Exogenous Substances Improved Salt Tolerance in Cotton
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Location and Materials
2.2. Experimental Design
2.3. Index Determination and Methods
2.3.1. Growth Index Determination
2.3.2. Physiological Index Measurement
2.4. Statistical Analysis
3. Results
3.1. Growth Characteristics
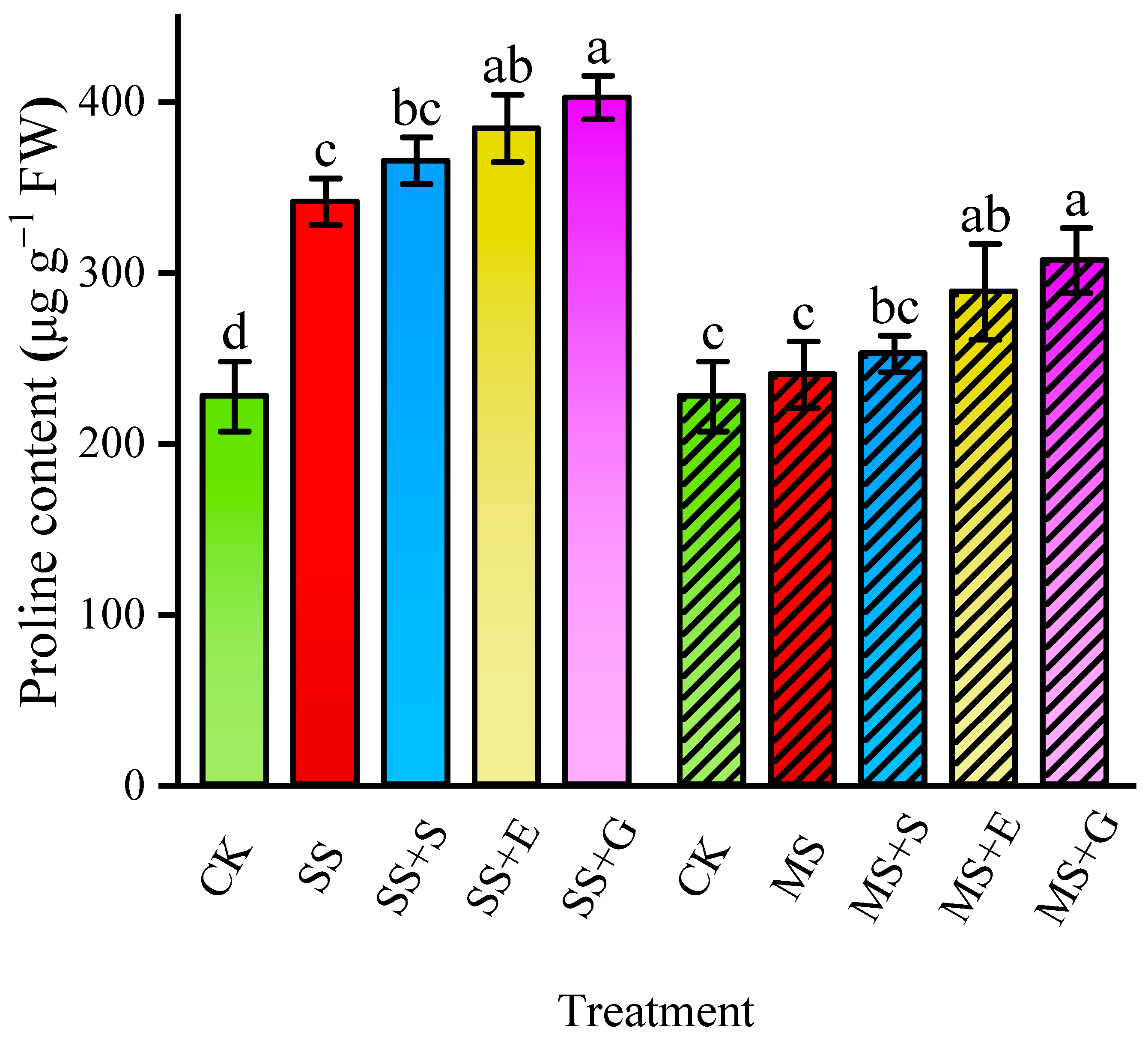
3.2. Proline Content
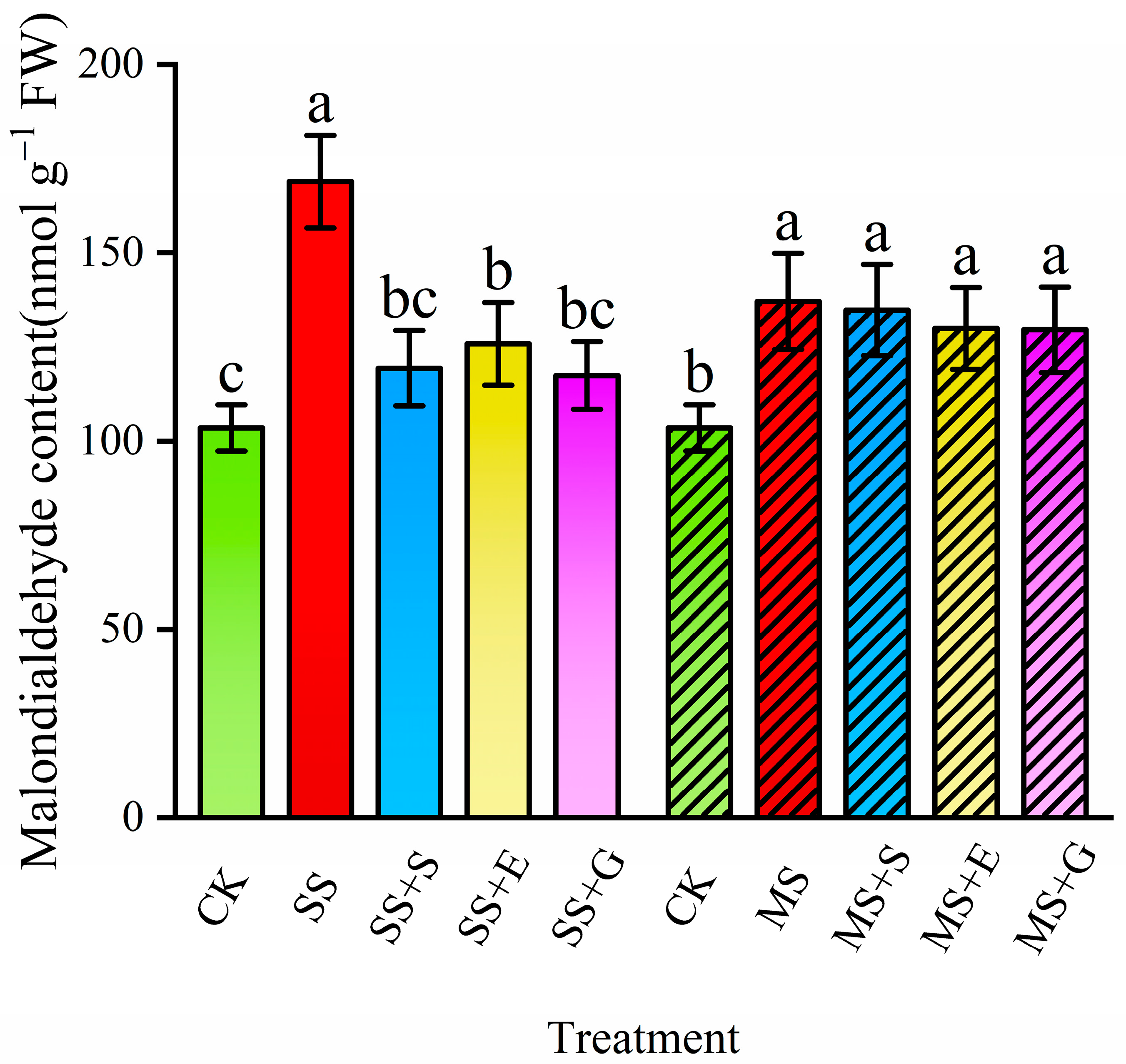
3.3. Malondialdehyde Content
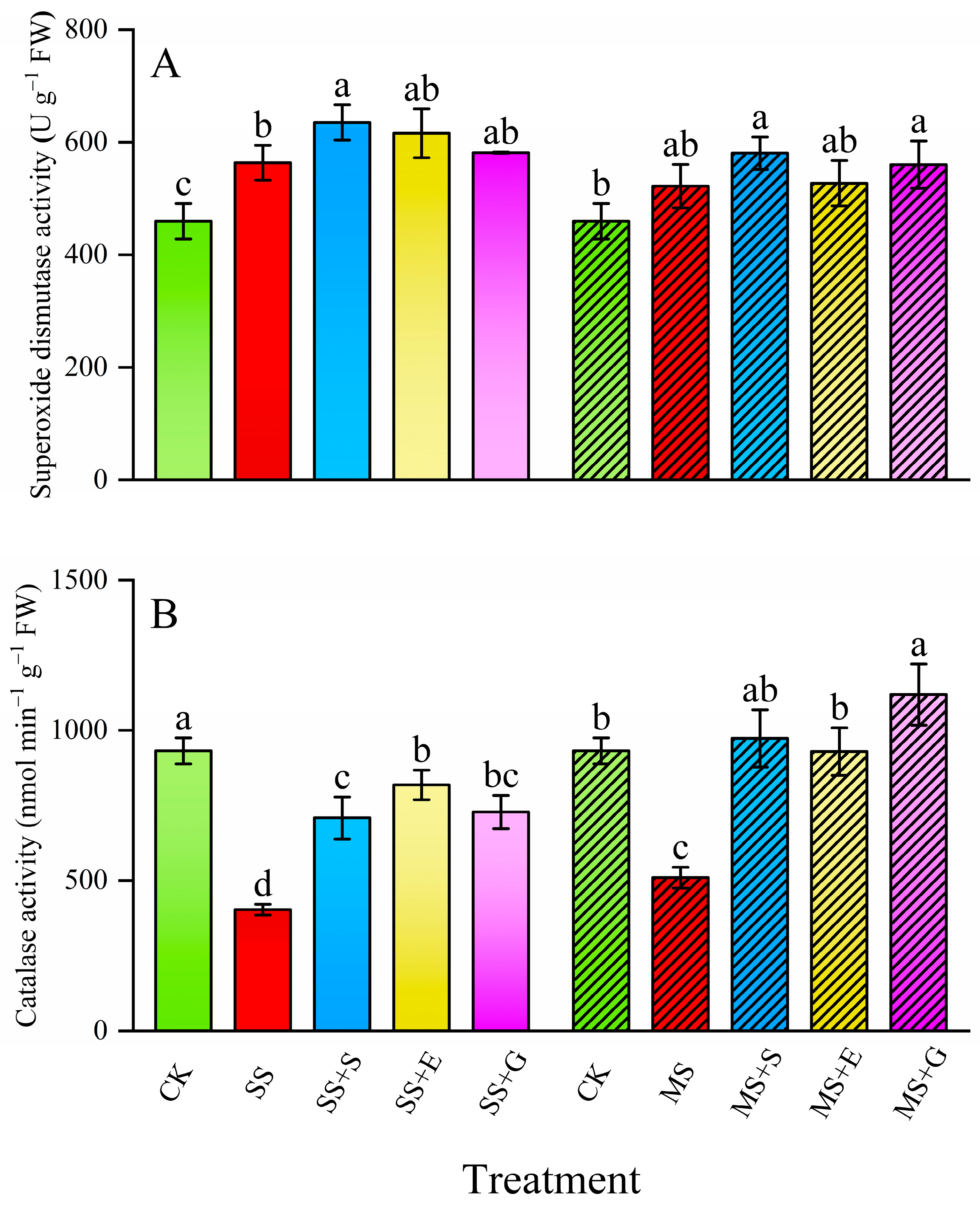
3.4. Antioxidant Enzyme Activity
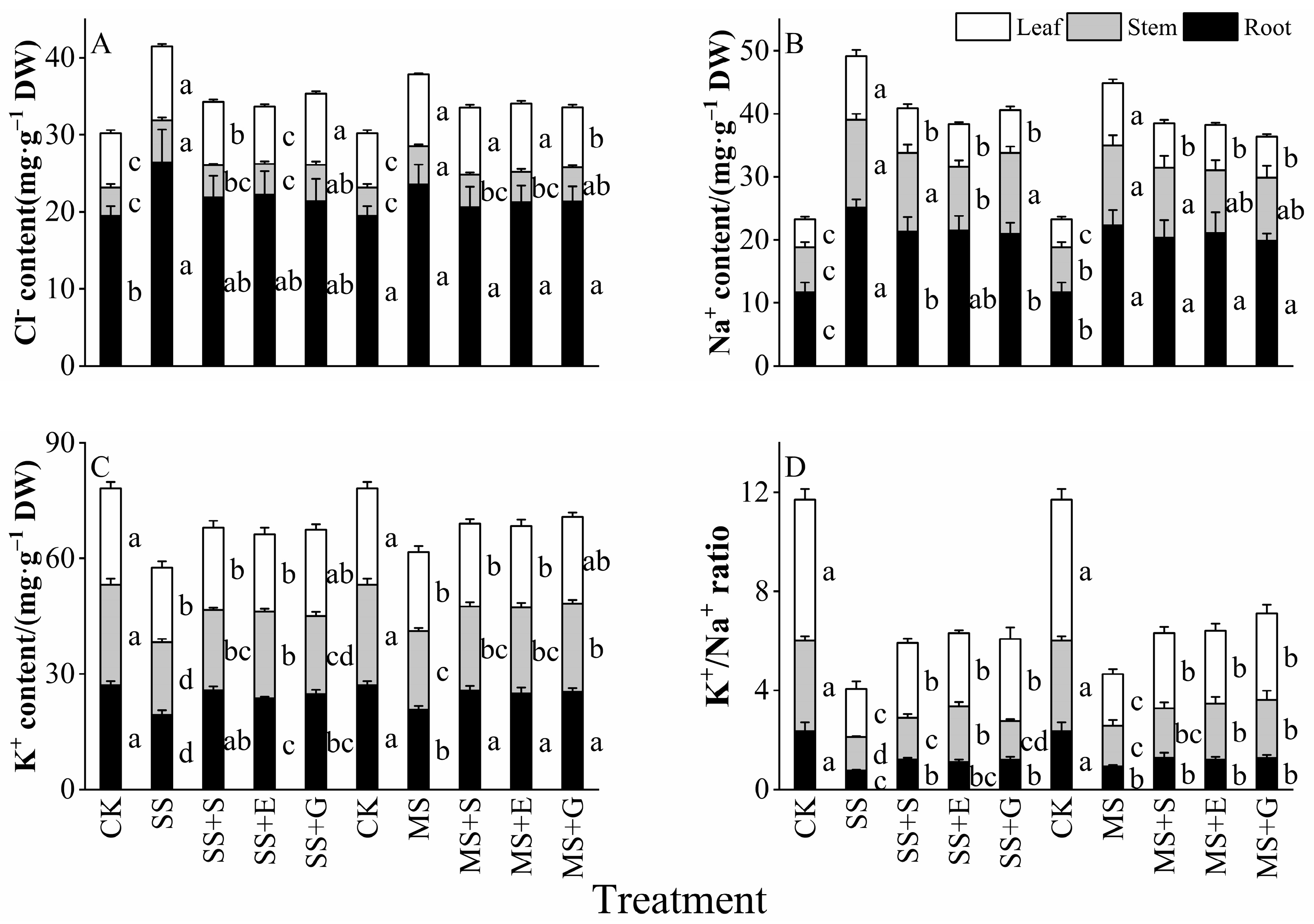
3.5. Ionic Content and K+/Na+ Ratio
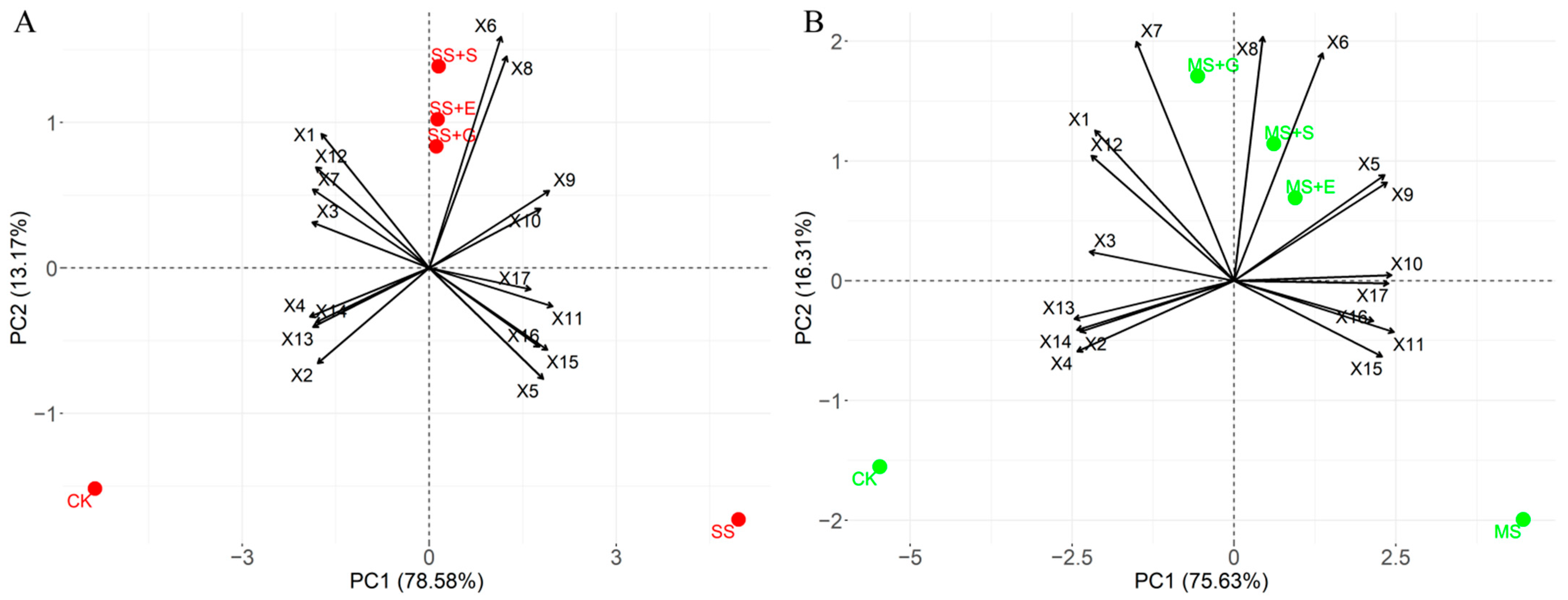
3.6. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, Z.T.; Bai, W.Q.; Xie, C.; Yu, J.; Dai, Y.L.; Pei, S.Z.; Zhang, F.c.; Li, Y.X.; Fan, J.L.; Yin, F. Irrigation depth and nitrogen rate effects on seed cotton yield, fiber quality and water-nitrogen utilization efficiency in southern Xinjiang, China. Agric. Water Manag. 2023, 290, 108583. [Google Scholar] [CrossRef]
- Wang, R.S.; Kang, Y.H.; Wan, S.Q. Effects of different drip irrigation regimes on saline–sodic soil nutrients and cotton yield in an arid region of Northwest China. Agric. Water Manag. 2015, 153, 1–8. [Google Scholar] [CrossRef]
- Lin, S.D.; Wang, Q.J.; Deng, M.J.; Su, L.J.; Wei, K.; Guo, Y.; Zhang, J.H. Assessing the influence of water fertilizer, and climate factors on seed cotton yield under mulched drip irrigation in Xinjiang Agricultural Regions. Eur. J. Agron. 2024, 152, 127034. [Google Scholar] [CrossRef]
- Hou, X.H.; Fan, J.L.; Zhang, F.C.; Hu, W.H.; Xiang, Y.Z. Optimization of water and nitrogen management to improve seed cotton yield, water productivity and economic benefit of mulched drip-irrigated cotton in southern Xinjiang, China. Field Crops Res. 2024, 308, 109301. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Sun, L.; Luo, Q.Y.; Chen, H.Y.; Yang, Y.D. Spatial optimization of cotton cultivation in Xinjiang: A climate change perspective. Int. J. Appl. Earth Obs. Geoinf. 2023, 124, 103523. [Google Scholar] [CrossRef]
- Xiao, C.; Ji, Q.Y.; Zhang, F.C.; Li, Y.; Fan, J.L.; Hou, X.H.; Yan, F.L.; Liu, X.Q.; Gong, K.Y. Effects of various soil water potential thresholds for drip irrigation on soil salinity, seed cotton yield and water productivity of cotton in northwest China. Agric. Water Manag. 2023, 279, 108172. [Google Scholar] [CrossRef]
- Ashraf, M. Salt tolerance of cotton: Some new advances. Crit. Rev. Plant Sci 2002, 21, 1–30. [Google Scholar] [CrossRef]
- Maryum, Z.; Luqman, T.; Nadeem, S.; Khan, S.M.U.D.; Wang, B.; Ditta, A.; Khan, M.K.R. An overview of salinity stress, mechanism of salinity tolerance and strategies for its management in cotton. Front. Plant Sci. 2022, 13, 907937. [Google Scholar] [CrossRef]
- Lu, K.S.; Yan, L.; Riaz, M.; Babar, S.; Hou, J.Y.; Zhang, Y.L.; Jiang, C. Exogenous boron alleviates salt stress in cotton by maintaining cell wall structure and ion homeostasis. Plant Physiol. Biochem. 2023, 201, 107858. [Google Scholar] [CrossRef]
- Chen, L.; Li, L.T.; Lu, B.; Ma, T.T.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.C.; Zhang, Y.J.; Bai, Z.Y.; et al. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar] [CrossRef]
- Ren, F.T.; Yang, G.; Li, W.J.; He, X.L.; Gao, Y.L.; Tian, L.J.; Li, F.D.; Wang, Z.L.; Liu, S.H. Yield-compatible salinity level for growing cotton (Gossypium hirsutum L.) under mulched drip irrigation using saline water. Agric. Water Manag. 2021, 250, 106859. [Google Scholar] [CrossRef]
- Sheri, V.; Kumar, M.; Jaconis, S.; Zhang, B.H. Antioxidant defense in cotton under environmental stresses: Unraveling the crucial role of a universal defense regulator for enhanced cotton sustainability. Plant Physiol. Biochem. 2023, 204, 108141. [Google Scholar] [CrossRef] [PubMed]
- Li, H.P.; Sun, H.C.; Ping, W.C.; Liu, L.T.; Zhang, Y.J.; Zhang, K.; Bai, Z.Y.; Li, A.C.; Zhu, J.J.; Li, C.D. Exogenous ethylene promotes the germination of cotton seeds under salt stress. J. Plant Growth Regul. 2023, 42, 3923–3933. [Google Scholar] [CrossRef]
- Cheng, B.Z.; Li, Z.; Liang, L.L.; Cao, Y.; Zeng, W.H.; Zhang, X.Q.; Ma, X.; Huang, L.K.; Nie, G.; Liu, W. The γ-aminobutyric acid (GABA) alleviates salt stress damage during seeds germination of white clover associated with Na+/K+ transportation, dehydrins accumulation, and stress-related genes expression in white clover. Int. J. Mol. Sci. 2018, 19, 2520. [Google Scholar] [CrossRef]
- Sousa, V.Q.; Messias, W.F.S.; Pereira, Y.C.; da Silva, B.R.S.; Lobato, E.M.S.G.; Alyemeni, M.N.; Ahmad, P.; Lobato, A.K.d.S. Pretreatment with 24-epibrassinolide synergistically protects root structures and chloroplastic pigments and upregulates antioxidant enzymes and biomass in Na+-stressed tomato plants. J. Plant Growth Regul 2021, 41, 2869–2885. [Google Scholar] [CrossRef]
- Wang, J.J.; Ye, W.W.; Zhou, D.Y.; LV, Y.J.; FAN, B.X.; Song, L.Y. Studies on germinationcharacteristics of diferent salinity-resistant cotton under salt stress. Cotton Sci. 2007, 19, 315–317. [Google Scholar] [CrossRef]
- Khan, M.O.; Irfan, M.; Muhammad, A.; Ullah, I.; Nawaz, S.; Khalil, M.K.; Ahmad, M. A practical and economical strategy to mitigate salinity stress through seed priming. Front. Environ. Sci. 2022, 10, 1–14. [Google Scholar] [CrossRef]
- Gupta, S.; Doležal, K.; Kulkarni, M.G.; Balázs, E.; Van Staden, J. Role of non-microbial biostimulants in regulation of seed germination and seedling establishment. Plant Growth Regul. 2022, 97, 271–313. [Google Scholar] [CrossRef]
- Batool, Z.; Ishfaq, M.; Akbar, N.; Zulfiqar, U.; Anjum, S.A.; Shafiq, M.; Nazir, S.; Aziz, A. Exogenous application of Atonik (sodium nitrophenolate) under skip irrigation regimes modulated the physiology, growth and productivity of Zea mays L. Arch. Agron. Soil Sci. 2023, 69, 2325–2339. [Google Scholar] [CrossRef]
- Swaefy, H.M.; El-Ziat, R.A. Calendula response to salinity stress. New Perspect. Agric. Crop Sci. 2020, 3, 978. [Google Scholar] [CrossRef]
- Mu, D.W.; Feng, N.J.; Zheng, D.F.; Zhou, H.; Liu, L.; Chen, G.J.; Mu, B.M. Physiological mechanism of exogenous brassinolide alleviating salt stress injury in rice seedlings. Sci. Rep. 2022, 12, 20439. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chai, J.L.; Liu, W.Y.; Zhu, X.L.; Liu, H.X.; Wei, X.H. Promotion of Ca2+ Accumulation in Roots by Exogenous Brassinosteroids as a Key Mechanism for Their Enhancement of Plant Salt Tolerance: A Meta-Analysis and Systematic Review. Int. J. Mol. Sci. 2023, 24, 16123. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.J.; Gong, J.Q.; Luo, S.T.; Zuo, Y.X.; Shen, Y.B. Role of gamma-aminobutyric acid in plant defense response. Metabolites 2023, 13, 741. [Google Scholar] [CrossRef]
- Alkharabsheh, H.M.; Seleiman, M.F.; Hewedy, O.A.; Battaglia, M.L.; Jalal, R.S.; Alhammad, B.A.; Schillaci, C.; Ali, N.; Al-Doss, A. Field crop responses and management strategies to mitigate soil salinity in modern agriculture: A review. Agronomy 2021, 11, 2299. [Google Scholar] [CrossRef]
- Hamani, A.K.M.; Wang, G.S.; Soothar, M.K.; Shen, X.J.; Gao, Y.; Qiu, R.J.; Mehmood, F. Responses of leaf gas exchange attributes, photosynthetic pigments and antioxidant enzymes in NaCl-stressed cotton (Gossypium hirsutum L.) seedlings to exogenous glycine betaine and salicylic acid. BMC Plant Biol. 2020, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Li, Y.M.; Yuan, Z.; Liu, X.H.; Shu, X.Y.; Liu, J.Y.; Guo, C.F. Cotton production pattern and contribution factors in Xinjiang from 1988 to 2020. J. Agric. Resour. Environ. 2024, 1–12. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.J.; Zhang, S.Y. Effects of silicon on growth andosmotic regulation of cotton seedlings under salt stress. Acta Agric. Boreali-Sin. 2019, 34, 110–117. [Google Scholar] [CrossRef]
- Duan, W.J.; Meng, Y.J.; Jiang, D.; Liu, L.T.; Zhang, K.; Zhang, Y.J.; Sun, H.C.; Ba, i.Z.Y.; Li, C.D. Effects of exogenous melatonin on the morphology and antioxidant enzyme activities of cotton seedlings under salt stress. Chin. J. Eco-Agric. 2022, 30, 92–104. [Google Scholar] [CrossRef]
- Keya, S.S.; Mostofa, M.G.; Rahman, M.M.; Das, A.K.; Sultana, S.; Ghosh, P.K.; Anik, T.R.; Ahsan, S.; Rahman, M.A.; Jahan, N. Salicylic Acid Application Improves Photosynthetic Performance and Biochemical Responses to Mitigate Saline Stress in Cotton. J. Plant Growth Regul. 2023, 42, 1–14. [Google Scholar] [CrossRef]
- Ahmadi, F.; Nazari, F.; Ghaderi, N.; Teixeira da Silva, J.A. Assessment of morpho-physiological and biochemical responses of perennial ryegrass to gamma-aminobutyric acid (GABA) application under salinity stress using multivariate analyses techniques. J. Plant Growth Regul. 2021, 42, 168–182. [Google Scholar] [CrossRef]
- Li, W.K.; Huang, B.; Li, M.L.; Wang, J.; Sun, M.T.; Yan, Y.; He, C.X.; Yu, X.C.; Li, Y.S. Studies on sodium naphthalene-1-acetate and compound sodium nitrophenolate enhancing pepper resistance to stress under intensity of sub-optimal temperature and light. China Veg. 2022, 2, 40–46. [Google Scholar] [CrossRef]
- Huang, B.; Li, W.K.; Sun, M.T.; Yan, Y.; Wang, J.; He, C.X.; Yu, X.C.; Li, Y.S. Effect of sodium nitrophenol on seed germination and cold tolerance of seedlings at low temperature. J. Nucl. Agric. Sci. 2022, 36, 845–855. [Google Scholar] [CrossRef]
- Shi, J.; Liu, D.Y.; Zhang, F.H. Physiological response and salt tolerance mechanism of cotton seedlings to salt stress. Acta Agric. Zhejiangensis 2020, 32, 1141–1148. [Google Scholar] [CrossRef]
- Feng, C.J.; Song, R.J.; Song, L.Y.; Zhang, S.; Qi, J.C. Effects of 2,4-epbrassinolide soaking on seed germination and physiological characteristics of Barley seedlings under drought stress. Xinjiang Agric. Sci. 2023, 60, 309–316. [Google Scholar] [CrossRef]
- Shu, X.; Su, X.L.; Yan, L.J.; Xiong, Y.L.; Xiong, Y.; Yu, Q.Q.; Ma, X. Effects of exogenous salicylic acid on seed germination and seedling of Elymus Sibiricus under NaCl stress. Chin. J. Grassl. 2024, 46, 87–96. [Google Scholar] [CrossRef]
- Ting, Z.T.; Le, L.Y.; Hong, C.; Xin, X.L.; Wei, C.X.; Heng, W.E.; Xin, Y.J. Effects of different exogenous substances on the seed germination, seedling growth, and physiology of Melilotus suaveolens under salt, alkali, and drought stress. Acta Prataculturae Sin. 2024, 33, 122–132. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.T.; Ma, T.T.; Jiang, D.; Sun, H.C.; Zhang, Y.J.; Zhang, K.; Bai, Z.Y.; Li, C.D. Effects of melatonin on the antioxidant enzyme activities and seed Germination of cotton (Gossypium hirsutum L.) under salt-stress conditions. Cotton Sci. 2019, 31, 438–447. [Google Scholar] [CrossRef]
- Muhammad, I.; Yang, L.; Ahmad, S.; Farooq, S.; Khan, A.; Muhammad, N.; Ullah, S.; Adnan, M.; Ali, S.; Liang, Q.P. Melatonin-priming enhances maize seedling drought tolerance by regulating the antioxidant defense system. Plant Physiol. 2023, 191, 2301–2315. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, X.L.; Lu, X.F.; Li, G.; Geng, Y.S.; Sun, Y.B.; Yang, X.Y.; Geng, J.B. Effects of soil-methylpiperine on leaf physiology and root morphology of cotton seedlings under salt stress. Jiangsu Agric. Sci. 2022, 50, 81–86. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Jiang, X.H.; Shin, X.J.; Sun, W.; Zhang, J.; Amin, A.S.; Gao, Y. The relationship between foliar K+ and Na+ concentrations and photosynthetic parameters of cotton seedlings under salt stress. Chin. J. Ecol. 2021, 40, 1716–1722. [Google Scholar] [CrossRef]
- Rattan, A.; Kapoor, D.; Kapoor, N.; Bhardwaj, R.; Sharma, A. Brassinosteroids regulate functional components of antioxidative defense system in salt stressed maize seedlings. J. Plant Growth Regul. 2020, 39, 1465–1475. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Halder, K.; Abdin, M.Z.; Majee, M.; Datta, A. Abiotic stress tolerance in plants: Brassinosteroids navigate competently. Int. J. Mol. Sci. 2022, 23, 14577. [Google Scholar] [CrossRef] [PubMed]
| Title | Unit | High-Salt Solution |
|---|---|---|
| pH | — | 8.61 |
| Salinity | g·kg−1 | 272.1 |
| Electrical conductivity | S·m−1 | 6.772 |
| HCO3− | g·kg−1 | 0.3942 |
| Cl− | g·kg−1 | 109.0058 |
| SO42− | g·kg−1 | 79.6118 |
| Ca2+ | g·kg−1 | 2.4125 |
| Mg2+ | g·kg−1 | 2.2074 |
| K+ | g·kg−1 | 0.4705 |
| Na+ | g·kg−1 | 37.15 |
| Treatment | Height (cm) | Root Dry Weight (mg·plant−1) | Stem Dry Weight (mg·plant−1) | Leaf Dry Weight (mg·plant−1) |
|---|---|---|---|---|
| CK | 7.2 ± 0.3 a | 86.7 ± 18.5 a | 165.1 ± 21 a | 155.3 ± 23 a |
| SS | 6.2 ± 0.2 b | 37.7 ± 17.8 b | 156.6 ± 28.8 a | 108.3 ± 20 b |
| SS+S | 7.1 ± 0.4 a | 48.7 ± 17.5 ab | 162.3 ± 15.9 a | 119.8 ± 20.8 ab |
| SS+E | 6.9 ± 0.1 a | 40.9 ± 19.4 b | 159.9 ± 17.8 a | 125.4 ± 24.5 ab |
| SS+G | 7.1 ± 0.5 a | 58.4 ± 21.3 ab | 162.2 ± 18.4 a | 132.6 ± 17.9 ab |
| CK | 7.2 ± 0.3 a | 86.8 ± 18.5 a | 165.1 ± 21 a | 155.3 ± 23 a |
| MS | 6.3 ± 0.2 b | 51.8 ± 20.3 a | 160.7 ± 25.3 a | 133.7 ± 21.5 a |
| MS+S | 7 ± 0.2 a | 57.8 ± 20.1 a | 163.7 ± 23.6 a | 137.4 ± 27.3 a |
| MS+E | 6.8 ± 0.2 a | 54.4 ± 17.2 a | 161.2 ± 22.2 a | 137.4 ± 26.2 a |
| MS+G | 7.2 ± 0.3 a | 69 ± 18 a | 163.2 ± 18.9 a | 141.6 ± 18.6 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Meng, A.; Qi, T.; Huang, J.; Yang, H.; Tayir, A.; Wang, B. Exogenous Substances Improved Salt Tolerance in Cotton. Agronomy 2024, 14, 2098. https://doi.org/10.3390/agronomy14092098
Dong Z, Meng A, Qi T, Huang J, Yang H, Tayir A, Wang B. Exogenous Substances Improved Salt Tolerance in Cotton. Agronomy. 2024; 14(9):2098. https://doi.org/10.3390/agronomy14092098
Chicago/Turabian StyleDong, Zhiduo, Ajing Meng, Tong Qi, Jian Huang, Huicong Yang, Aziguli Tayir, and Bo Wang. 2024. "Exogenous Substances Improved Salt Tolerance in Cotton" Agronomy 14, no. 9: 2098. https://doi.org/10.3390/agronomy14092098






