traits. LWT 2017, 85, 445–448. [Google Scholar] [CrossRef]
- Kazemi, S.; Zakerin, A.; Abdossi, V.; Moradi, P. Fruit yield and quality of the grafted tomatoes under different drought stress conditions. Asian J. Agric. Biol. 2021, 1, 1–14. [Google Scholar] [CrossRef]
- Medyouni, I.; Zouaoui, R.; Rubio, E.; Serino, S.; Ahmed, H.B.; Bertin, N. Effects of water deficit on leaves and fruit quality during the development period in tomato plant. Food Sci. Nutr. 2021, 9, 1949–1960. [Google Scholar] [CrossRef]
- Marín, A.; Rubio, J.S.; Martínez, V.; Gil, M.I. Antioxidant compounds in green and red peppers as affected by irrigation frequency, salinity and nutrient solution composition. J. Agric. Food Chem. 2009, 89, 1352–1359. [Google Scholar] [CrossRef]
- Nangare, D.D.; Singh, Y.; Kumar, P.S.; Minhas, P.S. Growth, fruit yield and quality of tomato (Lycopersicon esculentum Mill.) as affected by deficit irrigation regulated on phenological basis. Agric. Water Manag. 2016, 171, 73–79. [Google Scholar] [CrossRef]
- Jin, N.; Zhang, D.; Jin, L.; Wang, S.; Yang, X.; Lei, Y.; Meng, X.; Xu, Z.; Sun, J.; Lyu, J.; et al. Controlling water deficiency as an abiotic stress factor to improve tomato nutritional and flavour quality. Food Chem. 2023, 19, 100756. [Google Scholar] [CrossRef]
- Barbagallo, R.N.; Di Silvestro, I.; Patanè, C. Yield, physicochemical traits, antioxidant pattern, polyphenol oxidase activity and total visual quality of field-grown processing tomato cv. Brigade as affected by water stress in Mediterranean climate. J. Sci. Food Agric. 2013, 93, 1449–1457. [Google Scholar] [CrossRef]
- Ntagkas, N.; Woltering, E.; Nicole, C.; Labrie, C.; Marcelis, L.F.M. Light regulation of vitamin C in tomato fruit is mediated through photosynthesis. Environ. Exp. Bot. 2019, 158, 180–188. [Google Scholar] [CrossRef]
- Luthria, D.L.; Mukhopadhyay, S.; Krizek, D.T. Content of total phenolics and phenolic acids in tomato (Lycopersicon esculentum Mill.) fruits as influenced by cultivar and solar UV radiation. J. Food Compos. Anal. 2006, 19, 771–777. [Google Scholar] [CrossRef]
- Siracusa, L.; Patanè, C.; Rizzo, V.; Cosentino, S.L.; Ruberto, G. Targeted secondary metabolic and physico-chemical traits analysis to assess genetic variability within a germplasm collection of “long storage” tomatoes. Food Chem. 2018, 244, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Response of nutrients, minerals, antioxidant leaf pigments, vitamins, polyphenol, flavonoid and antioxidant activity in selected vegetable amaranth under four soil water content. Food Chem. 2018, 252, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Solankey, S.S. Functional physiology in drought tolerance of vegetable crops-An approach to mitigate climate change impact. In Climate Dynamics in Horticultural Science, Volume 1: The Principles and Applications; Chaudhary, M.L., Patel, V.B., Siddiqui, M.W., Mahdi, S.S., Eds.; CRC Press: Boca Raton, FL, USA, 2015; Chapter 9; pp. 150–171. [Google Scholar]



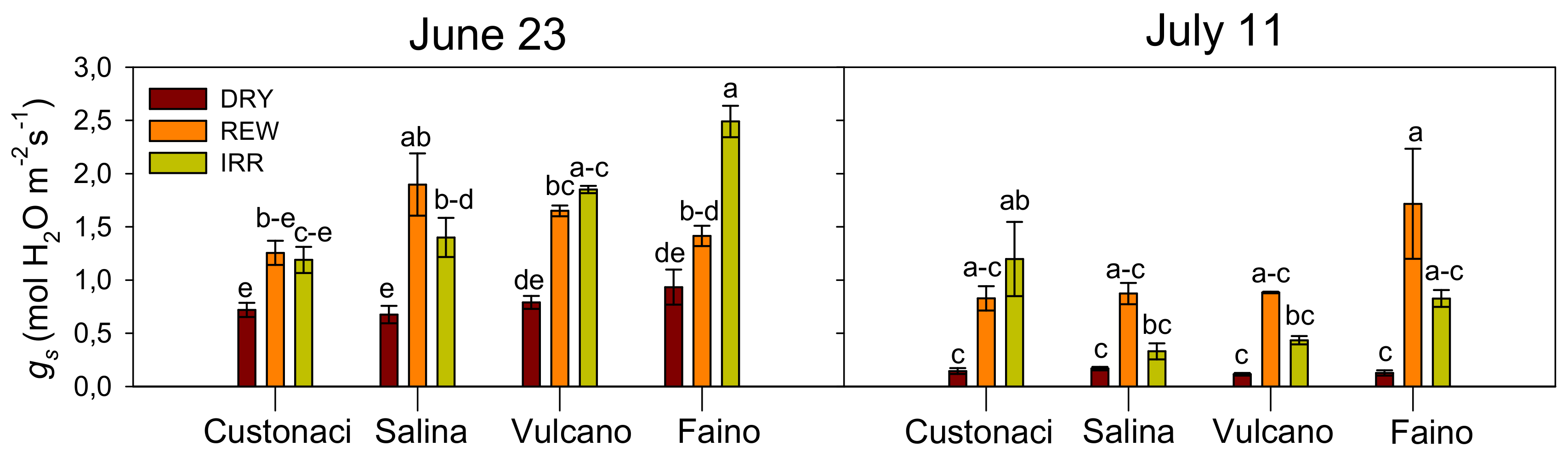

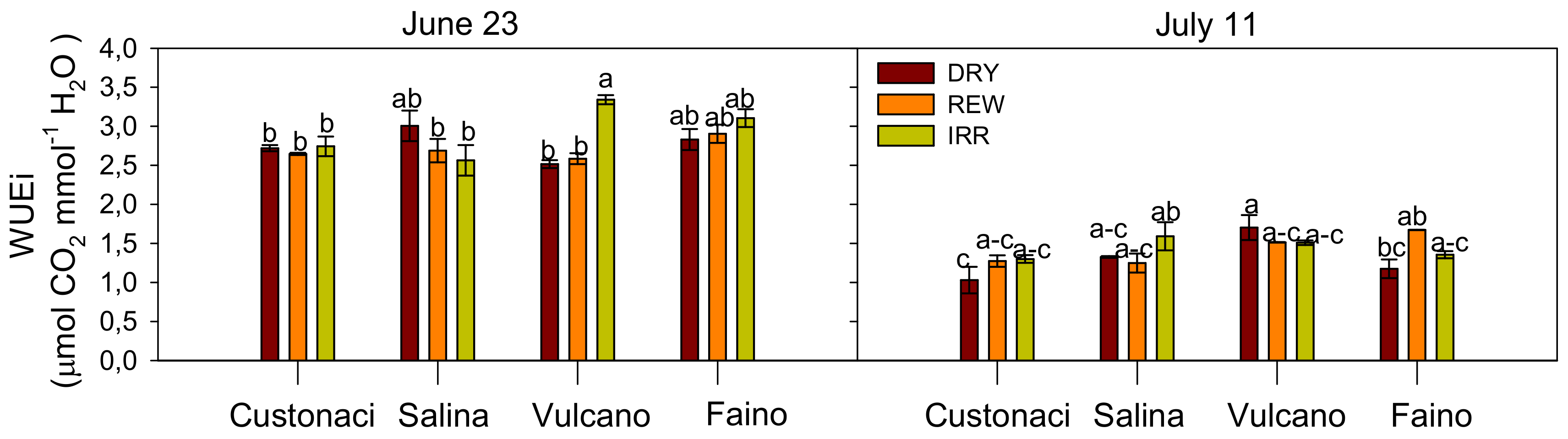

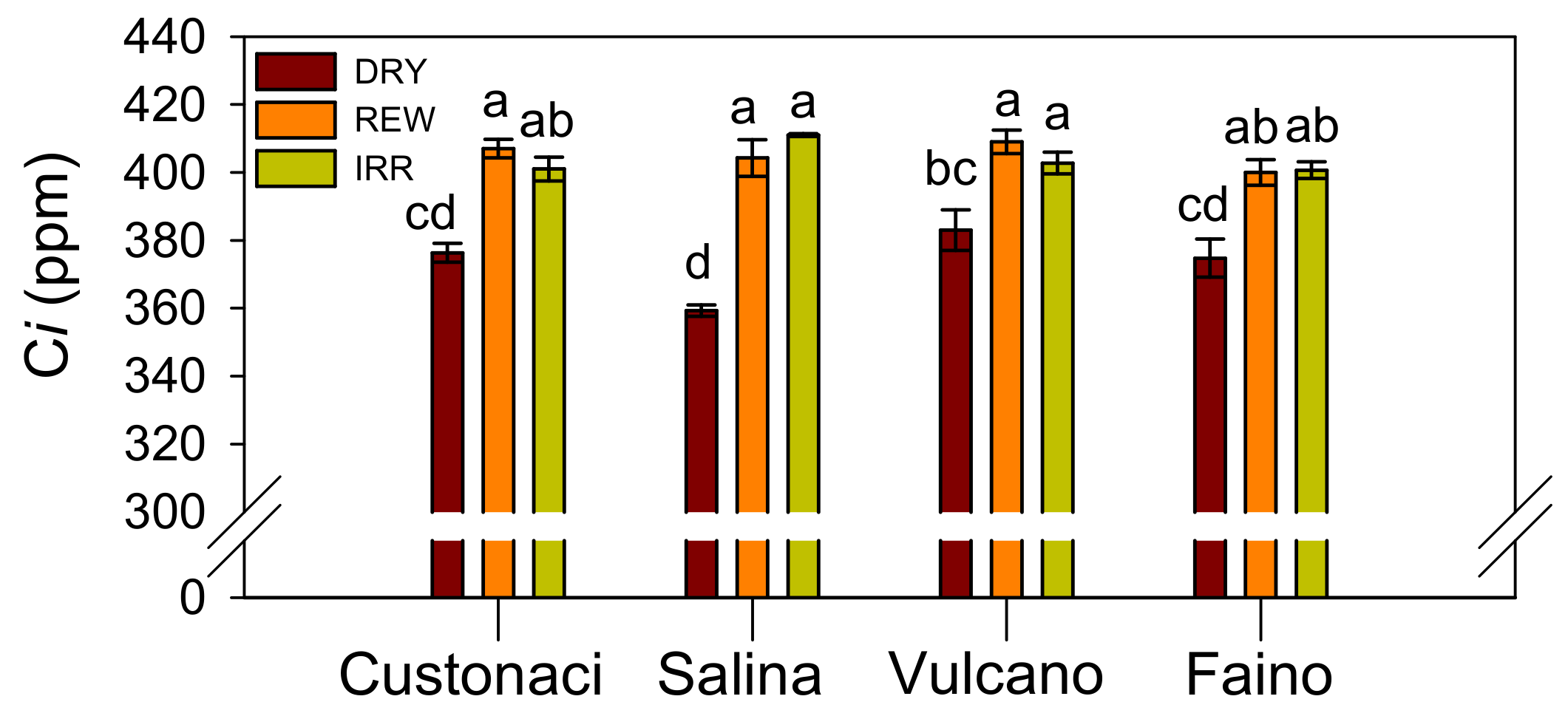
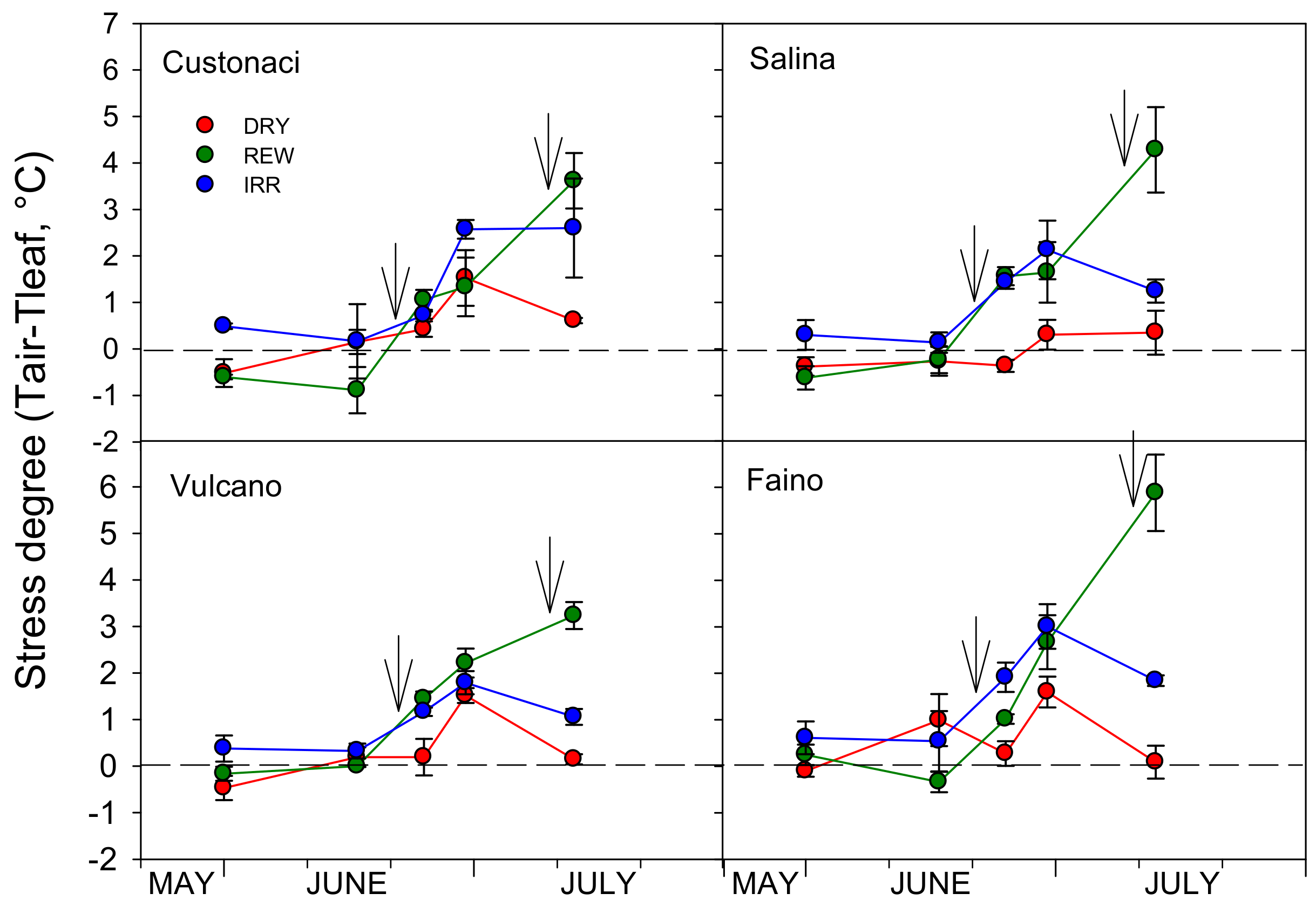

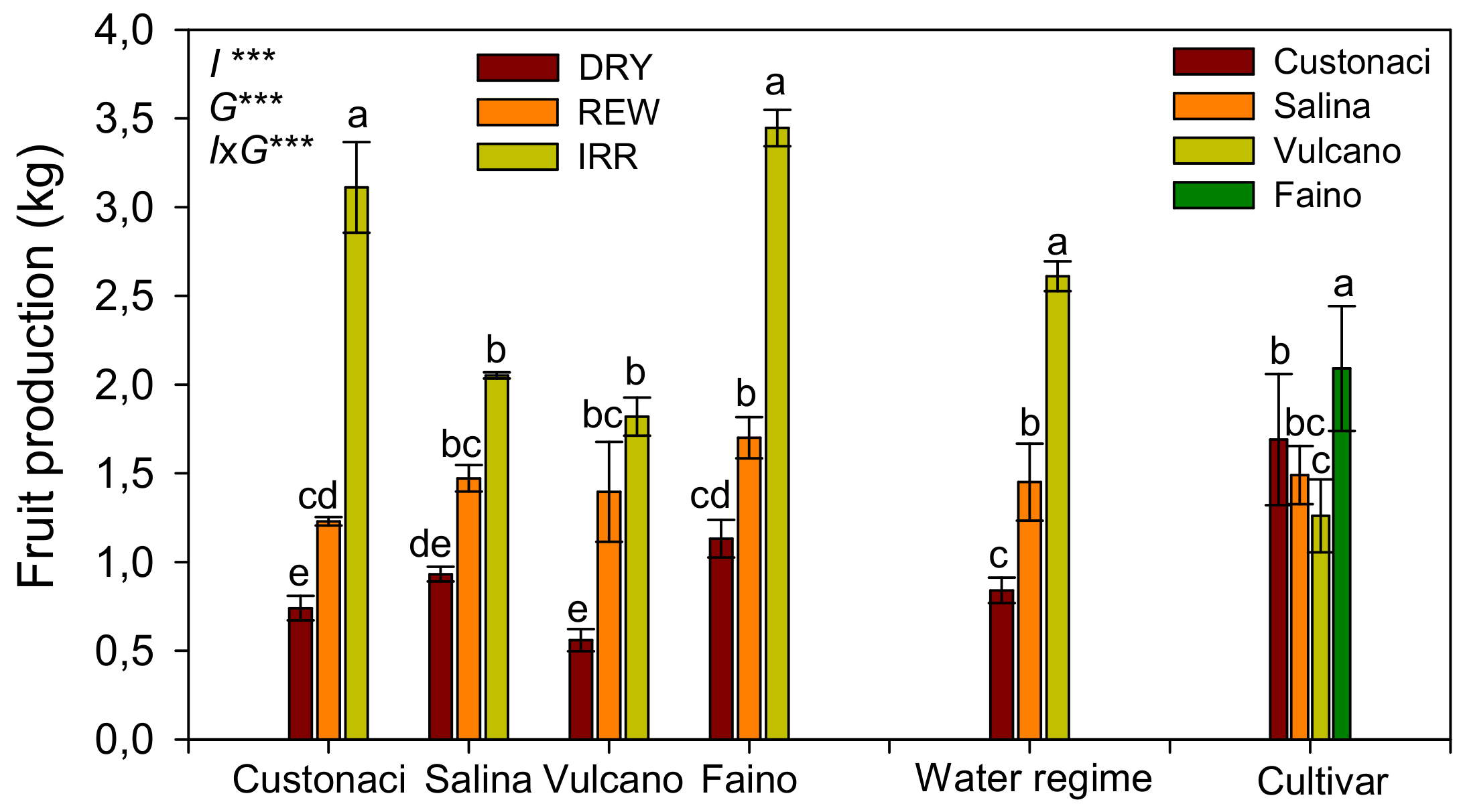

| Pn (μmol CO2 m−2s−1) | E (mmol H2O m−2s−1) | gs (mol H2O m−2s−1) | WUEi (μmol CO2 mmol−1 H2O) | Ci (ppm) | Ta−Tl (°C) | RWC (%) | ||
|---|---|---|---|---|---|---|---|---|
| DRY | 18.67 ± 0.39 b | 6.81 ± 0.19 b | 0.78 ± 0.05 b | 2.77 ± 0.07 ab | 373.4 ± 3.0 b | 0.13 ± 0.14 b | 75.5 ± 0.55 c | |
| Water regime | REW | 23.91 ± 0.46 a | 8.89 ± 0.24 a | 1.55 ± 0.10 a | 2.71 ± 0.05 b | 405.1 ± 2.0 a | 1.27 ± 0.10 a | 78.1 ± 0.48 b |
| (I) | IRR | 24.67 ± 1.10 a | 8.45 ± 0.30 a | 1.73 ± 0.14 a | 2.94 ± 0.10 a | 403.9 ± 1.6 a | 1.31 ± 0.14 a | 84.3 ± 0.59 a |
| Cultivar (G) | Custonaci (Local) | 20.04 ± 0.65 d | 7.44 ± 0.26 b | 1.05 ± 0.09 b | 2.70 ± 0.04 a | 394.8 ± 4.3 a | 0.74 ± 0.12 a | 80.3 ± 1.24 a |
| Salina (Local) | 21.56 ± 0.70 c | 8.01 ± 0.43 ab | 1.32 ± 0.19 ab | 2.75 ± 0.11 a | 391.5 ± 7.1 a | 0.88 ± 0.28 a | 80.1 ± 1.38 a | |
| Vulcano (Local) | 22.99 ± 0.98 b | 8.25 ± 0.38 a | 1.43 ± 0.14 a | 2.81 ± 0.12 a | 398.3 ± 4.1 a | 0.84 ± 0.21 a | 78.9 ± 1.22 ab | |
| Faino (Hy) | 25.08 ± 1.53 a | 8.49 ± 0.42 a | 1.61 ± 0.21 a | 2.95 ± 0.07 a | 391.8 ± 4.2 a | 1.07 ± 0.24 a | 77.8 ± 1.13 b | |
| Significance | I | *** | *** | *** | * | *** | *** | *** |
| G | *** | ** | *** | ns | ns | ns | * | |
| I × G | *** | *** | *** | *** | ** | ** | ns |
| Pn (μmol CO2 m−2s−1) | E (mmol H2O m−2s−1) | gs (mol H2O m−2s−1) | WUEi (μmol CO2 mmol−1 H2O) | Ci (ppm) | Ta-Tl (°C) | RWC (%) | ||
|---|---|---|---|---|---|---|---|---|
| DRY | 7.05 ± 0.41 c | 5.59 ± 0.32 c | 0.14 ± 0.01 c | 1.31 ± 0.06 a | 336.0 ± 5.9 b | 0.23 ± 0.15 c | 71.3 ± 1.19 c | |
| Water regime | REW | 23.51 ± 0.63 a | 16.69 ± 0.41 a | 1.07 ± 0.15 a | 1.43 ± 0.09 a | 362.0 ± 3.8 a | 4.19 ± 0.39 b | 77.5 ± 1.65 b |
| (I) | IRR | 20.68 ± 0.63 b | 14.68 ± 0.67 b | 0.72 ± 0.12 b | 1.44 ± 0.05 a | 350.5 ± 6.0 ab | 1.69 ± 0.33 b | 82.3 ± 1.12 a |
| Cultivar (G) | Custonaci (Local) | 15.66 ± 2.15 b | 12.81 ± 1.47 a | 0.72 ± 0.17 ab | 1.20 ± 0.07 b | 363.6 ± 6.2 a | 2.28 ± 0.53 a | 75.8 ± 1.74 b |
| Salina (Local) | 15.93 ± 1.78 b | 11.89 ± 1.53 a | 0.49 ± 0.10 b | 1.39 ± 0.08 ab | 347.7 ± 6.9 ab | 1.96 ± 0.64 a | 73.8 ± 1.53 b | |
| Vulcano (Local) | 17.77 ± 2.24 a | 11.62 ± 1.55 a | 0.48 ± 0.10 b | 1.58 ± 0.06 a | 334.4 ± 6.6 b | 1.48 ± 0.41 a | 74.1 ± 1.85 b | |
| Faino (Hy) | 18.95 ± 2.68 a | 12.95 ± 1.67 a | 0.89 ± 0.25 a | 1.40 ± 0.07 ab | 352.3 ± 5.2 ab | 2.42 ± 0.77 a | 84.5 ± 1.19 a | |
| Significance | I | *** | *** | *** | ns | *** | *** | *** |
| G | *** | ns | * | ** | ** | ns | *** | |
| I × G | *** | *** | * | ** | ns | ns | ** |
| TS (g 100 g−1 FW) | SS (°Brix) | RS (g 100 g−1) | AscA (mg 100 g−1 FW) | TP (mg GAE 100 g−1 FW) | ||
|---|---|---|---|---|---|---|
| DRY | 9.63 ± 0.18 a | 7.41 ± 0.26 a | 5.25 ± 0.16 a | 14.15 ± 0.93 a | 64.0 ± 2.51 a | |
| Water regime | REW | 9.13 ± 0.14 b | 6.55 ± 0.14 b | 4.52 ± 0.18 b | 13.32 ± 0.87 a | 56.2 ± 1.61 b |
| (I) | IRR | 7.36 ± 0.24 c | 6.07 ± 0.18 c | 4.34 ± 0.14 b | 11.21 ± 0.89 b | 53.7 ± 2.30 b |
| Cultivar (G) | Custonaci (Local) | 9.10 ± 0.19 a | 6.92 ± 1.01 c | 4.84 ± 0.22 a | 10.80 ± 0.67 b | 58.7 ± 2.33 b |
| Salina (Local) | 8.89 ± 0.14 a | 7.02 ± 1.09 b | 4.84 ± 0.17 a | 14.64 ± 1.17 a | 57.6 ± 1.78 b | |
| Vulcano (Local) | 8.98 ± 0.35 a | 7.15 ± 0.62 a | 4.97 ± 0.22 a | 15.01 ± 0.99 a | 65.2 ± 3.21 a | |
| Faino (Hy) | 7.85 ± 0.16 b | 5.63 ± 1.08 d | 4.15 ± 0.20 b | 11.12 ± 0.70 b | 50.5 ± 1.74 c | |
| Significance | I | *** | *** | *** | *** | *** |
| G | *** | *** | ** | ** | *** | |
| I × G | ns | *** | ns | ns | * |
| Pn | E | gs | RWC | Ci | Ta-Tl | WUEi | TS | SS | RS | AscA | TP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | 0.96 *** | - | - | - | - | - | - | - | - | - | - | - |
| gs | 0.85 *** | 0.80 ** | - | - | - | - | - | - | - | - | - | - |
| RWC | 0.64 * | 0.61 * | 0.62 * | - | - | - | - | - | - | - | - | - |
| Ci | 0.24 ns | 0.35 ns | 0.47 ns | 0.20 ns | - | - | - | - | - | - | - | - |
| Ta-Tl | 0.81 ** | 0.80 ** | 0.92 *** | 0.38 ns | 0.46 ns | - | - | - | - | - | - | - |
| WUEi | 0.37 ns | 0.12 ns | 0.27 ns | 0.05 ns | −0.40 ns | 0.20 ns | - | - | - | - | - | - |
| TS | −0.54 ns | −0.50 ns | −0.36 ns | −0.82 *** | 0.18 ns | −0.10 ns | −0.24 ns | - | - | - | - | - |
| SS | −0.61 * | −0.62 * | −0.55 ns | −0.95 *** | −0.29 ns | −0.37 ns | 0.07 ns | 0.76 ** | - | - | - | - |
| RS | −0.74 ** | −0.66 * | −0.76 ** | −0.88 *** | −0.27 ns | −0.55 ns | −0.21 ns | 0.69 * | 0.88 *** | - | - | - |
| AscA | −0.25 ns | −0.31 ns | −0.33 ns | −0.67 * | −0.06 ns | −0.12 ns | −0.26 ns | 0.57 ns | 0.60 * | 0.51 ns | - | - |
| TP | −0.60 * | −0.64 * | −0.57 * | −0.87 *** | −0.24 ns | −0.45 ns | −0.19 ns | 0.69 * | 0.93 *** | 0.86 *** | 0.61 * | |
| FP | 0.62 * | 0.65 * | 0.52 ns | 0.84 *** | 0.10 ns | 0.25 ns | 0.05 ns | −0.91 *** | −0.76 ** | −0.73 * | −0.61 * | −0.74 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patanè, C.; Siah, S.; Cafaro, V.; Cosentino, S.L.; Corinzia, S.A. Potential Impact of Drought and Rewatering on Plant Physiology and Fruit Quality in Long-Shelf-Life Tomatoes. Agronomy 2024, 14, 2045. https://doi.org/10.3390/agronomy14092045
Patanè C, Siah S, Cafaro V, Cosentino SL, Corinzia SA. Potential Impact of Drought and Rewatering on Plant Physiology and Fruit Quality in Long-Shelf-Life Tomatoes. Agronomy. 2024; 14(9):2045. https://doi.org/10.3390/agronomy14092045
Chicago/Turabian StylePatanè, Cristina, Sarah Siah, Valeria Cafaro, Salvatore L. Cosentino, and Sebastiano A. Corinzia. 2024. "Potential Impact of Drought and Rewatering on Plant Physiology and Fruit Quality in Long-Shelf-Life Tomatoes" Agronomy 14, no. 9: 2045. https://doi.org/10.3390/agronomy14092045







