Genotype and Nitrogen Source Influence Drought Stress Response in Oil Palm Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions and Experimental Design
2.2. Biomass, Root Length, Physiological Parameters, and Biochemical Indexes
2.3. Statistical Analysis
3. Results
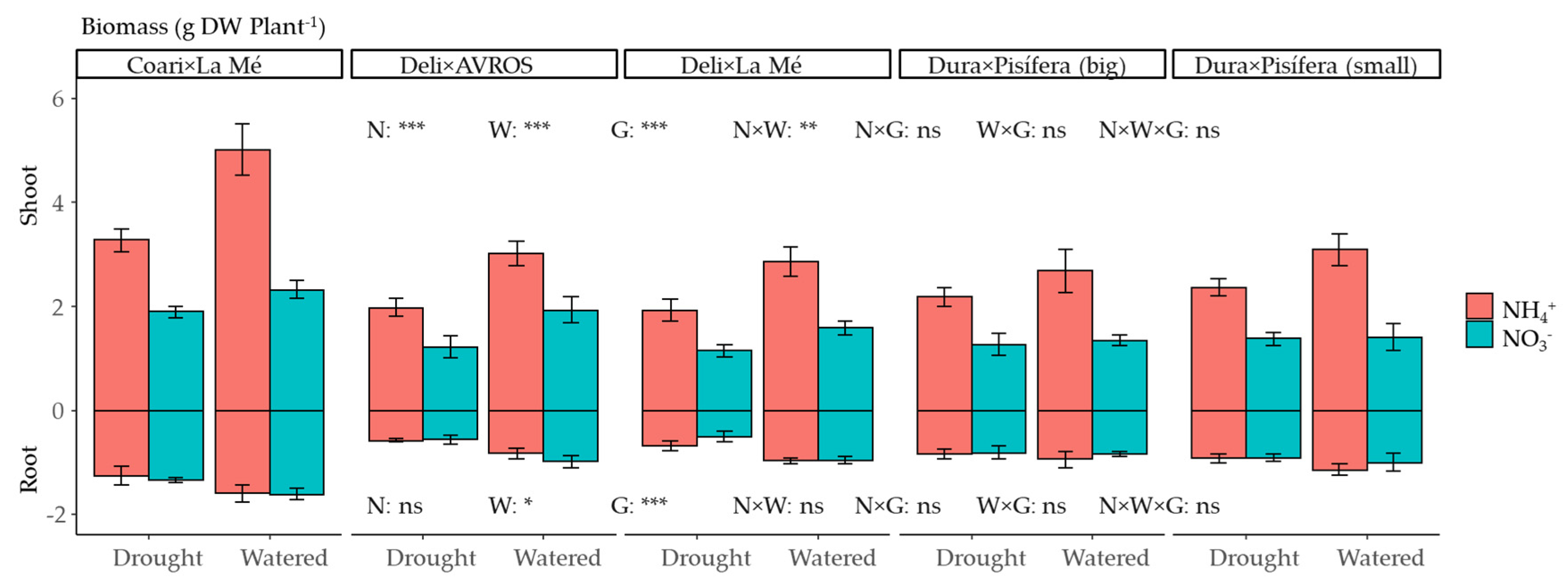
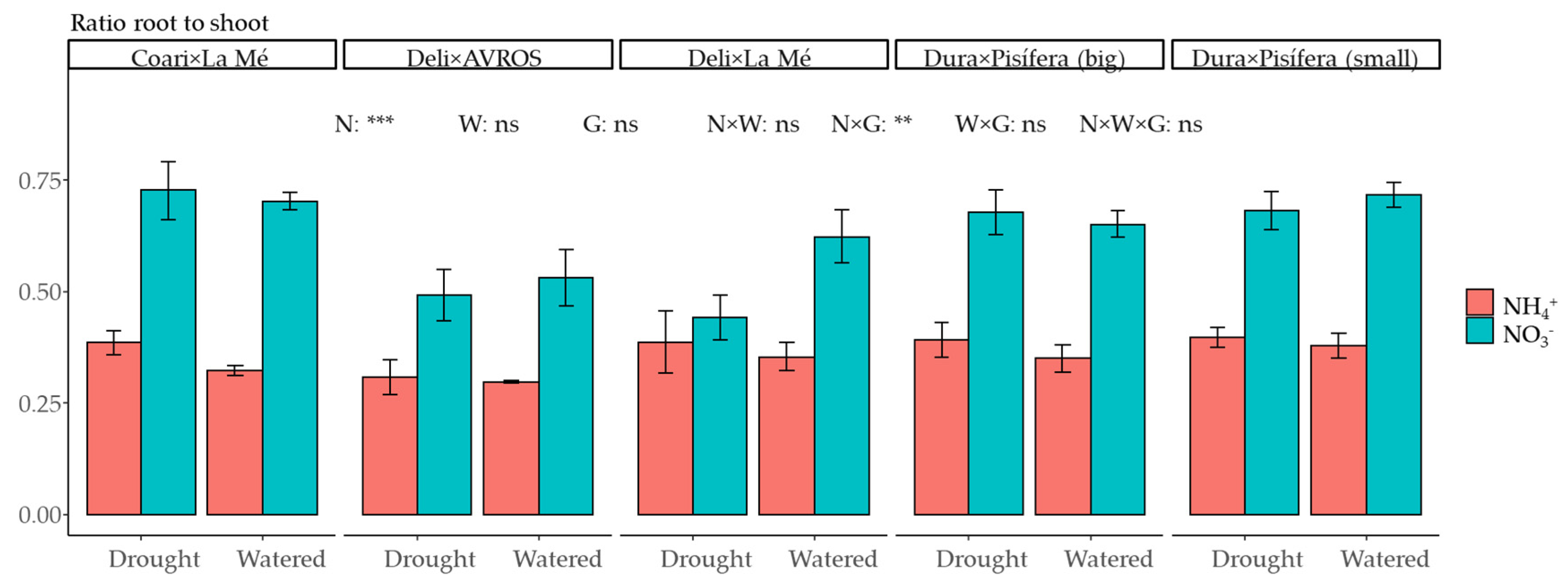
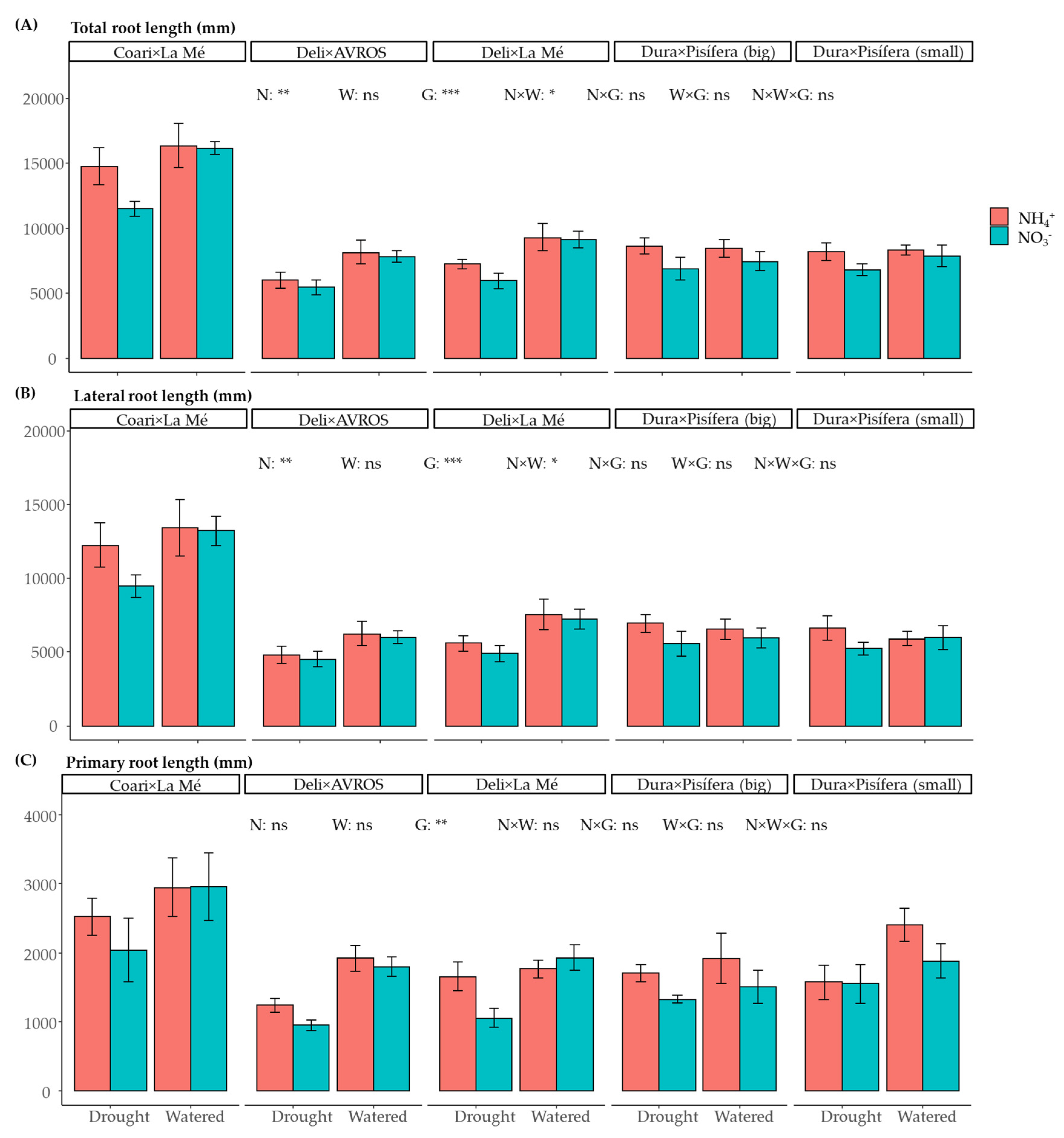
3.1. Growth and Development
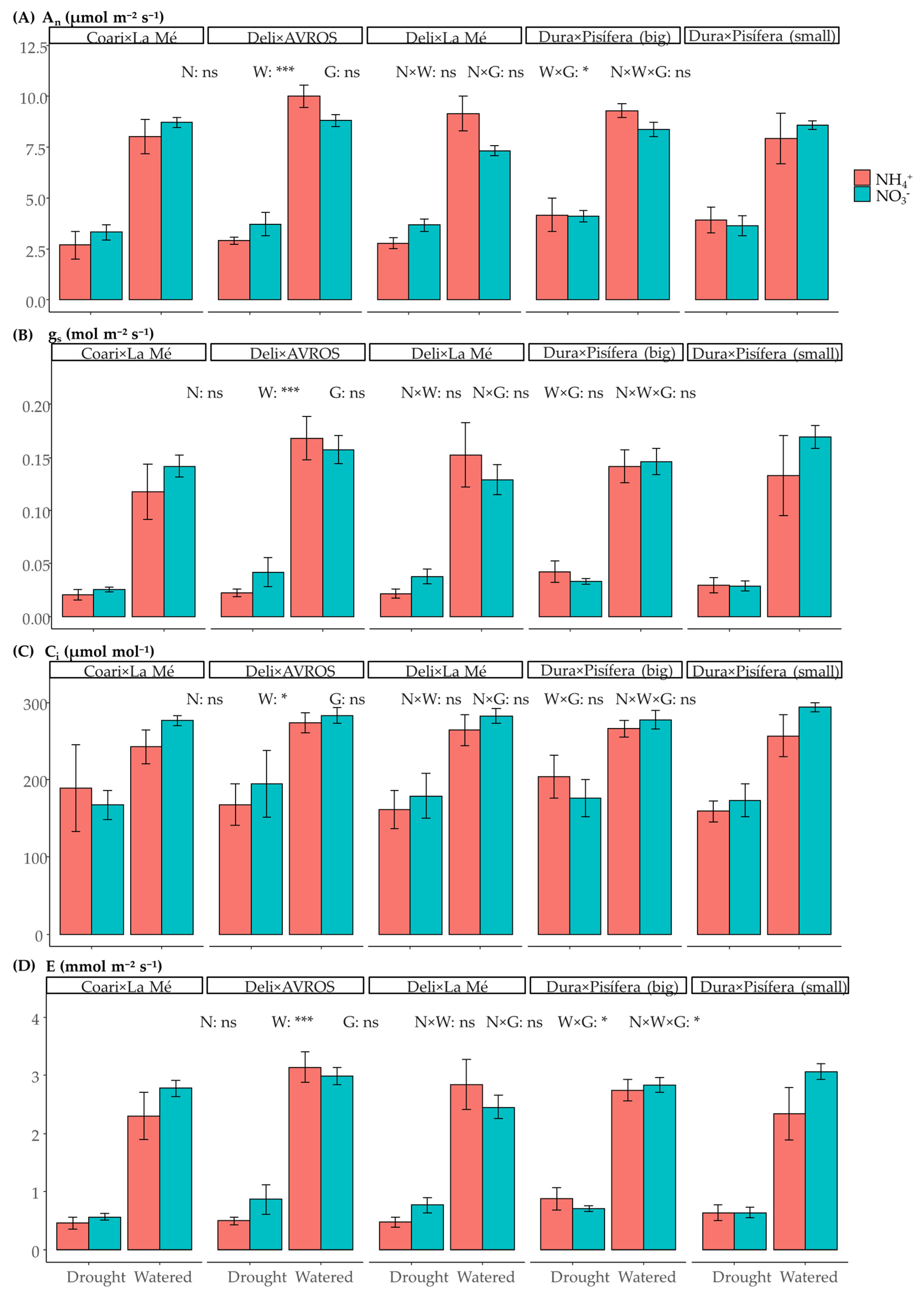
3.2. Leaf Gas Exchange and Fluorescence Parameters
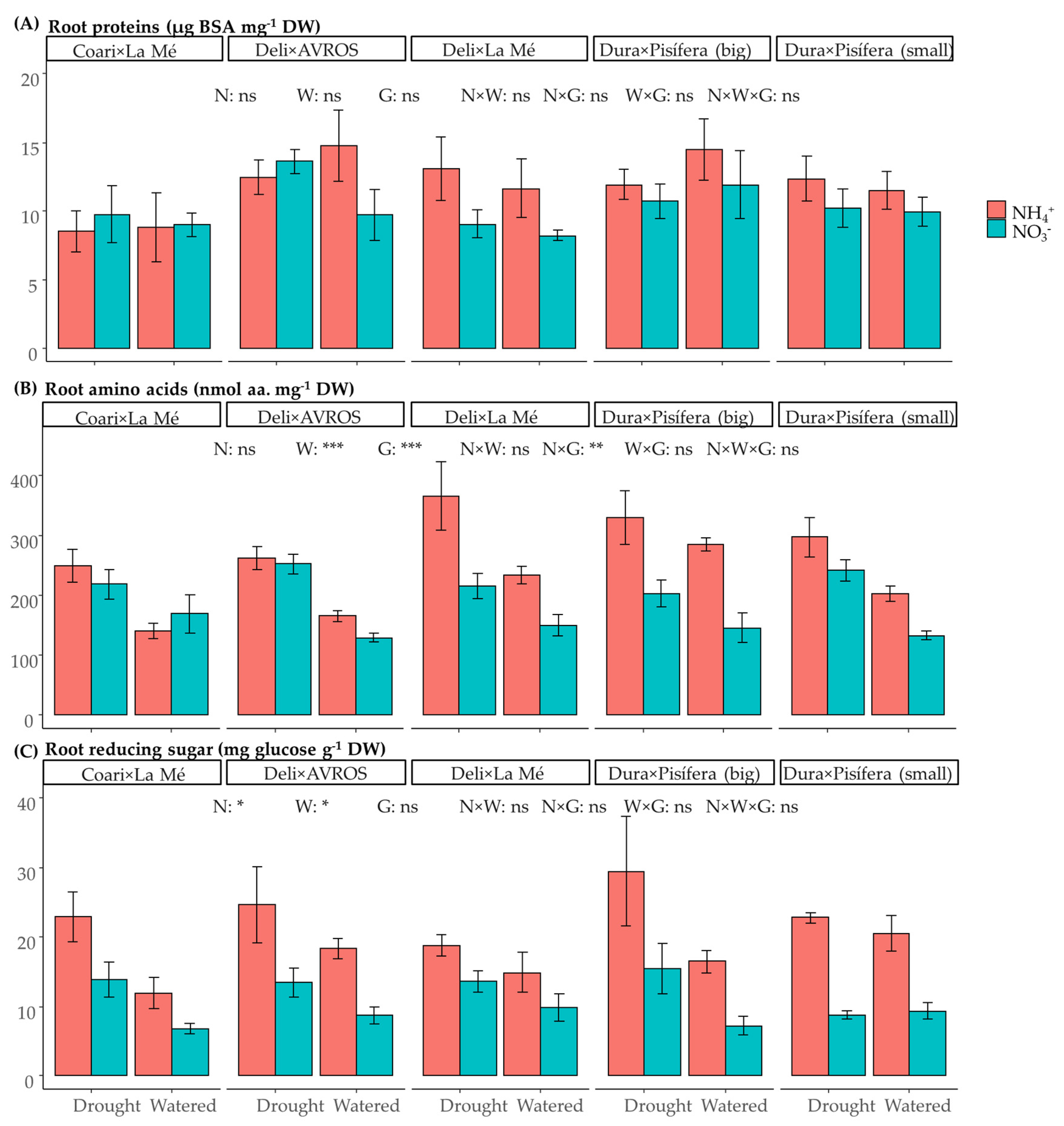
3.3. Metabolite Content
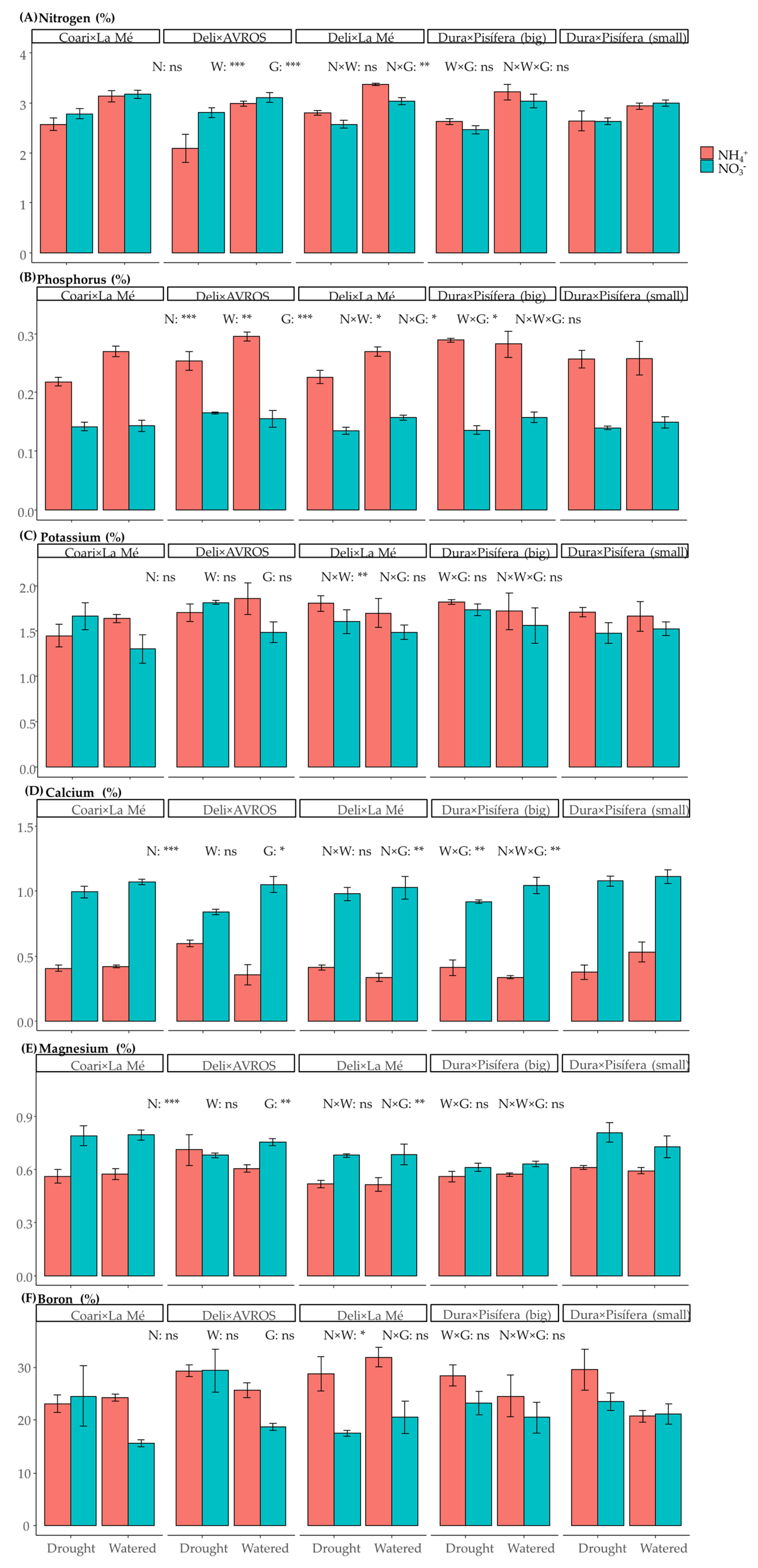
3.4. Foliar Content of Essential Minerals
4. Discussion
5. Conclusions
- 1.
- The choice of nitrogen fertilizer (ammonium vs. nitrate) can significantly impact the drought tolerance of oil palm seedlings, with potential long-term effects on plantation productivity under water-limited conditions.
- 2.
- The observed genotypic variations suggest that the careful selection of oil palm cultivars could simultaneously optimize the nitrogen use efficiency and drought tolerance.
- 3.
- The complex interactions between nitrogen nutrition and the root architecture under drought stress highlight the need for holistic approaches in breeding programs considering above-ground and below-ground plant responses.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture Foreign Agricultural Service. Oilseeds: World Markets and Trade; United States Department of Agriculture Foreign Agricultural Service: New York, NY, USA, 2024. [Google Scholar]
- Marulanda Pérez, N. Cosechando Historia, Sembrando Futuro. Palmas 2022, 43, 8–11. [Google Scholar]
- Arias-Arias, N.A. Indicadores y Eficiencia de La Nutrición Para La Toma de Decisiones. Palmas 2019, 40, 128–139. [Google Scholar]
- Mesa Dishington, J. Desafíos Para La Palmicultura Colombiana Challenges for the Oil Palm Agribusiness in Colombia. Palmas 2016, 37, 78–82. [Google Scholar]
- De la Peña, M.; Ruiz-Romero, R.; Romero, H.M. Nitrogen Use Efficiency in Oil Palm Seedlings: Unraveling the Untapped Potential of Elevated External Ammonium Supply. Plants 2023, 12, 2819. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.Y.; Tian, W.H.; Jin, C.W. Nitrogen in Plants: From Nutrition to the Modulation of Abiotic Stress Adaptation. Stress Biol. 2022, 2, 4. [Google Scholar] [CrossRef]
- Yang, X.; Rasheed, A.; Yan, H.; Zhou, C.; Shang, Q. Effects of Nitrogen Forms and Osmotic Stress on Root Aerenchyma Formation of Japonica Rice and Its Relationship with Water Absorption. Int. J. Agric. Biol. 2018, 20, 2287–2292. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Miller, A.J.; Cramer, M.D. Root Nitrogen Acquisition and Assimilation. Plant Soil 2005, 274, 1–36. [Google Scholar] [CrossRef]
- Iqbal, A.; Dong, Q.; Wang, X.; Gui, H.; Zhang, H.; Zhang, X.; Song, M. High Nitrogen Enhance Drought Tolerance in Cotton. Plants 2020, 9, 178. [Google Scholar] [CrossRef]
- Marino, D.; Moran, J.F. Can Ammonium Stress Be Positive for Plant Performance? Front. Plant Sci. 2019, 10, 1103. [Google Scholar] [CrossRef]
- De la Peña, M.; Ruiz-Romero, R.; Castro-Arza, L.I.; Romero, H.M. Changes in the Root Architecture of Oil Palm Seedlings in Response to Nitrogen Starvation. Agronomy 2024, 14, 409. [Google Scholar] [CrossRef]
- Cross, J.M.; Von Korff, M.; Altmann, T.; Bartzetko, L.; Sulpice, R.; Gibon, Y.; Palacios, N.; Stitt, M. Variation of Enzyme Activities and Metabolite Levels in 24 Arabidopsis Accessions Growing in Carbon-Limited Conditions. Plant Physiol. 2006, 142, 1574–1588. [Google Scholar] [CrossRef] [PubMed]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R.E. The Determination of Amino-Acids with Ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Somogyi, M. Notes on Sugar Determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar] [CrossRef]
- Spencer, R.R.; Erdmann, D.E. Azomethine H Colorimetric Method for Determining Dissolved Boron in Water. Environ. Sci. Technol. 1979, 13, 954–956. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.r-project.org/ (accessed on 8 September 2024).
- Ma, D.; Teng, W.; Yi, B.; Lin, Y.; Pan, Y.; Wang, L. Effects of the Nitrate and Ammonium Ratio on Plant Characteristics and Erythropalum Scandens Bl. Substrates. PLoS ONE 2023, 18, 0289659. [Google Scholar] [CrossRef]
- Salgado, F.F.; da Silva, T.L.C.; Vieira, L.R.; Silva, V.N.B.; Leão, A.P.; Costa, M.M.D.C.; Togawa, R.C.; Sousa, C.A.F.; Grynberg, P.; Souza, M.T. The Early Response of Oil Palm (Elaeis Guineensis Jacq.) Plants to Water Deprivation: Expression Analysis of MiRNAs and Their Putative Target Genes, and Similarities with the Response to Salinity Stress. Front. Plant Sci. 2022, 13, 970113. [Google Scholar] [CrossRef]
- Wang, L.; Lee, M.; Ye, B.; Yue, G.H. Genes, Pathways and Networks Responding to Drought Stress in Oil Palm Roots. Sci. Rep. 2020, 10, 21303. [Google Scholar] [CrossRef]
- Pangaribuan, I.F.; Akoeb, E.N. Analysis of Morphological Responses of Drought Stress Oil Palm in Nursery Phase. IOP Conf. Ser. Earth Environ. Sci. 2022, 977, 01213. [Google Scholar] [CrossRef]
- Jazayeri, S.M.; Rivera, Y.D.; Camperos-Reyes, J.E.; Romero, H.M. Physiological Effects of Water Deficit on Two Oil Palm (Elaeis Guineensis Jacq.) Genotypes. Agron. Colomb. 2015, 33, 164–173. [Google Scholar] [CrossRef]
- Silva, P.A.; Cosme, V.S.; Rodrigues, K.C.B.; Detmann, K.S.C.; Leão, F.M.; Cunha, R.L.; Festucci Buselli, R.A.; DaMatta, F.M.; Pinheiro, H.A. Drought Tolerance in Two Oil Palm Hybrids as Related to Adjustments in Carbon Metabolism and Vegetative Growth. Acta Physiol. Plant. 2017, 39, 58. [Google Scholar] [CrossRef]
- Cabrera-Bosquet, L.; Molero, G.; Bort, J.; Nogués, S.; Araus, J.L. The Combined Effect of Constant Water Deficit and Nitrogen Supply on WUE, NUE and Δ13C in Durum Wheat Potted Plants. Ann. Appl. Biol. 2007, 151, 277–289. [Google Scholar] [CrossRef]
- Frechilla, S.; Gonzalez, E.M.; Royuela, M.; Minchin, F.R.; Aparicio-Tejo, P.M.; Arrese-Igor, C. Source of Nitrogen Nutrition (Nitrogen Fixation or Nitrate Assimilation) Is a Major Factor Involved in Pea Response to Moderate Water Stress. J. Plant Physiol. 2000, 157, 609–617. [Google Scholar] [CrossRef]
- Gloser, V.; Dvorackova, M.; Mota, D.H.; Petrovic, B.; Gonzalez, P.; Geilfus, C.M. Early Changes in Nitrate Uptake and Assimilation Under Drought in Relation to Transpiration. Front. Plant Sci. 2020, 11, 602065. [Google Scholar] [CrossRef]
- Lu, Y.X.; Li, C.J.; Zhang, F.S. Transpiration, Potassium Uptake and Flow in Tobacco as Affected by Nitrogen Forms and Nutrient Levels. Ann. Bot. 2005, 95, 991–998. [Google Scholar] [CrossRef]
- Alencar, V.T.C.B.; Lobo, A.K.M.; Carvalho, F.E.L.; Silveira, J.A.G. High Ammonium Supply Impairs Photosynthetic Efficiency in Rice Exposed to Excess Light. Photosynth. Res. 2019, 140, 321–335. [Google Scholar] [CrossRef]
- Jin, E.J.; Yoon, J.H.; Lee, H.; Bae, E.J.; Yong, S.H.; Choi, M.S. Evaluation of Drought Stress Level in Sargent’s Cherry (Prunus Sargentii Rehder) Using Photosynthesis and Chlorophyll Fluorescence Parameters and Proline Content Analysis. PeerJ 2023, 11, e15954. [Google Scholar] [CrossRef]
- Molla, A.E.; Andualem, A.M.; Ayana, M.T.; Zeru, M.A. Effects of Drought Stress on Growth, Physiological and Biochemical Parameters of Two Ethiopian Red Pepper (Capsicum annum L.) Cultivars. J. Appl. Hortic. 2023, 25, 32–38. [Google Scholar] [CrossRef]
- González-Moro, M.B.; González-Moro, I.; de la Peña, M.; Estavillo, J.M.; Aparicio-Tejo, P.M.; Marino, D.; González-Murua, C.; Vega-Mas, I. A Multi-Species Analysis Defines Anaplerotic Enzymes and Amides as Metabolic Markers for Ammonium Nutrition. Front. Plant Sci. 2021, 11, 632285. [Google Scholar] [CrossRef]
- de la Peña, M.; González-Moro, M.B.; Marino, D. Providing Carbon Skeletons to Sustain Amide Synthesis in Roots Underlines the Suitability of Brachypodium Distachyon for the Study of Ammonium Stress in Cereals. AoB Plants 2019, 11, plz029. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Photosynthetic Nitrogen Assimilation: Inter-Pathway Control and Signaling. In Photosynthetic Nitrogen Assimilation and Associated Carbon and Respiratory Metabolism; Foyer, C.H., Noctor, G., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 1–22. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ Toxicity in Higher Plants: A Critical Review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, G.; Kinoshita, T.; Zhang, R.; Zhu, Y.; Shen, Q.; Xu, G. Stimulation of Phosphorus Uptake by Ammonium Nutrition Involves Plasma Membrane H+ ATPase in Rice Roots. Plant Soil 2012, 357, 205–214. [Google Scholar] [CrossRef]


| Genotype | Ammonium (MPa) | Nitrate (MPa) |
|---|---|---|
| C × LM | −1.85 ± 0.5 | −1.22 ± 0.56 |
| D × A | −1.05 ± 0.27 | −0.95 ± 0.48 |
| D × LM | −0.95 ± 0.27 | −0.98 ± 0.24 |
| D × P (large) | −1.26 ± 0.37 | −0.87 ± 0.34 |
| D × P (small) | −1.16 ± 0.32 | −0.95 ± 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Romero, R.; De la Peña, M.; Ayala-Díaz, I.; Montoya, C.; Romero, H.M. Genotype and Nitrogen Source Influence Drought Stress Response in Oil Palm Seedlings. Agronomy 2024, 14, 2082. https://doi.org/10.3390/agronomy14092082
Ruiz-Romero R, De la Peña M, Ayala-Díaz I, Montoya C, Romero HM. Genotype and Nitrogen Source Influence Drought Stress Response in Oil Palm Seedlings. Agronomy. 2024; 14(9):2082. https://doi.org/10.3390/agronomy14092082
Chicago/Turabian StyleRuiz-Romero, Rodrigo, Marlon De la Peña, Iván Ayala-Díaz, Carmenza Montoya, and Hernán Mauricio Romero. 2024. "Genotype and Nitrogen Source Influence Drought Stress Response in Oil Palm Seedlings" Agronomy 14, no. 9: 2082. https://doi.org/10.3390/agronomy14092082
APA StyleRuiz-Romero, R., De la Peña, M., Ayala-Díaz, I., Montoya, C., & Romero, H. M. (2024). Genotype and Nitrogen Source Influence Drought Stress Response in Oil Palm Seedlings. Agronomy, 14(9), 2082. https://doi.org/10.3390/agronomy14092082






