Combined Effects of Nitrogen Addition and Warming on Shrub Growth and Nutrient Uptake through Microbially Mediated Soil Fertility
Abstract
1. Introduction
2. Materials and Methods
2.1. Shrub Species and Soil Samples
2.2. Experimental Design, Shrub Growth, and Harvest
2.3. C, N, and P Analysis
2.4. Soil Microbial Analysis
2.4.1. Soil DNA Extraction, Amplification, and Sequencing
2.4.2. Bioinformatic Analyses
2.5. Data Analysis
3. Results
3.1. Response of Plant Growth to the Various Treatments
3.2. Response of Plant Nutrient Uptake to the Various Carbon (C), Nitrogen (N), and Phosphorus (P) Treatments within Plants and Soils
3.3. Response of Soil Fertility to the Various Treatments
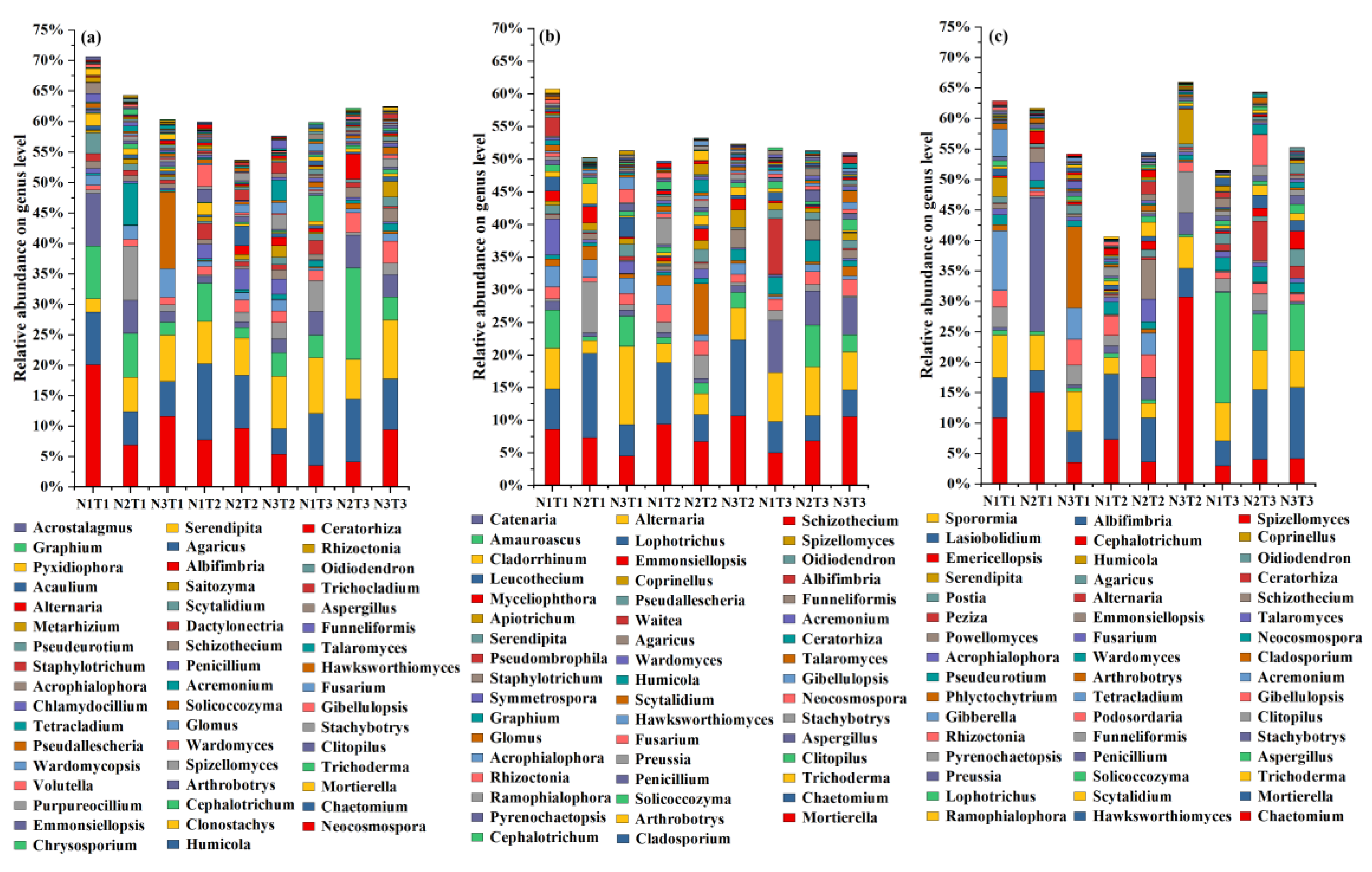
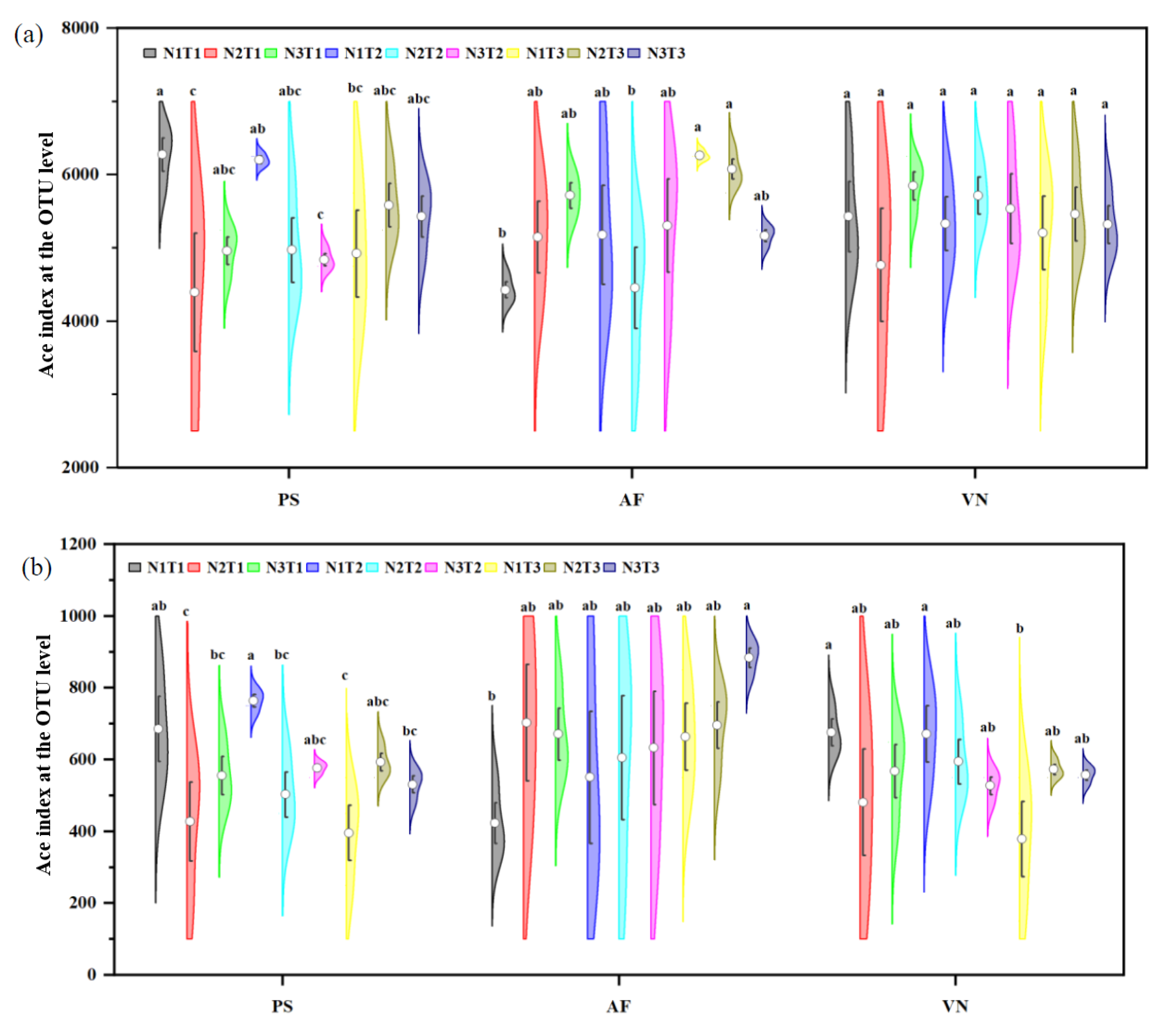
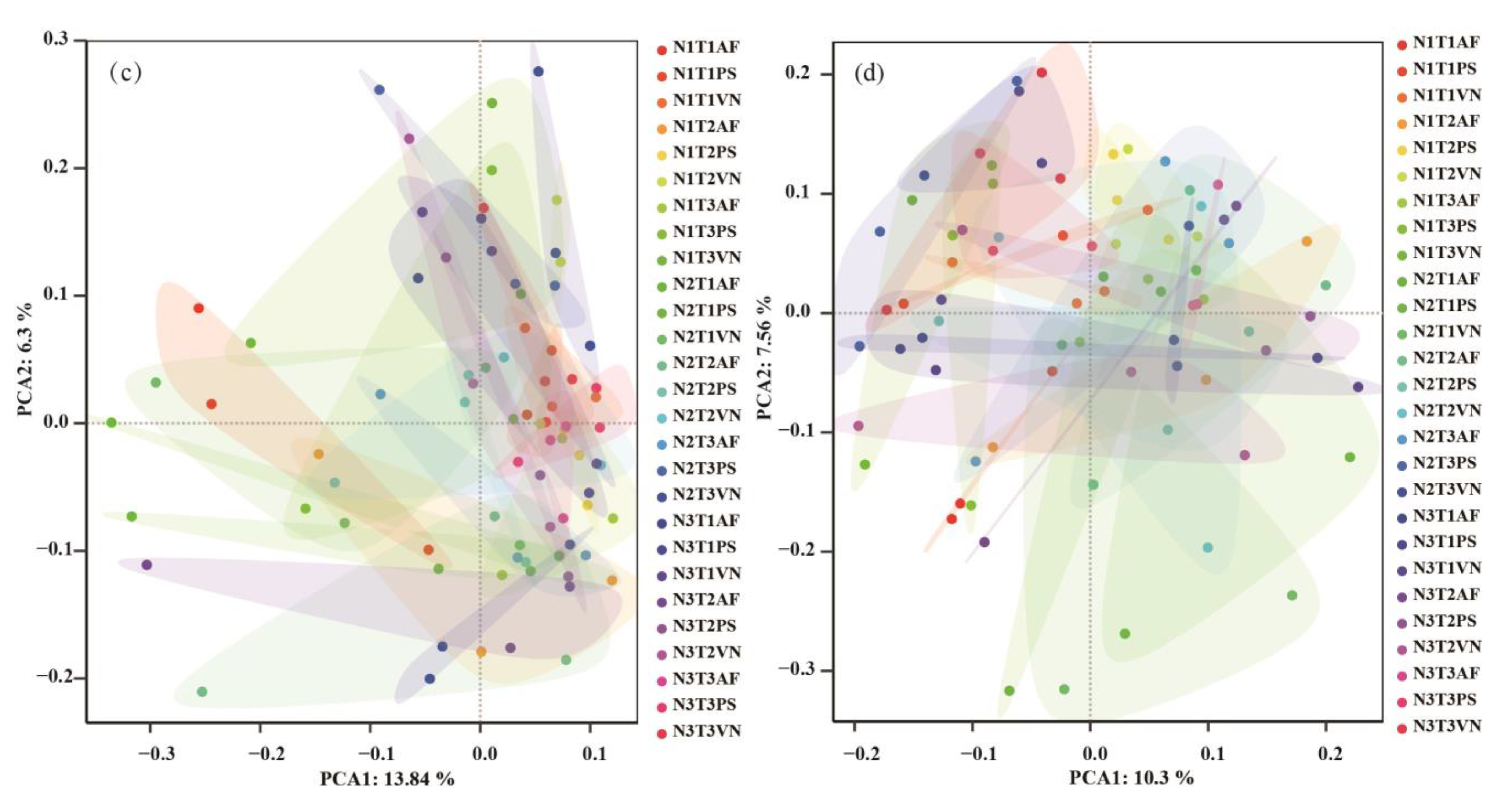
3.4. Response of Soil Microbial Community to the Various Treatments
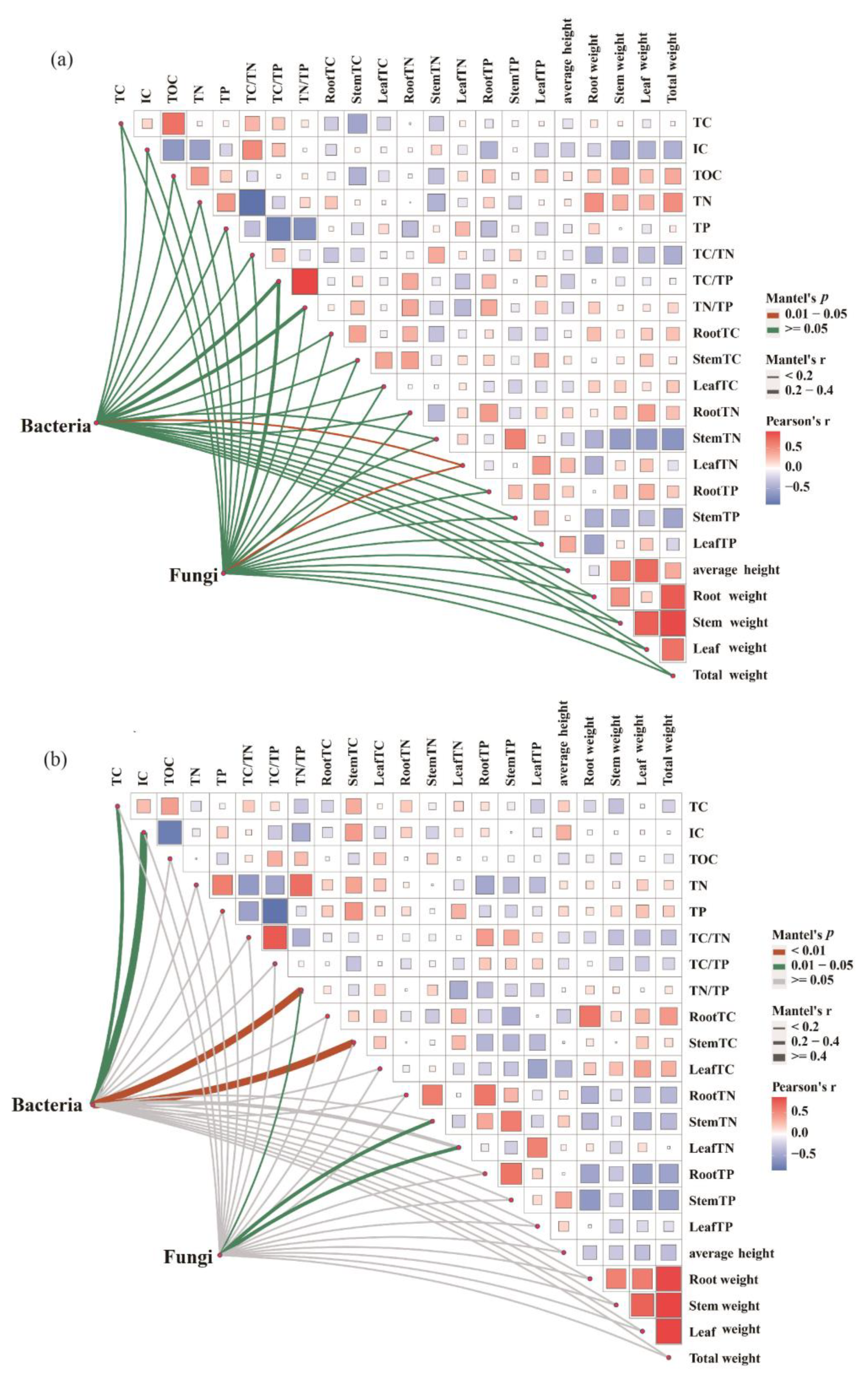
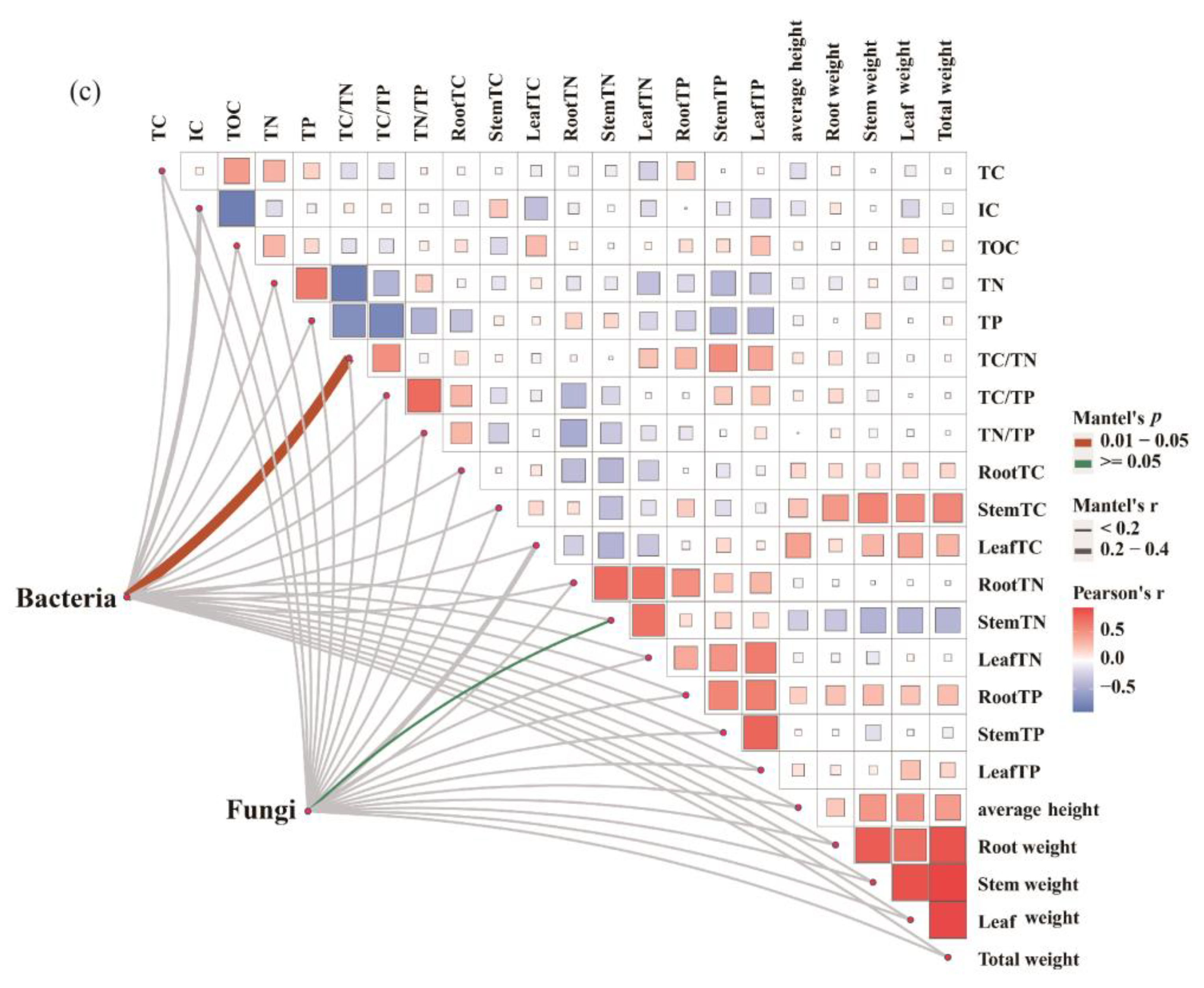
3.5. Relationship between Soil Microbial Community and C–N–P Stoichiometry of Plant and Soil
4. Discussion
4.1. Response of Shrub Growth and Nutrient Uptake to Diverse Nitrogen and Temperature Treatments
4.2. Soil Fertility and Microorganism Responses to Varied N and Temperature Treatments
4.3. The Mediating Role of Soil Microorganisms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, X.; Wang, B.; An, S.; Zeng, Q.; Zhang, H. Response of forest species to C:N:P in the plant-litter-soil system and stoichiometric homeostasis of plant tissues during afforestation on the Loess Plateau, China. Catena 2019, 183, 104186. [Google Scholar] [CrossRef]
- Fu, B.J.; Wang, S.; Liu, Y.; Liu, J.B.; Liang, W.; Miao, C.Y. Hydrogeomorphic ecosystem responses to natural and anthropogenic changes in the Loess Plateau of China. Ann. Rev. Earth Planet. Sci. 2017, 45, 223–243. [Google Scholar] [CrossRef]
- Kiryushin, V.I. The management of soil fertility and productivity of agrocenoses in adaptive-landscape farming systems. Eurasian Soil Sci. 2019, 52, 1137–1145. [Google Scholar] [CrossRef]
- Schjoerring, J.K.; Cakmak, I.; White, P.J. Plant nutrition and soil fertility: Synergies for acquiring global green growth and sustainable development. Plant Soil 2019, 434, 1–6. [Google Scholar] [CrossRef]
- Pang, X.P.; Guo, Z.G. Plateau pika disturbances alter plant productivity and soil nutrients in alpine meadows of the Qinghai-Tibetan Plateau, China. Rangel. J. 2017, 39, 133–144. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, T.; Li, Y.; Zheng, X. A critical review of the central role of microbial regulation in the nitrogen biogeochemical process: New insights for controlling groundwater nitrogen contamination. J. Environ. Manag. 2023, 328, 116959. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Rumpel, C.; Crème, A.; Ngo, P.T.; Velásquez, G.; Mora, M.L.; Chabbi, A. The impact of grassland management on biogeochemical cycles involving carbon, nitrogen and phosphorus. J. Soil Sci. Plant Nut. 2015, 15, 353–371. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, S.; Li, C.; Zhao, L.; Feng, H.; Yue, G.; Ren, Z.; Cheng, G. The soil carbon/nitrogen ratio and moisture affect microbial community structures in alkaline permafrost-affected soils with different vegetation types on the Tibetan plateau. Res. Microbiol. 2014, 165, 128–139. [Google Scholar] [CrossRef]
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M.A.; Santoyo, G. Unlocking the potential of plant growth-promoting rhizobacteria on soil health and the sustainability of agricultural systems. J. Environ. Manag. 2020, 273, 111118. [Google Scholar] [CrossRef]
- Das, P.P.; Singh, K.R.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef]
- Raymond, N.S.; Gómez-Muñoz, B.; van der Bom, F.J.; Nybroe, O.; Jensen, L.S.; Müller-Stöver, D.S.; Oberson, A.; Richardson, A.E. Phosphate-solubilising microorganisms for improved crop productivity: A critical assessment. New Phytol. 2021, 229, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Scotton, M. Using fertilization rates in semi-natural grasslands to produce seed for ecological restoration. Agric. Ecosyst. Environ. 2022, 340, 108165. [Google Scholar] [CrossRef]
- Pisani, O.; Frey, S.D.; Simpson, A.J.; Simpson, M.J. Soil warming and nitrogen deposition alter soil organic matter composition at the molecular-level. Biogeochemistry 2015, 123, 391–409. [Google Scholar] [CrossRef]
- Sun, Q.; Miao, C.; Hanel, M.; Borthwick, A.G.; Duan, Q.; Ji, D. Global heat stress on health, wildfires, and agricultural crops under different levels of climate warming. Environ. Int. 2019, 128, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhu, L.; Liang, J.; Yin, H.; Yin, C.; Li, D.; Zhang, N.; Liu, Q. Effects of experimental warming and nitrogen fertilization on soil microbial communities and processes of two subalpine coniferous species in Eastern Tibetan Plateau, China. Plant Soil 2014, 382, 189–201. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Li, X.; Ma, L.; Xiao, Y.; Zhang, C. Differential responses of plant primary productivity to nutrient addition in natural and restored alpine grasslands in the qinghai lake basin. Front. Plant Sci. 2021, 12, 792123. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Zhou, S.; Yang, X.; Fu, Y.; Miao, C.; Wang, S.; Zhang, G.; Wu, X.; Yang, C.; et al. A comprehensive review on coupled processes and mechanisms of soil-vegetation-hydrology, and recent research advances. China Earth Sci. 2022, 65, 2083–2114. [Google Scholar] [CrossRef]
- Yu, B.; Liu, G.; Liu, Q. Effects of land use changes for ecological restoration on soil moisture on the Chinese Loess Plateau: A meta-analytical approach. J. For. Res. 2020, 31, 443–452. [Google Scholar] [CrossRef]
- Ren, M.; Wang, S.; Zhang, X.; Wang, Z.; Yang, M. Rhizosheath soil microbial functional diversity of two typical Gramineae plants in the southern margin of the Junggar basin. Acta Ecol. Sin. 2017, 37, 5630–5639. [Google Scholar]
- Burylo, M.; Rey, F.; Mathys, N.; Dutoit, T. Plant root traits affecting the resistance of soils to concentrated flow erosion. Earth Surf. Process. Landf. 2012, 37, 1463–1470. [Google Scholar] [CrossRef]
- Gondek, K.; MierzwaHersztek, M. Effect of Soil-Applied L-tryptophan on the Amount of Biomass and Nitrogen and Sulfur Utilization by Maize. Agronomy 2021, 11, 2582. [Google Scholar] [CrossRef]
- Yusoff, M.Z.M.; Hu, A.; Feng, C.; Maeda, T.; Shirai, Y.; Hassan, M.A.; Yu, C.P. Influence of pretreated activated sludge for electricity generation in microbial fuel cell application. Bioresour. Technol. 2013, 145, 90–99. [Google Scholar] [CrossRef]
- Adams, R.P.; Boratynski, A.; Arista Palmero, M.; Schwarzbach, A.E.; Leschner, H.; Liber, Z.; Minissale, P.; Mataraci, T.; Manolis, A. Analysis of Juniperus phoenicea from throughout its range in the Mediterranean using DNA sequence data from nrDNA and petN-psbM: The case for the recognition of J. turbinata Guss. Phytologia 2013, 95, 202–209. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; Pennanen, T.; et al. The UNITE database for molecular identification of fungi–recent updates and future perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Brown, S.P.; Veach, A.M.; Rigdon-Huss, A.R.; Grond, K.; Lickteig, S.K.; Lothamer, K.; Oliver, A.K.; Jumpponen, A. Scraping the bottom of the barrel: Are rare high throughput sequences artifacts? Fungal Ecol. 2015, 13, 221–225. [Google Scholar] [CrossRef]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.D.; Petchey, O.L.; et al. Biodiversity and resilience of ecosystem functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Heaton, E.; Voigt, T.; Long, S.P. A quantitative review comparing the yields of two candidate C4 perennial biomass crops in relation to nitrogen, temperature and water. Biomass Bioenergy 2004, 27, 21–30. [Google Scholar] [CrossRef]
- Burton, A.; Pregitzer, K.; Ruess, R.; Hendrick, R.; Allen, M. Root respiration in North American forests: Effects of nitrogen concentration and temperature across biomes. Oecologia 2002, 131, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Reich, P.; Bolstad, P. Acclimation of leaf respiration to temperature is rapid and related to specific leaf area, soluble sugars and leaf nitrogen across three temperate deciduous tree species. Funct. Ecol. 2005, 19, 640–647. [Google Scholar] [CrossRef]
- Martinez del Castillo, E.; Zang, C.S.; Buras, A.; Hacket-Pain, A.; Esper, J.; Serrano-Notivoli, R.; Hartl, C.; Weigel, R.; Klesse, S.; Resco de Dios, V.; et al. Climate-change-driven growth decline of European beech forests. Commun. Biol. 2022, 5, 163. [Google Scholar] [CrossRef]
- Christie, E.K.; Delting, J.K. Analysis of interferences between C (3) and C (4) grasses in relation to temperature and soil nitrogen supply. Ecology 1982, 63, 1277–1284. [Google Scholar] [CrossRef]
- Zani, D.; Crowther, T.W.; Mo, L.; Renner, S.S.; Zohner, C.M. Increased growing-season productivity drives earlier autumn leaf senescence in temperate trees. Science 2020, 370, 1066–1071. [Google Scholar] [CrossRef]
- Mooney, H.; Ferrar, P.J.; Slatyer, R. Photosynthetic capacity and carbon allocation patterns in diverse growth forms of Eucalyptus. Oecologia 1978, 36, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef]
- Palmroth, S.; Holm Bach, L.; Nordin, A.; Palmqvist, K. Nitrogen-addition effects on leaf traits and photosynthetic carbon gain of boreal forest understory shrubs. Oecologia 2014, 175, 457–470. [Google Scholar] [CrossRef]
- Dai, Z.; Yu, M.; Chen, H.; Zhao, H.; Huang, Y.; Su, W. Elevated temperature shifts soil N cycling from microbial immobilization to enhanced mineralization, nitrification and denitrification across global terrestrial ecosystems. Glob. Chang. Biol. 2020, 26, 5267–5276. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Lewis, S.L.; Affum-Baffoe, K.; Castilho, C.; Costa, F.; Sanchez, A.C.; Ewango, C.E.; Hubau, W.; Marimon, B.; Monteagudo-Mendoza, A.; et al. Long-term thermal sensitivity of Earth’s tropical forests. Science 2020, 368, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Powlson, D.S.; Hirsch, P.R.; Brookes, P.C. The role of soil microorganisms in soil organic matter conservation in the tropics. Nutr. Cycl. Agroecosystems 2001, 61, 41–51. [Google Scholar] [CrossRef]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Firestone, M.K.; Foley, M.M.; Hestrin, R.; Hungate, B.A.; et al. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef]
- Zak, D.R.; Pregitzer, K.S.; King, J.S.; Holmes, W.E. Elevated atmospheric CO2, fine roots and the response of soil microorganisms: A review and hypothesis. New Phytol. 2000, 147, 201–222. [Google Scholar] [CrossRef]
- Rincón, A.; Valladares, F.; Gimeno, T.E.; Pueyo, J.J. Water stress responses of two Mediterranean tree species influenced by native soil microorganisms and inoculation with a plant growth promoting rhizobacterium. Tree Physiol. 2008, 28, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Thoms, C.; Gleixner, G. Seasonal differences in tree species’ influence on soil microbial communities. Soil Biol. Biochem. 2013, 66, 239–248. [Google Scholar] [CrossRef]
- Ren, C.; Chen, J.; Deng, J.; Zhao, F.; Han, X.; Yang, G.; Tong, X.; Feng, Y.; Shelton, S.; Ren, G. Response of microbial diversity to C:N:P stoichiometry in fine root and microbial biomass following afforestation. Biol. Fertil. Soils 2017, 53, 457–468. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Fierer, N.; Morse, J.L.; Berthrong, S.T.; Bernhardt, E.S.; Jackson, R.B. Environmental controls on the landscape-scale biogeography of stream bacterial communities. Ecology 2007, 88, 2162–2173. [Google Scholar] [CrossRef]
- Ward, N.L.; Challacombe, J.F.; Janssen, P.H.; Henrissat, B.; Coutinho, P.M.; Wu, M.; Xie, G. Three genomes from the phylum Acidobacteria provide insight into the lifestyles of these microorganisms in soils. Appl. Environ. Microb. 2009, 75, 2046–2056. [Google Scholar] [CrossRef]
- Magdoff, F.; Bartlett, R. Soil pH buffering revisited. Soil Sci. Soc. Am. J. 1985, 49, 145–148. [Google Scholar] [CrossRef]
- Castro, H.F.; Classen, A.T.; Austin, E.E.; Norby, R.J.; Schadt, C.W. Soil microbial community responses to multiple experimental climate change drivers. Appl Environ. Microbiology 2010, 76, 999–1007. [Google Scholar] [CrossRef]
- Bennett, A.C.; Murugapiran, S.K.; Hamilton, T.L. Temperature impacts community structure and function of phototrophic Chloroflexi and Cyanobacteria in two alkaline hot springs in Yellowstone National Park. Environ. Microbiol. Rep. 2020, 12, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, D.Y.; Lücker, S.; Vejmelkova, D.; Kostrikina, N.A.; Kleerebezem, R.; Rijpstra, W.I.C.; Damsté, J.S.S.; Paslier, D.L.; Muyzer, G.; Wagner, M.; et al. Nitrification expanded: Discovery, physiology and genomics of a nitrite-oxidizing bacterium from the phylum Chloroflexi. ISME J. 2012, 6, 2245–2256. [Google Scholar] [CrossRef]
- Miura, Y.; Watanabe, Y.; Okabe, S. Significance of Chloroflexi in performance of submerged membrane bioreactors (MBR) treating municipal wastewater. Environ. Sci. Technol. 2007, 41, 7787–7794. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, J.; You, Y.; Wang, R.; Chu, S.; Chi, Y. When nanoparticle and microbes meet: The effect of multi-walled carbon nanotubes on microbial community and nutrient cycling in hyperaccumulator system. J. Hazard. Mater. 2022, 423, 126947. [Google Scholar] [CrossRef]
- Courty, P.E.; Buée, M.; Diedhiou, A.G.; Frey-Klett, P.; Le, T.F.; Rineau, F. The role of ectomycorrhizal communities in forest ecosystem processes: New perspectives and emerging concepts. Soil Biol. Biochem. 2010, 42, 679–698. [Google Scholar] [CrossRef]
- Ortiz-Bobea, A.; Ault, T.R.; Carrillo, C.M.; Chambers, R.G.; Lobell, D.B. Anthropogenic climate change has slowed global agricultural productivity growth. Nat. Clim. Chang. 2021, 11, 306–312. [Google Scholar] [CrossRef]
- Herms, C.H.; Hennessy, R.C.; Bak, F.; Dresbøll, D.B.; Nicolaisen, M.H. Back to our roots: Exploring the role of root morphology as a mediator of beneficial plant–microbe interactions. Environ. Microbiol. 2022, 24, 3264–3272. [Google Scholar] [CrossRef]
- Tang, M.; Li, L.; Wang, X.; You, J.; Li, J.; Chen, X. Elevational is the main factor controlling the soil microbial community structure in alpine tundra of the Changbai Mountain. Sci. Rep. 2020, 10, 12442. [Google Scholar] [CrossRef]
- Calvaruso, C.; Turpault, M.P.; Frey-Klett, P. Root-associated bacteria contribute to mineral weathering and to mineral nutrition in trees: A budgeting analysis. Appl. Environ. Microbiology 2006, 72, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Li, W.; Pang, X.; Liu, Q.; Yin, C. Soil properties and root traits are important factors driving rhizosphere soil bacterial and fungal community variations in alpine Rhododendron nitidulum shrub ecosystems along an altitudinal gradient. Sci. Total Environ. 2023, 864, 161048. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, M.; Raza, S.T.; Yang, S.; Liu, J.; Cai, M.; Xue, S.; Wu, J. Fungal but not bacterial β-diversity decreased after 38-year-long grazing in a southern grassland. Plant Soil. 2023, 485, 1–13. [Google Scholar] [CrossRef]
- An, F.; Niu, Z.; Liu, T.; Su, Y. Succession of soil bacterial community along a 46-year choronsequence artificial revegetation in an arid oasis-desert ecotone. Sci. Total Environ. 2022, 814, 152496. [Google Scholar] [CrossRef]
- Kumar, A.; Mukhia, S.; Kumar, R. Microbial community dynamics from a fast-receding glacier of Western Himalayas highlight the importance of microbes in primary succession, nutrient recycling, and xenobiotics degradation. Ecol. Indic. 2022, 144, 109565. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M. Mycorrhizal types differ in ecophysiology and alter plant nutrition and soil processes. Biol. Rev. 2019, 94, 1857–1880. [Google Scholar] [CrossRef]
- Kashkak, E.S.; Khakhinov, V.V.; Danilova, E.V.; Oorzhak, U.S.; Dagurova, O.P. Chemical component depositions in the microbial mats and travertine in the Khoito-Gol thermal spring (Eastern Sayan). IOP Conf. Ser. Earth Environ. Sci. 2021, 908, 012004. [Google Scholar] [CrossRef]




| Root | Stem | Leaf | Root | Stem | Leaf | Root | Stem | Leaf | |
|---|---|---|---|---|---|---|---|---|---|
| TC (%) | TN (g/kg) | TP (g/kg) | |||||||
| A. fruticosa, AF | |||||||||
| N1T1 | 45.77 ± 0.35 bc | 44.31 ± 0.84 b | 43.68 ± 0.62 a | 18.57 ± 6.62 ab | 22.63 ± 5.93 ab | 17.82 ± 3.39 b | 2.50 ± 0.83 a | 1.93 ± 0.26 ab | 2.54 ± 0.44 b |
| N2T1 | 47.06 ± 0.31 ab | 44.55 ± 0.76 b | 44.44 ± 0.43 a | 17.92 ± 2.59 ab | 12.27 ± 2.59 b | 23.07 ± 1.46 ab | 1.12 ± 0.28 a | 0.67 ± 0.15 c | 0.91 ± 0.20 cd |
| N3T1 | 46.32 ± 0.90 bc | 44.00 ± 0.56 b | 44.64 ± 0.73 a | 22.75 ± 4.62 ab | 15.13 ± 1.00 ab | 21.82 ± 6.12 ab | 0.90 ± 0.39 a | 0.82 ± 0.07 bc | 0.52 ± 0.33 d |
| N1T2 | 45.00 ± 0.38 c | 45.17 ± 0.20 ab | 45.36 ± 0.41 a | 19.82 ± 2.53 ab | 22.99 ± 6.68 ab | 24.85 ± 7.04 ab | 1.68 ± 0.34 a | 2.59 ± 0.38 a | 2.42 ± 0.47 b |
| N2T2 | 48.47 ± 0.27 a | 47.08 ± 1.04 a | 44.65 ± 0.31 a | 29.42 ± 3.65 a | 16.46 ± 0.15 ab | 21.45 ± 3.94 ab | 2.46 ± 0.48 a | 1.57 ± 0.20 abc | 1.96 ± 0.42 bc |
| N3T2 | 46.85 ± 0.71 b | 44.99 ± 1.48 ab | 44.47 ± 0.23 a | 24.03 ± 2.14 ab | 16.54 ± 0.81 ab | 33.81 ± 2.60 a | 1.23 ± 0.60 a | 1.08 ± 0.13 bc | 2.51 ± 0.59 b |
| N1T3 | 46.04 ± 0.42 bc | 44.13 ± 0.65 b | 43.29 ± 1.86 a | 14.34 ± 0.48 b | 23.78 ± 1.66 a | 24.75 ± 2.81 ab | 1.47 ± 0.36 a | 2.51 ± 0.77 a | 2.29 ± 0.53 bc |
| N2T3 | 44.97 ± 0.32 c | 45.02 ± 0.26 ab | 43.95 ± 0.38 a | 27.07 ± 2.17 a | 14.01 ± 1.16 ab | 32.92 ± 1.10 a | 2.09 ± 0.11 a | 1.52 ± 0.27 abc | 3.15 ± 0.60 ab |
| N3T3 | 45.72 ± 0.32 bc | 45.69 ± 0.36 ab | 44.38 ± 0.21 a | 23.27 ± 1.95 ab | 15.64 ± 2.54 ab | 32.24 ± 2.25 a | 2.36 ± 0.65 a | 1.47 ± 0.35 abc | 4.41 ± 0.27 a |
| P. sepium, PS | |||||||||
| N1T1 | 47.16 ± 0.40 a | 42.07 ± 0.12 abc | 45.54 ± 0.65 a | 10.03 ± 1.07 ab | 10.66 ± 0.79 ab | 24.68 ± 2.54 a | 1.72 ± 0.35 ab | 1.63 ± 0.12 bc | 2.55 ± 0.25 abc |
| N2T1 | 46.36 ± 0.78 ab | 41.94 ± 0.55 abc | 43.27 ± 0.05 ab | 6.09 ± 2.13 b | 5.21 ± 1.74 c | 20.69 ± 2.41 a | 0.95 ± 0.50 b | 1.19 ± 0.37 c | 2.48 ± 0.26 abc |
| N3T1 | 47.17 ± 0.45 a | 43.63 ± 0.61 a | 44.79 ± 0.86 a | 11.89 ± 1.29 ab | 10.42 ± 1.46 b | 24.16 ± 2.39 a | 1.09 ± 0.19 ab | 1.38 ± 0.44 bc | 2.15 ± 0.42 bc |
| N1T2 | 45.68 ± 0.49 b | 41.67 ± 0.27 bc | 42.31 ± 1.01 b | 9.06 ± 0.94 ab | 11.96 ± 1.11 ab | 23.78 ± 1.18 a | 1.95 ± 0.43 ab | 3.08 ± 0.45 a | 3.53 ± 0.27 a |
| N2T2 | 45.54 ± 0.37 b | 42.19 ± 0.40 abc | 43.68 ± 1.14 ab | 14.33 ± 2.12 a | 12.25 ± 1.05 ab | 24.01 ± 1.36 a | 2.35 ± 0.22 a | 2.42 ± 0.21 ab | 3.32 ± 0.64 ab |
| N3T2 | 46.58 ± 0.29 ab | 43.53 ± 0.29 a | 44.98 ± 0.19 a | 12.33 ± 1.46 a | 12.08 ± 0.25 ab | 22.48 ± 1.26 a | 1.98 ± 0.42 ab | 2.42 ± 0.47 ab | 1.69 ± 0.41 c |
| N1T3 | 45.67 ± 0.26 b | 41.16 ± 1.00 c | 44.65 ± 0.67 ab | 11.25 ± 1.00 ab | 13.50 ± 1.91 ab | 17.13 ± 5.11 a | 2.42 ± 0.37 a | 3.25 ± 0.16 a | 2.44 ± 0.32 abc |
| N2T3 | 45.33 ± 0.12 b | 42.52 ± 0.30 abc | 43.57 ± 0.63 ab | 12.03 ± 1.78 ab | 15.09 ± 1.58 a | 16.90 ± 3.85 a | 2.27 ± 0.21 ab | 3.49 ± 0.27 a | 1.58 ± 0.56 c |
| N3T3 | 45.54 ± 0.08 b | 42.94 ± 0.62 ab | 44.66 ± 0.41 ab | 14.18 ± 3.17 a | 14.16 ± 1.51 ab | 18.47 ± 3.67 a | 1.36 ± 0.72 ab | 1.91 ± 0.51 c | 1.22 ± 0.33 c |
| V. negundo, VN | |||||||||
| N1T1 | 45.89 ± 0.21 a | 44.12 ± 0.30 a | 46.16 ± 0.77 ab | 12.16 ± 1.00 b | 10.54 ± 0.70 b | 16.44 ± 0.33 b | 1.52 ± 0.24 a | 1.33 ± 0.04 abc | 1.85 ± 0.43 ab |
| N2T1 | 45.03 ± 1.71 a | 45.31 ± 0.10 a | 44.27 ± 0.43 ab | 16.98 ± 0.84 a | 15.15 ± 0.50 a | 27.31 ± 0.94 a | 1.61 ± 0.42 a | 1.39 ± 0.17 abc | 2.22 ± 0.12 a |
| N3T1 | 45.83 ± 0.61 a | 44.57 ± 0.75 a | 45.66 ± 0.79 ab | 8.30 ± 1.48 c | 8.07 ± 0.71 b | 10.69 ± 1.78 c | 0.54 ± 0.11 a | 0.40 ± 0.20 c | 0.51 ± 0.12 b |
| N1T2 | 47.09 ± 0.23 a | 44.64 ± 0.59 a | 44.68 ± 0.74 ab | 9.70 ± 3.10 c | 8.53 ± 0.59 b | 22.04 ± 0.57 b | 1.74 ± 0.56 a | 1.81 ± 0.29 ab | 3.28 ± 0.28 a |
| N2T2 | 46.46 ± 0.33 a | 45.58 ± 1.19 a | 46.76 ± 0.56 a | 11.43 ± 3.25 b | 7.19 ± 2.17 b | 18.71 ± 3.67 b | 1.44 ± 0.68 a | 0.77 ± 0.33 bc | 1.81 ± 0.71 ab |
| N3T2 | 45.52 ± 0.57 a | 44.46 ± 0.23 a | 44.01 ± 1.47 b | 15.93 ± 0.79 a | 14.94 ± 1.19 a | 25.08 ± 2.55 a | 1.45 ± 0.27 a | 1.06 ± 0.22 bc | 1.90 ± 0.98 ab |
| N1T3 | 46.28 ± 0.42 a | 45.24 ± 0.27 a | 46.63 ± 0.33 ab | 9.23 ± 2.98 c | 7.40 ± 1.19 b | 19.72 ± 2.93 b | 1.57 ± 0.62 a | 2.15 ± 0.46 a | 3.15 ± 0.59 a |
| N2T3 | 45.43 ± 0.78 a | 45.29 ± 1.03 a | 46.67 ± 0.77 ab | 14.64 ± 1.03 a | 9.78 ± 2.19 b | 25.62 ± 1.67 a | 1.70 ± 0.25 a | 1.78 ± 0.59 ab | 3.33 ± 0.35 a |
| N3T3 | 45.26 ± 0.31 a | 44.38 ± 0.30 a | 45.12 ± 0.80 ab | 16.27 ± 0.16 a | 15.71 ± 0.87 a | 29.83 ± 1.53 a | 0.83 ± 0.13 a | 1.35 ± 0.13 abc | 2.96 ± 0.47 a |
| Soil Property | Treatments | AF | PS | VN |
|---|---|---|---|---|
| TC (%) | N1T1 | 1.77 ± 0.03 Aa | 1.83 ± 0.02 Aa | 1.77 ± 0.04 Aa |
| N2T1 | 1.71 ± 0.04 Aab | 1.72 ± 0.05 Aa | 1.75 ± 0.02 Aab | |
| N3T1 | 1.78 ± 0.01 Aa | 1.73 ± 0.02 Aa | 1.76 ± 0.02 Aab | |
| N1T2 | 1.65 ± 0.13 Aab | 1.73 ± 0.05 Aa | 1.70 ± 0.02 Aab | |
| N2T2 | 1.51 ± 0.10 Bb | 1.77 ± 0.01 Aa | 1.77 ± 0.01 Aa | |
| N3T2 | 1.74 ± 0.01 Aa | 1.74 ± 0.05 Aa | 1.74 ± 0.03 Aab | |
| N1T3 | 1.68 ± 0.05 Aab | 1.72 ± 0.06 Aa | 1.72 ± 0.02 Aab | |
| N2T3 | 1.78 ± 0.03 Aa | 1.76 ± 0.02 Aa | 1.73 ± 0.01 Aab | |
| N3T3 | 1.66 ± 0.03 Aab | 1.78 ± 0.01 Aa | 1.65 ± 0.09 Ab | |
| IC (%) | N1T1 | 1.40 ± 0.09 Aa | 1.32 ± 0.07 Aa | 1.33 ± 0.05 Aa |
| N2T1 | 1.34 ± 0.09 Aa | 1.42 ± 0.04 Aa | 1.45 ± 0.02 Aa | |
| N3T1 | 1.39 ± 0.07 Aa | 1.38 ± 0.07 Aa | 1.34 ± 0.05 Aa | |
| N1T2 | 1.42 ± 0.05 Aa | 1.35 ± 0.03 Aa | 1.34 ± 0.04 Aa | |
| N2T2 | 1.37 ± 0.07 Aa | 1.42 ± 0.05 Aa | 1.33 ± 0.09 Aa | |
| N3T2 | 1.38 ± 0.07 Aa | 1.42 ± 0.03 Aa | 1.39 ± 0.02 Aa | |
| N1T3 | 1.35 ± 0.05 Aa | 1.33 ± 0.11 Aa | 1.35 ± 0.01 Aa | |
| N2T3 | 1.40 ± 0.06 Aa | 1.44 ± 0.05 Aa | 1.28 ± 0.15 Aa | |
| N3T3 | 1.21 ± 0.01 Ba | 1.39 ± 0.02 Aa | 1.23 ± 0.07 Ba | |
| TOC (%) | N1T1 | 0.38 ± 0.08 Aab | 0.46 ± 0.07 Aa | 0.44 ± 0.04 Aa |
| N2T1 | 0.37 ± 0.10 Aab | 0.30 ± 0.04 Aa | 0.31 ± 0.03 Aa | |
| N3T1 | 0.39 ± 0.07 Aab | 0.35 ± 0.08 Aa | 0.42 ± 0.06 Aa | |
| N1T2 | 0.23 ± 0.09 Aab | 0.38 ± 0.04 Aa | 0.37 ± 0.05 Aa | |
| N2T2 | 0.15 ± 0.15 Ab | 0.36 ± 0.06 Aa | 0.39 ± 0.10 Aa | |
| N3T2 | 0.37 ± 0.07 Aab | 0.32 ± 0.05 Aa | 0.35 ± 0.10 Aa | |
| N1T3 | 0.33 ± 0.07 Aab | 0.39 ± 0.06 Aa | 0.42 ± 0.02 Aa | |
| N2T3 | 0.38 ± 0.05 Aab | 0.32 ± 0.06 Aa | 0.45 ± 0.01 Aa | |
| N3T3 | 0.46 ± 0.04 Aa | 0.44 ± 0.03 Aa | 0.42 ± 0.14 Aa | |
| TN (g/kg) | N1T1 | 1.72 ± 0.35 Ab | 0.74 ± 0.03 Bd | 1.15 ± 0.18 ABab |
| N2T1 | 1.98 ± 0.22 Aa | 1.32 ± 0.47 ABbcd | 0.71 ± 0.15 Bbc | |
| N3T1 | 2.31 ± 0.18 Aa | 2.66 ± 0.22 Aa | 1.15 ± 0.16 Bab | |
| N1T2 | 1.74 ± 0.12 Ab | 1.17 ± 0.17 Bbcd | 0.35 ± 0.04 Cc | |
| N2T2 | 1.79 ± 0.23 Ab | 0.79 ± 0.14 Bcd | 1.51 ± 0.16 Aba | |
| N3T2 | 1.71 ± 0.24 Ab | 1.72 ± 0.20 Abc | 0.82 ± 0.15 Bbc | |
| N1T3 | 1.63 ± 0.07 Ab | 1.36 ± 0.63 Abcd | 0.87 ± 0.28 Abc | |
| N2T3 | 1.83 ± 0.05 Ab | 0.61 ± 0.11 Bd | 0.58 ± 0.10 Bbc | |
| N3T3 | 2.06 ± 0.28 Aab | 1.81 ± 0.02 ABab | 1.10 ± 0.25 Bab | |
| TP (g/kg) | N1T1 | 6.68 ± 2.07 Ab | 7.48 ± 2.44 Abc | 9.16 ± 0.38 Aa |
| N2T1 | 11.87 ± 1.05 Aa | 6.01 ± 0.85 Cbc | 9.06 ± 0.15 Ba | |
| N3T1 | 9.79 ± 0.39 Bab | 12.32 ± 0.19 Aa | 9.93 ± 0.45 Ba | |
| N1T2 | 9.71 ± 1.22 Aab | 9.29 ± 0.38 Aab | 2.52 ± 0.83 Bb | |
| N2T2 | 7.73 ± 1.48 Ab | 6.36 ± 0.83 Abc | 8.84 ± 1.01 Aa | |
| N3T2 | 9.30 ± 1.10 Aab | 9.27 ± 0.16 Aab | 7.86 ± 0.24 Aa | |
| N1T3 | 8.82 ± 0.23 Aab | 4.39 ± 1.49 Acd | 7.26 ± 2.19 Aa | |
| N2T3 | 8.96 ± 0.92 Aab | 6.69 ± 0.40 Abc | 6.75 ± 1.17 Aa | |
| N3T3 | 7.91 ± 0.79 Ab | 2.10 ± 1.88 Bd | 9.00 ± 0.19 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Z.; Li, Y.; Chen, S.; Wang, Y.; Jing, G.; Wei, Y.; Shang, H.; Yue, M. Combined Effects of Nitrogen Addition and Warming on Shrub Growth and Nutrient Uptake through Microbially Mediated Soil Fertility. Agronomy 2024, 14, 2167. https://doi.org/10.3390/agronomy14092167
Mao Z, Li Y, Chen S, Wang Y, Jing G, Wei Y, Shang H, Yue M. Combined Effects of Nitrogen Addition and Warming on Shrub Growth and Nutrient Uptake through Microbially Mediated Soil Fertility. Agronomy. 2024; 14(9):2167. https://doi.org/10.3390/agronomy14092167
Chicago/Turabian StyleMao, Zhuxin, Yang Li, Siyu Chen, Yuchao Wang, Guanghua Jing, Ying Wei, Huiying Shang, and Ming Yue. 2024. "Combined Effects of Nitrogen Addition and Warming on Shrub Growth and Nutrient Uptake through Microbially Mediated Soil Fertility" Agronomy 14, no. 9: 2167. https://doi.org/10.3390/agronomy14092167
APA StyleMao, Z., Li, Y., Chen, S., Wang, Y., Jing, G., Wei, Y., Shang, H., & Yue, M. (2024). Combined Effects of Nitrogen Addition and Warming on Shrub Growth and Nutrient Uptake through Microbially Mediated Soil Fertility. Agronomy, 14(9), 2167. https://doi.org/10.3390/agronomy14092167





