Abstract
Cold stress severely impairs plant growth, development, and yields. L-ascorbic acid (ASA), a crucial antioxidant, is pivotal in mitigating stress-induced damage. Previous research found that 2-keto-L-gulonic acid (2KGA), a precursor of ASA in its industrial production, effectively enhances the endogenous ASA content in plants. We hypothesized that 2KGA might alleviate chilling stress and tried to verify it through a cultivation experiment of Arabidopsis thaliana. The results demonstrate that the application of 2KGA significantly increased ASA content (24.58%) and up-regulated ASA biosynthetic genes in Arabidopsis at 4 °C for 24 h. Furthermore, 2KGA alleviated the decrease in fresh weight (17.05%) and total chlorophyll content (15.85%) caused by low temperatures. The contents of proline, soluble sugar (SS), soluble protein (SP), and the activities of antioxidant enzymes including superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) were significantly increased under the 2KGA treatment at low temperatures, while the malondialdehyde (MDA) content was reduced. Moreover, 2KGA up-regulated the ICE-CBF-COR signaling pathway in response to cold stress. These collective findings strongly support the involvement of 2KGA in enhancing cold tolerance in Arabidopsis, presenting an innovative approach for agricultural practices aimed at enhancing crop resilience to environmental stresses.
1. Introduction
Cold stress is a major abiotic factor that severely impacts the growth and development of plants. As climate change leads to more unseasonable temperatures, the frequency and intensity of cold stress may increase, posing a growing threat to global food security [1]. When exposed to low temperatures, reactive oxygen species (ROS) in plants are rapidly accumulated, leading to oxidative damage to necessary cellular ingredients such as proteins, lipids, nucleic acids, and carbohydrates, ultimately affecting plant growth, development, and yield [2]. To mitigate the adverse impacts induced by the over-generated ROS, plants have evolved antioxidant defense systems to resist injuries, including both enzymatic antioxidants, such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) [3], as well as non-enzymatic antioxidants, such as ascorbic acid (ASA) and glutathione (GSH) [4]. At the molecular level, plants respond to cold stress by activating a series of gene expression and signal transduction pathways, whereas the ICE-CBF-COR signaling pathway is considered to be the critical mechanism [5,6]. The inducers of CBF expression (ICE) genes are activated by cold signals and subsequently induce the expression of the C-repeat binding factor (CBF) genes, which in turn up-regulate the expression of cold-regulated (COR) genes, thereby enhancing plant cold tolerance [7]. Additionally, transcription factors such as calmodulin-binding transcription activators (CAMTA3), zinc-finger protein (ZAT12), and R2R3 type transcription factor (MYB15) also contribute to the modulation of cold-responsive gene expression [8].
L-ascorbic acid (ASA, vitamin C) is a major antioxidant in plants, playing a vital role in scavenging ROS to mitigate the damaging effects induced by stressful environments [9]. ASA can directly remove ROS through redox reactions and also serves as a substrate for APX to facilitate the detoxification of ROS [10]. The accumulation of ASA in plants is a complex process governed by a series of enzymatic reactions, including the biosynthetic pathway and the recycling pathways [11]. The importance of ASA in enhancing plant stress tolerance has been highlighted in numerous studies, which suggest that its accumulation can significantly improve the resistance of plants to abiotic stress [12,13,14,15]. The accumulation of ASA under cold stress has been observed to be a common phenomenon in plants, suggesting that ASA is involved in the tolerance to cold stress [16]. Previous studies have indicated that exogenous application of ascorbic acid can improve the cold tolerance of various plants by improving photosynthesis, regulating cellular osmotic pressure, and enhancing the antioxidant defense system [17,18,19]. Moreover, the accumulation of ASA in plants through gene engineering could efficiently increase plant stress tolerance [20]. Current evidence shows that higher levels of endogenous ASA could enhance cold stress tolerance in plants [21]. Although several findings suggest that exogenous application of ASA or genetic editing can increase endogenous ASA levels in plants, the instability of ASA and the controversies surrounding genetically engineered crops present challenges for its application in agricultural production.
2-keto-L-gulonic acid (2KGA), mainly produced via a two-step microbial fermentation process, is an exclusive precursor utilized in the chemical synthesis of ASA [22]. In the industrial production of ASA, a residue after evaporation (RAE) generated during the crystallization of 2KGA from fermentation liquor contains a range of low-molecular-weight organic acids (LMWOAs) such as 2KGA (approximately 25%), oxalic acid, formic acid, and acetic acid [23]. Previous studies have demonstrated that the application of RAE can effectively improve the yield and quality (particularly the ASA content) of pak choi (Brassica rapa L.) [24], purslane (Portulaca oleracea L.) [25], and cotton (Gossypium hirsutum L.) [26], showing a future potential of RAE in agricultural application. Remarkably, as the main component in RAE, 2KGA may be the key to effectively improving the endogenous ASA levels in plants [27]. In addition, the results of our previous findings suggest that 2KGA can relieve the negative impacts of salt stress on plant growth by promoting the accumulation of endogenous ASA [28].
Due to the positive impact of 2KGA in the ASA synthesis of plants and the essential impact of ASA in plant growth and development, we hypothesized that exogenous 2KGA could increase the endogenous ASA content in plants and thereafter enhance resistance to cold stress. So far, there is no report on whether 2KGA can enhance cold stress tolerance in plants. In this study, Arabidopsis thaliana, a model organism extensively used for plant biology research [29], was chosen as the research material to evaluate the effects of 2KGA on plant growth, physiology, antioxidant system, ASA metabolism-related gene expression, and cold stress-related gene expression under low-temperature stress conditions. The results improve our understanding of the protective mechanisms of 2KGA under cold stress in plants and may offer a new strategy to enhance cold tolerance in economically important crops, ensuring sustainable agricultural practices amid climate change.
2. Materials and Methods
2.1. Plant Growth and Experimental Design
The Arabidopsis thaliana ecotype Columbia-0 (Col-0) was used in this study. Seeds were surface-sterilized with 5% sodium hypochlorite solution for 10 min and then thoroughly washed with sterile water. The sterilized seeds were cultured on half-strength Murashige and Skoog (MS) medium containing 0.8% agar and 1% sucrose and kept at 4 °C in the dark for 3 days. After vernalization, the seeds were transferred to a growth chamber under the following conditions: 16 h light/8 h dark cycles, a light intensity of 7500 lx, a temperature of 22 °C ± 1 °C, and a relative humidity of 60 ± 10%. Subsequently, 10-day-old Arabidopsis seedlings were transplanted into square plastic pots (7 cm in length, width, and depth), containing approximately 250 g of sterilized nutrient soil. Four seedlings were placed in each pot and grown under the same conditions.
The experiment included two groups: the CK group (control group) and the 2KGA group (2KGA treatment group). For the 2KGA treatment, 5 mM 2KGA solution was pretreated by adjusting the pH to 6.5 using KOH solution. KCl solution with the same K+ content was used as a control to eliminate the potential interference of K+. Next, 4-week-old Arabidopsis were divided into two groups and treated with either KCl solution (CK group) or 5 mM 2KGA solution (2KGA group) by irrigation at a dose of 10 mL per pot at six-day intervals, three times. The quantity of 2KGA (0.04 g/kg soil) was determined as the optimal dosage based on preliminary experiments (not published). For cold stress treatment, 7-week-old Arabidopsis (3 days after the final treatment) were transferred to 4 °C conditions. Each experimental group consisted of six biological replicates, with each replicate containing 12 plants harvested from three pots. Fresh leaf samples were collected at 0 h (before cold stress), 12 h, 24 h, and 48 h for the measurement of growth parameters and ASA content. Other samples were rapidly frozen in liquid nitrogen and stored at −80 °C until subsequent analysis.
2.2. Growth Parameters and ASA Content
Growth parameters of Arabidopsis, including leaf length, leaf width, and maximum rosette radius were measured using a Vernier caliper (Deli, Ningbo, China). Fresh weight was determined on an analytical balance (Sartorius, Goettingen, Germany) by weighing the whole rosette. For ASA analysis, ascorbic acid was extracted from fresh leaves (0.15 g) homogenized in 1.5 mL of 1% (w/v) metaphosphate on ice. The homogenate was centrifuged at 6000 rpm for 10 min at 4 °C, and the supernatant was filtered using a 0.22 μm injection filter (Millipore, Billerica, MA, USA). The extract was measured by high-performance liquid chromatography (HPLC) equipped with a C18 column as previously reported [24,28]. The detection conditions were as follows: mobile phase, 95% NaH2PO4 solution (20 mM, pH 2.8 ± 0.1), and 5% acetonitrile; flow rate, 1 mL/min; temperature, 40 °C; and detection wavelength, 243 nm.
2.3. Photosynthetic Pigments
Photosynthetic pigments were extracted from leaf samples (0.2 g) homogenized in 5 mL of 95% ethanol on ice in the dark as previously described [30]. The homogenate was centrifuged at 6000 rpm for 10 min and the supernatant was collected. The absorbance of the supernatant was measured at 665, 649, and 470 nm, respectively. The contents of chlorophyll a (Chla), chlorophyll b (Chlb), total chlorophyll (Chlab), and carotenoids (Car) were calculated using the following formulas:
2.4. Contents of Malondialdehyde, Proline, Soluble Carbohydrate, and Soluble Protein
The content of malondialdehyde (MDA) was determined using the 2-thiobarbituric acid (TBA) method by measuring the absorbance at 450, 532, and 600 nm [31]. Proline content was quantified through the acid–ninhydrin method, with absorbance recorded at 520 nm [32]. The content of soluble sugar (SS) was assessed using the phenol–sulfuric acid method [33] by reading the absorbance at 490 nm. Soluble protein (SP) content was determined by the Bradford assay with absorbance measured at 595 nm [34].
2.5. Antioxidant Enzyme Activity
Leaf samples (0.2 g) were homogenized in 2 mL of 50 mM PBS buffer (pH 7.8) containing 1 mM EDTA-2Na, 0.5 mM ascorbic acid, and 1% (w/v) polyvinylpyrrolidone (PVP) in an ice bath. The homogenate was centrifuged at 10,000× g for 20 min at 4 °C, and the supernatant was collected to estimate enzyme activities [35]. SOD activity was assessed by observing the photoinhibition of nitroblue tetrazolium (NBT) at 560 nm [36]. POD activity was determined by recording the extension of guaiacol at 470 nm [37]. CAT was observed by measuring the decomposition of hydrogen peroxide at 240 nm [38]. APX activity was evaluated by monitoring the oxidation of ascorbate at 290 nm [39].
2.6. Gene Expression Analysis
Total RNA was extracted from the Arabidopsis leaves using the SteadyPure Universal RNA Extraction Kit (Accurate Bio, Inc., Changsha, China) according to the instructions of the manufacturer. cDNA was prepared using the Evo M-MLV RT Premix for qPCR (Accurate Bio, Inc., Changsha, China) following the manufacturer’s instructions. Real-time quantitative PCR (RT-qPCR) was performed using the SYBR Green Premix Pro Taq HS qPCR Kit (Accurate Bio, Inc., Changsha, China). The reference gene in this study was Actin and the primers are listed in Table S1. The relative expression values were calculated by the 2−ΔΔCt method [40].
2.7. Statistical Analysis
All the statistical analyses were performed using GraphPad Prism Version 9.0.0 (GraphPad Software Inc., La Jolla, CA, USA). Data were expressed as means ± standard deviation (SD) of six biological replicates. Two-tailed Student’s t-test was used to identify significant variations. p < 0.05 was considered to indicate a statistically significant difference.
3. Results
3.1. Effect of 2KGA on Plant Growth Parameters under Cold Stress
The application of 2KGA positively impacted the growth of Arabidopsis at room temperature (0 h). As presented in Table 1, leaf length, and maximum rosette radius in the 2KGA group were significantly increased by 7.31 and 7.12%, respectively, compared with the control (p < 0.05), while leaf width was not significantly different among treatments. Furthermore, the 2KGA treatment (0.49 ± 0.04 g/plant) markedly improved (p < 0.05) the fresh weight of Arabidopsis by 13.71% compared with the control (0.43 ± 0.02 g/plant) (Figure 1a). It was observed that cold stress had a depressive effect on all plant growth parameters. The fresh weight of Arabidopsis was decreased by 24.94 and 30.80% in the CK group and by 21.92 and 28.76% in the 2KGA group kept at 4 °C for 12 h and 24 h, respectively (Figure 1a). Notably, despite exposure to low temperatures causing a reduction in fresh weight, the application of 2KGA markedly enhanced the fresh weight at 12 h and 24 h by 18.27 and 17.05% (p < 0.05), respectively, compared to the control (Figure 1a). Consequently, 2KGA promoted plant growth at normal temperatures and further counteracted the negative effects of cold stress (such as fresh weight loss) on plant growth.

Table 1.
Effect of 2KGA on the leaf length, leaf width, and maximum rosette radius of Arabidopsis under cold stress for 0 h, 12 h, and 24 h. Data represent the means ± SD (n = 6). Different letters indicate statistically significant differences (p < 0.05).
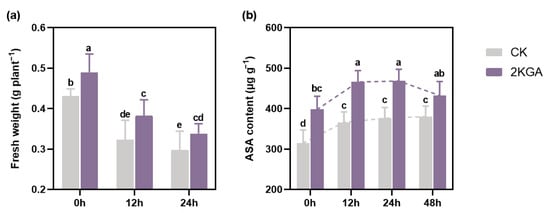
Figure 1.
Effect of 2KGA on the (a) fresh weight and (b) ASA content of Arabidopsis under cold stress for 0 h, 12 h, and 24 h. The error bars represent the means ± SD (n = 6). The dotted lines indicate the variation in ASA content during 48h. Different letters indicate statistically significant differences (p < 0.05).
3.2. Effect of 2KGA on ASA Content under Cold Stress
As shown in Figure 1b, 2KGA treatment (397.93 ± 29.88 µg/g) significantly increased the ASA content of Arabidopsis by 26.31% at normal temperature (p < 0.05) in comparison with the control (315.04 ± 29.74 µg/g). To understand the potential effect of 2KGA on ASA accumulation, we detected the change in ASA content at 4 °C for 48 h. Under chilling conditions, both groups exhibited an accumulation of ASA, with the 2KGA group showing a significantly higher ASA content (p < 0.05) of 27.2, 24.58, and 13.71% at 12 h, 24 h, and 48 h compared to the CK group, respectively (Figure 1b). However, the pattern of temporal variation in ASA content is different between the two groups during 48 h at low temperatures. The ASA content exhibited a steady increase in the CK group at 4 °C, from 366.23 ± 23.5 µg/g at 12 h to 376.17 ± 24.45 µg/g at 24 h, and further to 380.54 ± 23.64 µg/g at 48 h. The ASA content in the 2KGA group increased rapidly at 12 h (465.97 ± 25.74 µg/g), peaked at 24 h (468.62 ± 26.33 µg/g), followed by a gradual decline at 48 h (432.72 ± 31.56 µg/g) under low temperature. Remarkably, the ASA content in the 2KGA group was significantly higher (p < 0.05) than in the control group whether exposed to cold stress or not (Figure 1b), suggesting that 2KGA effectively accelerated the accumulation of endogenous ASA content in Arabidopsis.
3.3. Effect of 2KGA on Photosynthetic Pigments under Cold Stress
To assess the protective roles of 2KGA on the photosynthetic performance under cold stress, the levels of photosynthetic pigments in Arabidopsis leaves were determined. Figure 2 illustrates that the application of 2KGA significantly enhanced the content of photosynthetic pigments at normal temperature (p < 0.05). The contents of Chla, Chlb, and total Chlab in the 2KGA group (0.80 ± 0.01, 0.33 ± 0.01, and 1.13 ± 0.01 mg/g) exhibited a significant increase of 12.06, 5.68, and 10.14%, respectively, when compared with the CK group (0.72 ± 0.02, 0.31 ± 0.01, and 1.02 ± 0.02 mg/g) (Figure 2a–c). However, the two groups did not show any disparity in terms of the Car content (Figure 2d). The results indicate that 2KGA treatment has a promoting effect on chlorophyll accumulation. It was shown that low temperature adversely affected photosynthetic performance, as evidenced by a substantial decrease in photosynthetic pigments (Figure 2). During the period of chilling condition (12 h and 24 h), there was a significant decrease in the contents of Chla (19.95 and 21.80%), Chlb (20.63 and 20.72%), Chlab (20.16 and 21.48%), and Car (16.95 and 19.25%) in the CK group (p < 0.05) (Figure 2a–d). While 2KGA treatment improved the contents of Chla (16.92 and 15.07%), Chlb (17.85 and 16.22%), and Chlab (17.20 and 15.85%) at 4 °C for 12 h and 24 h, respectively, compared with the control (Figure 2a–c), it considerably alleviated the suppression caused by cold stress. Likewise, there was no difference in the Car content between the two groups at low temperatures (Figure 2d). The application of 2KGA could potentially mitigate the destruction of photosynthetic performance in response to low temperatures.
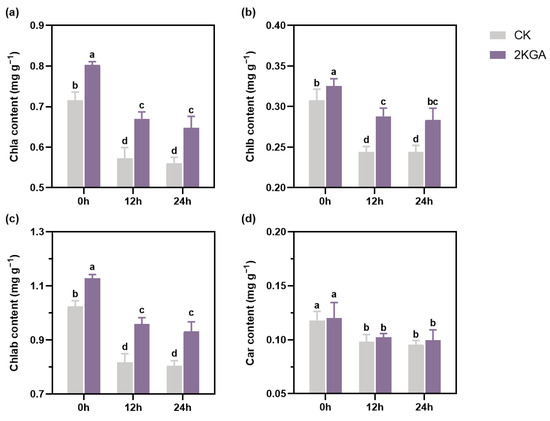
Figure 2.
Effect of 2KGA on the contents of (a) Chla, (b) Chlb, (c) Chlab, and (d) Car of Arabidopsis under cold stress for 0 h, 12 h and 24 h. The error bars represent the means ± SD (n = 6). Different letters indicate statistically significant differences (p < 0.05). Chla, chlorophyll a; Chlb, chlorophyll b; Chlab, total chlorophyll; and Car, carotenoids.
3.4. Effect of 2KGA on MDA, Proline, SS, and SP under Cold Stress
When exposed to low temperatures, the MDA content exhibited a significant increase. However, cold stress-induced enhancement in MDA content at 24 h was reduced to 15.98% with the application of 2KGA (Figure 3a). The proline content rapidly increased under cold stress, with considerably higher (p < 0.05) content in the 2KGA group at 24 h compared to the CK group (Figure 3b). Furthermore, the 2KGA treatment had a negligible effect on MDA and proline contents at normal temperatures (Figure 3a,b). These findings suggest that the application of 2KGA could be an efficacious approach for reducing MDA content and increasing proline content under low-temperature conditions.
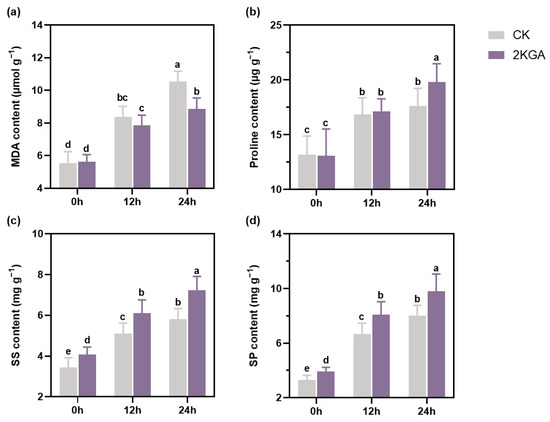
Figure 3.
Effect of 2KGA on the contents of (a) MDA, (b) proline, (c) SS, and (d) SP of Arabidopsis under cold stress for 0 h, 12 h, and 24 h. The error bars represent the means ± SD (n = 6). Different letters indicate statistically significant differences (p < 0.05). MDA, malondialdehyde; SS, soluble sugar; and SP, soluble protein.
It was observed that the application of 2KGA significantly enhanced (p < 0.05) the contents of SS and SP at room temperature (Figure 3c,d). Both SS and SP contents in the leaves of Arabidopsis increased after exposure to low temperatures. The SS content increased by 47.86 and 68.78% in the control group at 12 h and 24 h, respectively, and by 49.47 and 76.97% in the 2KGA group at the same time points (Figure 3c). Similarly, the SP content showed a 103.28 and 143.97% increase in the CK group, and a 105.43 and 149.66% increase in the 2KGA group at 12 h and 24 h, respectively (Figure 3d). The increase in SS and SP content was greater in the 2KGA group under chilling conditions. Compared to the control group, the contents of SS and SP increased by 19.70 and 20.95% in the 2KGA group under cold stress at 12 h, and by 24.15 and 22.48% at 24 h, respectively (Figure 3c,d). The findings therefore suggest that treatment with 2KGA could effectively enhance the accumulation of SS and SP at low temperatures.
3.5. Effect of 2KGA on Enzymatic Antioxidant System under Cold Stress
Low temperatures caused a significant increase (p < 0.05) in the activity of antioxidant enzymes (Figure 4). No significant differences in antioxidant enzyme activities were observed between the 2KGA treatment and the control at normal conditions (Figure 4). Under the chilling condition, the activities of SOD, POD, CAT, and APX in the CK group were enhanced by 9.25, 3.87, 85.84, and 87.25% at 12 h and by 14.97, 8.53, 173.74, and 168.46% at 24 h, respectively. Concurrently, the activities of SOD, POD, CAT, and APX in the 2KGA group were increased by 16.95, 8.47, 211.26, and 167.15% at 12 h and by 20.05, 48,81, 248,58, and 385.30% at 24 h over the same duration (Figure 4). It was observed that the activities of SOD, POD, CAT, and APX were notably higher (p < 0.05) in the 2KGA group in comparison to the CK group under cold stress (Figure 4). Specifically, the activities of SOD, POD, CAT, and APX under 2KGA treatment were enhanced by 9.24, 8.99, 62.05, and 32.54% at 12 h, and by 6.56, 43.11, 23.20, and 67.94% at 24 h, respectively, compared with the control (Figure 4). The results demonstrate the efficacy of 2KGA application in enhancing the activity of antioxidant enzymes for combating cold stress.
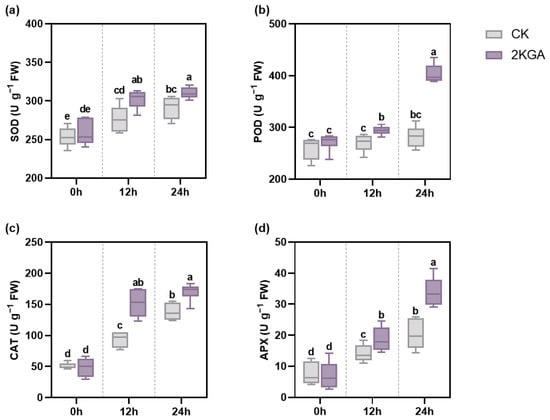
Figure 4.
Effect of 2KGA on the activities of (a) SOD, (b) POD, (c) CAT, and (d) APX of Arabidopsis under cold stress for 0 h, 12 h and 24 h. The error bars represent the means ± SD (n = 6). Different letters indicate statistically significant differences (p < 0.05). SOD, superoxide dismutase; POD, peroxidase; CAT, catalase; and APX, ascorbate peroxidase.
3.6. Effect of 2KGA on ASA Metabolism-Related Gene Expression under Cold Stress
The expression levels of the ASA metabolism-related genes under cold stress including GDP-mannose pyrophosphorylase (GMP), L-galactose-1,4-lactone dehydrogenase (GLDH), myo-inositol monophosphatase (MIXO), L-gulono-1,4-lactone oxidase (GLO), APX, dehydroascorbate reductase (DHAR), and monodehydroascorbate reductase (MDHAR) are presented in Figure 5. In the ASA biosynthesis pathway, 2KGA treatment resulted in a significant increase (p < 0.05) of 1.87, 4.55, 3.41, and 8.23-fold at 12 h (Figure 5a), and 1.87, 3.09, 2.14, and 7.74-fold at 24 h in the expression levels of GMP, GLDH, MIXO, and GLO under cold stress, respectively (Figure 5b), compared to the control group. In the ASA recycling pathway, 2KGA treatment significantly increased (p < 0.05) the expression levels of APX, DHAR1, DHAR2, and MDHAR in Arabidopsis by 2.34, 3.08, 1.68, and 10.46-fold, respectively, compared to the control group after exposure to low temperature for 12 h (Figure 5a). Moreover, after 24 h under cold stress, 2KGA treatment significantly increased the expression levels of APX by 2.31-fold, and decreased the expression levels of MDHAR by 0.29-fold in Arabidopsis compared to the control (Figure 5b). No significant difference was found among treatments in the expression levels of DHAR1 and DHAR2 at 24 h under low temperatures (Figure 5b). The aforementioned results put forth a hypothesis suggesting that the enhancement of cold stress tolerance could potentially be linked to the accumulation of ASA.
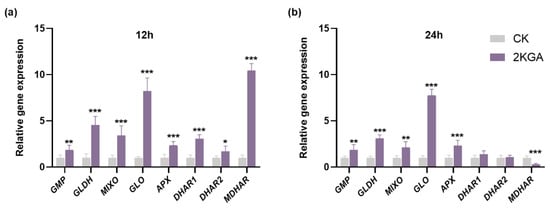
Figure 5.
Expression levels of ASA metabolism-related genes of Arabidopsis under cold stress for (a) 12 h and (b) 24 h. The error bars represent the means ± SD (n = 6). Asterisks indicate statistically significant differences (*** p < 0.001, ** p < 0.01, and * p < 0.05).
3.7. Effect of 2KGA on Cold Stress-Related Gene Expression under Cold Stress
To further elucidate the molecular mechanisms underlying the enhancement of cold tolerance in Arabidopsis by 2KGA, the expression levels of genes associated with cold stress were determined. The results in Figure 6a show that 2KGA treatment significantly up-regulated the expression levels of ICE1, CBF1, CBF2, CBF3, COR15b, and CAMTA3 in Arabidopsis by 1.61, 1.68, 2.82, 2.96, 1.48, and 2.94-fold, respectively, while down-regulating the expression levels of ZAT12 by 0.61-fold (p < 0.05) compared to the control after 12 h under cold stress. There was no significant difference in the expression levels of ICE2, COR15a, and MYB15 in Arabidopsis at 4 °C for 12 h. After 24 h at low temperature, 2KGA treatment, respectively, resulted in a remarkable increase of 2.65, 1.83, 1.43, 2.14, 3.80, 1.71, 3.94, and 2.52-fold in the expression levels of ICE1, ICE2, CBF1, CBF2, CBF3, COR15a, COR15b, and CAMTA3 (p < 0.05) compared with the control (Figure 6b). Additionally, 2KGA treatment significantly decreased (p < 0.05) the expression levels of ZAT12 and MYB15 in Arabidopsis by 0.32 and 0.64-fold, respectively, after 24 h under cold stress (Figure 6b).
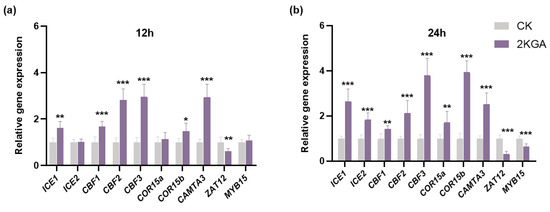
Figure 6.
Expression levels of cold stress-related genes of Arabidopsis under cold stress for (a) 12 h and (b) 24 h. The error bars represent the means ± SD (n = 6). Asterisks indicate statistically significant differences (*** p < 0.001, ** p < 0.01, and * p < 0.05).
4. Discussion
Chilling stress, as a consequence of abrupt climate change, significantly jeopardizes the health and yield of crops, posing serious risks to agricultural food production [41]. Low temperatures induce the accumulation of ROS, which impacts plant growth by causing cellular injury, reducing membrane fluidity, and affecting metabolic activity. The enhancement of crop cold tolerance is imperative for ensuring agricultural productivity in the face of escalating global temperature fluctuations. ASA, a well-known important antioxidant, is crucial in mitigating stress-induced damage [42]. Our previous studies have shown that 2KGA, a precursor in the industrial production of ASA, potentially affects ASA metabolism in plants [25,28]. Therefore, this study aimed to investigate the role of 2KGA in enhancing the tolerance of Arabidopsis thaliana to low temperatures by examining various physiological parameters and molecular mechanisms related to cold stress tolerance.
Initially, our results demonstrated that 2KGA treatment had a positive impact on the growth of Arabidopsis under normal conditions. Leaf length, maximum rosette radius, fresh weight, and chlorophyll contents were significantly increased in the 2KGA group compared to the control group. These findings are consistent with previous studies that have demonstrated the growth-promoting effects of 2KGA in plants [23,24,28]. Exposure to cold conditions inhibited the growth of Arabidopsis in this study, as reflected by a significant reduction in both fresh weight and chlorophyll contents, which is consistent with prior findings [17,43,44]. However, it was observed that the fresh weight in 2KGA-treated plants was significantly higher than in the control group at low temperatures, indicating that 2KGA treatment efficiently alleviates the adverse effects caused by cold stress. Furthermore, the increased chlorophyll contents exhibited in the 2KGA group under chilling stress further supports the role of 2KGA in maintaining photosynthetic efficiency during low-temperature conditions to sustain plant growth [15]. The observed enhancement in plant growth and photosynthetic pigment content under cold stress may be attributed to the enhanced antioxidant function of the 2KGA application, which sustains metabolic activity.
Subsequently, we investigated the levels of ASA, an important non-enzymatic antioxidant that protects photosynthesis and cell membrane stability from oxidative damage by reducing ROS [45]. The results of this study reveal an increase in ASA content in Arabidopsis following exposure to chilling conditions for 12 h, 24 h, and 48 h. This finding aligns with previous research that has observed widespread conservation of cold-induced ASA accumulation in plants. [16]. Various studies have shown that augmenting endogenous ASA levels contributes to the enhancement of plant stress tolerance [46]. Strikingly, the application of 2KGA resulted in higher ASA content in plants subjected to cold stress compared to the control group, indicating the effective augmentation of antioxidant capacity by 2KGA application. Interestingly, we observed a decline in the ASA content of the 2KGA group after peaking at 24 h under chilling stress, as opposed to the gradual accumulation observed in the CK group for 48 h. We suspect that this may be ascribed to the rapid initial accumulation of endogenous ASA being sufficient to withstand the cold stress, leading to a subsequent higher utilization of endogenous ASA for antioxidative defense. In addition, genes involved in ASA biosynthesis, such as GMP, GLDH, MIXO, and GLO exhibited significant up-regulation, suggesting that 2KGA promotes the biosynthesis of ASA under cold stress [21]. Meanwhile, the application of 2KGA also impacts gene expression in the ASA recycling pathway. The up-regulation of APX, DHAR1, DHAR2, and MDHAR at 12 h regulates the regeneration of ASA, thereby maintaining its antioxidative capacity. Nevertheless, the down-regulation of MDHAR at 24 h may be associated with the feedback regulation of ASA content, which may account for the decline in ASA content in the 2KGA group at 48 h. Overall, these findings are consistent with our previous explorations, which revealed that 2KGA promotes ASA accumulation in plants under stress conditions [28]. Based on these results, 2KGA may enhance the antioxidant capacity of Arabidopsis by promoting the biosynthesis and accumulation of ASA, thereby accelerating the ROS scavenging and mitigating the negative impacts on plant growth under cold conditions.
Furthermore, MDA content was measured as a reliable biomarker for assessing lipid peroxidation, thus providing valuable insights into the extent of damage to cellular membranes and the presence of oxidative stress [47]. The results of this study show that the MDA content was significantly increased at low temperatures, which is consistent with the results of other studies [35]. Remarkably, 2KGA treatment efficiently reduced the MDA content at 24 h under cold stress, suggesting a decrease in lipid peroxidation and oxidative damage in plants treated with 2KGA. Moreover, the further determination of osmoprotectant content showed a significant accumulation of proline at 24 h, as well as soluble sugar (SS) and soluble protein (SP) contents at 12 h and 24 h under cold stress with 2KGA treatment. These compounds play a crucial role in stabilizing cellular structures, maintaining osmotic balance, and supporting metabolic processes, thereby enhancing plant resilience under stressful conditions [48]. Some studies have shown that the increase in osmoprotectant content maintains cellular osmotic balance and reduces cellular water loss, thereby improving the resistance of plants to low-temperature stress [17,35]. Increased levels of proline, SP, and SS revealed that 2KGA enhanced the osmotic adjustment, energy reserves, and metabolic activity in Arabidopsis, which may be related to improved cold stress tolerance through the accumulation of antioxidants.
Additionally, we evaluated the activities of antioxidant enzymes, including SOD, POD, CAT, and APX, which play critical roles in detoxifying ROS and protecting cellular components against oxidative damage [49]. In the present study, cold stress induced an increase in SOD, POD, CAT, and APX activities in Arabidopsis, suggesting the activation of antioxidant systems. Antioxidant enzymes act as a protective enzyme system, limiting the levels of free radicals and preventing damage while maintaining a balance between antioxidants and free radicals [2]. The application of 2KGA increased the activities of SOD, POD, CAT, and APX under low-temperature stress, thereby strengthening the enzymatic defense system against ROS. Notably, the up-regulation of APX consists of the improved activity of APX, implying that 2KGA may induce the expression of antioxidant enzyme coding genes, thus improving the activity of the antioxidant enzymes in plants under cold stress. Similarly, the application of 2KGA was also observed to enhance the activity of antioxidant enzymes in leaves under salt stress [28]. Our results provide evidence that 2KGA potentially serves as an effective antioxidant defense system enhancer, improving the protective mechanism to maintain cell membrane integrity and osmotic adjustment to resist cold stress.
Moreover, the gene expression levels of the ICE-CBF-COR regulatory cascade, a well-defined pathway in response to cold stress in plants, were measured to further understand the role of 2KGA under cold stress [50]. The transcription factors ICE1, ICE2, MYB15, CAMTA3, and ZAT12 are upstream regulators of the CBF genes (CBF1, CBF2, and CBF3). The CBF genes regulate the expression of downstream target genes COR15a and COR15b [6]. In this study, 2KGA treatment significantly up-regulated the expression levels of ICE1, ICE2, CBF1, CBF2, CBF3, COR15a, and COR15b under cold stress at 24 h. This indicates that 2KGA has a significant positive effect on the regulation of the whole ICE-CBF-COR pathway. In Arabidopsis, CAMTA3 is a positive regulator, whereas MYB15 and ZAT12 act as negative regulators of the CBF genes [51,52,53]. The results show that the expression of CAMTA3 was significantly increased in the 2KGA group at 12 h and 24 h, and the expression levels of MYB15 and ZAT12 were significantly decreased at 24 h, compared to the control at low temperature, resulting in the up-regulation of CBF genes. Under cold stress, the ICE-CBF-COR signaling pathway activates the expression of downstream genes, which encode a series of osmoregulation substances to help plant cells maintain stability [54]. The up-regulation of the ICE1-CBF-COR pathway further demonstrates that 2KGA enhances the expression of genes involved in cold tolerance at the transcriptional level, thereby safeguarding plants against low temperatures.
Our study provides several lines of evidence clearly suggesting that 2KGA enhanced the cold stress tolerance in Arabidopsis. Firstly, the application of 2KGA significantly elevated the content of ASA (non-enzymatic antioxidants) and the up-regulated ASA biosynthesis genes under chilling conditions, as well as enhanced the activities of enzymatic antioxidants, including SOD, POD, CAT, and APX. These results fully demonstrate the positive effect of 2KGA on enhancing the performance of the antioxidant system. Secondly, the enhancement of the antioxidant defense system was further reflected in mitigating the adverse impacts induced by low temperatures, including the alleviation of the decrease in fresh weight and chlorophyll content. Thirdly, the reduction in MDA content and the higher levels of osmoprotectants (proline, SS, and SP) in the 2KGA group under cold stress indicate lower lipid peroxidation and cellular damage, and enhanced osmotic regulation and cellular protection, suggesting the role of 2KGA in enhancing antioxidant capacity. Moreover, the up-regulation of the ICE-CBF-COR pathway demonstrates that the application of 2KGA efficiently improved the cold stress tolerance in plants. Taken together, these interconnected results collectively validate our hypothesis from various aspects, demonstrating that 2KGA enhances cold tolerance in Arabidopsis thaliana by reinforcing the antioxidant defense system.
The findings of this study have significant implications for agricultural practices. Enhancing the endogenous ASA levels in plants through 2KGA supplementation represents a novel and efficient strategy to improve plant tolerance to cold stress. Although previous studies reported the positive effects of exogenous ASA application on plant growth and stress adaptation [55], the easy oxidation and instability of ASA limit its effective application in agriculture. As a precursor in industrial ASA production, 2KGA is chemically stable, readily available, and relatively inexpensive, making it a viable option for large-scale agricultural applications [27]. Different methods have been utilized to enhance abiotic stress tolerance in crop plants. Conventional breeding, for instance, is particularly time-consuming, while genetically engineered crop plants present ecological and genetic risks and face public acceptance challenges [56]. Recent studies suggest that enhancing plant tolerance against abiotic stresses by pretreatment with chemical compounds, such as melatonin [57] and phytohormones [58,59], has emerged as a promising avenue in the management of crop stress [60]. The usage of low-cost compounds for a remarkable yield enhancement will provide more advantages in field applications. The application of 2KGA or its fermentation residue could be a cost-effective strategy for enhancing crop resilience and maintaining growth and productivity under cold stress in agriculture.
In the ASA industrial production process, RAE, a residue generated after extraction of 2KGA from fermentation liquor, poses a major environmental challenge due to its high chemical oxygen demand (COD) and low pH [23]. With an annual discharge of approximately 60,000 tons of RAE in China, efficient disposal or resource utilization methods are necessary. Previous studies have demonstrated that the utilization of RAE in agriculture can significantly enhance soil nutrient content, improve soil microbial community structure, and promote plant growth [24,26]. Thus, the reutilization of RAE reduces waste disposal burdens and offers a cost-effective source of valuable LMWOAs for agriculture. This dual benefit underscores the potential of RAE as a resource in sustainable agricultural practices, aligning with environmental conservation and economic efficiency. To assess the suitability of RAE, it is essential to investigate the mechanism of 2KGA (primary constituent in RAE) on plant growth, nutrient uptake, and stress resistance [28]. Additionally, exploring the metabolic pathways of 2KGA in plants may elucidate its overall impact on crop productivity and quality [25]. Deepening our understanding of 2KGA can provide valuable insights into the eco-friendly implementation of RAE in agriculture.
The underlying mechanisms of cold stress response are conserved across many plant species [61,62]. Therefore, although the focus of this study is Arabidopsis thaliana, the observed benefits of 2KGA treatment in Arabidopsis have the potential to be extrapolated to other crops. The efficacy of 2KGA in enhancing cold tolerance in economically significant crops, such as wheat, rice, maize, and cotton, should be further investigated to validate its utility in agriculture. The experiments in this study were conducted under controlled laboratory conditions to assess the role of 2KGA in cold stress, which may not fully replicate the complex environmental factors present in the field. The efficacy of 2KGA under agricultural production facing cold stress should be validated through field trials in future studies. Moreover, the molecular mechanisms underlying the observed effects of 2KGA remain to be fully elucidated. Our study demonstrates the involvement of ASA metabolism and the ICE1-CBF-COR pathway. Further research is needed to identify other potential pathways and molecular targets influenced by 2KGA under cold stress.
5. Conclusions
This study provides compelling evidence that 2KGA enhances cold stress tolerance in Arabidopsis thaliana, primarily by increasing endogenous ASA content and thereafter improving the antioxidant defense system. Moreover, 2KGA up-regulates the expression of cold-related genes, highlighting its protective role under cold stress. These findings underscore the potential of 2KGA or its fermentation residue as a novel and promising strategy for enhancing cold stress tolerance in plants, with significant implications for future applications in agriculture aimed at improving crop resilience.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy14092149/s1, Table S1: Primers for real-time quantitative PCR.
Author Contributions
Conceptualization, Q.W. and H.X.; methodology, Q.W. and M.G.; software, Q.W. and H.S.; formal analysis, Q.W.; investigation, Q.W., M.G. and W.Y.; resources, W.Y. and H.X.; data curation, Q.W. and M.G.; writing—original draft preparation, Q.W.; writing—review and editing, H.X.; visualization, Q.W. and H.S.; supervision, H.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China, grant number 2020YFA0907800; the Science and Technology Plan Project of Liaoning Province, grant number 2023JH2/101700358; and the Central Government Guides Local Funds for Science and Technology Development, grant number 2022ZY0104.
Data Availability Statement
All data presented in this study are contained within the article and the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fedoroff, N.V.; Battisti, D.S.; Beachy, R.N.; Cooper, P.J.M.; Fischhoff, D.A.; Hodges, C.N.; Knauf, V.C.; Lobell, D.; Mazur, B.J.; Molden, D.; et al. Radically Rethinking Agriculture for the 21st Century. Science 2010, 327, 833–834. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Li, S. Novel Insight into Functions of Ascorbate Peroxidase in Higher Plants: More than a Simple Antioxidant Enzyme. Redox Biol. 2023, 64, 102789. [Google Scholar] [CrossRef]
- Rudenko, N.N.; Vetoshkina, D.V.; Marenkova, T.V.; Borisova-Mubarakshina, M.M. Antioxidants of Non-Enzymatic Nature: Their Function in Higher Plant Cells and the Ways of Boosting Their Biosynthesis. Antioxidants 2023, 12, 2014. [Google Scholar] [CrossRef]
- Bajwa, V.S.; Shukla, M.R.; Sherif, S.M.; Murch, S.J.; Saxena, P.K. Role of Melatonin in Alleviating Cold Stress in Arabidopsis thaliana. J. Pineal Res. 2014, 56, 238–245. [Google Scholar] [CrossRef]
- Hwarari, D.; Guan, Y.; Ahmad, B.; Movahedi, A.; Min, T.; Hao, Z.; Lu, Y.; Chen, J.; Yang, L. ICE-CBF-COR Signaling Cascade and Its Regulation in Plants Responding to Cold Stress. Int. J. Mol. Sci. 2022, 23, 1549. [Google Scholar] [CrossRef]
- Gusain, S.; Joshi, S.; Joshi, R. Sensing, Signalling, and Regulatory Mechanism of Cold-Stress Tolerance in Plants. Plant Physiol. Biochem. 2023, 197, 107646. [Google Scholar] [CrossRef]
- Wang, L.; Ma, R.; Yin, Y.; Jiao, Z. Role of Carbon Ion Beams Irradiation in Mitigating Cold Stress in Arabidopsis thaliana. Ecotoxicol. Environ. Saf. 2018, 162, 341–347. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Jalili, I.; Ebadi, A.; Askari, M.A.; KalatehJari, S.; Aazami, M.A. Foliar Application of Putrescine, Salicylic Acid, and Ascorbic Acid Mitigates Frost Stress Damage in Vitis vinifera Cv. ‘Giziluzum’. BMC Plant Biol. 2023, 23, 135. [Google Scholar] [CrossRef]
- Broad, R.C.; Bonneau, J.P.; Hellens, R.P.; Johnson, A.A.T. Manipulation of Ascorbate Biosynthetic, Recycling, and Regulatory Pathways for Improved Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 1790. [Google Scholar] [CrossRef] [PubMed]
- Lisko, K.A.; Aboobucker, S.I.; Torres, R.; Lorence, A. Engineering Elevated Vitamin C in Plants to Improve Their Nutritional Content, Growth, and Tolerance to Abiotic Stress. In Phytochemicals—Biosynthesis, Function and Application; Jetter, R., Ed.; Springer International Publishing: Cham, Switzerland, 2014; Volume 44, pp. 109–128. ISBN 978-3-319-04045-5. [Google Scholar]
- Ali, S.; Nawaz, A.; Hussain, S.; Khan, S.M.; Ejaz, S.; Ahmad, S. Abiotic Stress Tolerance in Plants by Priming and Pretreatments with Ascorbic Acid. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer: Singapore, 2019; pp. 459–493. ISBN 9789811386251. [Google Scholar]
- Noreen, S.; Sultan, M.; Akhter, M.S.; Shah, K.H.; Ummara, U.; Manzoor, H.; Ulfat, M.; Alyemeni, M.N.; Ahmad, P. Foliar Fertigation of Ascorbic Acid and Zinc Improves Growth, Antioxidant Enzyme Activity and Harvest Index in Barley (Hordeum vulgare L.) Grown under Salt Stress. Plant Physiol. Biochem. 2021, 158, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Roy, S.; Satya, P.; Alam, N.M.; Goswami, T.; Barman, D.; Bera, A.; Saha, R.; Mitra, S.; Mitra, J. Exogenous Ascorbic Acid Application Ameliorates Drought Stress through Improvement in Morpho-Physiology, Nutrient Dynamics, Stress Metabolite Production and Antioxidant Activities Recovering Cellulosic Fibre Production in Jute (Corchorus olitorius L.). Ind. Crops Prod. 2024, 217, 118808. [Google Scholar] [CrossRef]
- Liu, X.; Bulley, S.M.; Varkonyi-Gasic, E.; Zhong, C.; Li, D. Kiwifruit bZIP Transcription Factor AcePosF21 Elicits Ascorbic Acid Biosynthesis during Cold Stress. Plant Physiol. 2023, 192, 982–999. [Google Scholar] [CrossRef]
- Elkelish, A.; Qari, S.H.; Mazrou, Y.S.A.; Abdelaal, K.A.A.; Hafez, Y.M.; Abu-Elsaoud, A.M.; Batiha, G.E.-S.; El-Esawi, M.A.; El Nahhas, N. Exogenous Ascorbic Acid Induced Chilling Tolerance in Tomato Plants Through Modulating Metabolism, Osmolytes, Antioxidants, and Transcriptional Regulation of Catalase and Heat Shock Proteins. Plants 2020, 9, 431. [Google Scholar] [CrossRef]
- Xiang, N.; Hu, J.; Wen, T.; Brennan, M.A.; Brennan, C.S.; Guo, X. Effects of Temperature Stress on the Accumulation of Ascorbic Acid and Folates in Sweet Corn (Zea mays L.) Seedlings. J. Sci. Food Agric. 2020, 100, 1694–1701. [Google Scholar] [CrossRef]
- Fu, Q.; Cao, H.; Wang, L.; Lei, L.; Di, T.; Ye, Y.; Ding, C.; Li, N.; Hao, X.; Zeng, J.; et al. Transcriptome Analysis Reveals That Ascorbic Acid Treatment Enhances the Cold Tolerance of Tea Plants through Cell Wall Remodeling. Int. J. Mol. Sci. 2023, 24, 10059. [Google Scholar] [CrossRef]
- Jha, U.C.; Bohra, A.; Jha, R. Breeding Approaches and Genomics Technologies to Increase Crop Yield under Low-Temperature Stress. Plant Cell Rep. 2017, 36, 1–35. [Google Scholar] [CrossRef]
- Bulley, S.M.; Cooney, J.M.; Laing, W. Elevating Ascorbate in Arabidopsis Stimulates the Production of Abscisic Acid, Phaseic Acid, and to a Lesser Extent Auxin (IAA) and Jasmonates, Resulting in Increased Expression of DHAR1 and Multiple Transcription Factors Associated with Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 6743. [Google Scholar] [CrossRef]
- Yang, W.; Han, L.; Mandlaa, M.; Zhang, H.; Zhang, Z.; Xu, H. A Plate Method for Rapid Screening of Ketogulonicigenium vulgare Mutants for Enhanced 2-Keto-l-Gulonic Acid Production. Braz. J. Microbiol. 2017, 48, 397–402. [Google Scholar] [CrossRef]
- Kong, T.; Xu, H.; Wang, Z.; Sun, H.; Wang, L. Effect of a Residue after Evaporation from Industrial Vitamin C Fermentation on Chemical and Microbial Properties of Alkali-Saline Soil. Pak. J. Pharm. Sci. 2014, 27, 1069–1074. [Google Scholar] [PubMed]
- Wang, B.; Sun, H.; Yang, W.; Gao, M.; Zhong, X.; Zhang, L.; Chen, Z.; Xu, H. Potential Utilization of Vitamin C Industrial Effluents in Agriculture: Soil Fertility and Bacterial Community Composition. Sci. Total Environ. 2022, 851, 158253. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhang, Z.; Yang, W.; Sun, H.; Xu, H. Application of Organic Waste Derived from Vitamin C Industry Increases Yield and Bioactive Constituents of Medicinal Food Plant Purslane (Portulaca oleracea L.). Horticulturae 2024, 10, 683. [Google Scholar] [CrossRef]
- Gao, M.; Han, X.; Yang, W.; Sun, H.; Zhang, L.; Xu, H. A Strategy for Improving Saline-Alkali Soil Properties and Cotton Stress Tolerance Using Vitamin C Industrial Wastes: A “Prebiotics-Probiotics” Interaction between Organic Acids and Bacillus Endophyticus. Ind. Crops Prod. 2024, 220, 119187. [Google Scholar] [CrossRef]
- Shi, M.; Gao, M.; Sun, H.; Yang, W.; Zhao, H.; Zhang, L.; Xu, H. Exogenous 2-Keto-L-Gulonic Acid Supplementation as a Novel Approach to Enhancing L-Ascorbic Acid Biosynthesis in Zebrafish (Danio rerio). Animals 2023, 13, 2502. [Google Scholar] [CrossRef]
- Gao, M.; Sun, H.; Shi, M.; Wu, Q.; Ji, D.; Wang, B.; Zhang, L.; Liu, Y.; Han, L.; Ruan, X.; et al. 2-Keto-L-Gulonic Acid Improved the Salt Stress Resistance of Non-Heading Chinese Cabbage by Increasing L-Ascorbic Acid Accumulation. Front. Plant Sci. 2021, 12, 697184. [Google Scholar] [CrossRef]
- Sivasubramanian, R.; Mukhi, N.; Kaur, J. Arabidopsis Thaliana: A Model for Plant Research. In Plant Biology and Biotechnology: Volume II: Plant Genomics and Biotechnology; Bahadur, B., Venkat Rajam, M., Sahijram, L., Krishnamurthy, K.V., Eds.; Springer India: New Delhi, India, 2015; pp. 1–26. ISBN 978-81-322-2283-5. [Google Scholar]
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: London, UK, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Ali, S.; Gill, R.A.; Ulhassan, Z.; Zhang, N.; Hussain, S.; Zhang, K.; Huang, Q.; Sagir, M.; Tahir, M.B.; Gill, M.B.; et al. Exogenously Applied Melatonin Enhanced the Tolerance of Brassica Napus against Cobalt Toxicity by Modulating Antioxidant Defense, Osmotic Adjustment, and Expression of Stress Response Genes. Ecotoxicol. Environ. Saf. 2023, 252, 114624. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Zhang, W.H.; Wu, J.; Weng, L.; Zhang, H.; Zhang, J.; Wu, A. An Improved Phenol-Sulfuric Acid Method for the Determination of Carbohydrates in the Presence of Persulfate. Carbohydr. Polym. 2020, 227, 115332. [Google Scholar] [CrossRef]
- Mm, B. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Xiang, H.; Wang, S.; Liang, X.; Wang, X.; Xie, H.; Wang, D.; Gai, Z.; Wang, N.; Xiang, P.; Han, D.; et al. Foliar Spraying of Exogenous Uniconazole (S3307) at the Flowering Stage as an Effective Method to Resist Low-Temperature Stress on Mung Bean [Vigna radiata (L.) Wilczek]. Sci. Rep. 2023, 13, 22331. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Castillo, F.J.; Penel, C.; Greppin, H. Peroxidase Release Induced by Ozone in Sedum album Leaves: Involvement of Ca2+. Plant Physiol. 1984, 74, 846–851. [Google Scholar] [CrossRef]
- Anderson, M.D.; Prasad, T.K.; Stewart, C.R. Changes in Isozyme Profiles of Catalase, Peroxidase, and Glutathione Reductase during Acclimation to Chilling in Mesocotyls of Maize Seedlings. Plant Physiol. 1995, 109, 1247–1257. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Raza, A.; Bashir, S.; Khare, T.; Karikari, B.; Copeland, R.G.R.; Jamla, M.; Abbas, S.; Charagh, S.; Nayak, S.N.; Djalovic, I.; et al. Temperature-Smart Plants: A New Horizon with Omics-Driven Plant Breeding. Physiol. Plant. 2024, 176, e14188. [Google Scholar] [CrossRef]
- Celi, G.E.A.; Gratão, P.L.; Lanza, M.G.D.B.; Reis, A.R.D. Physiological and Biochemical Roles of Ascorbic Acid on Mitigation of Abiotic Stresses in Plants. Plant Physiol. Biochem. 2023, 202, 107970. [Google Scholar] [CrossRef]
- Cheng, P.; Feng, L.; Zhang, S.; Li, L.; Guan, R.; Long, W.; Xian, Z.; Zhang, J.; Shen, W. Ammonia Borane Positively Regulates Cold Tolerance in Brassica Napus via Hydrogen Sulfide Signaling. BMC Plant Biol. 2022, 22, 585. [Google Scholar] [CrossRef]
- Dai, T.; Ban, S.; Han, L.; Li, L.; Zhang, Y.; Zhang, Y.; Zhu, W. Effects of Exogenous Glycine Betaine on Growth and Development of Tomato Seedlings under Cold Stress. Front. Plant Sci. 2024, 15, 1332583. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, H.; Huang, R.; Ye, R.; Luo, Y.; Guo, Z.; Lu, S. AIR12 Confers Cold Tolerance through Regulation of the CBF Cold Response Pathway and Ascorbate Homeostasis. Plant Cell Environ. 2021, 44, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, T.; Yan, W.; Yu, P.; Fu, W.; Li, J.; Su, X.; Chen, T.; Fu, G.; Wu, Z.; et al. Transcriptome and Metabolome Analyses Reveal Ascorbic Acid Ameliorates Cold Tolerance in Rice Seedling Plants. Agronomy 2024, 14, 659. [Google Scholar] [CrossRef]
- Heidari, P.; Reza Amerian, M.; Barcaccia, G. Hormone Profiles and Antioxidant Activity of Cultivated and Wild Tomato Seedlings under Low-Temperature Stress. Agronomy 2021, 11, 1146. [Google Scholar] [CrossRef]
- Rahman, M.d.M.; Mostofa, M.G.; Rahman, M.d.A.; Islam, M.d.R.; Keya, S.S.; Das, A.K.; Miah, M.d.G.; Kawser, A.Q.M.R.; Ahsan, S.M.; Hashem, A.; et al. Acetic Acid: A Cost-Effective Agent for Mitigation of Seawater-Induced Salt Toxicity in Mung Bean. Sci. Rep. 2019, 9, 15186. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive Oxygen Gene Network of Plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Tang, K.; Zhao, L.; Ren, Y.; Yang, S.; Zhu, J.-K.; Zhao, C. The Transcription Factor ICE1 Functions in Cold Stress Response by Binding to the Promoters of CBF and COR Genes. J. Integr. Plant Biol. 2020, 62, 258–263. [Google Scholar] [CrossRef]
- Vogel, J.T.; Zarka, D.G.; Van Buskirk, H.A.; Fowler, S.G.; Thomashow, M.F. Roles of the CBF2 and ZAT12 Transcription Factors in Configuring the Low Temperature Transcriptome of Arabidopsis. Plant J. 2005, 41, 195–211. [Google Scholar] [CrossRef]
- Doherty, C.J.; Van Buskirk, H.A.; Myers, S.J.; Thomashow, M.F. Roles for Arabidopsis CAMTA Transcription Factors in Cold-Regulated Gene Expression and Freezing Tolerance. Plant Cell 2009, 21, 972–984. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.; Li, Z.; Shi, Y.; Wang, J.; Hua, J.; Gong, Z.; Zhou, J.-M.; Yang, S. PUB25 and PUB26 Promote Plant Freezing Tolerance by Degrading the Cold Signaling Negative Regulator MYB15. Dev. Cell 2019, 51, 222–235.e5. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Chen, S. Physiological and Molecular Mechanism Involved in Cold Stress Tolerance in Plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, B. Exogenous Ascorbic Acid Mediated Abiotic Stress Tolerance in Plants. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Hossain, M.A., Munné-Bosch, S., Burritt, D.J., Diaz-Vivancos, P., Fujita, M., Lorence, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 233–253. ISBN 978-3-319-74057-7. [Google Scholar]
- Liang, C.; Prins, T.W.; van de Wiel, C.C.M.; Kok, E.J. Safety Aspects of Genetically Modified Crops with Abiotic Stress Tolerance. Trends Food Sci. Technol. 2014, 40, 115–122. [Google Scholar] [CrossRef]
- Hu, Z.; Fan, J.; Xie, Y.; Amombo, E.; Liu, A.; Gitau, M.M.; Khaldun, A.B.M.; Chen, L.; Fu, J. Comparative Photosynthetic and Metabolic Analyses Reveal Mechanism of Improved Cold Stress Tolerance in Bermudagrass by Exogenous Melatonin. Plant Physiol. Biochem. 2016, 100, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, D.; Wang, Q.; Song, X.; Wang, Y.; Yang, X.; Qin, D.; Xie, T.; Yang, D. Exogenous Salicylic Acid Improves Chilling Tolerance in Maize Seedlings by Improving Plant Growth and Physiological Characteristics. Agronomy 2021, 11, 1341. [Google Scholar] [CrossRef]
- Sun, H.; Rang, X.; Han, H.; Pei, Z.; Zhao, J.; Zhu, Z.; Li, J.; Zhang, P.; Zhao, Y.; Duan, Y. Exogenous Gibberellic Acid Ameliorates Chilling Injury in Peach (Prunus persica L.) by Improving the Antioxidant System. Agronomy 2024, 14, 816. [Google Scholar] [CrossRef]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical Priming of Plants Against Multiple Abiotic Stresses: Mission Possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef]
- Jaglo, K.R.; Kleff, S.; Amundsen, K.L.; Zhang, X.; Haake, V.; Zhang, J.Z.; Deits, T.; Thomashow, M.F. Components of the Arabidopsis C-Repeat/Dehydration-Responsive Element Binding Factor Cold-Response Pathway Are Conserved inBrassica Napus and Other Plant Species. Plant Physiol. 2001, 127, 910–917. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, F.; Wu, Z.; Li, Y.; Shi, G.; Hu, J.; Hou, X. Components of the Arabidopsis CBF Cold-Response Pathway Are Conserved in Non-Heading Chinese Cabbage. Plant Mol. Biol. Rep. 2011, 29, 525–532. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).