Combination of Nitrogen-Enriched Zeolite and Arbuscular Mycorrhizal Symbiosis to Improve Growth of Maize (Zea mays L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zeolite and Biological Materials
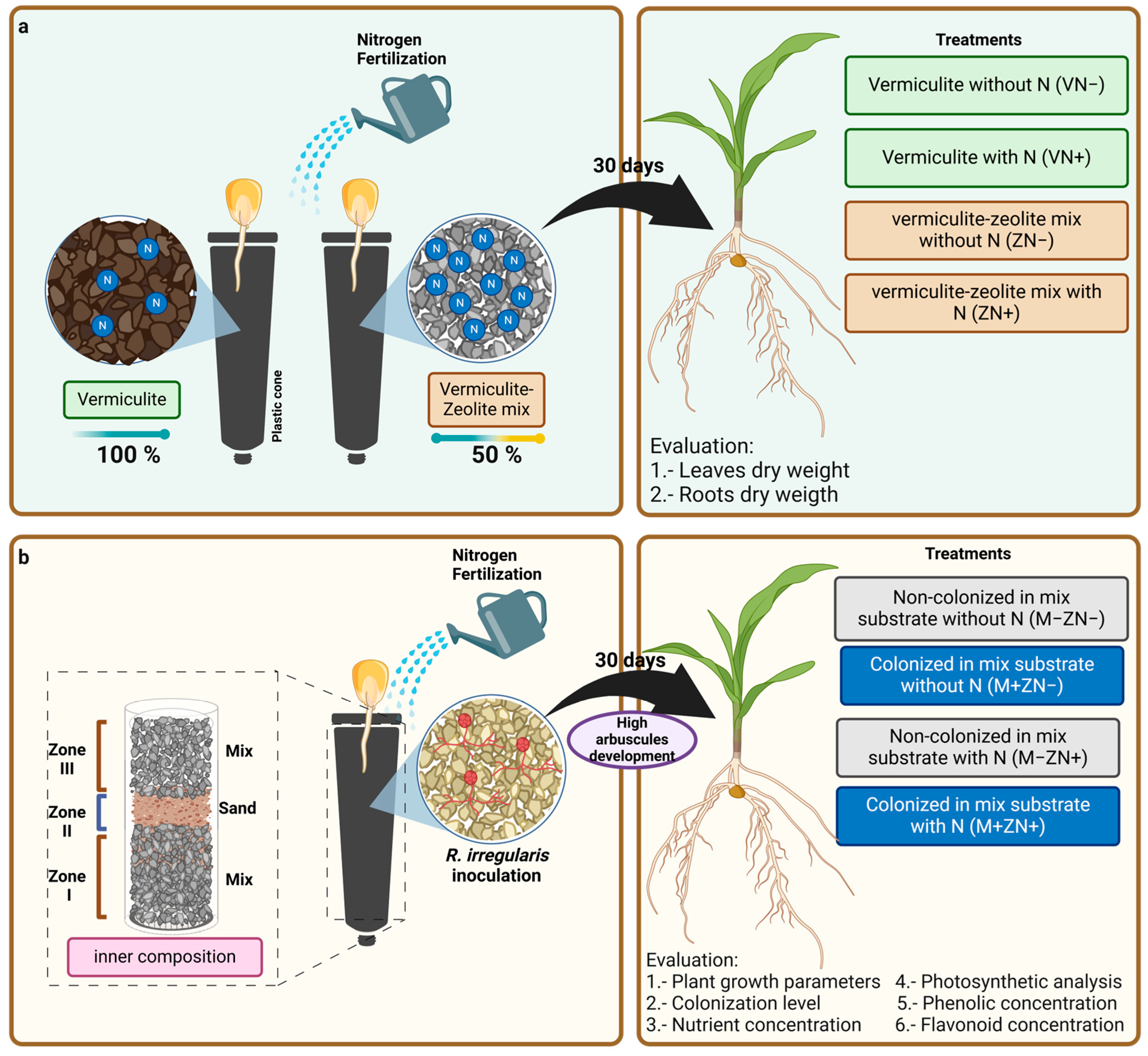
2.2. Experimental Design
2.3. Characterization and Chemical Properties of Zeolite
2.4. Determination of Plant Growth
2.5. Staining Procedures and Quantification of Mycorrhizal Colonization
2.6. Nitrogen, Phosphorus, and Potassium Content Analysis
2.7. Photosynthetic Pigment Concentration
2.8. Photosynthetic Performance Analysis
2.9. Quantification of Phenolic and Flavonoid Metabolites
2.10. Statistical Analysis
3. Results
3.1. Particle Size and Elemental Composition of Zeolite
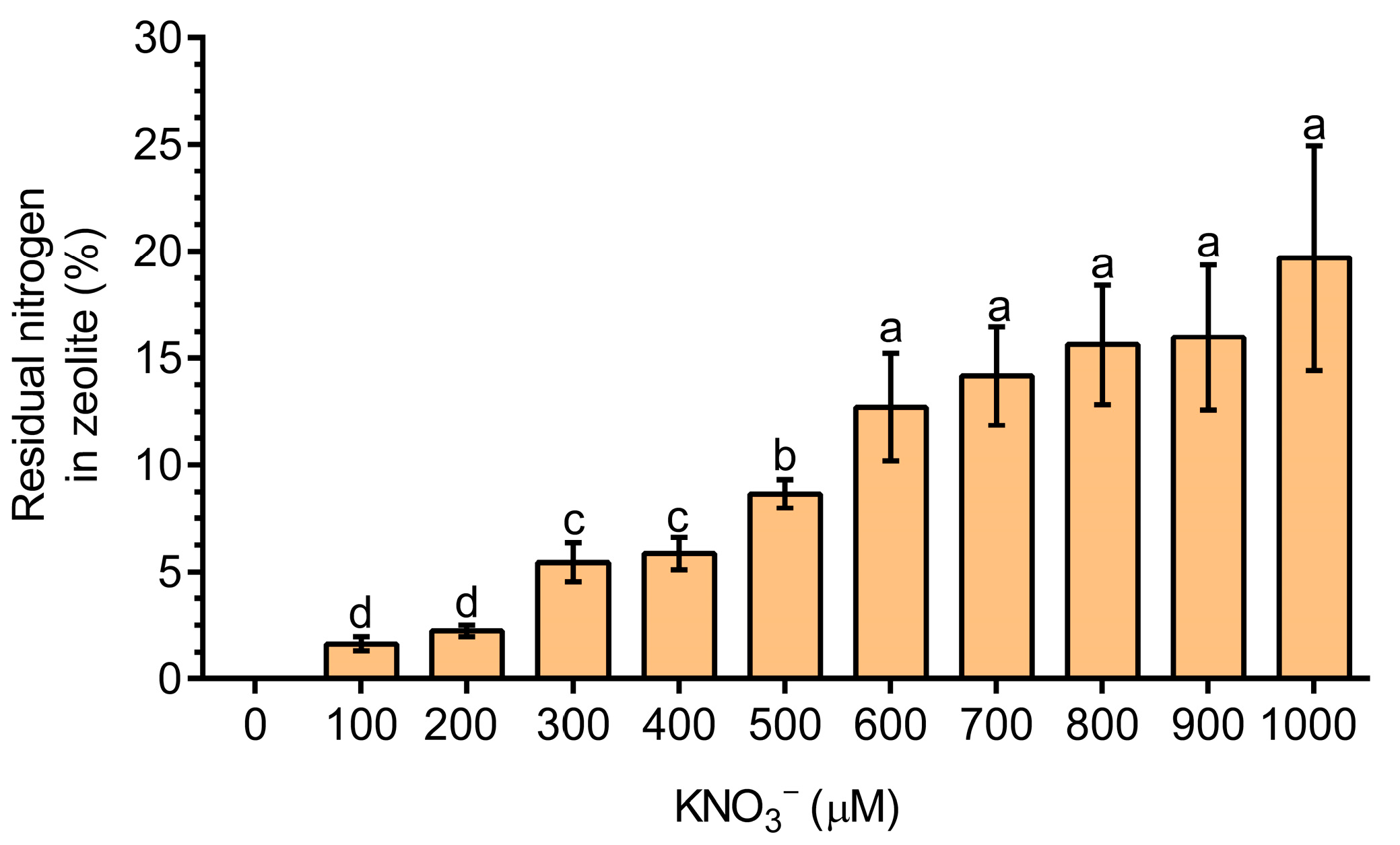
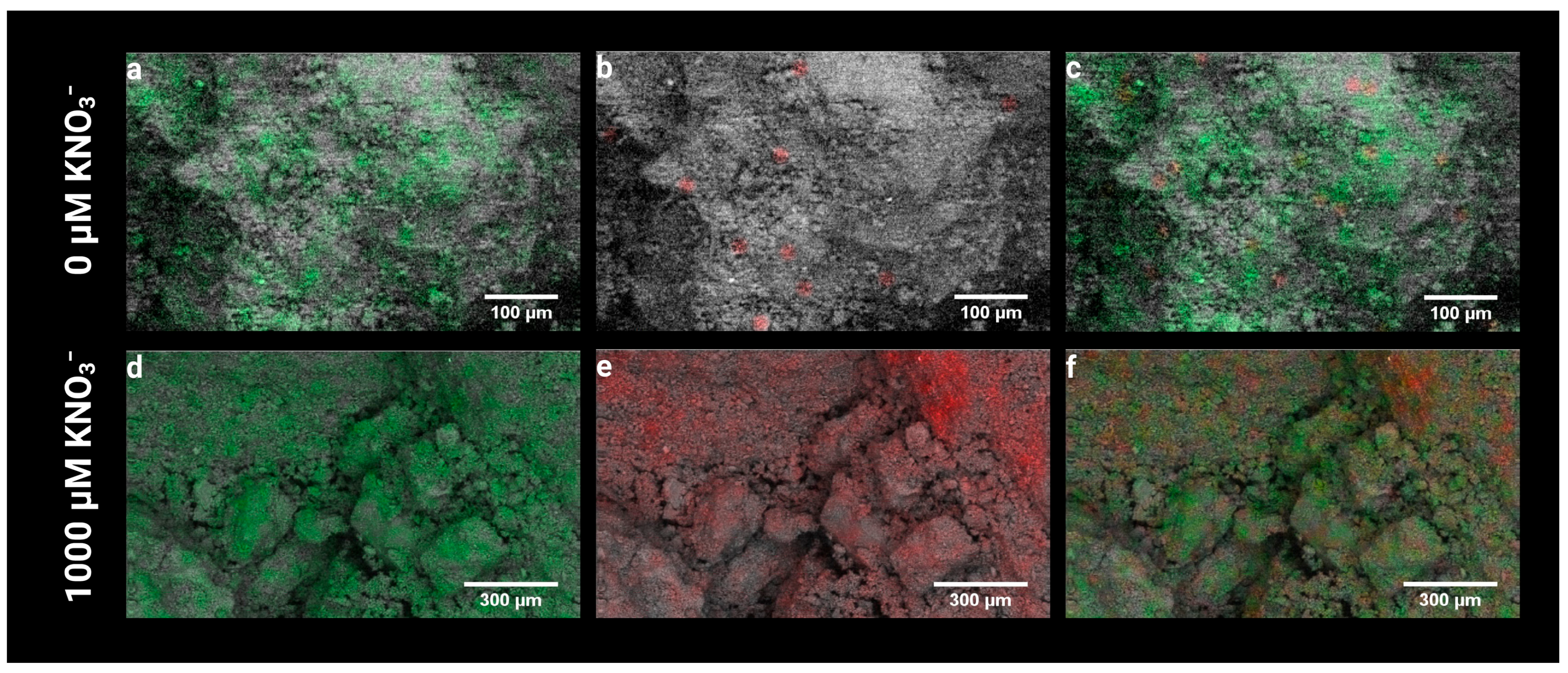
3.2. Residual N Is Retained in the Zeolite Structure
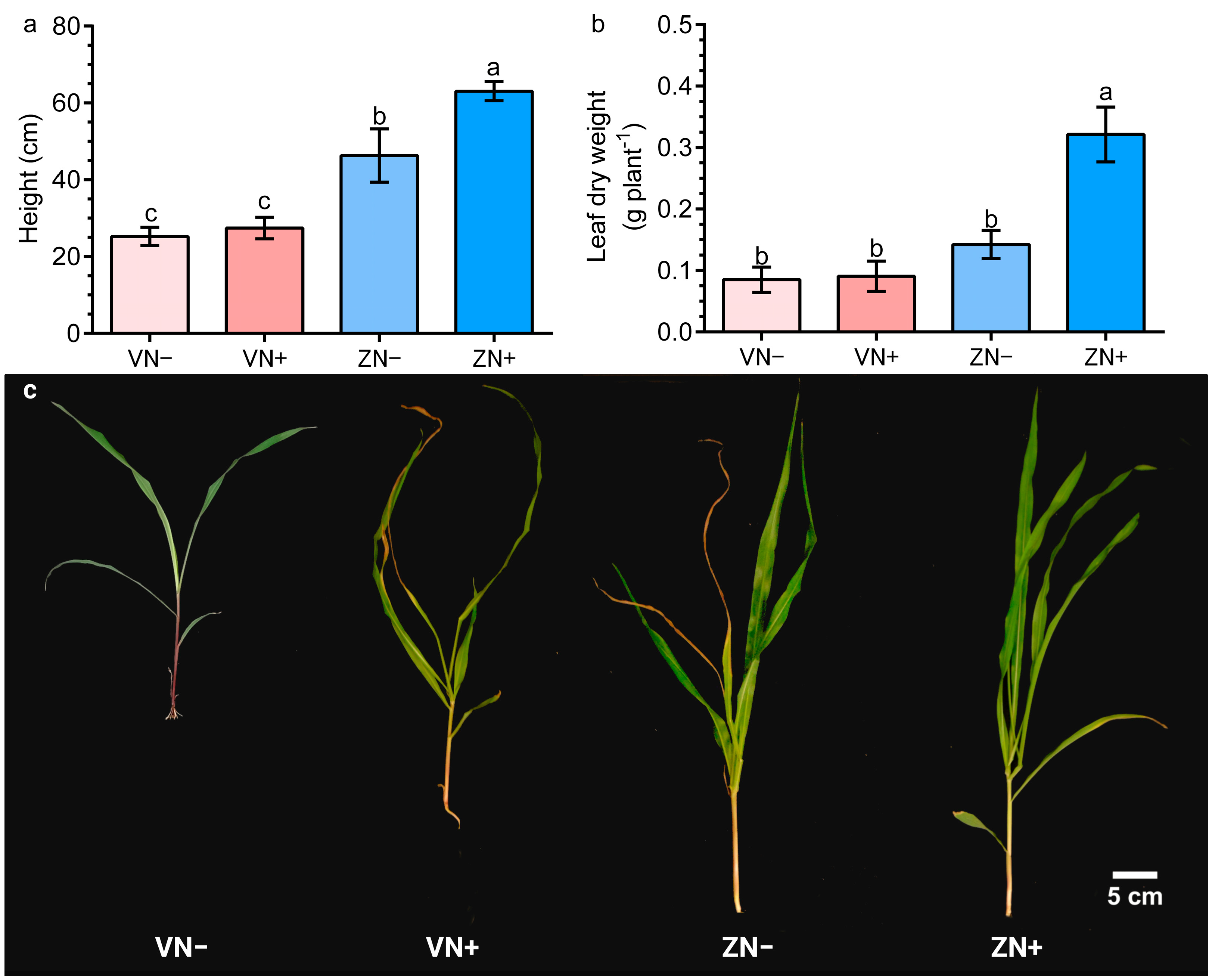
3.3. N-Enriched Zeolite Improves Maize Growth
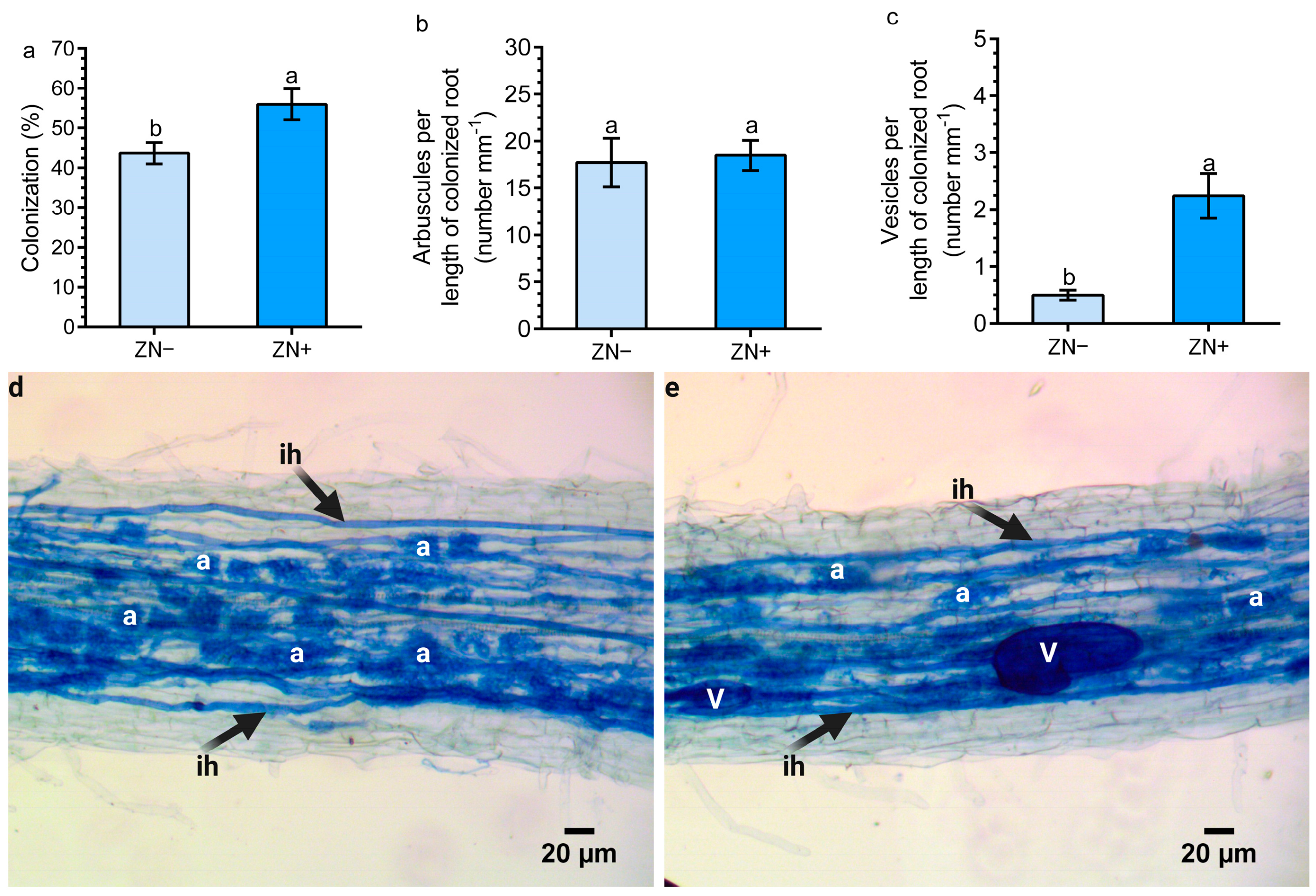
3.4. N-Enriched Zeolite Enhance Mycorrhizal Colonization in Maize
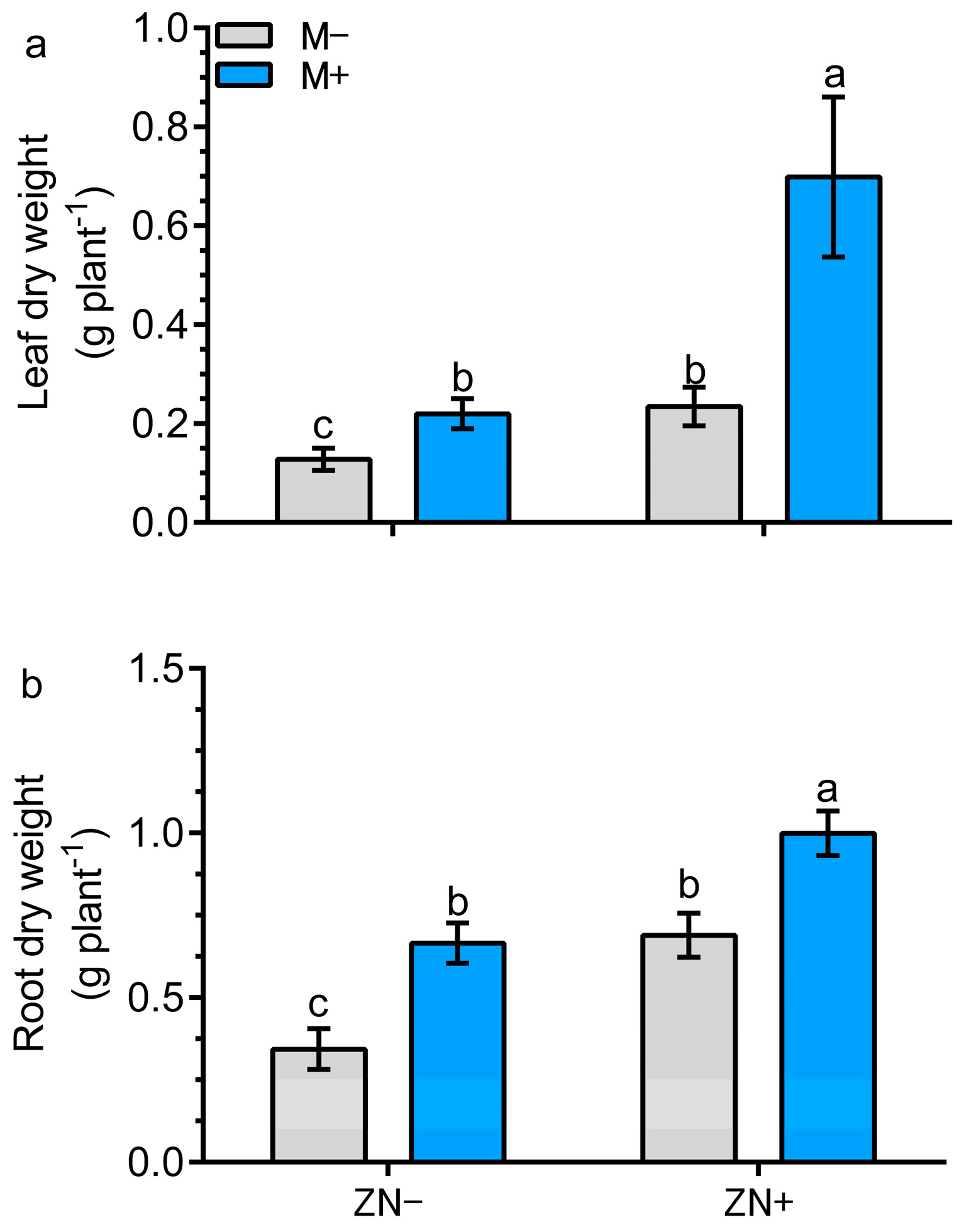
3.5. AM Colonization in Combination with N-Enriched Zeolite Improves Plant Growth in Maize
3.6. The N Concentration Increases in the Leaves of Colonized Maize Plants Under N-Enriched Zeolite Condition
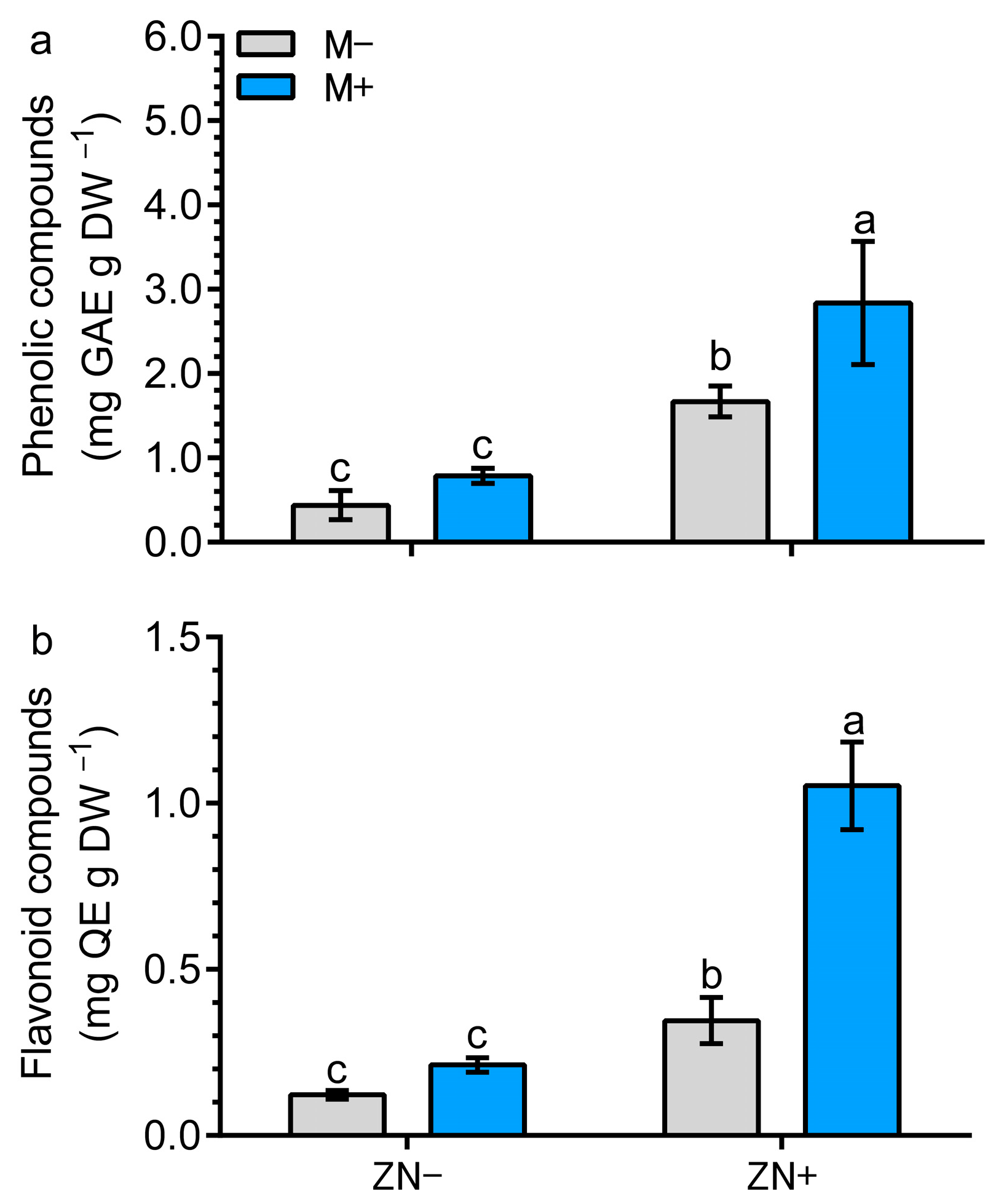
3.7. Specialized Metabolites Increase by AM Symbiosis in Combination with N-Enriched Zeolite in Maize
3.8. Multivariate Principal Component Analysis
4. Discussion
4.1. N-Enriched Zeolite Is an Effective Alternative for Promoting Plant Growth in Maize
4.2. The Synergistic Combination of N-Enriched Zeolite and AM Symbiosis Improves the Physiological and Nutritional Fitness of Maize Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Verma, J.P. Does Plant—Microbe Interaction Confer Stress Tolerance in Plants: A Review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, J.; Xie, Q.; Jiang, Y.; Yu, N.; Wang, E. Nutrient Exchange and Regulation in Arbuscular Mycorrhizal Symbiosis. Mol. Plant 2017, 10, 1147–1158. [Google Scholar] [CrossRef]
- Suman, J.; Rakshit, A.; Ogireddy, S.D.; Singh, S.; Gupta, C.; Chandrakala, J. Microbiome as a Key Player in Sustainable Agriculture and Human Health. Front. Soil Sci. 2022, 2, 821589. [Google Scholar] [CrossRef]
- Harrison, M.J. Cellular Programs for Arbuscular Mycorrhizal Symbiosis. Curr. Opin. Plant Biol. 2012, 15, 691–698. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D. Speak, Friend, and Enter: Signalling Systems That Promote Beneficial Symbiotic Associations in Plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A Phylum-Level Phylogenetic Classification of Zygomycete Fungi Based on Genome-Scale Data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef]
- Gutjahr, C.; Parniske, M. Cell and Developmental Biology of Arbuscular Mycorrhiza Symbiosis. Annu. Rev. Cell Dev. Biol. 2013, 29, 593–617. [Google Scholar] [CrossRef]
- Calabrese, S.; Pérez-Tienda, J.; Ellerbeck, M.; Arnould, C.; Chatagnier, O.; Boller, T.; Schüßler, A.; Brachmann, A.; Wipf, D.; Ferrol, N.; et al. GintAMT3—A Low-Affinity Ammonium Transporter of the Arbuscular Mycorrhizal Rhizophagus irregularis. Front. Plant Sci. 2016, 7, 679. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, A.; Xie, K.; Yang, X.; Luo, Z.; Chen, J.; Zeng, D.; Ren, Y.; Yang, C.; Wang, L.; et al. Functional Analysis of the OsNPF4.5 Nitrate Transporter Reveals a Conserved Mycorrhizal Pathway of Nitrogen Acquisition in Plants. Proc. Natl. Acad. Sci. USA 2020, 117, 16649–16659. [Google Scholar] [CrossRef]
- Frey, B.; Schüepp, H. Acquisition of Nitrogen by External Hyphae of Arbuscular Mycorrhizal Fungi Associated with Zea mays L. New Phytol. 1993, 124, 221–230. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen Uptake, Assimilation and Remobilization in Plants: Challenges for Sustainable and Productive Agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Shen, R.F.; Zhao, X.Q. Nitrogen Source Preference in Maize at Seedling Stage Is Mainly Dependent on Growth Medium PH. Agronomy 2022, 12, 2149. [Google Scholar] [CrossRef]
- McCoy, J.M.; Kaur, G.; Golden, B.R.; Orlowski, J.M.; Cook, D.R.; Bond, J.A.; Cox, M.S. Nitrogen Fertilization of Soybean Affects Root Growth and Nodulation on Two Soil Types in Mississippi. Commun. Soil Sci. Plant Anal. 2018, 49, 181–187. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Uhart, S.A.; Andrade, F.H. Nitrogen Deficiency in Maize: I. Effects on Crop Growth, Development, Dry Matter Partitioning, and Kernel Set. Crop Sci. 1995, 35, 1376–1383. [Google Scholar] [CrossRef]
- Ren, N.; Wang, Y.; Ye, Y.; Zhao, Y.; Huang, Y.; Fu, W.; Chu, X. Effects of Continuous Nitrogen Fertilizer Application on the Diversity and Composition of Rhizosphere Soil Bacteria. Front. Microbiol. 2020, 11, 1948. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, N.; Emekci, M.; Athanassiou, C.G. Applications of Natural Zeolites on Agriculture and Food Production. J. Sci. Food Agric. 2017, 97, 3487–3499. [Google Scholar] [CrossRef] [PubMed]
- Vinaches, P.; Bernardo-Gusmão, K.; Pergher, S.B.C. An Introduction to Zeolite Synthesis Using Imidazolium-Based Cations as Organic Structure-Directing Agents. Molecules 2017, 22, 1307. [Google Scholar] [CrossRef]
- Soudejani, H.T.; Kazemian, H.; Inglezakis, V.J.; Zorpas, A.A. Application of Zeolites in Organic Waste Composting: A Review. Biocatal. Agric. Biotechnol. 2019, 22, 101396. [Google Scholar] [CrossRef]
- Gholamhoseini, M.; Ghalavand, A.; Khodaei-Joghan, A.; Dolatabadian, A.; Zakikhani, H.; Farmanbar, E. Zeolite-Amended Cattle Manure Effects on Sunflower Yield, Seed Quality, Water Use Efficiency and Nutrient Leaching. Soil Tillage Res. 2013, 126, 193–202. [Google Scholar] [CrossRef]
- Latifah, O.; Ahmed, O.H.; Majid, N.M.A. Enhancing Nitrogen Availability from Urea Using Clinoptilolite Zeolite. Geoderma 2017, 306, 152–159. [Google Scholar] [CrossRef]
- Iskander, A.L.; Khald, E.M.; Sheta, A.S. Zinc and Manganese Sorption Behavior by Natural Zeolite and Bentonite. Ann. Agric. Sci. 2011, 56, 43–48. [Google Scholar] [CrossRef]
- Lim, S.S.; Lee, D.S.; Kwak, J.H.; Park, H.J.; Kim, H.Y.; Choi, W.J. Fly Ash and Zeolite Amendments Increase Soil Nutrient Retention but Decrease Paddy Rice Growth in a Low Fertility Soil. J. Soils Sediments 2016, 16, 756–766. [Google Scholar] [CrossRef]
- Conversa, G.; Pacifico, S.; La Rotonda, P.; Lazzizera, C.; Bonasia, A.; Elia, A. Foliar Application of Natural Zeolites Affects the Growth and Productivity of Processing Tomato. Eur. J. Agron. 2024, 154, 127100. [Google Scholar] [CrossRef]
- Amirahmadi, E.; Ghorbani, M.; Moudrý, J. Effects of Zeolite on Aggregation, Nutrient Availability, and Growth Characteristics of Corn (Zea mays L.) in Cadmium-Contaminated Soils. Water Air. Soil Pollut. 2022, 233, 436. [Google Scholar] [CrossRef]
- Li, Y.; Xia, G.; Wu, Q.; Chen, W.; Lin, W.; Zhang, Z.; Chen, Y.; Chen, T.; Siddique, K.H.M.; Chi, D. Zeolite Increases Grain Yield and Potassium Balance in Paddy Fields. Geoderma 2021, 405, 115397. [Google Scholar] [CrossRef]
- Ibrahim, H.M.S.; Mahmoud, A.W.M.; Soliman, M.M.; Heider, S.M.; Abdel Mottaleb, S. Assessing Biochar, Clinoptilolite Zeolite and Zeo-Char Loaded Nano-Nitrogen for Boosting Growth Performance and Biochemical Ingredients of Peace Lily (Spathiphyllum wallisii) Plant under Water Shortage. BMC Plant Biol. 2024, 24, 924. [Google Scholar] [CrossRef] [PubMed]
- Domenico, P. Optimised Fertilisation with Zeolitites Containing Plant Growth Promoting Rhizobacteria (PGPR) in Ranunculus Asiaticus. GSC Biol. Pharm. Sci. 2020, 10, 096–102. [Google Scholar] [CrossRef]
- Real-Santillán, R.O.; del-Val, E.; Cruz-Ortega, R.; Contreras-Cornejo, H.Á.; González-Esquivel, C.E.; Larsen, J. Increased Maize Growth and P Uptake Promoted by Arbuscular Mycorrhizal Fungi Coincide with Higher Foliar Herbivory and Larval Biomass of the Fall Armyworm Spodoptera frugiperda. Mycorrhiza 2019, 29, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Belay, M.K. Growth, Yield-Related Traits and Yield of Lowland Maize (Zea mays L.) Varieties as Influenced by Inorganic NPS and N Fertilizer Rates at Babile, Eastern Ethiopia. Int. J. Agron. 2020, 2020, 8811308. [Google Scholar] [CrossRef]
- Ndayisaba, P.C.; Kuyah, S.; Midega, C.A.O.; Mwangi, P.N.; Khan, Z.R. Intercropping Desmodium and Maize Improves Nitrogen and Phosphorus Availability and Performance of Maize in Kenya. Field Crops Res. 2021, 263, 108067. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Dweikat, I.; Huber, D.M.; Warren, H.L. Interrelationship of Nitrogen Nutrition with Maize (Zea mays) Grain Yield, Nitrogen Use Efficiency and Grain Quality. J. Sci. Food Agric. 1992, 58, 1–8. [Google Scholar] [CrossRef]
- Sun, C.X.; Hao, L.; Wang, D.; Li, C.; Zhang, C.; Chen, X.; Fu, J.; Zhang, Y.L. Nitrogen Utilisation and Metabolism in Maize (Zea mays L.) Plants under Different Rates of Biochar Addition and Nitrogen Input Conditions. Plant Biol. 2019, 21, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Berta, G.; Copetta, A.; Gamalero, E.; Bona, E.; Cesaro, P.; Scarafoni, A.; D’Agostino, G. Maize Development and Grain Quality Are Differentially Affected by Mycorrhizal Fungi and a Growth-Promoting Pseudomonad in the Field. Mycorrhiza 2014, 24, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Leyva, J.F.; Segura-Castruita, M.A.; Hernández-Cuevas, L.V.; Íñiguez-Rivas, M. Arbuscular Mycorrhizal Fungi Associated with Maize (Zea mays L.) in the Formation and Stability of Aggregates in Two Types of Soil. Microorganisms 2023, 11, 2615. [Google Scholar] [CrossRef]
- Li, M.; Perez-Limón, S.; Ramírez-Flores, M.R.; Barrales-Gamez, B.; Meraz-Mercado, M.A.; Ziegler, G.; Baxter, I.; Olalde-Portugal, V.; Sawers, R.J.H. Mycorrhizal Status and Host Genotype Interact to Shape Plant Nutrition in Field Grown Maize (Zea mays ssp. mays). Mycorrhiza 2023, 33, 345–358. [Google Scholar] [CrossRef]
- Wang, Z.; Lian, J.; Liang, J.; Wei, H.; Chen, H.; Hu, W.; Tang, M. Arbuscular Mycorrhizal Symbiosis Modulates Nitrogen Uptake and Assimilation to Enhance Drought Tolerance of Populus cathayana. Plant Physiol. Biochem. 2024, 210, 108648. [Google Scholar] [CrossRef]
- Hui, J.; An, X.; Li, Z.; Neuhäuser, B.; Ludewig, U.; Wu, X.; Schulze, W.X.; Chen, F.; Feng, G.; Lambers, H.; et al. The Mycorrhiza-Specific Ammonium Transporter ZmAMT3;1 Mediates Mycorrhiza-Dependent Nitrogen Uptake in Maize Roots. Plant Cell 2022, 34, 4066–4087. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Wu, J.; Xie, K.; Li, X. LjAMT2;2 Promotes Ammonium Nitrogen Transport during Arbuscular Mycorrhizal Fungi Symbiosis in Lotus japonicus. Int. J. Mol. Sci. 2022, 23, 9522. [Google Scholar] [CrossRef] [PubMed]
- Nouri, E.; Breuillin-Sessoms, F.; Feller, U.; Reinhardt, D. Phosphorus and Nitrogen Regulate Arbuscular Mycorrhizal Symbiosis in Petunia hybrida. PLoS ONE 2014, 9, e90841. [Google Scholar] [CrossRef]
- Blanke, V.; Renker, C.; Wagner, M.; Füllner, K.; Held, M.; Kuhn, A.J.; Buscot, F. Nitrogen Supply Affects Arbuscular Mycorrhizal Colonization of Artemisia vulgaris in a Phosphate-Polluted Field Site. New Phytol. 2005, 166, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Drijber, R.A.; Zhang, J.L.; Li, X.L. Impact of Long-Term Nitrogen Fertilization and Rotation with Soybean on the Diversity and Phosphorus Metabolism of Indigenous Arbuscular Mycorrhizal Fungi within the Roots of Maize (Zea mays L.). Agric. Ecosyst. Environ. 2013, 164, 53–61. [Google Scholar] [CrossRef]
- Estrada-Navarrete, G.; Alvarado-Affantranger, X.; Olivares, J.E.; Guillén, G.; Díaz-Camino, C.; Campos, F.; Quinto, C.; Gresshoff, P.M.; Sanchez, F. Fast, Efficient and Reproducible Genetic Transformation of Phaseolus Spp. by Agrobacterium rhizogenes. Nat. Protoc. 2007, 2, 1819–1824. [Google Scholar] [CrossRef]
- Bécard, G.; Fortin, J.A. Early Events of Vesicular–Arbuscular Mycorrhiza Formation on Ri T-DNA Transformed Roots. New Phytol. 1988, 108, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-López, L.G.; López-Meyer, M.; Sepúlveda-Jiménez, G.; Cárdenas, L.; Rodríguez-Monroy, M. Photosynthetic Performance and Stevioside Concentration Are Improved by the Arbuscular Mycorrhizal Symbiosis in Stevia rebaudiana under Different Phosphate Concentrations. PeerJ 2020, 8, e10173. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. Soil Sci. 1950, 48, 356. [Google Scholar] [CrossRef]
- Harrison, M.J. A Phosphate Transporter from Medicago truncatula Involved in the Acquisition of Phosphate Released by Arbuscular Mycorrhizal Fungi. Plant Cell Online 2002, 14, 2413–2429. [Google Scholar] [CrossRef]
- Volpe, V.; Giovannetti, M.; Sun, X.G.; Fiorilli, V.; Bonfante, P. The Phosphate Transporters LjPT4 and MtPT4 Mediate Early Root Responses to Phosphate Status in Non Mycorrhizal Roots. Plant Cell Environ. 2016, 39, 660–671. [Google Scholar] [CrossRef]
- Guerrero-Molina, M.F.; Lovaisa, N.C.; Salazar, S.M.; Díaz-Ricci, J.C.; Pedraza, R.O. Elemental Composition of Strawberry Plants Inoculated with the Plant Growth-Promoting Bacterium Azospirillum brasilense REC3, Assessed with Scanning Electron Microscopy and Energy Dispersive X-Ray Analysis. Plant Biol. 2014, 16, 726–731. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Gronlund, M.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Phosphorus Nutrition: Interactions between Pathways of Phosphorus Uptake in Arbuscular Mycorrhizal Roots Have Important Implications for Understanding and Manipulating Plant Phosphorus Acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An Evaluation of Techniques for Measuring Vesicular Arbuscular Mycorrhizal Infection in Roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Sarmiento-López, L.G.; López-Meyer, M.; Sepúlveda-Jiménez, G.; Cárdenas, L.; Rodríguez-Monroy, M. Arbuscular Mycorrhizal Symbiosis in Stevia rebaudiana Increases Trichome Development, Flavonoid and Phenolic Compound Accumulation. Biocatal. Agric. Biotechnol. 2021, 31, 101889. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Oviedo-Pereira, D.G.; López-Meyer, M.; Evangelista-Lozano, S.; Sarmiento-López, L.G.; Sepúlveda-Jiménez, G.; Rodríguez-Monroy, M. Enhanced Specialized Metabolite, Trichome Density, and Biosynthetic Gene Expression in Stevia rebaudiana (Bertoni) Bertoni Plants Inoculated with Endophytic Bacteria Enterobacter Hormaechei. PeerJ 2022, 10, e13675. [Google Scholar] [CrossRef] [PubMed]
- Abdelsattar, A.M.; El-Esawi, M.A.; Elsayed, A.; Heikal, Y.M. Comparison between Bacterial Bio-Formulations and Gibberellic Acid Effects on Stevia rebaudiana Growth and Production of Steviol Glycosides through Regulating Their Encoding Genes. Sci. Rep. 2024, 14, 24130. [Google Scholar] [CrossRef] [PubMed]
- Latifah, O.; Ahmed, O.H.; Majid, N.M.A. Short Term Enhancement of Nutrients Availability in Zea mays L. Cultivation on an Acid Soil Using Compost and Clinoptilolite Zeolite. Compost Sci. Util. 2017, 25, 22–35. [Google Scholar] [CrossRef]
- Lamhamedi, M.S.; Renaud, M.; Auger, I.; Fortin, J.A. Granular Calcite Stimulates Natural Mycorrhization and Growth of White Spruce Seedlings in Peat-Based Substrates in Forest Nursery. Microorganisms 2020, 8, 1088. [Google Scholar] [CrossRef] [PubMed]
- Shoumkova, A.; Stoyanova, V. Zeolites Formation by Hydrothermal Alkali Activation of Coal Fly Ash from Thermal Power Station “Maritsa 3”, Bulgaria. Fuel 2013, 103, 533–541. [Google Scholar] [CrossRef]
- De Campos Bernardi, A.C.; Anchão Oliviera, P.P.; De Melo Monte, M.B.; Souza-Barros, F. Brazilian Sedimentary Zeolite Use in Agriculture. Microporous Mesoporous Mater. 2013, 167, 16–21. [Google Scholar] [CrossRef]
- Torma, S.; Vilcek, J.; Adamisin, P.; Huttmanova, E.; Hronec, O. Influence of Natural Zeolite on Nitrogen Dynamics in Soil. Turkish J. Agric. For. 2014, 38, 739–744. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9780123705266. [Google Scholar]
- Sylvia, D.M.; Neal, L.H. Nitrogen Affects the Phosphorus Response of VA Mycorrhiza. New Phytol. 1990, 115, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Azcón, R.; Ambrosano, E.; Charest, C. Nutrient Acquisition in Mycorrhizal Lettuce Plants under Different Phosphorus and Nitrogen Concentration. Plant Sci. 2003, 165, 1137–1145. [Google Scholar] [CrossRef]
- Javot, H.; Penmetsa, R.V.; Breuillin, F.; Bhattarai, K.K.; Noar, R.D.; Gomez, S.K.; Zhang, Q.; Cook, D.R.; Harrison, M.J. Medicago truncatula Mtpt4 Mutants Reveal a Role for Nitrogen in the Regulation of Arbuscule Degeneration in Arbuscular Mycorrhizal Symbiosis. Plant J. 2011, 68, 954–965. [Google Scholar] [CrossRef]
- Yang, Y.; Han, X.; Liang, Y.; Ghosh, A.; Chen, J.; Tang, M. The Combined Effects of Arbuscular Mycorrhizal Fungi (AMF) and Lead (Pb) Stress on Pb Accumulation, Plant Growth Parameters, Photosynthesis, and Antioxidant Enzymes in Robinia pseudoacacia L. PLoS ONE 2015, 10, e0145726. [Google Scholar] [CrossRef] [PubMed]
- Schoefs, B.; Uddling, J.; Spetea, C.; Adolfsson, L.; Keresztes, Á.; Andersson, M.X.; Solymosi, K. Mycorrhiza Symbiosis Increases the Surface for Sunlight Capture in Medicago truncatula for Better Photosynthetic Production. PLoS ONE 2015, 10, e0115314. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of Arbuscular Mycorrhizae on Photosynthesis and Water Status of Maize Plants under Salt Stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef]
- Santana, L.R.; da Silva, L.N.; Tavares, G.G.; Batista, P.F.; Cabral, J.S.R.; Souchie, E.L. Arbuscular Mycorrhizal Fungi Associated with Maize Plants during Hydric Deficit. Sci. Rep. 2023, 13, 1519. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Chen, Y. The Physiological Response of Photosynthesis to Nitrogen Deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Zhu, X.C.; Song, F.B.; Liu, S.Q.; Liu, T.D. Effects of Arbuscular Mycorrhizal Fungus on Photosynthesis and Water Status of Maize under High Temperature Stress. Plant Soil 2011, 346, 189–199. [Google Scholar] [CrossRef]
- Porcel, R.; Redondo-Gómez, S.; Mateos-Naranjo, E.; Aroca, R.; Garcia, R.; Ruiz-Lozano, J.M. Arbuscular Mycorrhizal Symbiosis Ameliorates the Optimum Quantum Yield of Photosystem II and Reduces Non-Photochemical Quenching in Rice Plants Subjected to Salt Stress. J. Plant Physiol. 2015, 185, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.M.; Niemeyer, E.D. Effects of Nitrogen Fertilization on the Phenolic Composition and Antioxidant Properties of Basil (Ocimum basilicum L.). J. Agric. Food Chem. 2008, 56, 8685–8691. [Google Scholar] [CrossRef] [PubMed]
- Coronado, C.; Zuanazzi, J.; Sallaud, C.; Quirion, J.C.; Esnault, R.; Husson, H.P.; Kondorosi, A.; Ratet, P. Alfalfa Root Flavonoid Production Is Nitrogen Regulated. Plant Physiol. 1995, 108, 533–542. [Google Scholar] [CrossRef]
- Avio, L.; Sbrana, C.; Giovannetti, M.; Frassinetti, S. Arbuscular Mycorrhizal Fungi Affect Total Phenolics Content and Antioxidant Activity in Leaves of Oak Leaf Lettuce Varieties. Sci. Hortic. 2017, 224, 265–271. [Google Scholar] [CrossRef]
- Liu, X.Q.; Cheng, S.; Aroca, R.; Zou, Y.N.; Wu, Q.S. Arbuscular Mycorrhizal Fungi Induce Flavonoid Synthesis for Mitigating Oxidative Damage of Trifoliate Orange under Water Stress. Environ. Exp. Bot. 2022, 204, 105089. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarmiento-López, L.G.; Matos-Alegria, A.; Cesario-Solis, M.E.; Tapia-Maruri, D.; Goodwin, P.H.; Quinto, C.; Santana, O.; Cardenas, L. Combination of Nitrogen-Enriched Zeolite and Arbuscular Mycorrhizal Symbiosis to Improve Growth of Maize (Zea mays L.). Agronomy 2025, 15, 156. https://doi.org/10.3390/agronomy15010156
Sarmiento-López LG, Matos-Alegria A, Cesario-Solis ME, Tapia-Maruri D, Goodwin PH, Quinto C, Santana O, Cardenas L. Combination of Nitrogen-Enriched Zeolite and Arbuscular Mycorrhizal Symbiosis to Improve Growth of Maize (Zea mays L.). Agronomy. 2025; 15(1):156. https://doi.org/10.3390/agronomy15010156
Chicago/Turabian StyleSarmiento-López, Luis G., Arny Matos-Alegria, Mariana E. Cesario-Solis, Daniel Tapia-Maruri, Paul H. Goodwin, Carmen Quinto, Olivia Santana, and Luis Cardenas. 2025. "Combination of Nitrogen-Enriched Zeolite and Arbuscular Mycorrhizal Symbiosis to Improve Growth of Maize (Zea mays L.)" Agronomy 15, no. 1: 156. https://doi.org/10.3390/agronomy15010156
APA StyleSarmiento-López, L. G., Matos-Alegria, A., Cesario-Solis, M. E., Tapia-Maruri, D., Goodwin, P. H., Quinto, C., Santana, O., & Cardenas, L. (2025). Combination of Nitrogen-Enriched Zeolite and Arbuscular Mycorrhizal Symbiosis to Improve Growth of Maize (Zea mays L.). Agronomy, 15(1), 156. https://doi.org/10.3390/agronomy15010156





