The Influence of Microalgae Fertilizer on Soil Water Conservation and Soil Improvement: Yield and Quality of Potted Tomatoes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Experimental Site
2.2. Experimental Material
2.3. Experimental Design
2.4. Measurement Items and Methods
2.4.1. Measurement Methods
2.4.2. Determination of Soil Moisture
2.4.3. Measurement of Tomato Growth Indicators
2.4.4. Measurement of Tomato Yield and Quality Indicators
2.4.5. Water-Use Efficiency
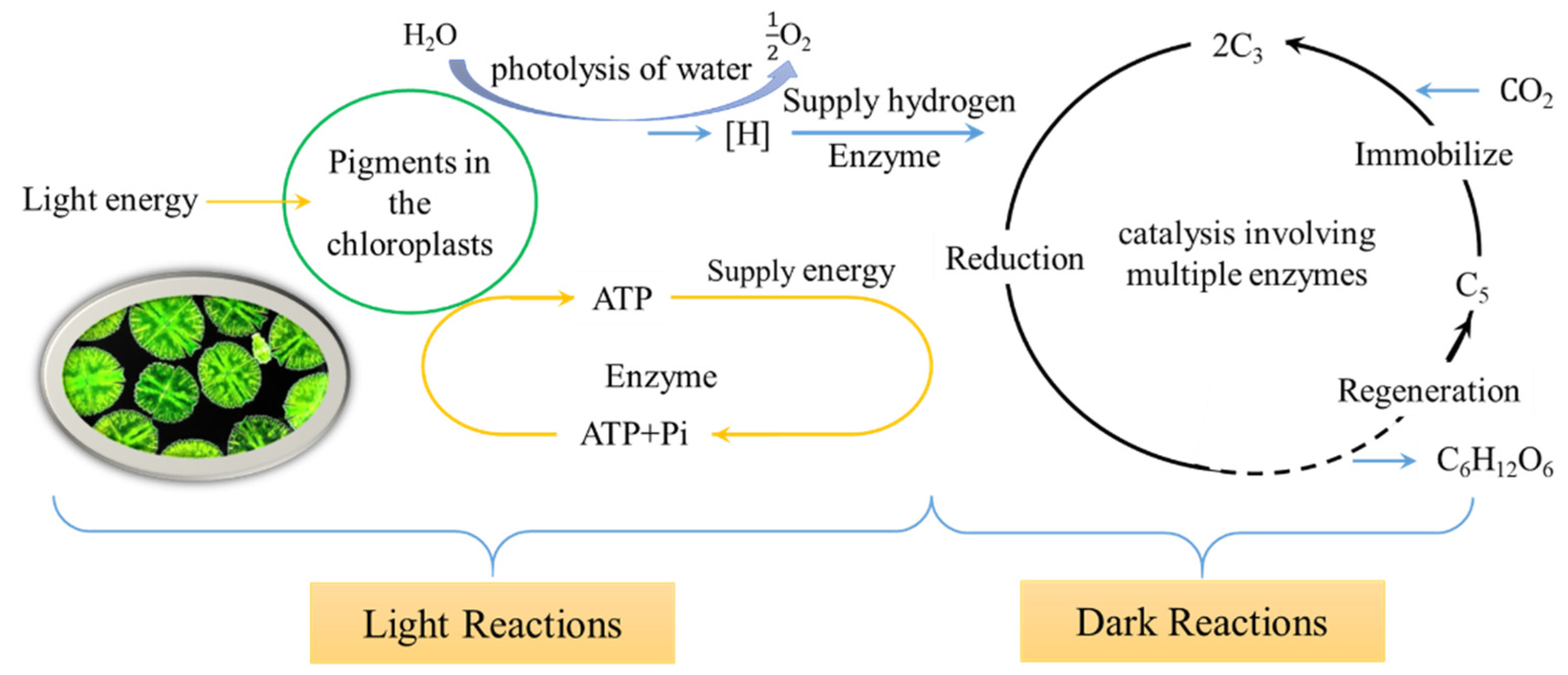
2.4.6. Measurement of Tomato Photosynthetic Indicators
2.5. Data Processing and Analysis
3. Results and Analysis
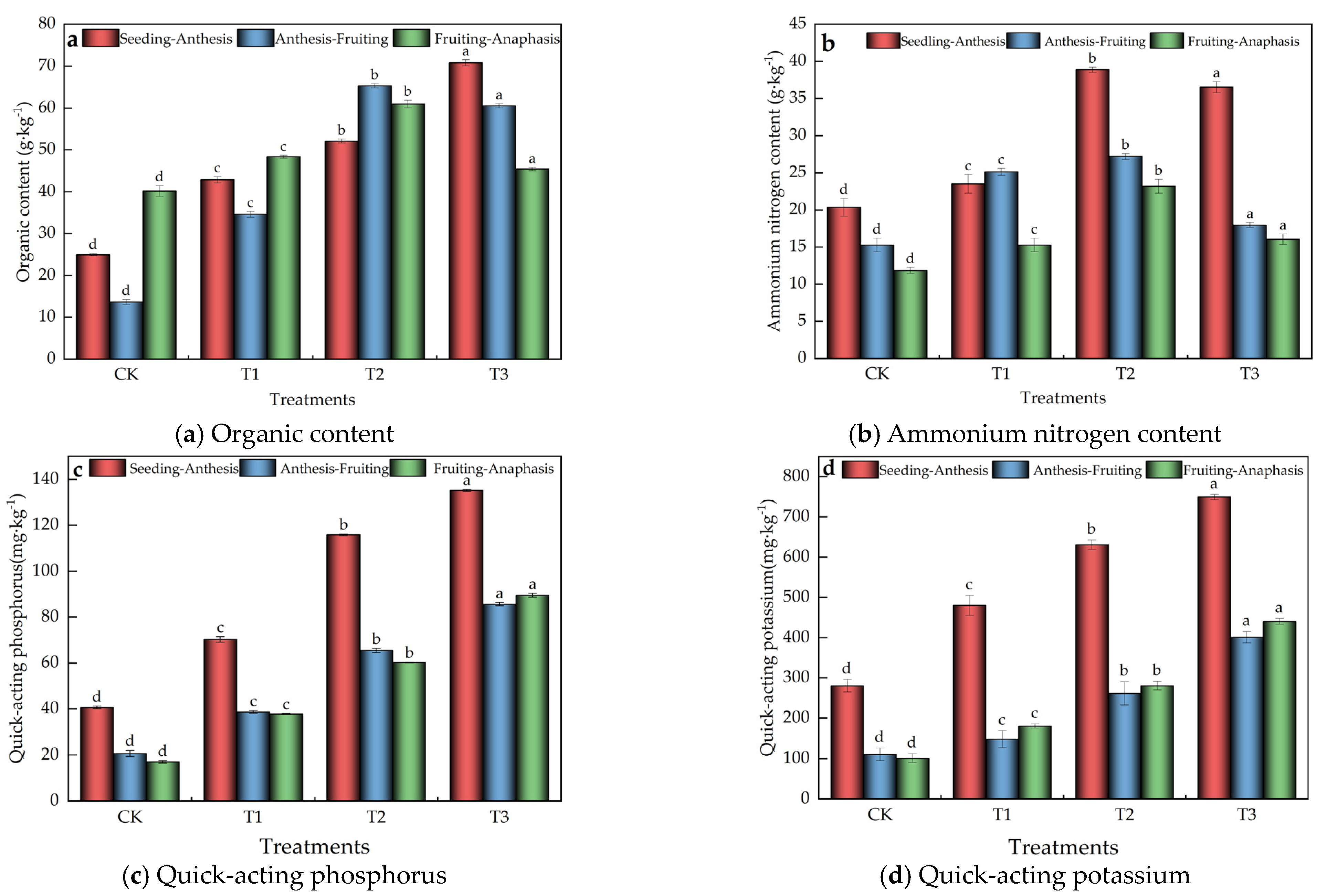
3.1. Impact of Microalgae Fertilizer on Soil Physicochemical Properties
3.1.1. Soil Nutrients
3.1.2. Soil Moisture Content
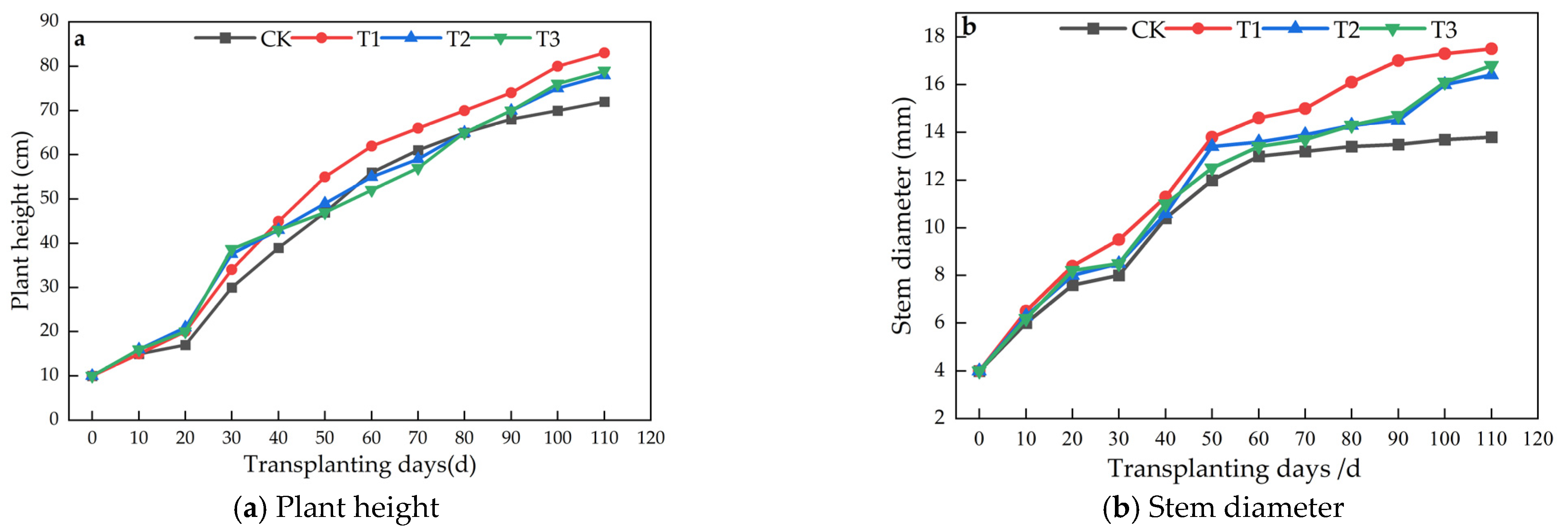
3.2. Impact of Microalgae Fertilizer on Tomato Plant Height and Stem Thickness
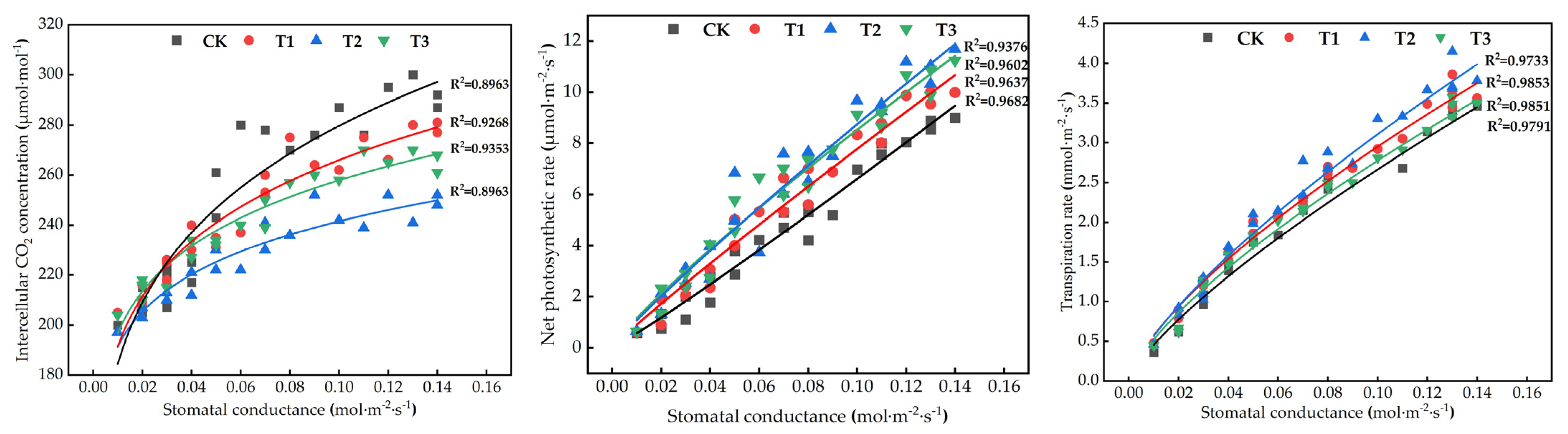
3.3. Effect of Microalgae Fertilizer on Tomato Leaf Photosynthetic Parameters
3.4. Impact of Microalgae Fertilizer on Tomato Yield and Water-Use Efficiency
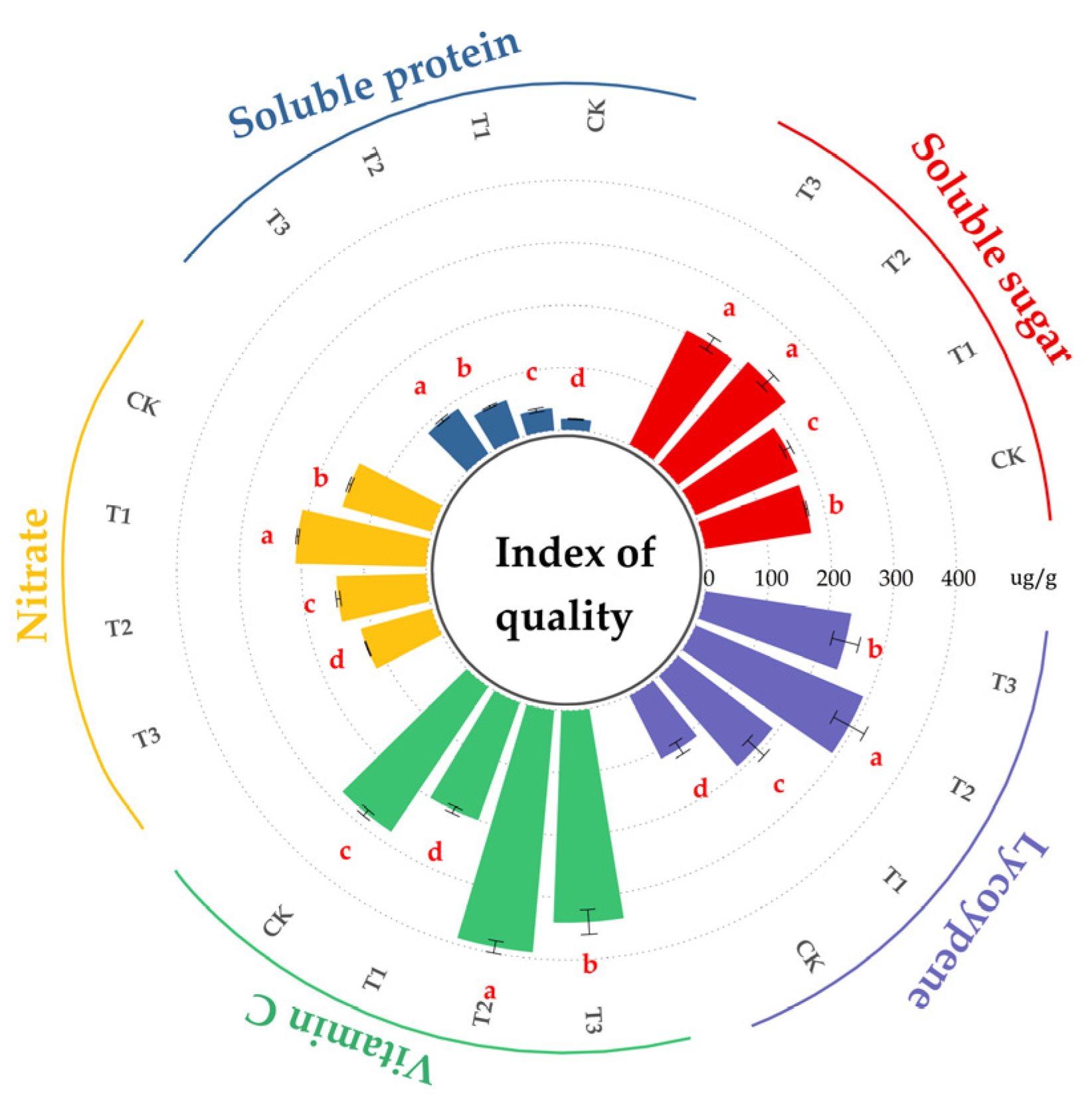
3.5. Impact of Microalgae Fertilizer on Tomato Nutritional Quality
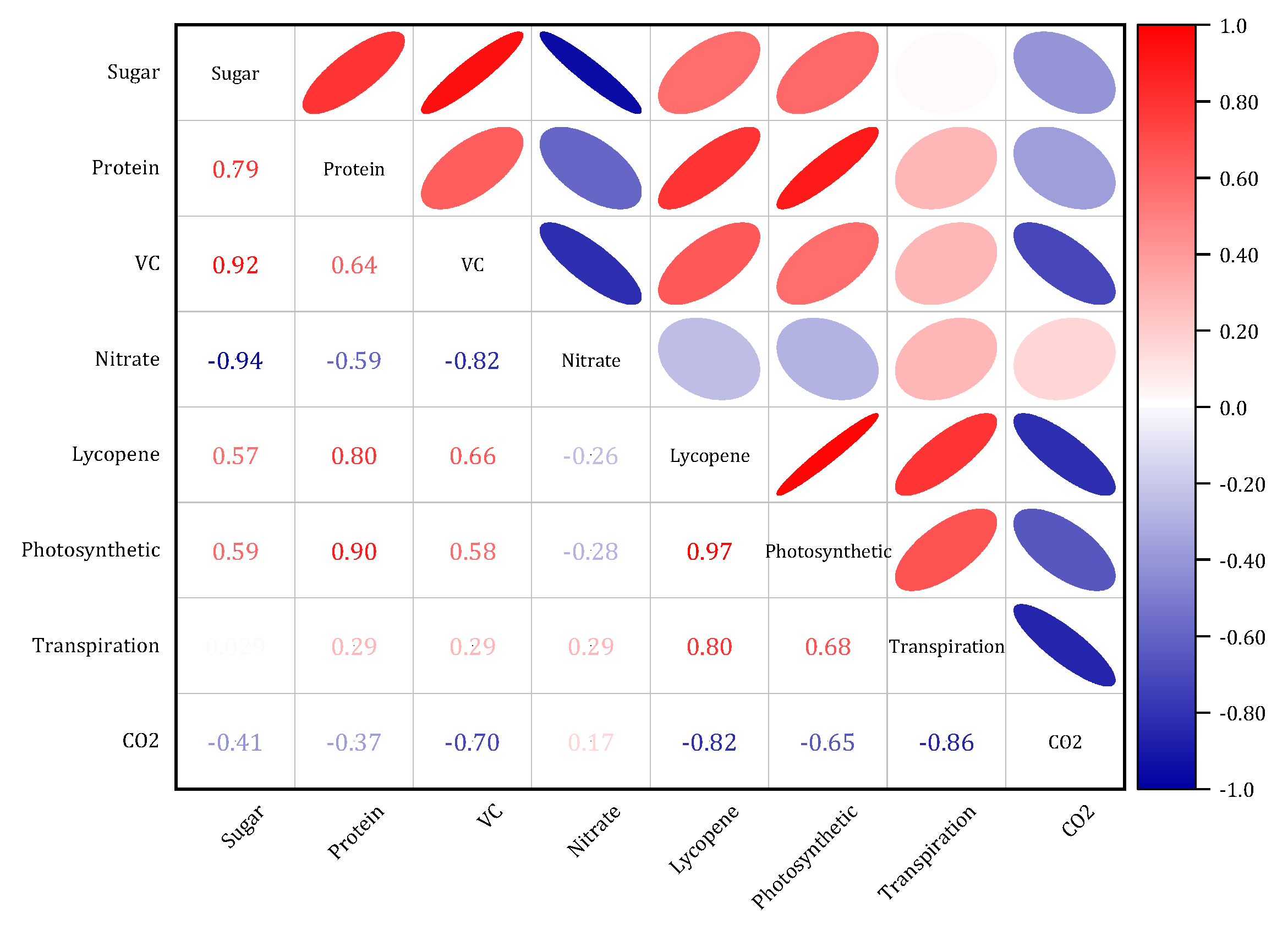
3.6. Factors Affecting Tomato Yield and Quality and Correlation Analysis
3.6.1. Correlation Analysis
3.6.2. Principal Component Analysis
3.6.3. Membership Function Analysis
4. Discussion
4.1. Impact of Microalgae Fertilizer on Soil Quality and Soil Moisture Content
4.2. Impact of Microalgae Fertilizer on Crop Growth and Fruit Quality
4.3. Impact of Microalgae Fertilizer on Yield and Water-Use Efficiency
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Su, S.; Zhou, Y.; Zhou, X. Spatio-temporal evolution and influencing factors of chemical fertilizer application intensity of vegetable planting in China. J. China Agric. Univ. 2022, 27, 248–263. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Su, T.; Li, H.; Tan, B.; Yang, W. Effects of Partial Substitution of Chemical Fertilizers by Organic Fertilizers on Lettuce Growth and Soil Environment. J. Southwest Univ. (Nat. Sci.) 2022, 44, 41–49. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, Y.; Hu, C.; Dong, W.; Li, X.; Liu, X.; Zhang, L.; Wen, H. Effects of long-term nutrient recycling pathways on soil nutrient dynamics and fertility in farmland. Chin. J. Eco-Agric. 2022, 30, 937–951. [Google Scholar] [CrossRef]
- Jiang, L.; Xie, Y.; Li, T.; Liu, K.; Zhang, Q.; Cao, J.; Shao, J. Effects of Different Organic Substitutes on Soil Organic Carbon and Nitrogen Fractions and Winter Wheat Yield in Dryland of Loess Plateau. J. Soil Water Conserv. 2022, 36, 286–293. [Google Scholar]
- Reardon, C.; Klein, A.; Melle, C.; Hagerty, C.; Klarer, E.; Machado, S.; Paulitz, T.; Pritchett, L.; Schlatter, D.; Smith, S.; et al. Enzyme activities distinguish long-term fertilizer effects under different soil storage methods. Appl. Soil Ecol. 2022, 177, 104518. [Google Scholar] [CrossRef]
- Deng, X.Y. Applied Microalgae Biology; Oceanpress: Beijing, China, 2016; ISBN 978-7-5027-9615-0. [Google Scholar]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial Applications of Microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Coppens, J.; Grunert, O.; Van Den Hende, S.; Vanhoutte, I.; Boon, N.; Haesaert, G.; De Gelder, L. The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J. Appl. Phycol. 2016, 28, 2367–2377. [Google Scholar] [CrossRef]
- Du, Y.; Du, Y.R.; Zhu, S.P.; Zeng, Q. Research Progress of Microalgae in Environmental Remediation. Environ. Sci. Technol. 2014, 37, 316–320. [Google Scholar]
- Colica, G.; Li, H.; Rossi, F.; Li, D.; Liu, Y.; De Philippis, R. Microbial secreted exopolysaccharides affect the hydrological behavior of induced biological soil crusts in desert sandy soils. Soil Biol. Biochem. 2014, 68, 62–70. [Google Scholar] [CrossRef]
- De, P.K. The role of blue-green algae in nitrogen fixation in rice-fields. Proc. R. Soc. 1938, 127, 121–139. Available online: https://s.dic.cool/S/AonjlsqZ (accessed on 29 May 2024).
- Shang-Hao, L.; Tsing-Chuan, Y.; Fu-Jui, L.; Lih-Mei, W.; Shi-Kiung, T. The effects of nitrogen-fixing blue-green algae on theyields of rice plant. J. Aquat. Biol. 1959, 4, 440–444. [Google Scholar]
- Tang, D.S.; Qing, R.W.; Fu, H.L. Studies on Soil Micro-algae’s Ameliorative Effection to Sterile Soil. J. Sichuan Univ. (Nat. Sci. Ed.) 2003, 40, 352–355. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wang, D.L. Response characteristics of root morphology of blueberry to different fertilizers and fuzzy comprehensive evaluation. Non-Wood For. Res. 2019, 37, 110–118. [Google Scholar] [CrossRef]
- Ye, Z.C. Comparative Study on Comprehensive Characters and Quality of 24 Tomato Varieties. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2022. [Google Scholar]
- Liang, Y.L. The Mechanism of Tomato ASA-GSH Cycle Key Enzymes Involved in Alleviating Nitrate Stress by NO. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2018. [Google Scholar]
- Zhu, Y.; Zhang, Y.Y.; Zhu, H.B.; Yue, L.M.; Song, Z.Y.; Wu, R.H.; Liu, G.H. Advances in Research on Biological Functions of Lycopene. Food Res. Dev. 2020, 41, 202–207. [Google Scholar]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672. [Google Scholar] [CrossRef]
- Muller, A.; Schader, C.; El-Hage Scialabba, N.; Brüggemann, J.; Isensee, A.; Erb, K.-H.; Smith, P.; Klocke, P.; Leiber, F.; Stolze, M.; et al. Strategies for feeding the world more sustainably with organic agriculture. Nat. Commun. 2017, 8, 1290. [Google Scholar] [CrossRef] [PubMed]
- Renuka, N.; Prasanna, R.; Sood, A.; Bansal, R.; Bidyarani, N.; Singh, R.; Shivay, Y.S.; Nain, L.; Ahluwalia, A.S. Wastewater grown microalgal biomass as inoculants for improving micronutrient availability in wheat. Rhizosphere 2017, 3, 150–159. [Google Scholar] [CrossRef]
- Ding, S.; Xu, X.; Zhu, F.; Wang, R.; Pan, G.; Cui, Y.; Hu, X.; Mostafa; Huo, S. Distribution characteristics and physiological and ecological functions of soil algae in different habitats. Ecol. Environ. Sci. 2023, 32, 1873–1888. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, Z.; Sun, Y.; Cai, K.; Wang, W. Effects of Seaweed Fertilizer and Other Different Fertilization on Soil Nitrogen Mineralization and Nitrogen Use Efficiency. Chin. Agric. Sci. Bull. 2012, 28, 103–107. [Google Scholar] [CrossRef]
- Du, Q.; Kong, W.B.; Han, R.; Niu, S. Research progress on species and functions of soil microalgae. Bull. Biol. 2015, 50, 1–5. [Google Scholar]
- Liu, S.; Lu, J.; Feng, J.; Liu, Q.; Guo, J.; Jiao, X.; Xie, S. Effect of Administering Microalgae on Growth of Cucumber and Quality of Soil. J. Shanxi Agric. Sci. 2016, 44, 1312–1315, 1319. [Google Scholar] [CrossRef]
- Ling, L.L.; Qing, R.W.; Fu, H.L.; Mo, Y.; Nie, D.; Wen, C.; Lan, L. The Algae’s Influence on Soil Fertility. J. Sichuan Univ. (Nat. Sci. Ed.) 2003, 40, 135–138. [Google Scholar] [CrossRef]
- Wang, M.Y.; Lan, L.Q.; Qing, R.W.; Fu, H.L. Studies of Soil Microalgae Influence on Soil Quality. J. Soil Water Conserv. 2001, 15, 103–106. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, Y.L.; Zhao, Y.C.; Guo, S.; Cui, Z.; Shi, W. Effect of fertilizer reduction combined with organic fertilizer on soil quality and yield and quality of eggplant in solar greenhouse. North China J. Agron. 2023, 38, 188–198. [Google Scholar] [CrossRef]
- Bian, J.; Cui, Y.; Yang, S.; Meng, X.; Luo, G.; Wang, Z. Research progress in agricultural application of microalgae bio-fertilizer. Soils Fertil. Sci. China 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Cui, Y.; Bian, J.W.; Liu, Y.; Luo, G. Research Progress of Microalgae Polysaccharides and Their Derivatives in Agriculture. Chin. J. Soil Sci. 2023, 54, 1226–1236. [Google Scholar] [CrossRef]
- Zou, L.-G.; Zheng, D.-L.; Yao, Y.-T.; Wen, F.-F.; Li, D.-W.; Yang, Y.-F.; Yang, W.-D.; Balamurugan, S.; Kwok, H.F.; Li, H.-Y. Polyphenols modulate microalgae metabolism with a particular increment in lipid accumulation. Fuel 2023, 325, 129085. [Google Scholar] [CrossRef]
- Zhang, J.J. Study on the Effect of Microalgae Biofertilizer on Improving Soil Fertility and Root Microflora and Improving Yield and Quality of Small Rapeseed. Master’s Thesis, Shanxi Agricultural University, Jinzhong, China, 2020. [Google Scholar]
- Issa, O.M.; Défarge, C.; Le Bissonnais, Y.; Marin, B.; Duval, O.; Bruand, A.; D’acqui, L.P.; Nordenberg, S.; Annerman, M. Effects of the inoculation of cyanobacteria on the microstructure and the structural stability of a tropical soil. Plant Soil 2007, 290, 209–219. [Google Scholar] [CrossRef]
- Yilmaz, E.; Sönmez, M. The role of organic/bio–fertilizer amendment on aggregate stability and organic carbon content in different aggregate scales. Soil Tillage Res. 2017, 168, 118–124. [Google Scholar] [CrossRef]
- Wuang, S.C.; Khin, M.C.; Chua, P.Q.D.; Luo, Y.D. Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algae Res. 2016, 15, 59–64. [Google Scholar] [CrossRef]
- Ji, C.; Li, S.; Li, S. Effects of Fertilization, Variety and Seed Size on Photosynthesis and Chlorophyll Fluorescence of Winter Wheat. Acta Bot. Boreali-Occident. Sin. 2007, 27, 2522–2530. [Google Scholar] [CrossRef]
- Li, C.; Zhang, L.; Ma, S.; Shao, Y.; Han, R.; Wang, W.; Li, H.; Cui, J. Effects of Organic Fertilization Combined with Reduced Nitrogen Fertilizer on Wheat Photosynthetic Characteristics, Nitrogen Uptake and Grain Yield. Acta Bot. Boreali-Occident. Sin. 2017, 37, 943–951. [Google Scholar]
- Del Amor, F.M.; Cuadra-Crespo, P.; Walker, D.J.; Cámara, J.M.; Madrid, R. Effect of foliar application of antitranspirant on photosynthesis and water relations of pepper plants under different levels of CO2 and water stress. J. Plant Physiol. 2010, 167, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.W.; Zhang, X.C.; Zhang, C.J.; Li, P.; Zhao, T.K. Effect of application methods of nitrogen fertilizer on the nitrogen utilization of tomato in green house. J. Beijing Univ. Agric. 2017, 32, 62–68. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, M.; Fan, Y.; Zhang, L.; Wang, H. Using microalgae to reduce the use of conventional fertilizers in hydroponics and soil-based cultivation. Sci. Total Environ. 2024, 912, 169424. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.F.; Xie, W.J.; Chen, J.C. Comparison of Difulai algae active cell biological fertilizer on winter potato in the same field. Agric. Technol. Commun. 2018, 11, 146–148. [Google Scholar]
- Pang, M.; Ou, X.; Bao, X.; Yang, Z.; Lin, Y.; Liang, Z.; Chen, J.; Zhu, B. Effects of Several New Fertilizers on Yield and Quality of Muskmelon in Greenhouse. China Fruit Avegetable 2020, 40, 66–69. [Google Scholar] [CrossRef]
- Li, W.Y.; Cui, Y.; Meng, X.G.; Luo, G.H. Research progress on preparation and agricultural application of microalgae biological fertilizer. Shandong Agric. Sci. 2022, 54, 144–150. [Google Scholar] [CrossRef]
- Hachiya, T.; Sakakibara, H. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J. Exp. Bot. 2017, 68, 2501–2512. [Google Scholar] [CrossRef]
- Wang, R.; Peng, B.; Huang, K. The research progress of CO2 sequestration by algal bio-fertilizer in China. J. CO2 Util. 2015, 11, 67–70. [Google Scholar] [CrossRef]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F.; Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as multifunctional options in modern agriculture: Current trends, prospects and challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef] [PubMed]



| Treatment | Treatment Mode | Fertilizing Amount |
|---|---|---|
| CK | CF100% | CF: 1.8 g per pot |
| T1 | MF25% + CF75% | MF: 25 mL per pot + CF: 1.35 g per pot |
| T2 | MF75% + CF25% | MF: 75 mL per pot + CF: 0.45 g per pot |
| T3 | MF100% | MF: 100 mL per pot |
| Treatment | Seedling Stage to Flowering Stage | Flowering Stage to Fruitful Stage | Fruitful Stage to Later Stage |
|---|---|---|---|
| CK | 8.3 ± 0.2 a | 8.4 ± 0.02 b | 7.7 ± 0.12 c |
| T1 | 8.1 ± 0.09 b | 8.5 ± 0.02 a | 7.6 ± 0.03 b |
| T2 | 8.1 ± 0.06 b | 8.4 ± 0.04 b | 7.5 ± 0.08 a |
| T3 | 8.1 ± 0.05 b | 8.3 ± 0.03 b | 7.5 ± 0.06 a |
| Treatment | Water Consumption/mm | Individual Plant Yield/kg | Water-Use Efficiency/kg·m3 |
|---|---|---|---|
| CK | 324 ± 9.07 a | 0.485 ± 0.005 c | 12.21 ± 0.31 c |
| T1 | 295 ± 6.56 b | 0.477 ± 0.007 c | 13.21 ± 0.46 b |
| T2 | 273 ± 6.02 c | 0.630 ± 0.011 a | 18.81 ± 0.41 ab |
| T3 | 266 ± 6.11 c | 0.521 ± 0.008 b | 15.98 ± 0.24 a |
| PC | Eigenvalue | Variance Contribution Rate/% | Cumulative Variance Contribution Rate/% |
|---|---|---|---|
| 1 | 6.461 | 71.785 | 71.785 |
| 2 | 2.274 | 25.266 | 97.051 |
| Treatment | Principal Component Values | Comprehensive Score | Ranking | |
|---|---|---|---|---|
| 1 | 2 | |||
| CK | −2.13 | −0.71 | −1.71 | 4 |
| T1 | −0.97 | 0.04 | −0.69 | 3 |
| T2 | 1.87 | −0.38 | 1.25 | 1 |
| T3 | 1.23 | 1.05 | 1.15 | 2 |
| Participating Indicators | CK | T1 | T2 | T3 |
|---|---|---|---|---|
| Organic matter | 0.462 | 0.471 | 0.692 | 0.571 |
| Ammonium nitrogen | 0.412 | 0.359 | 0.303 | 0.051 |
| Available phosphorus | 0.153 | 0.029 | 0.096 | 0.079 |
| Available potassium | 0.054 | 0.088 | 0.079 | 0.123 |
| Soil moisture content | 0.248 | 0.219 | 0.217 | 0.177 |
| Plant height | 0.594 | 0.577 | 0.574 | 0.644 |
| Stem diameter | 0.689 | 0.667 | 0.653 | 0.638 |
| Net photosynthetic rate | 0.485 | 0.538 | 0.519 | 0.532 |
| Transpiration rate | 0.544 | 0.577 | 0.578 | 0.55 |
| Intercellular CO2 concentration | 0.476 | 0.474 | 0.508 | 0.527 |
| Soluble protein | 0.5 | 0.667 | 0.75 | 0.667 |
| Soluble sugar | 0.045 | 0.231 | 0.389 | 0.5 |
| Vitamin C | 0.5 | 0.5 | 0.5 | 0.5 |
| Nitrate | 0.715 | 0.734 | 0.652 | 0.429 |
| Lycopene | 0.144 | 0.396 | 0.288 | 0.805 |
| Average membership degree | 0.401 | 0.435 | 0.453 | 0.452 |
| Ranking | 4 | 3 | 1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Liang, Y.; Miao, Q.; Ji, X.; Duan, P.; Quan, D. The Influence of Microalgae Fertilizer on Soil Water Conservation and Soil Improvement: Yield and Quality of Potted Tomatoes. Agronomy 2024, 14, 2102. https://doi.org/10.3390/agronomy14092102
Li C, Liang Y, Miao Q, Ji X, Duan P, Quan D. The Influence of Microalgae Fertilizer on Soil Water Conservation and Soil Improvement: Yield and Quality of Potted Tomatoes. Agronomy. 2024; 14(9):2102. https://doi.org/10.3390/agronomy14092102
Chicago/Turabian StyleLi, Chao, Yaqi Liang, Qingfeng Miao, Xiang Ji, Pengcheng Duan, and Dong Quan. 2024. "The Influence of Microalgae Fertilizer on Soil Water Conservation and Soil Improvement: Yield and Quality of Potted Tomatoes" Agronomy 14, no. 9: 2102. https://doi.org/10.3390/agronomy14092102
APA StyleLi, C., Liang, Y., Miao, Q., Ji, X., Duan, P., & Quan, D. (2024). The Influence of Microalgae Fertilizer on Soil Water Conservation and Soil Improvement: Yield and Quality of Potted Tomatoes. Agronomy, 14(9), 2102. https://doi.org/10.3390/agronomy14092102






