Abstract
The action-to-reaction dynamics of next-generation nanofertilizers (NFs) towards field crops are currently being addressed in precision and sustainable agriculture. Therefore, our aim was to evaluate the effects of foliar application of ZnO nanoparticles (ZnO-NPs) or their combination with hybrid nanoporous biosilica mixed with yeast (ZnSi-bio) for soybean plants’ selected production and physiological indices in comparison to an NF-free control. The experiment was conducted at eco-friendly concentrations in Veľký Krtíš, Slovakia, a location within the Central European agronomical region. The ZnSi-bio variant had an improved number of pods, seed count, and yield, while the ZnO-NPs variant had an enhanced seed bulk density compared to the NF-free control, which had a greater effect on thousand-seed weight (TSW). Significant differences were found in the final quality components of soybeans with respect to phosphorus content without ZnO-NP biofortification. In the case of the ZnSi-bio variant, soybeans were biofortified with zinc. Both leaf-applied NFs markedly improved nutritional and energetic values for soybeans. NFs continued to positively affect seasonal physiology, such as the stomatal conductance (Ig) and crop water stress index (CWSI), compared to the control. The results suggest that the ZnO-NPs, especially when combined with hybrid biosilica and yeast, open new avenues for interdisciplinary research in agro-food science.
1. Introduction
At present, a range of nanotechnological disciplines and smart solutions are being incorporated into agricultural practices. This includes electronic bio/nanosensors, functional nano-agrochemicals, nanoproducts for plant protection, and nanofertilizers (NFs), among other innovations [1]. Many of these nanodomains are associated with silica- or metal-based nanosystems that are up to 100 nm in size [2]. Due to their variable crystallinity, diverse morphology with nanoporous-specific reactive surfaces [3], and plant essentiality, they exhibit various physicochemical and biological effects, such as hypothetically improved photosynthesis, abiotic stress tolerance, productivity, and microbial community structure [4,5]. When these nanoporous-silica hybrid biomaterials are combined with different types of microorganisms and micronutrient-based NPs, they offer a novel approach to NF technology. The synergistic effects of these hybrid formulations, such as those combining zinc oxide nanoparticles (ZnO-NPs) with biosilica and yeast, may enhance nutrient delivery and plant uptake. This combination may not only improve plant growth and stress resistance but also significantly boost the nutritional profile and physiological parameters, leading to increased yield and quality. Biosilica may act as an adjuvant and desiccant while also slowly releasing Si ions that improve plant health [6]. ZnO-NPs provide the Zn nutrient essential for many plant enzymes, reduce the damage to plant leaf organs from UV irradiation, and provide antimicrobial protection [7]. ZnO-NP foliar application has been beneficial in diverse plant species. Here, NPs could be absorbed, taken up through leaf stomata, and gradually distributed, transformed, or accumulated in leaves [8], stems [9], and whole plants via the vascular system. ZnO-NPs were also used for Zn biofortification in seeds, e.g., in wheat [10]. Foliar spray applications of yeast, depending on the species, dosage, and environmental conditions, have improved plant production, protection, stress resistance, and the potential nutritional value of the produce. However, they have also led to negative effects, such as pathogen growth and development or changes in microbial–leaf balance [11]. Although the use of nanoporous bio-silica was recently researched for soil applications [12], the combination may provide synergistic effects and reduce the potential negative effects compared to the foliar application of just the single component of the hybrid NF.
The effects of ZnO-NPs or nanoporous biogenic silica particles have been observed at low concentrations in sunflower [13], foxtail millet [14], and lentil [15], but their impact on production, physiological, or nutritional values in soybean within the Central European agronomic region remains unknown.
From a global-nutritional perspective, soybeans are a significant model crop that is also used in experiments with ZnO-NPs [16,17], but there is a lack of academic studies on the positive effects of hybrid silica-based NFs. Soybean (Glycine max), a species within the Fabaceae family, is notable for its protein-rich seeds, which are extensively used in food production, animal feed—due to their energy value—and biofuel manufacturing [18,19].
Currently, studies on the effects of NPs have predominantly focused on toxicological responses at high concentrations in laboratory or greenhouse settings [20,21], with less emphasis on their application in real-world agricultural environments. The influence of bio-silica with yeasts on the absorption of Zn and soybean health has not been evaluated yet. This study addresses this gap by investigating the foliar application of ZnO-NPs combined with a nanoporous hybrid bio-SiO2 material and yeast (ZnSi-bio), in comparison with ZnO-NPs alone and a NF-free control. This research evaluates the impact of these treatments on soybean production, nutritional components, and potential zinc biofortification under real-world conditions in the Central European agronomic region. The hypothesis proposes more efficient utilization and an active role for selected nanofertilizers in foliar applications with a dispersed aqueous medium as a control. The changes resulting from NFs and aqueous medium-spray deposition could be observed through the overall response of the production process. Key indicators include increased yield, changes in yield-contributing components, and shifts in final harvest quality. Physiologically, a decrease in crop water stress index and an increase in stomatal conductance values are expected. The hypothesis also seeks to confirm that ZnSi-bio has a greater synergistic effect than ZnO-NPs in improving seed quality, yield, and physiological response.
2. Materials and Methods
2.1. Utilized Nanoparticles, Nanoporous Biosilica, and Food-Grade Yeast
The ZnO-NPs for spray application to soybean leaves were obtained from Sigma-Aldrich (Saint Louis, MO, USA). Nanoporous biosilica was sourced from Radegast Brewery (Plzeňský Prazdroj, Nošovice, Czech Republic), which is used for beer filtration, and commercially purchased baker’s yeast was used for dispersion.
2.2. Soybean Variety Used
The “Korus” soybean variety was employed to examine its effects on NPs. This variety is characterized by its intensive initial growth (00+), medium plant height, and resistance to lodging and pests, including Sclerotinia. It presents a high protein content of up to 42% at 12% moisture, coupled with excellent yield, making it ideal for tofu production [22].
2.3. Description of Natural Conditions, Weather, and Climate at the Experimental Site
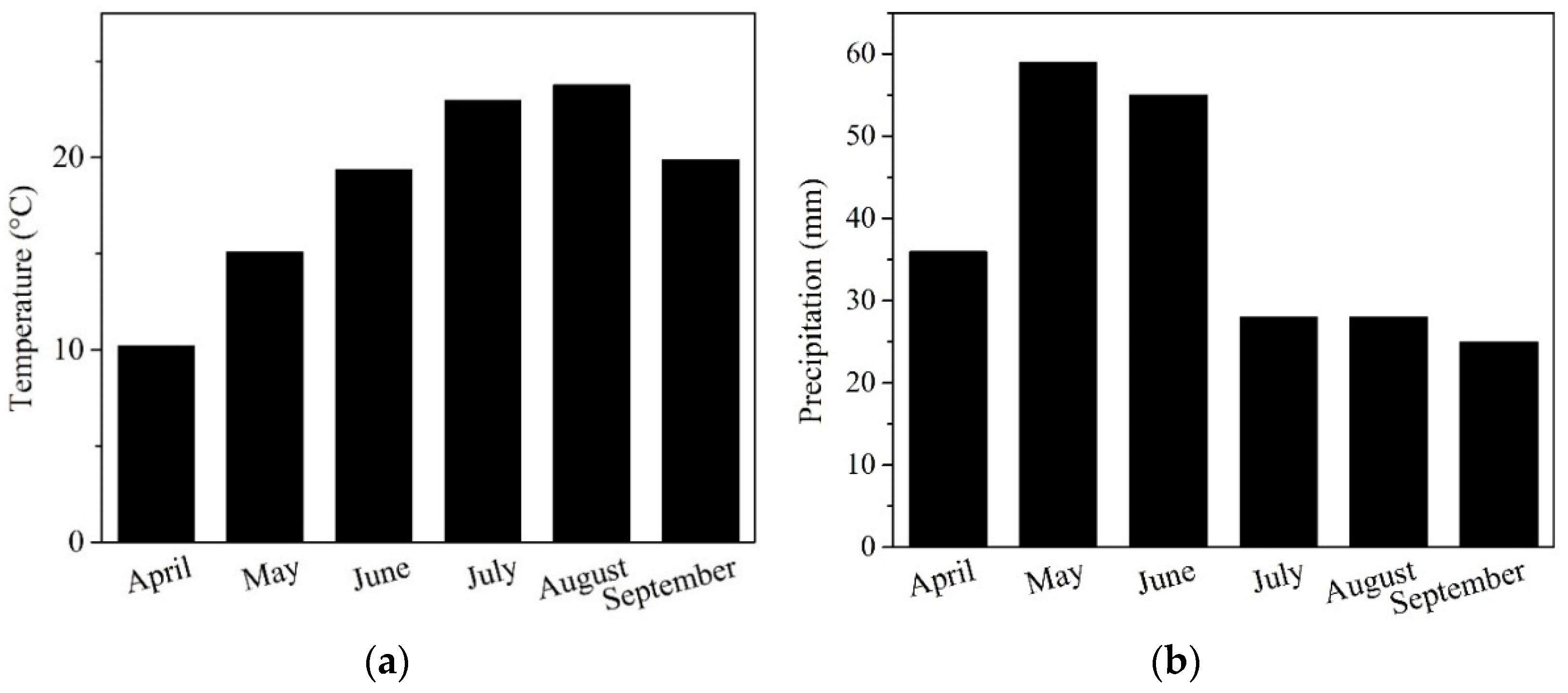
The experimental site is located near Pôtor village with coordinates 48.238290° N, 19.413773° E in the Veľký Krtíš, in the southern part of central Slovakia, at an altitude of 204 m above sea level. The soil type is classified as Fluvisol, and the locality is situated near the alluvium of the “Stará rieka” stream [23]. Generally, the site belongs to a warm, very dry basin with a Central European continental climate. Meteorological data from Meteoblue [24] show recorded variability in precipitation (mm) (Figure 1a) and monthly temperature (°C) (Figure 1b) during the 2023 vegetation season.
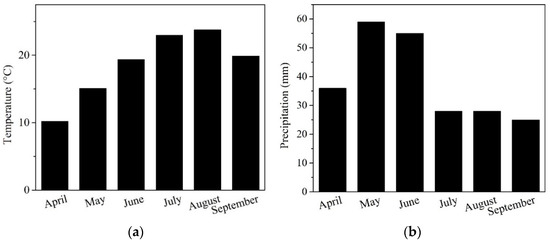
Figure 1.
Monthly variations in (a) air temperature and (b) precipitation at the experimental site in Veľký Krtíš, Slovakia, Central Europe.
2.4. Agronomic Practices Establishing the Soybean Experiment
The experiment was conducted using a conventional soil-management system, with winter rye as a forecrop. The autumn soil preparation for 2022 was carried out in October, involving deep tillage to a depth of up to 30 cm. The soil procedure in early spring was carried out in two phases, with leveling of the land and a pre-sow seedbed preparation. Here, a fertilizer was integrated into the soil at a dosage of 80 kg·ha−1, specifically consisting of 50 kg of Physio Mescal G18 fertilizer (containing 18% P2O5, 6% SO3, 65% CaO, and 5% MgO) with 30 kg of ammonium sulfate. Soybean sowing was conducted in April using a seed drill (Väderstad Rapid RDA 600, Väderstad, Sweden) with an inter-row spacing of 12.5 cm and a sowing depth of 4 cm.
The first preemergence herbicide application was carried out using Escort® Nový (containing 16.7 g·L−1 imazamox and 1250 g·L−1 pendimethalin) at a rate of 3 L·ha−1 (BASF SE, Ludwigshafen, Germany). The second application used Pulsar® 40 (containing 40 g·L−1 imazamox) at a rate of 1 L·ha−1 (BASF Agrochemical Products de PuertoRico, Manati, PR, USA), along with the NP application. During the soybean vegetation, all treatments, including the NP-free control, were treated with the foliar fertilizer VermiVital® (VermiVital s.r.o., Záhorce, Slovakia) at a concentration of 5 L per hectare. The fertilizer composition was as follows: 10.5–13% N, 0.64% S, 0.33% Mg, 0.33% Mn, 0.25% Cu, 0.58% B, and 0.33% Zn.
The experiment was conducted following the methodology of Duflo and Banerjee [25] on an area of approximately 1.8 ha with three treatments, including a control, each with dimensions of 180 × 24 m, resulting in individual treatment plots of 4320 m2. The foliar application variants for soybean consisted of (i) ZnO-NPs; (ii) ZnO-NPs combined with silicon nanoporous recrystallized diatomite and food-grade yeast (ZnSi-bio); and (iii) NF-free control. Each variant was applied in three replications. For the ZnO-NPs, the concentration was 10 mg·L−1 for both variants, nanoporous biosilica corresponded to 15 mg·L−1, and, for yeasts, the concentration was 7.5 g·L−1. The NP dispersion was applied early in the morning under windless conditions, ensuring that the soybean leaves were completely wet 45 days after sowing. Prior to leaf deposition, each colloidal dispersion was subjected to ultrasonication for 15 min.
2.5. Evaluation of Selected Seasonal Physiological Indices of Soybean
For the seasonal physiology determination, indexes such as the stomatal conductance index (Ig) and the crop water stress index (CWSI) were selected and measured using an EasIR-4 thermal camera (Bibus AG, Fehraltorf, Switzerland) following the methodology described by Jones et al. [26].
In this context, various thermal-image characteristics are related, such as leaf temperature parameters (Tleaf), which include dry (Tdry) and wet (Twet) leaf surface temperatures, as well as atmospheric moisture. The stomatal conductance index (Ig) and the crop water stress index (CWSI) were calculated according to the following equations:
Ig = (Tdry − Tleaf)/(Tleaf − Twet),
CWSI = (Tleaf − Twet)/(Tdry − Twet)
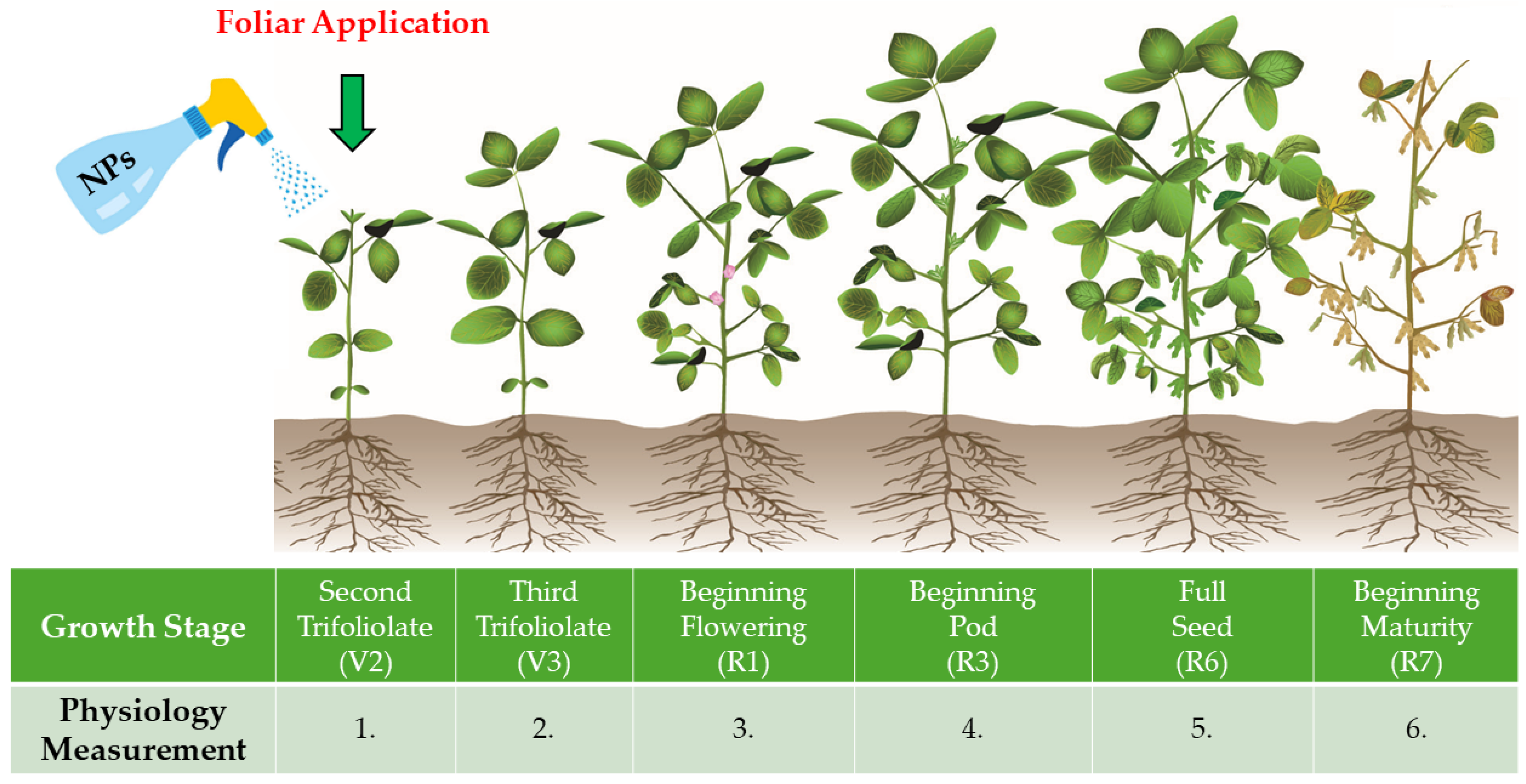
Temperature images were captured using the thermal camera at a diagonal angle relative to the soybean leaves, from a distance of 2 m, at a height of 1.5 m, and with a resolution of 20.6° × 15.5° in auto-focus mode. To ensure consistency and accuracy, all measurements were performed on the same day on equivalent plants with fully developed leaves at a similar developmental stage (Figure 2). Measurements were carried out between 11 a.m. and 1 p.m.
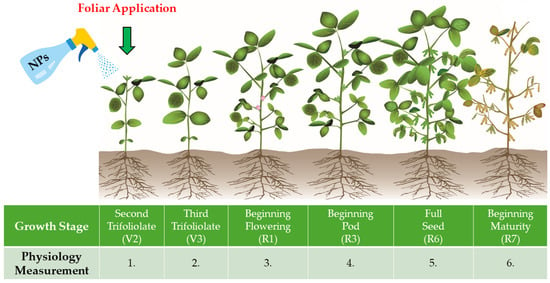
Figure 2.
Illustrative diagram of soybean development with the period of NF application and seasonal physiological indices measurements.
2.6. Analysis of Soybean Production
When the soybean seeds reached full maturity and the required moisture, they were harvested with a combine Claas Lexion 670 (CLAAS GmbH & Co. KGaA, Harsewinkel, Germany). After that, the quantitative parameters were assessed for each variant, where the number of pods was manually counted. The number of seeds per plant was evaluated using a Numirex seed count device (MEZOS spol. s.r.o., Hradec Králové, Czech Republic); weight of thousand-seed (TSW) and bulk density were measured according to STN standard 461011 [27] which involves filling a standard container with grain, leveling the surface, and weighing the grain using the Kern PCB3500-2 laboratory scale (KERN & Sohn GmbH, Balingen, Germany). The soybean yield was calculated in tons per hectare (t·ha−1).
2.7. Analysis of Selected Nutritional Parameters and Energy Value of Soybean Seeds
The mature soybean seeds were evaluated for nutritional parameters. Initially, they were dried at 105 °C in a drying oven. The gross energy (GE), expressed in MJ·kg−1, was assessed using a bomb calorimeter Parr 6400 Calorimeter (Parr Instrument Company, Moline, IL, USA) with benzoic acid as the standard. Procedures and calculations for other energy parameters, specifically digestible energy (DG), metabolizable energy (ME), net energy (NE) for pig growth, and protein solubility in KOH, were conducted according to the STN standard 2300-7/94 [28]. The standardized method 945.39 was used for the ether extracts.
The seed samples weighing 0.15–0.30 g were digested in a mixture of HNO3 and HClO4 (2:1, v/v). For soybean zinc analysis, flame atomic absorption spectrometry (F-AAS) was used (Perkin-Elmer Model 1100, Waltham, MA, USA) according to the method described by Losak et al. [29], while phosphorus content was determined colorimetrically using a spectrophotometer at a wavelength of 660 nm.
2.8. Statistical Analysis
The obtained results were statistically evaluated using one-way analysis of variance (ANOVA) at a significance level of 95% with Tukey’s Honestly Significant Difference (HSD) post-hoc test (TIBCO Statistica™ 14.0.0).
3. Results and Discussion
During the initial NFs foliar application in the growing season 2023 at the Veľký Krtíš location, as was expected, no negative stress reactions were observed in soybeans at our eco-friendly concentration. Inappropriate NFs concentration and timing of application often lead to a reduction in individual plant numbers, surface leaf destruction, the appearance and spread of pathogens [1,30], or genetic modifications in soil applications [31]. Negative growth effects in crops are generally associated with excessive concentrations of NFs, such as 2000 mg·L−1 in habanero peppers [32]. In our study, however, the adaptability and beneficial effect of NFs were demonstrated through improvements in several quantitative parameters (Table 1).

Table 1.
Analysis of selected quantitative parameters and yield of soybean.
The ZnO-NP application had the most pronounced effect on the bulk density of soybean seeds, while other yield-forming factors did not show statistically significant differences. This result can be attributed to a lower number of pods per plant yet a relatively larger number of smaller seeds, leading to a reduced seed size and a lower thousand-seed weight (Table 1). This presents an interesting agronomic outcome, as it suggests a higher potential for seed quality despite reduced seed size. The functional relationship between soybean production and the dynamics of Zn absorption, uptake, or deficiency can manifest in either optimal growth or, conversely, the onset of chlorosis, which directly reduces seed production and indirectly affects overall yield [33]. In this case, the physicochemical nature of ZnO-NPs provided sufficient and gradual Zn release, offering a more targeted effect than conventional fertilizers [34]. The statistically non-significant negative results of the number of pods per plant and weight of thousand seeds associated with ZnO-NPs application can be explained by the higher number of inflorescences and the pollen-favorable development as reproductive organs [35,36]. These improved the bulk density of seeds rather than their weight and yield (Table 1). A similar stimulatory effect, with an increase in the number of pods per plant and a slight increase in soybean biomass achieved through soil application, was also reported by Priester et al. [37], while Yusefi-Tanha et al. [38] observed comparable effects on yield.
During the interval of inflorescence formation and fruit ripening, there exists an important functional dependence on other non-ideal environmental factors, which leads to thermal stress and bioenergetically limits soil nutrient uptake and utilization [39].
Regarding production parameters, the ZnSi-bio application produced the highest yield, albeit at a statistically non-significant level (Table 1). Silica, compared to zinc, is less essential for plants; however, it enhanced soybean yield in nanoformulations with oligochitosan [40]. The combination of ZnO-NP with nanoporous biosilica and food yeasts has confirmed the active role of next-generation nanofertilizers in integrating micronutrients, nanoporous hybrid materials, and microorganisms to enhance agronomical functional properties through foliar spray application [41].
Although the cultivar we used was genetically treated for higher average yields [22], our results highlight that all three variants, including the control, exceeded FAO standards for maximum yield, i.e., more than 3.5 t·ha−1 [42]. Here, the ZnSi-bio variant led to a yield that surpassed the threshold of 4 tons per hectare (Table 1), posting an excellent output in the context of the moderate climate of Central European agronomy.
Generating sufficient energy is the most economically demanding nutritional component in plant-based feed for pigs [43]. In general, soybean seeds contain 7.5–10% moisture, 3–10% sugars (including sucrose, stachyose, raffinose, and polysaccharides), 16.3–21% fats (such as palmitic, stearic, oleic, linoleic, and linolenic acids), 34–40% proteins (primarily lysine and methionine), 4–5.24% mineral nutrients, and approximately 8% fiber (in the form of cellulose, hemicellulose, and lignin) [44,45]. The ratio of synthesized nutritional components in seeds, e.g., soybeans, enables the development of grain-energy productivity with feeding strategies based on their bioenergetic value for individual animals with ideal marked prices [46].
Compared to the individual soybean variants, the higher energy-caloric content is associated with increased concentrations of digestible proteins, essential amino acids, fats (especially oil and fatty acids), soluble carbohydrates, and oligosaccharides, as well as soluble fiber (pectin), and a reduction in anti-nutritional factors, such as trypsin inhibitors and phytic acid [18,19]. Our findings reveal that both the ZnO-NPs and ZnSi-bio variants are more effective than the control across all observed energy parameters at statistically significant levels (Table 2).

Table 2.
Comparison of selected energy parameters of soybeans after NF application.
It is most likely that zinc plays a direct role as a cofactor in carbon and nitrogen metabolism, such as enhancing carbohydrate formation, facilitating atmospheric N-fixation during amino acid synthesis, glycerol utilization, and the biosynthesis of growth hormones and nucleic acids, thereby intensifying their bioenergetic value [19,47].
The results for the energy parameters are directly related to the obtained ether extract (Table 3), which generally correlates with the quantitative oil content in soybeans and impacts the total energy value. However, this only partially correlates with the complex relationship of the protein digestibility profile (Table 3), where the ZnSi-bio variant was indicatively more effective than the control and ZnO variants. In this context, it could be interpreted as a basic relationship between more intense oil synthesis and protein production, which was also demonstrated to be dependent on soil characteristics [48].

Table 3.
Comparison of final qualitative-nutritional parameters of soybeans with potential zinc biofortification after spray deposition of ZnO-NPs and ZnSi-bio nanofertilizers.
In the case of Zn-positive biofortification affecting the final quality of soybean seeds, no increased concentrations were observed (Table 3) in the case of the ZnO-NPs variant. Zinc showed a stimulatory biofortification effect on soybean seeds in the ZnSi-bio variant.
However, the Zn levels do not exceed the average concentrations found in soybean seeds [49]. It is likely that Zn was redistributed throughout the plant, similarly to what has been observed in tomatoes [50], and could have had a physiological–metabolic effect in the soybeans as well.
This is partially supported by p values, where higher P content can reduce the final quality of the product because, after digestion, it binds to divalent and trivalent macronutrients in the form of phytic acid [51]. In our case, the highest values were observed in the control and, surprisingly, in the ZnO-NPs variant, while the lowest value corresponded to the ZnSi-bio variant, likely due to the relative positive physiological effects of nanoporous biosilica or applied food yeasts.
A concentration-adequate, bioavailable, and well-distributed Zn form plays significant roles in plants, including as a growth regulator, enzyme activator, protein synthesis facilitator, environmental stress-tolerance enhancer, root developer, pollinator attractant, and seed producer, as well as in maintaining membrane integrity, stability, and chlorophyll activation [34].
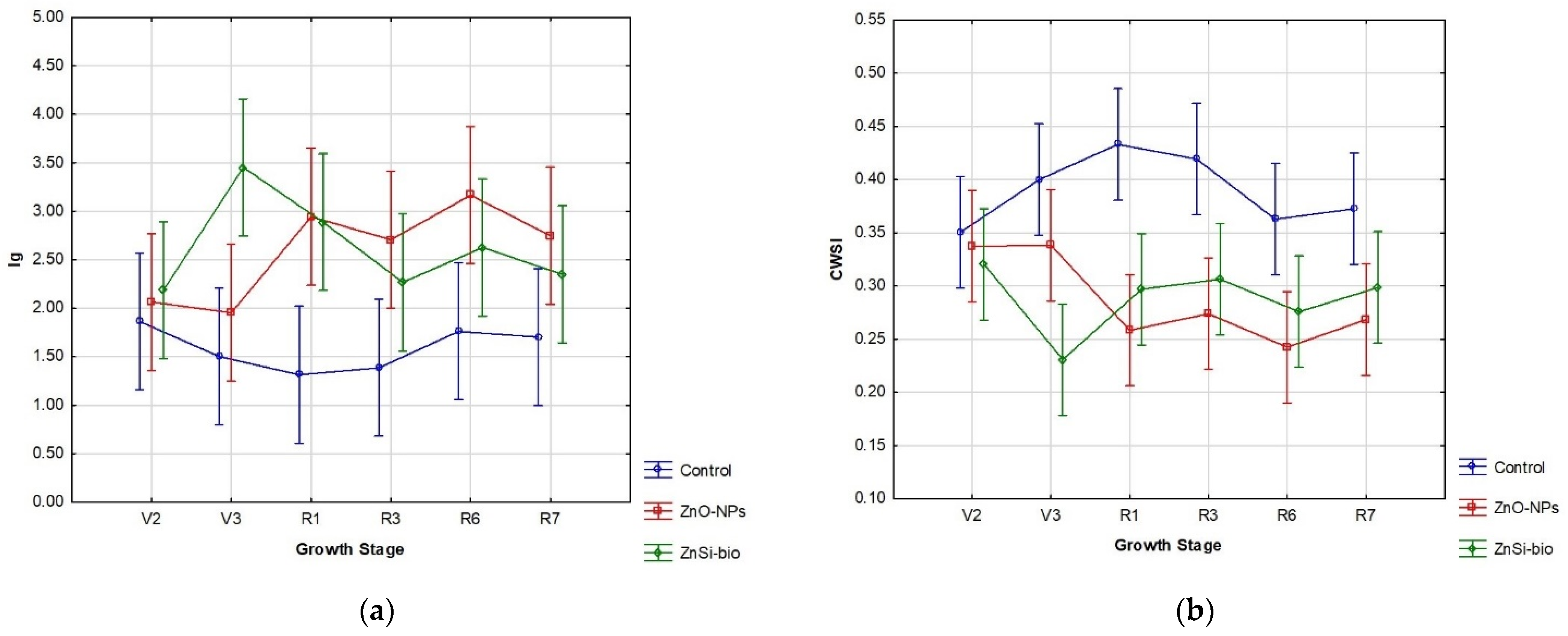
In our case, the foliar application of nano-domain NFs showed a positive response in overall physiological indices (Table 4) at a high significance level, particularly in Ig and CWSI (Table 4). However, the physiological dynamics throughout the growing season (Figure 3) reveal statistically significant changes in both applied variants, specifically during the vegetative growth stage V3 (third trifoliolate) and reproductive growth stage R6 (full seed development).

Table 4.
Comparison of physiological indices in soybeans after NFs-foliar deposition on soybean leaves.
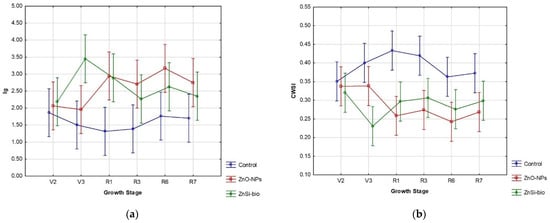
Figure 3.
Comparison of seasonal physiological indices (a) Ig and (b) CWSI in soybeans after NFs-foliar deposition on leaves in the Central European agronomic region for the 2023 vegetation season.
In this context, our applied NFs can be considered a relative functional tool for mitigating climate changes, as the thermal requirements for soybeans aligned with a relative seasonal optimum at around 2500 °C (Figure 1a) with an interactive effect of CO2 on plant growth [52]. However, the water management supply (Figure 1b) was non-ideal during the growing season [53], with the NF-treated variants showing better physiological adaptation in our agronomical region (Table 4, Figure 3).
The study conducted by Sedghi et al. [54] found that ZnO-NPs can mitigate the adverse effects of drought, thereby helping soybeans to better manage thermal stress and increase their drought tolerance [55]. Tripathi et al. [56] observed that SiO2 accumulation facilitates the opening of stomata, leading to increased transpiration and CO2 uptake. The SiO2 improves the utilization of available water to achieve higher net assimilation.
Principally, ZnO-NPs in soybean spray applications may act as a Zn2+ micronutrient or photosynthesis-encouraging agent. Despite soybeans being classified as a plant with a critical photoperiod of about 13 h of daylight, they initiate flowering below this photoperiod and reach optimal photosynthetic efficiency at temperatures of up to 30 °C in certain developmental stages [39]. In this case, theoretically, the energy provided by the transformation of solar irradiation through the NP’s photocatalytic activity could enhance photosynthesis, similar to the effect observed with TiO2-NPs in spinach [57,58]. It is similar in terms of size and crystallography to the ZnO-NPs we applied. The authors Hong et al. [57] and Hong et al. [58] evidenced that, in addition to enhancing photosynthesis, this approach also increased enzymatic activity in catalases and peroxidases, as well as oxygen release. Furthermore, exposure of ZnO-NPs to sunlight radiation leads to gradual photocorrosion [59], with the Zn ions released adsorbing into plant tissues and subsequently complexing with oxalates, citrates, phytates, or hydrated Zn2⁺ ions, thereby positively influencing plant’s metabolism [8].
The ZnSi-bio variant utilizes the synergistic positive physiological effect of silica in soybeans [56] with ZnO-NPs and yeasts. In general, crystalline SiO2 particles, either alone or combined with other oxides, serve as significant photocatalytic materials widely used in industry and the environment [60]. The porous structure of the biosilica used may have improved the rate at which Zn ions were released to the leaf surface and may have mitigated the potential negative effects yeast can have on plants [6].
In our previous short-term empirical outcomes with other plants, ZnO-NPs positive physiological responses were observed typically one month after the second foliar application of NFs at a statistically significant level in sunflower [13] and lentil [15], as well as in extending critical metabolic–energetic processes during the vegetation period in foxtail millet [14]. Results of this agronomic study demonstrated that a single foliar application of nanofertilizers (NFs) was sufficient for soybeans, potentially lowering the financial costs associated with fertilizer use.
4. Conclusions
Our study in the Central Europe agronomical region found interesting results in the foliar application of environmentally friendly concentrations of NFs on soybeans. Although the production parameters were generally inconclusive, the ZnSi-bio variant showed a beneficial effect on the number of pods, seed count, and yield, while the ZnO-NPs variant excelled in terms of seed bulk density compared to the NF-free control. In terms of seed weight, the NF-free control variant showed a relatively greater effect, so the hypothesis that foliar application of NFs would lead to a more effective yield was only partially confirmed.
Our work focused on optimizing the final harvesting quality of soybean seeds, and our results have demonstrated improved outcomes at a statistically significant level for ether extract content and bulk density of seeds but without Zn biofortification with ZnO-NP application. However, in the case of the ZnSi-bio variant, zinc exhibited a stimulatory biofortification effect on the quality of soybeans. This was most likely due to the synergistic effect of ZnO-NPs with bio-silica and food yeast sprayed onto soybean leaves once per season for agronomic and economic benefit.
Both foliar NFs showed statistically significant increases in the highest nutritional-energetic values of soybean beans. Additionally, the seasonally intensified physiology was observed in stomatal conductance (Ig) and the crop water stress index (CWSI) compared to the control variant, fully confirming the hypothesis.
Author Contributions
Investigation, writing—original draft preparation, supervision, proposed the topic, methodology, D.E. and M.K. (Marek Kolenčík); formal analysis, figures, tables, and software—statistical implementations, and validation, L.D., I.Č. and V.S.; writing—review and editing, grammar, review and editing, M.Š., Y.Q. and J.G.S.; performed analysis, M.K. (Michal Kupec); funding acquisition and project administration, D.E. and M.K. (Marek Kolenčík). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Grant Agency of the Slovak Republic Ministry of Education and the Slovak Academy of Sciences under contract VEGA 1/0655/23, VEGA 1/0331/23, also the project of Grant Agency of the Faculty of Agrobiology and Food Resources, SUA in Nitra, GAFAPZ 9/2023 with title “Investigation of Effect of Foliar Application of Nanofertilizers on Selected Field Crop Production Indicators”, and by the European Union foundation (Erasmus Plus Programme for academic staff mobility), and postgraduate program sponsored by National Scholarship Programme of the Slovak Republic through SAIA Organization.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Yadav, A.; Yadav, K.; Abd-Elsalam, K. Nanofertilizers: Types, Delivery and Advantages in Agricultural Sustainability. Agrochemicals 2023, 2, 296–336. [Google Scholar] [CrossRef]
- Rajiv, P.; Chen, X.; Li, H.; Rehaman, S.; Vanathi, P.; Abd-Elsalam, K.A.; Li, X. Silica-Based Nanosystems: Their Role in Sustainable Agriculture. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 437–459. ISBN 978-0-12-821354-4. [Google Scholar]
- Liu, R.; Lal, R. Potentials of Engineered Nanoparticles as Fertilizers for Increasing Agronomic Productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Mousavi Kouhi, S.M.; Lahouti, M.; Ganjeali, A.; Entezari, M.H. Comparative Phytotoxicity of ZnO Nanoparticles, ZnO Microparticles, and Zn2+ on Rapeseed (Brassica napus L.): Investigating a Wide Range of Concentrations. Toxicol. Environ. Chem. 2014, 96, 861–868. [Google Scholar] [CrossRef]
- Afzal, S.; Singh, N.K. Effect of Zinc and Iron Oxide Nanoparticles on Plant Physiology, Seed Quality and Microbial Community Structure in a Rice-Soil-Microbial Ecosystem. Environ. Pollut. 2022, 314, 120224. [Google Scholar] [CrossRef]
- Constantinescu-Aruxandei, D.; Lupu, C.; Oancea, F. Siliceous Natural Nanomaterials as Biorationals—Plant Protectants and Plant Health Strengtheners. Agronomy 2020, 10, 1791. [Google Scholar] [CrossRef]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc Oxide Nanoparticles for Revolutionizing Agriculture: Synthesis and Applications. Sci. World J. 2014, 2014, 925494. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Van Der Ent, A.; Cheng, M.; Jiang, H.; Lund Read, T.; Lombi, E.; Tang, C.; De Jonge, M.D.; Menzies, N.W.; et al. Absorption of Foliar-Applied Zn in Sunflower (Helianthus annuus): Importance of the Cuticle, Stomata and Trichomes. Ann. Bot. 2019, 123, 57–68. [Google Scholar] [CrossRef]
- Gao, X.; Kundu, A.; Bueno, V.; Rahim, A.A.; Ghoshal, S. Uptake and Translocation of Mesoporous SiO2-Coated ZnO Nanoparticles to Solanum lycopersicum Following Foliar Application. Environ. Sci. Technol. 2021, 55, 13551–13560. [Google Scholar] [CrossRef]
- Du, W.; Yang, J.; Peng, Q.; Liang, X.; Mao, H. Comparison tudy of Zinc Nanoparticles and Zinc Sulphate on Wheat Growth: From Toxicity and Zinc Biofortification. Chemosphere 2019, 227, 109–116. [Google Scholar] [CrossRef]
- Kowalska, J.; Krzymińska, J.; Tyburski, J. Yeasts as a Potential Biological Agent in Plant Disease Protection and Yield Improvement—A Short Review. Agriculture 2022, 12, 1404. [Google Scholar] [CrossRef]
- Yadav, M.; Dwibedi, V.; Sharma, S.; George, N. Biogenic Silica Nanoparticles from Agro-Waste: Properties, Mechanism of Extraction and Applications in Environmental Sustainability. J. Environ. Chem. Eng. 2022, 10, 108550. [Google Scholar] [CrossRef]
- Kolenčík, M.; Ernst, D.; Urík, M.; Ďurišová, Ľ.; Bujdoš, M.; Šebesta, M.; Dobročka, E.; Kšiňan, S.; Illa, R.; Qian, Y.; et al. Foliar Application of Low Concentrations of Titanium Dioxide and Zinc Oxide Nanoparticles to the Common Sunflower under Field Conditions. Nanomaterials 2020, 10, 1619. [Google Scholar] [CrossRef] [PubMed]
- Kolenčík, M.; Ernst, D.; Komár, M.; Urík, M.; Šebesta, M.; Dobročka, E.; Černý, I.; Illa, R.; Kanike, R.; Qian, Y.; et al. Effect of Foliar Spray Application of Zinc Oxide Nanoparticles on Quantitative, Nutritional, and Physiological Parameters of Foxtail Millet (Setaria italica L.) under Field Conditions. Nanomaterials 2019, 9, 1559. [Google Scholar] [CrossRef] [PubMed]
- Kolenčík, M.; Ernst, D.; Komár, M.; Urík, M.; Šebesta, M.; Ďurišová, Ľ.; Bujdoš, M.; Černý, I.; Chlpík, J.; Juriga, M.; et al. Effects of Foliar Application of ZnO Nanoparticles on Lentil Production, Stress Level and Nutritional Seed Quality under Field Conditions. Nanomaterials 2022, 12, 310. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Bindraban, P.S.; Fugice, J.; Agyin-Birikorang, S.; Singh, U.; Hellums, D. Composite Micronutrient Nanoparticles and Salts Decrease Drought Stress in Soybean. Agron. Sustain. Dev. 2017, 37, 5. [Google Scholar] [CrossRef]
- Dola, D.; Mannan, M. Foliar Application Effects of Zinc Oxide Nanoparticles on Growth, Yield and Drought Tolerance of Soybean. Bangladesh Agron. J. 2023, 25, 73–82. [Google Scholar] [CrossRef]
- Chauhan, G.; Joshi, O.P. Soybean (Glycine max)-the 21st century crop. Indian J. Agric. Sci. 2005, 75. [Google Scholar]
- Ali, W.; Ahmad, M.M.; Iftikhar, F.; Qureshi, M.; Ceyhan, A. Nutritive potentials of Soybean and its significance for humans health and animal production: A Review. Eurasian J. Food Sci. Technol. 2020, 4, 41–53. [Google Scholar]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative Study of the Phytotoxicity of ZnO Nanoparticles and Zn Accumulation in Nine Crops Grown in a Calcareous Soil and an Acidic Soil. Sci. Total Environ. 2018, 644, 770–780. [Google Scholar] [CrossRef]
- García-López, J.; Zavala-García, F.; Olivares-Sáenz, E.; Lira-Saldívar, R.; Díaz Barriga-Castro, E.; Ruiz-Torres, N.; Ramos-Cortez, E.; Vázquez-Alvarado, R.; Niño-Medina, G. Zinc Oxide Nanoparticles Boosts Phenolic Compounds and Antioxidant Activity of Capsicum Annuum L. during Germination. Agronomy 2018, 8, 215. [Google Scholar] [CrossRef]
- Prograin, S. KORUS. Available online: https://www.ipkagro.sk/en/soja-ponuka-osiv/ (accessed on 7 June 2024).
- Džatko, M.; Sobocká, J.; Granec, M.; Bezák, P. Príručka pre používanie máp pôdnoekologických jednotiek. Inovovaná Príručka Pre Bonitáciu A Hodnot. Poľnohospodárskych Pôd Slovenska. Výskumný Ust. Pôdoznalectva A Ochr. Pôdy 2009. [Google Scholar]
- Meteoblue. Available online: https://www.meteoblue.com/sk/ (accessed on 3 April 2023).
- Banerjee, A.; Duflo, E. (Eds.) Handbook of Economic Field Experiments; Handbooks in Economics; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-444-63324-8. [Google Scholar]
- Jones, H.G.; Serraj, R.; Loveys, B.R.; Xiong, L.; Wheaton, A.; Price, A.H. Thermal Infrared Imaging of Crop Canopies for the Remote Diagnosis and Quantification of Plant Responses to Water Stress in the Field. Funct. Plant Biol. 2009, 36, 978. [Google Scholar] [CrossRef] [PubMed]
- STN standard 461011; Testing of Cereals, Legumes and Oil-Bearing Crops. Slovak Office of Standards, Metrology and Testing: Bratislava, Slovakia, 1988.
- STN standard 2300-7/94; Oilseeds. Soya Seed. Slovak Office of Standards, Metrology and Testing: Bratislava, Slovakia, 2002.
- Losak, T.; Hlusek, J.; Martinec, J.; Jandak, J.; Szostkova, M.; Filipcik, R.; Manasek, J.; Prokes, K.; Peterka, J.; Varga, L.; et al. Nitrogen Fertilization Does Not Affect Micronutrient Uptake in Grain Maize (Zea mays L.). Acta Agric. Scand. Sect. B-Soil Plant Sci. 2011, 61, 543–550. [Google Scholar] [CrossRef]
- Hong, J.; Wang, C.; Wagner, D.C.; Gardea-Torresdey, J.L.; He, F.; Rico, C.M. Foliar Application of Nanoparticles: Mechanisms of Absorption, Transfer, and Multiple Impacts. Environ. Sci. Nano 2021, 8, 1196–1210. [Google Scholar] [CrossRef]
- López-Moreno, M.L.; De La Rosa, G.; Hernández-Viezcas, J.Á.; Castillo-Michel, H.; Botez, C.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Evidence of the Differential Biotransformation and Genotoxicity of ZnO and CeO2 Nanoparticles on Soybean (Glycine max) Plants. Environ. Sci. Technol. 2010, 44, 7315–7320. [Google Scholar] [CrossRef]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef]
- Martínez Cuesta, N.; Carciochi, W.; Wyngaard, N.; Sainz Rozas, H.; Silva, S.; Salvagiotti, F.; Barbieri, P. Zinc Fertilization Strategies in Soybean: Plant Uptake, Yield, and Seed Concentration. J. Plant Nutr. 2023, 46, 1134–1144. [Google Scholar] [CrossRef]
- Sturikova, H.; Krystofova, O.; Huska, D.; Adam, V. Zinc, Zinc Nanoparticles and Plants. J. Hazard. Mater. 2018, 349, 101–110. [Google Scholar] [CrossRef]
- Pandey, N.; Gupta, B.; Pathak, G.C. Foliar Application of Zn at Flowering Stage Improves Plant’s Performance, Yield and Yield Attributes of Black Gram. Indian J. Exp. Biol. 2013, 51, 548–555. [Google Scholar]
- Pandey, N.; Pathak, G.C.; Sharma, C.P. Zinc Is Critically Required for Pollen Function and Fertilisation in Lentil. J. Trace Elem. Med. Biol. 2006, 20, 89–96. [Google Scholar] [CrossRef]
- Priester, J.H.; Ge, Y.; Mielke, R.E.; Horst, A.M.; Moritz, S.C.; Espinosa, K.; Gelb, J.; Walker, S.L.; Nisbet, R.M.; An, Y.-J.; et al. Soybean Susceptibility to Manufactured Nanomaterials with Evidence for Food Quality and Soil Fertility Interruption. Proc. Natl. Acad. Sci. USA 2012, 109, E2451–E2456. [Google Scholar] [CrossRef] [PubMed]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Responses of Soybean (Glycine max [L.] Merr.) to Zinc Oxide Nanoparticles: Understanding Changes in Root System Architecture, Zinc Tissue Partitioning and Soil Characteristics. Sci. Total Environ. 2022, 835, 155348. [Google Scholar] [CrossRef] [PubMed]
- Staniak, M.; Szpunar-Krok, E.; Kocira, A. Responses of Soybean to Selected Abiotic Stresses—Photoperiod, Temperature and Water. Agriculture 2023, 13, 146. [Google Scholar] [CrossRef]
- Phu, D.V.; Du, B.D.; Tuan, L.N.A.; Tam, H.V.; Hien, N.Q. Preparation and Foliar Application of Oligochitosan—Nanosilica on the Enhancement of Soybean Seed Yield. Int. J. Environ. Agric. Biotechnol. 2017, 2, 421–428. [Google Scholar] [CrossRef]
- Kolenčík, M.; Šebesta, M.; Ďurišová, Ľ.; Ďúranová, H.; Ernst, D.; Kšiňan, S.; Kósa, P.; Illa, R.; Baby, M.K.; Zapletalová, A.; et al. Complex Study of Foliar Application of Inorganic Nanofertilizers in Field Conditions: Impact on Crop Production and Environmental–Ecological Assessment. In Nanofertilizers for Sustainable Agroecosystems; Abd-Elsalam, K.A., Alghuthaymi, M.A., Eds.; Nanotechnology in the Life Sciences; Springer Nature: Cham, Switzerland, 2024; pp. 507–560. ISBN 978-3-031-41328-5. [Google Scholar]
- Sobean. Available online: https://www.fao.org/land-water/databases-and-software/crop-information/soybean/en/ (accessed on 6 March 2024).
- Shurson, G.C.; Kerr, B.J.; Hanson, A.R. Evaluating the Quality of Feed Fats and Oils and Their Effects on Pig Growth Performance. J. Anim. Sci. Biotechnol. 2015, 6, 10. [Google Scholar] [CrossRef]
- Rahman, A.; Cho, B.-K. Assessment of Seed Quality Using Non-Destructive Measurement Techniques: A Review. Seed Sci. Res. 2016, 26, 285–305. [Google Scholar] [CrossRef]
- Priester, J.H.; Moritz, S.C.; Espinosa, K.; Ge, Y.; Wang, Y.; Nisbet, R.M.; Schimel, J.P.; Susana Goggi, A.; Gardea-Torresdey, J.L.; Holden, P.A. Damage Assessment for Soybean Cultivated in Soil with Either CeO2 or ZnO Manufactured Nanomaterials. Sci. Total Environ. 2017, 579, 1756–1768. [Google Scholar] [CrossRef]
- Mandal, K.G.; Saha, K.P.; Ghosh, P.K.; Hati, K.M.; Bandyopadhyay, K.K. Bioenergy and Economic Analysis of Soybean-Based Crop Production Systems in Central India. Biomass Bioenergy 2002, 23, 337–345. [Google Scholar] [CrossRef]
- Cabrera-Orozco, A.; Jimenez-Martinez, C.; Davila-Ortiz, G. Soybean: Non-Nutritional Factors and Their Biological Functionality. In Soybean—Bio-Active Compounds; El-Shemy, H., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-0977-8. [Google Scholar]
- Anthony, P.; Malzer, G.; Sparrow, S.; Zhang, M. Soybean Yield and Quality in Relation to Soil Properties. Agron. J. 2012, 104, 1443–1458. [Google Scholar] [CrossRef]
- Shute, T.; Macfie, S.M. Cadmium and Zinc Accumulation in Soybean: A Threat to Food Safety? Sci. Total Environ. 2006, 371, 63–73. [Google Scholar] [CrossRef]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.-N.; Biswas, P. Mechanistic Evaluation of Translocation and Physiological Impact of Titanium Dioxide and Zinc Oxide Nanoparticles on the Tomato (Solanum lycopersicum L.) Plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-Y.; El Sheikha, A.F.; Hu, D.-M. The Positive Impacts of Microbial Phytase on Its Nutritional Applications. Trends Food Sci. Technol. 2019, 86, 553–562. [Google Scholar] [CrossRef]
- Baker, J.T.; Allen, L.H.; Boote, K.J.; Jones, P.; Jones, J.W. Response of Soybean to Air Temperature and Carbon Dioxide Concentration. Crop Sci. 1989, 29, 98–105. [Google Scholar] [CrossRef]
- Zanon, A.J.; Streck, N.A.; Grassini, P. Climate and Management Factors Influence Soybean Yield Potential in a Subtropical Environment. Agron. J. 2016, 108, 1447–1454. [Google Scholar] [CrossRef]
- Sedghi, M.; Sheikhnavaz Jahed, P.; Gholi-Tolouie, S. Zinc oxide nano particles alleviate drought stress effects on soybean antioxidant system during germination. Iran. J. Plant Physiol. 2021, 11, 3769–3778. [Google Scholar]
- Linh, T.M.; Mai, N.C.; Hoe, P.T.; Lien, L.Q.; Ban, N.K.; Hien, L.T.T.; Chau, N.H.; Van, N.T. Metal-Based Nanoparticles Enhance Drought Tolerance in Soybean. J. Nanomater. 2020, 2020, 4056563. [Google Scholar] [CrossRef]
- Tripathi, P.; Na, C.-I.; Kim, Y. Effect of Silicon Fertilizer Treatment on Nodule Formation and Yield in Soybean (Glycine max L.). Eur. J. Agron. 2021, 122, 126172. [Google Scholar] [CrossRef]
- Hong, F.; Yang, F.; Liu, C.; Gao, Q.; Wan, Z.; Gu, F.; Wu, C.; Ma, Z.; Zhou, J.; Yang, P. Influences of Nano-TiO2 on the Chloroplast Aging of Spinach Under Light. Biol. Trace Elem. Res. 2005, 104, 249–260. [Google Scholar] [CrossRef]
- Hong, F.; Zhou, J.; Liu, C.; Yang, F.; Wu, C.; Zheng, L.; Yang, P. Effect of Nano-TiO2 on Photochemical Reaction of Chloroplasts of Spinach. Biol. Trace Elem. Res. 2005, 105, 269–280. [Google Scholar] [CrossRef]
- Ma, H.; Wallis, L.K.; Diamond, S.; Li, S.; Canas-Carrell, J.; Parra, A. Impact of Solar UV Radiation on Toxicity of ZnO Nanoparticles through Photocatalytic Reactive Oxygen Species (ROS) Generation and Photo-Induced Dissolution. Environ. Pollut. 2014, 193, 165–172. [Google Scholar] [CrossRef]
- Yamashita, H.; Mori, K.; Kuwahara, Y.; Kamegawa, T.; Wen, M.; Verma, P.; Che, M. Single-Site and Nano-Confined Photocatalysts Designed in Porous Materials for Environmental Uses and Solar Fuels. Chem. Soc. Rev. 2018, 47, 8072–8096. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).