Physiological Indexes in Seed Germination and Seedling Growth of Rangpur Lime (Citrus limonia L. Osbeck) under Plant Growth Regulators
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site, Seed Acquisition, Design, and Treatments
2.2. Seed Imbibition and Treatment Application
2.3. Evaluations
2.3.1. Laboratory Tests
2.3.2. Greenhouse Tests
2.4. Statistical Analyses
3. Results
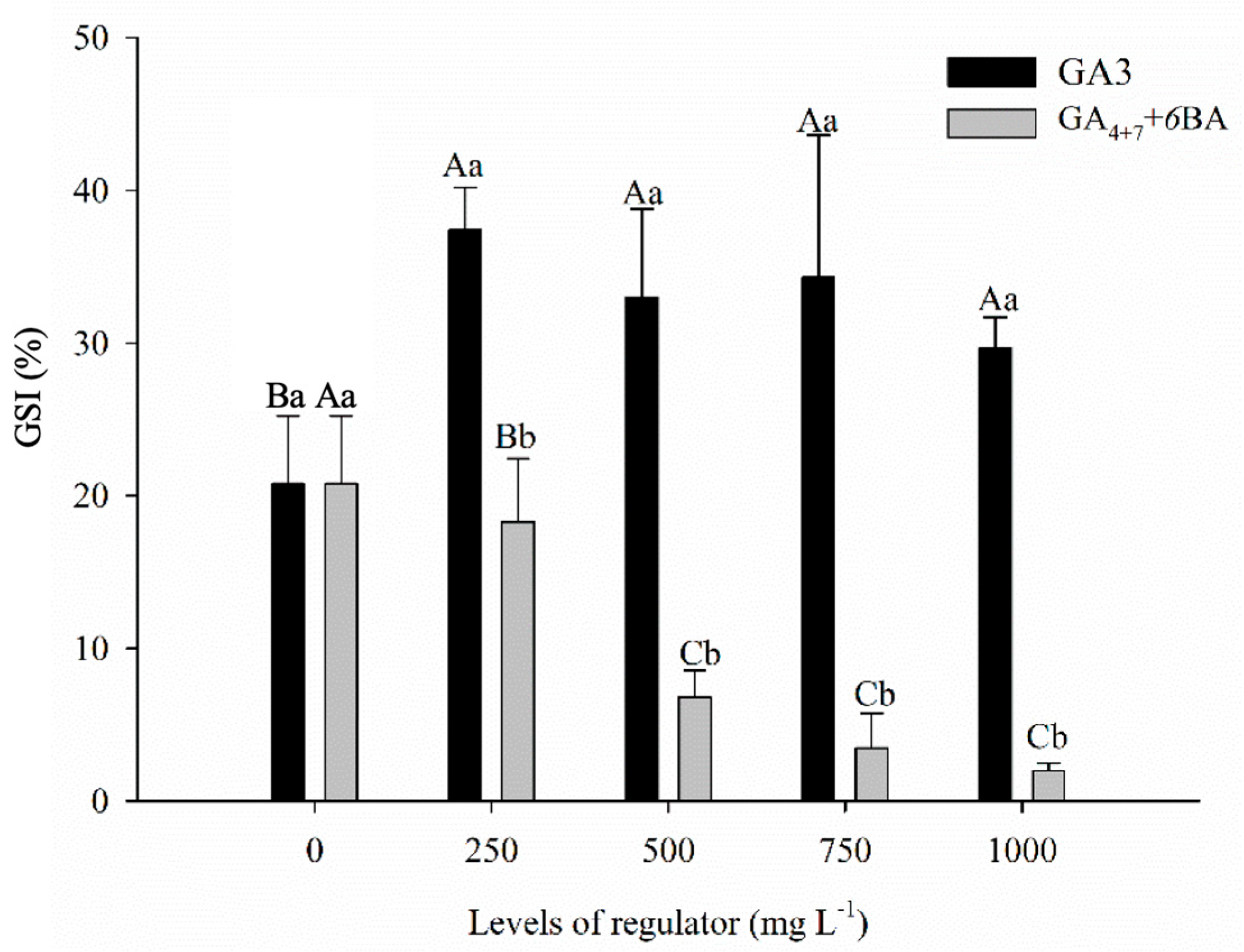
3.1. Germination Seeds
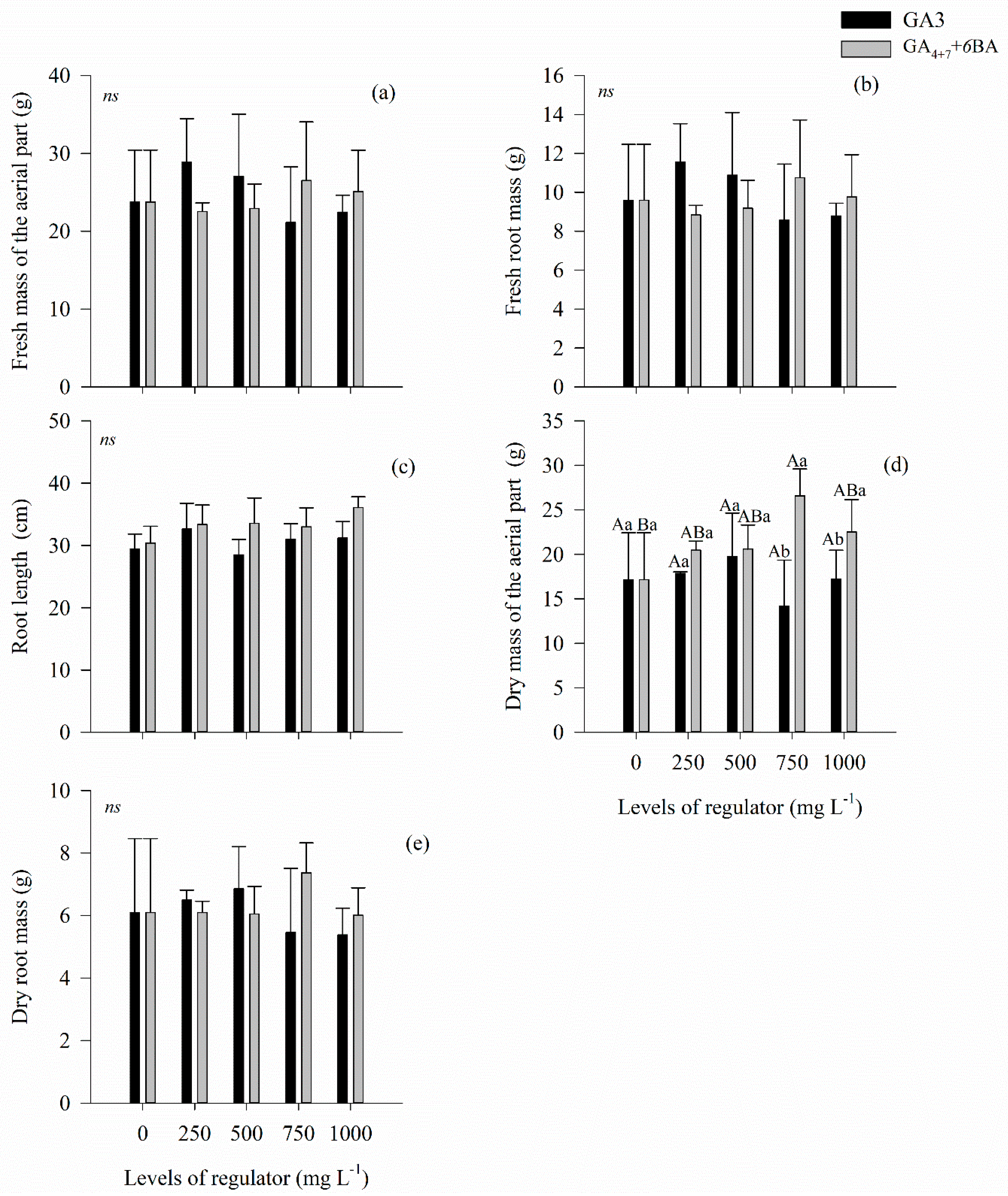
3.2. Growth and Development of Seedlings
3.3. Protein Level and Oxidative Enzymes of Seedlings
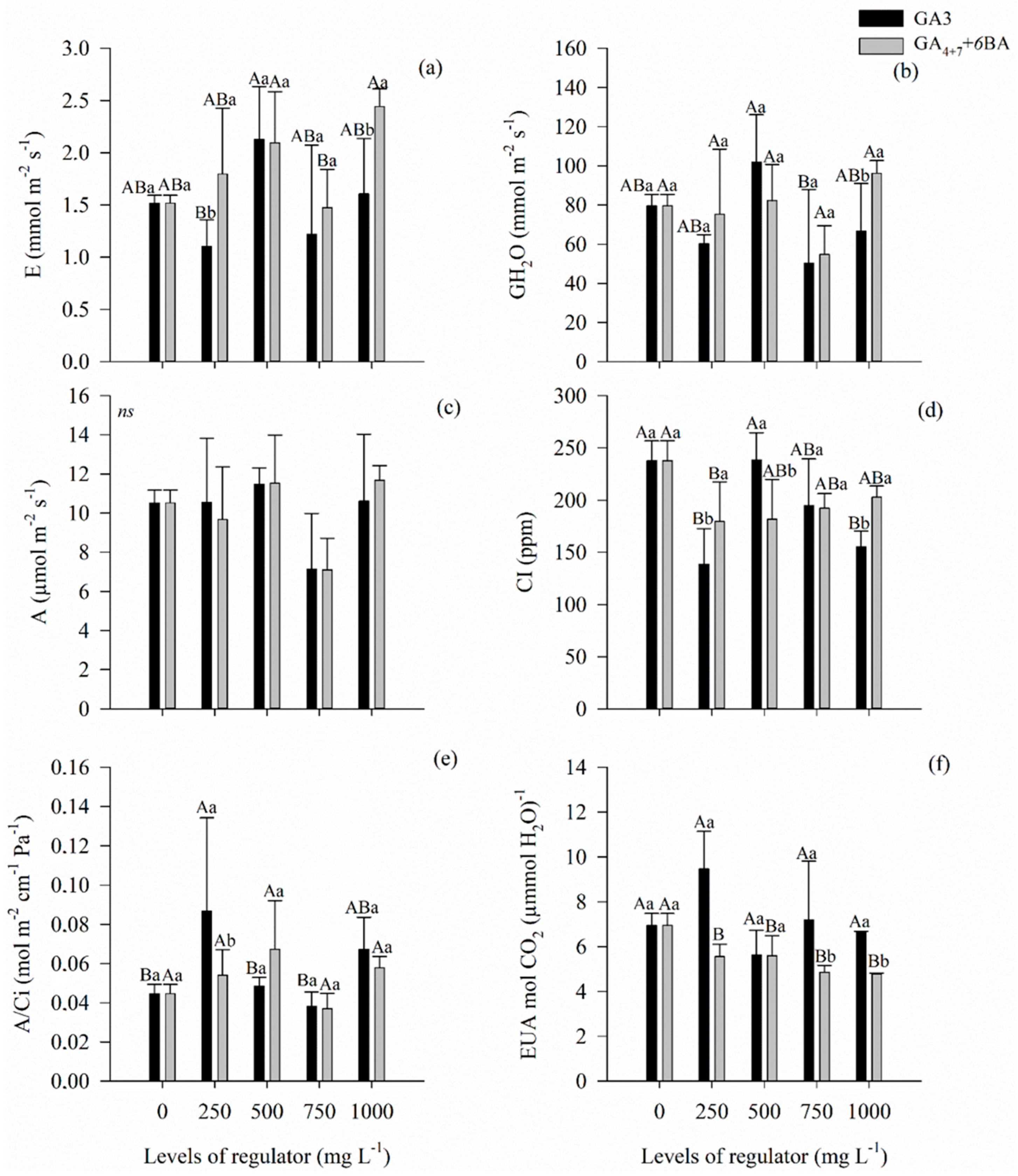
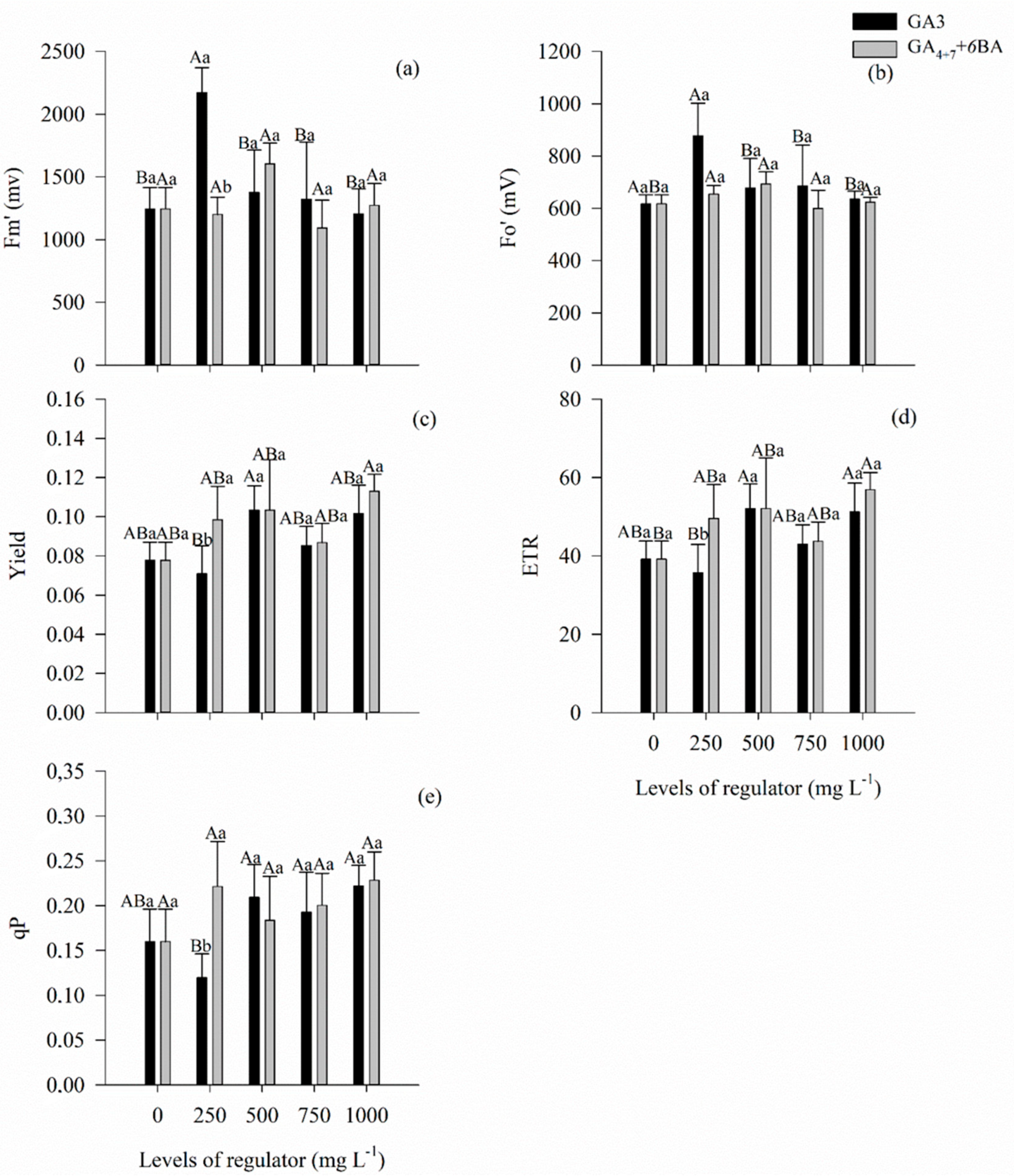
3.4. Gas Exchange, Water Use Efficiency, and Fluorescence
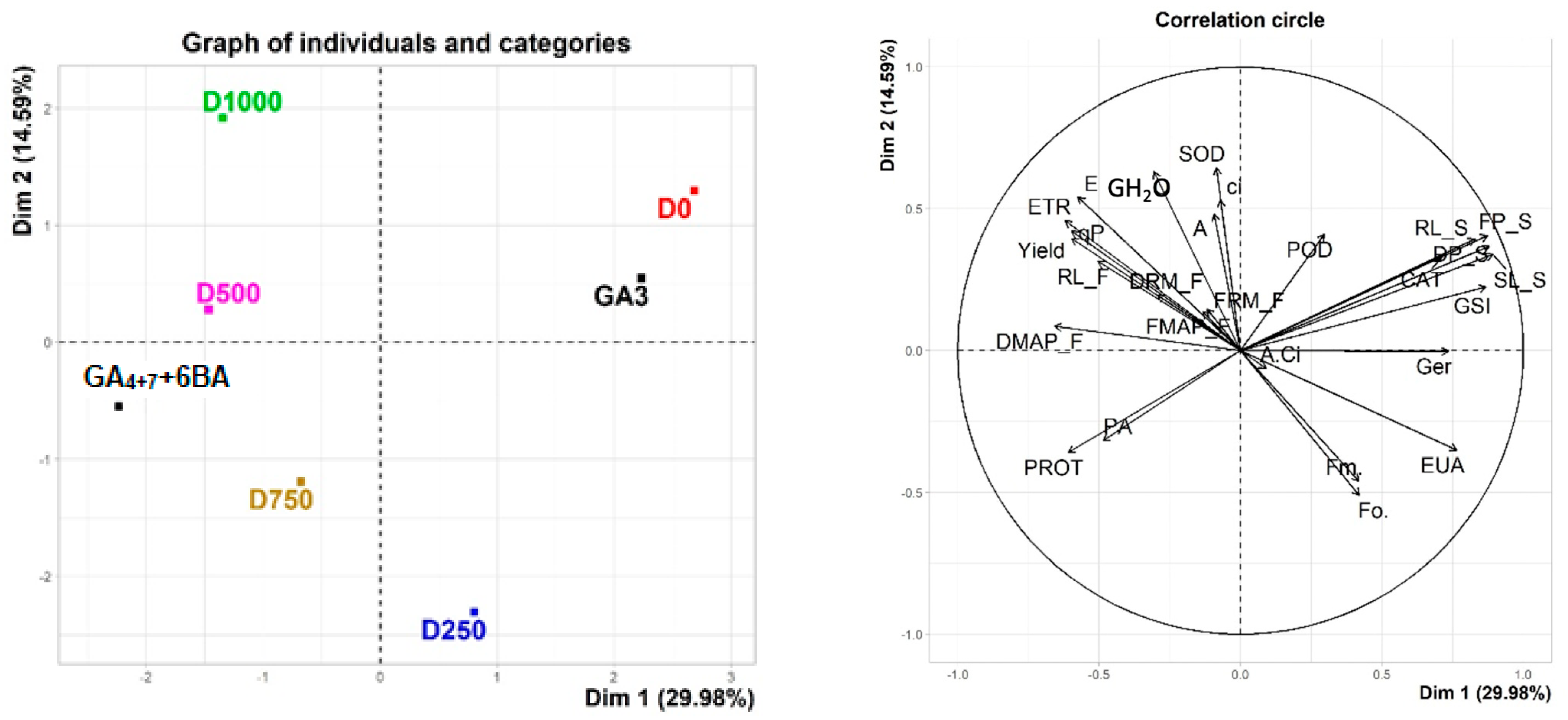
3.5. Principal Component Analysis
4. Discussion
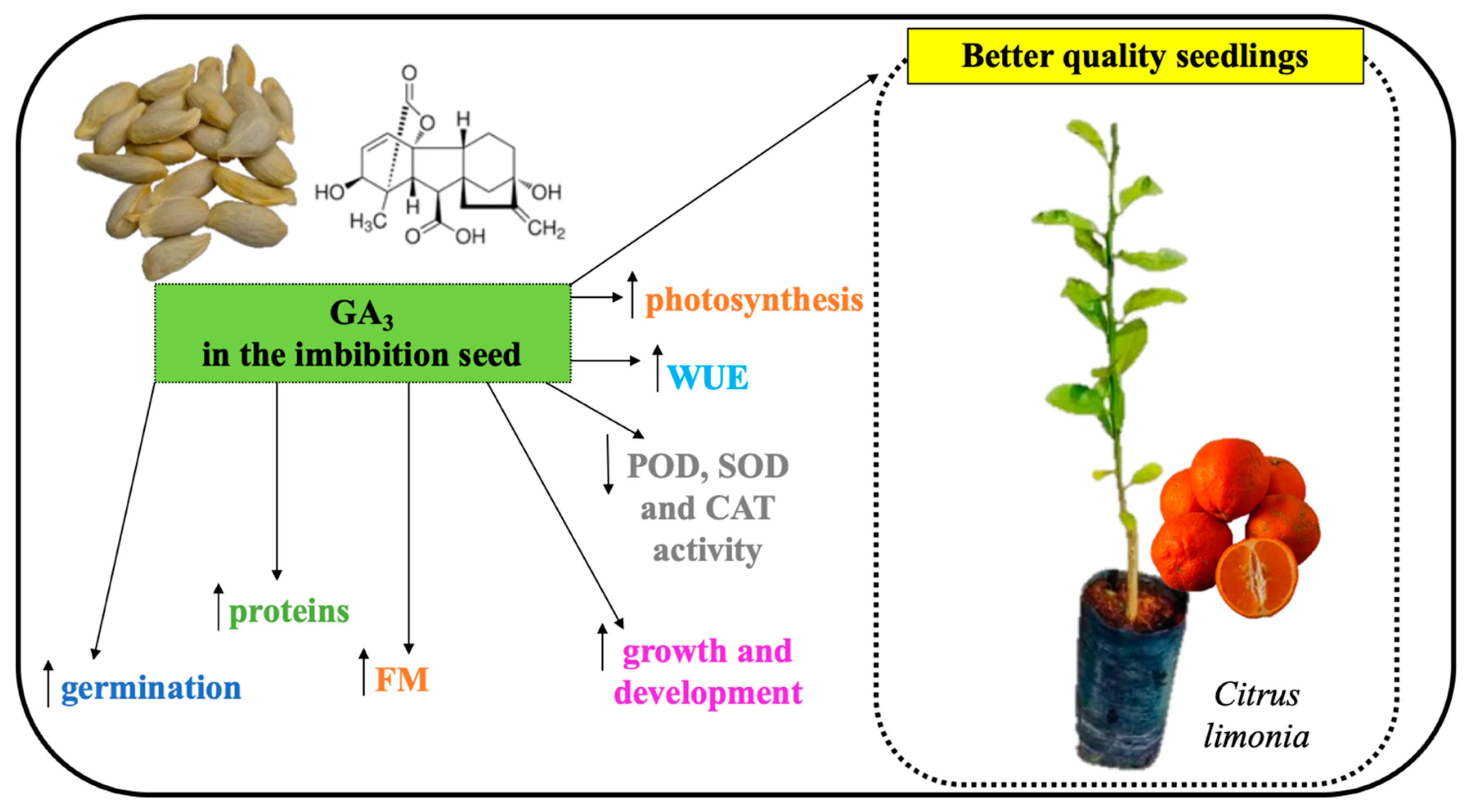
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ávila, M.R.; Barbosa, J.; Fonseca Júnior, N.S.; Nagashima, G.T.; Oliveira, C.M.G.; Garcia, E.B. Dynamics of the rangpur lime seed germination test conducted under different temperatures. J. Seed Sci. 2019, 41, 344–351. [Google Scholar] [CrossRef]
- Liberato, E.M.S.; Leonel, S.; Souza, J.M.A.; Napoleão, G.M. Substrate mixing formulations for Citrus nursery management. Nativa 2021, 9, 500–507. [Google Scholar] [CrossRef]
- Curk, F.; Olitrautt, F.; Garcia-Lor, A.; Luro, F.; Navarro, L.; Ollitraut, P. Phylogenetic origin of limes and lemons revealed by cytoplasmic and nuclear markers. Ann. Bot. 2016, 117, 1–19. [Google Scholar] [CrossRef]
- Oliveira, C.R.M.; Mello-Farias, P.C.; Oliveira, D.S.C.; Chaves, A.L.S.; Herter, F.G. Water availability effect on gas exchanges and on phenology of ‘Cabula’ orange. Acta Hortic. 2017, 1150, 133–138. [Google Scholar] [CrossRef]
- Fadel, A.L.; Mourão Filho, F.A.A.; Stuchi, E.S.; Wulff, N.A.; Couto, H.T.Z. Citrus sudden death-associated virus (CSDaV) and citrus tristeza virus (CTV) in eleven rootstocks for ‘Valência’ sweet orange. Rev. Bras. De Frutic. 2018, 40, e-788. [Google Scholar] [CrossRef]
- Ferrarezi, R.S. Citrus Nursery Production Guide, Chapter 8: Stock and Plant Tree Production. Irrigation and Fertilization; HS1333; UF/IFAS Extension, University of Florida: Gainesville, FL, USA, 2019. [Google Scholar]
- Merlin, T.P.A.; Lima, G.P.P.; Leonel, S.; Vianelo, F. Peroxidase activity and total phenol content in citrus cuttings treated with different copper sources. S. Afr. J. Bot. 2012, 83, 159–164. [Google Scholar] [CrossRef]
- Dorji, K.; Lakey, L. Citrus Nursery Management—A Technical Guide; Department of Agriculture: Thimphu, Bhutan, 2015; 67p. [Google Scholar]
- Conceição, P.M.; Azevedo, F.A.; Souza, A.J.B.; Próspero, A.G.; Morelli, M.; Forti, V.A. Ideal harvesting point of ‘Limeira-IAC382’ trifoliate orange fruits for seed extraction. Rev. Bras. De Frutic. 2020, 42, e-535. [Google Scholar] [CrossRef]
- Morelli, M.; Azevedo, F.A.; Conceição, P.M.; Souza, A.J.B. Maturation and physiological quality of IAC-863 Rangpur lime seeds. Comun. Sci. Hort. J. 2019, 10, 454–460. [Google Scholar] [CrossRef]
- Bons, H.K.; Kaur, N.; Rattampal, H.S. Quality and quantity improvement of citrus: Role of plant growth regulators. Int. J. Agric. Environ. Biotechnol. 2015, 8, 433–447. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Fisiologia e Desenvolvimento Vegetal; ARTMED: Porto Alegre, Brazil, 2017; 888p. [Google Scholar]
- Marcos Filho, J. Fisiologia de Sementes de Plantas Cultivadas; Fealq: Piracicaba, Brazil, 2005; 495p. [Google Scholar]
- Silva, E.A.A.; Toorop, P.E.; Nijsse, J.; Bewley, J.D.; Hilhorst, W.M. Exogenous gibberellins inhibit coffee (Coffea arabica cv. Rubi) seed germination and cause cell death in the embryo. J. Exp. Bot. 2005, 56, 1029–1038. [Google Scholar] [CrossRef]
- Domingues Neto, F.J.; Dalanhol, S.J.; Machry, M.; Pimentel Junior, A.; Rodrigues, J.D.; Ono, E.O. Effects of plant growth regulators on eggplant seed germination and seedling growth. Aust. J. Crop Sci. 2017, 11, 1277–1282. [Google Scholar] [CrossRef]
- Hartmann, H.; Kester, D.; Davies, F.; Geneve, R.; Wilson, S. Plant Propagation: Principles and Practices; Prentice Hall: New York, NY, USA, 2011; 915p. [Google Scholar]
- BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Regras para Análise de Sementes; Ministério Da Agricultura: Brasília, Brazil, 2009; 395p. [Google Scholar]
- Hadas, A. Water uptake germination of leguminous seeds under changing external water potential in osmotic solution. J. Exp. Bot 1976, 27, 480–489. [Google Scholar] [CrossRef]
- Maguire, J.D. Speed of germination aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Teisseire, H.; Guy, V. Copper-induced changes in antioxidant enzymes activities in fronds of duckweed (Lemna minor). Plant Sci. 2000, 153, 65–72. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, H.P.P.; Cambraia, J.; Sant’ana, R.; Mosquim, P.R.; Moreira, A.M. Aluminium effects on lipid peroxidation and the activities of enzymes of oxidative metabolism in sorghum. Rev. Bras. Fisiol. Veg. 1999, 11, 137–143. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photo chemistry in chloroplasts by dibromo thymoquinone. Biochim. Biophys. Acta 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Barker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Richet, N.; Afif, D.; Huber, F.; Pollet, B.; Banvoy, J.; Zein, R.E.; Lapierre, C.; Dizengremel, P.; Perré, P.; Cabané, M. Cellulose and lignin biosynthesis is altered by ozone in wood of hybrid poplar (Populus tremula × alba). J. Exp. Bot. 2011, 62, 3575–3586. [Google Scholar] [CrossRef]
- Borghetti, F.; Ferreira, A.G. Interpretação de resultados de germinação. In Germinação: Do Básico ao Aplicado; Ferreira, A.G., Borghetti, F., Eds.; Artmed: Porto Alegre, Brazil, 2004; pp. 209–222. [Google Scholar]
- Shant, P.S.; Rao, S.N. Note on the effect of Gibberellic acid on seed germination and seedling growth of Acid lime. Prog. Hortic. 1973, 5, 63–65. [Google Scholar]
- Misra, R.S.; Verma, V.K. Studies on the seed germination of kinnow orange in the central Himalayas. Prog. Hortic. 1979, 12, 79–84. [Google Scholar]
- Dilip, W.S.; Singh, D.; Moharana, D.; Rout, S.; Patra, S.S. Effect of gibberellic acid (GA) different concentrations at different time intervals on seed germination and seedling growth of Rangpur lime. J. Agro. Nat. Res. Man. 2017, 4, 157–165. [Google Scholar]
- Misra, R.S.; Singh, S.B. Effect of plant growth regulators and Ascorbic acid on germination and growth of Malta common seedlings in Garhwal hills. Prog. Hortic. 1982, 14, 165–168. [Google Scholar]
- Fu, Y.; Ma, L.; Li, J.; Hou, D.; Zeng, B.; Zhang, L.; Liu, C.; Bi, Q.; Tan, J.; Yu, X. Factors Influencing Seed Dormancy and Germination and Advances in Seed Priming Technology. Plants 2024, 13, 1319. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chien, C.T.; Chung, J.D.; Yang, Y.S.; Kuo, S.R. Dormancy-break and germination in seeds of Prunus campanulata (Rosaceae): Role of covering layers and changes in concentration of abscisic acid and gibberellins. Seed Sci. Res. 2007, 17, 21–32. [Google Scholar]
- Chen, S.Y.; Chien, C.T.; Baskin, J.M.; Baskin, C.C. Storage behavior and changes in concentrations of abscisic acid and gibberellins during dormancy break and germination in seeds of Phellodendron amurense var. wilsonii (Rutaceae). Tree Phys. 2010, 30, 275–284. [Google Scholar] [CrossRef]
- Levitt, J. Introduction to Plant Physiology, 2nd ed.; C.V. Mosby: Saint Louis, MO, USA, 1974; 447p. [Google Scholar]
- Kiran, S.D.C.; Shrishail, M.V.; Saini, S.M.; Prakash, H.T. Effect of Gibberellic acid on seed germination and seedling vigour of Indi’s Kagzi Lime [Citrus aurantifolia (Christmas) Swingle]. Int. J. Adv. Bioc. Res. 2024, 8, 355–358. [Google Scholar]
- Chaudhary, A.; Ahlawat, T.R.; Kumar, S.; Jena, S.; Patel, D. Effect of Gibberellic Acid on Germination and Vigour of Kagzi Lime Seedlings. Curr. J. Appl. Sci. Technol. 2019, 38, 1–8. [Google Scholar] [CrossRef]
- Ho, T.H.D.; Gomez, A.G.; Zentella, R.; Casaretto, J. Crosstalk between gibberellin and abscisic acid in cereal aleurone. J. Plant Growth Regul. 2003, 222, 185–194. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Wang, W. Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol. 2001, 127, 315–323. [Google Scholar] [CrossRef]
- Salisbury, F.B.; Ross, C.W. Plant Physiology; Wadsworth: Belmont, CA, USA, 1991; 759p. [Google Scholar]
- Zhao, Q.; Wang, H.; Yin, Y.; Xu, Y.; Chen, F.; Dixon, R.A. Syringyl lignin biosynthesis is directly regulated by a secondary cell wall master switch. Proc. Natl. Acad. Sci. USA 2010, 107, 14496–14501. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Shannon, J.C. Effect of gibberellin on growth, protein secretion and starch accumulation in maize endosperm suspension cells. J. Plant Growth Regul. 1997, 16, 37–140. [Google Scholar] [CrossRef]
- Van Huizen, R.; Ozga, J.A.; Reinecke, D.M. Influence of auxin and gibberellin on in vivo protein synthesis during early pea fruit growth. Plant Physiol. 1996, 112, 53–59. [Google Scholar] [CrossRef][Green Version]
- Patil, S.R.; Waskar, D.P.; Sonkamble, A.M. Effect of gibberellic acid, urea and neem cake on growthof Rangpur lime (Citrus limonia, Osbeck) seedlings. Asian J. Hortic. 2013, 8, 285–287. [Google Scholar]
- Meshram, P.C.; Joshi, P.S.; Bhoyar, R.K.; Sahoo, A.K. Effect of different plant growth regulators on seedling growth of acid lime. Res. Environ. Life Sci. 2015, 8, 725–728. [Google Scholar]
- Hardham, A.R.; Jones, D.A.; Takemoto, D. Cytoskeleton and cell wall function in penetration resistance. Curr. Opin. Plant Biol. 2007, 10, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.R.; Kandratavicius, L.; Leitte, J.P. O papel das proteínas do citoesqueleto na fisiologia celular normal e em condições patológicas. J. Epilepsy Clin. Neurophysiol. 2011, 17, 17–23. [Google Scholar] [CrossRef]
- Larcher, W. Ecofisiologia Vegetal; RIMA: São Carlos, Brazil, 2004. [Google Scholar]
- Caserta, R.; Teixeira-Silva, N.S.; Granato, L.M.; Dorta, S.O.; Rodrigues, C.M.; Mitre, L.K.; Yochikawa, J.T.H.; Fischer, E.R.; Nascimento, C.A.; Souza-Neto, R.R.; et al. Citrus biotechnology: What has been done to improve disease resistance in such an important crop? Biotechnol. Res. Innov. 2019, 3, 95–109. [Google Scholar] [CrossRef]
- Pinto, L.S.R.C. Avaliação do metabolismo fotossintético de plantas transgênicas de tabaco (Nicotiana tabacum) que expressam o gene Lhcb1*2 constitutivamente em condições de alta luminosidade. Tese de Doutorado, Universidade de São Paulo, Piracicaba, Brazil, 2000; 160p. [Google Scholar] [CrossRef]
- Crozier, A.; Kamiya, K.; Bishop, G.; Yokota, T. Biosynthesis of hormones and elicitor molecules. Biochem. Mol. Biol. Plants 2001, 850–929. [Google Scholar]
- Huerta, L.; Forment, J.; Gadea, J.; Fagoaga, C.; Peña, L.; Perez-Amador, M.A.; Garcia-Martinez, J. Gene expression analysis in citrus reveals the role of gibberellins on photosynthesis and stress. Plant Cell Environ. 2008, 31, 1620–1633. [Google Scholar] [CrossRef]
- Rodrigues, A.L. Respostas Fisiológicas e Estruturais em Plantas Submetidas a Estresse Hídrico Recorrente em Diferentes Condições de Luz. Tese De Doutorado, Univ. Estadual Paul., São Paulo, Brazil, 2018; 114p. Available online: https://bdtd.ibict.br/vufind/Record/UNSP_8eedb62a24a05f9f51b97502072b506f (accessed on 2 August 2024).
- Budanov, A.V.; Lee, J.H.; Karin, M. Stressin’ Sestrins take an aging fight. EMBO Mol. Med. 2010, 1, 1–13. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Rodríguez-Serrano, M.; Corpas, F.J.; Gomez, M.D.; Del Río, L.A.; Sandalio, L.M. Cadmium-induced subcellular accumulation of O2− and H2O2 in pea leaves. Plant Cell Environ. 2004, 27, 1122–1134. [Google Scholar] [CrossRef]
- Mandal, M.; Sarkar, M.; Khan, A.; Biswas, M.; Masi, A.; Rakwal, R.; Agrawal, G.K.; Srivastava, A.; Sarkar, A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) in plants– maintenance of structural individuality and functional blend. Adv. Redox Res. 2022, 5, 100039. [Google Scholar] [CrossRef]
- Zhou, Y.; He, R.; Guo, Y.; Liu, K.; Huang, G.; Peng, C.; Liu, Y.; Zhang, M.; Li, Z.; Duan, L. A novel ABA functional analogue B2 enhances drought tolerance in wheat. Sci. Rep. 2019, 9, 2887. [Google Scholar] [CrossRef]
- Sharp, R.E. Interaction with ethylene: Changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell Environ. 2002, 25, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Thorne, E.T.; Sharp, R.E.; Cosgrove, D.J. Modification of expansin transcript levels in the maize primary root at low water potentials. Plant Physiol. 2001, 126, 1471–1479. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingues Neto, F.J.; Carneiro, D.C.d.S.; Putti, F.F.; Rodrigues, J.D.; Tecchio, M.A.; Leonel, S.; Silva, M.d.S. Physiological Indexes in Seed Germination and Seedling Growth of Rangpur Lime (Citrus limonia L. Osbeck) under Plant Growth Regulators. Agronomy 2024, 14, 2066. https://doi.org/10.3390/agronomy14092066
Domingues Neto FJ, Carneiro DCdS, Putti FF, Rodrigues JD, Tecchio MA, Leonel S, Silva MdS. Physiological Indexes in Seed Germination and Seedling Growth of Rangpur Lime (Citrus limonia L. Osbeck) under Plant Growth Regulators. Agronomy. 2024; 14(9):2066. https://doi.org/10.3390/agronomy14092066
Chicago/Turabian StyleDomingues Neto, Francisco José, Débora Cavalcante dos Santos Carneiro, Fernando Ferrari Putti, João Domingos Rodrigues, Marco Antonio Tecchio, Sarita Leonel, and Marcelo de Souza Silva. 2024. "Physiological Indexes in Seed Germination and Seedling Growth of Rangpur Lime (Citrus limonia L. Osbeck) under Plant Growth Regulators" Agronomy 14, no. 9: 2066. https://doi.org/10.3390/agronomy14092066
APA StyleDomingues Neto, F. J., Carneiro, D. C. d. S., Putti, F. F., Rodrigues, J. D., Tecchio, M. A., Leonel, S., & Silva, M. d. S. (2024). Physiological Indexes in Seed Germination and Seedling Growth of Rangpur Lime (Citrus limonia L. Osbeck) under Plant Growth Regulators. Agronomy, 14(9), 2066. https://doi.org/10.3390/agronomy14092066







