Assessment of the Effects of Newly Fabricated CaO, CuO, ZnO Nanoparticles on Callus Formation Maintenance of Alfalfa (Medicago sativa L.) Under In Vitro Salt Stress
Abstract
:1. Introduction
2. Materials and Methods
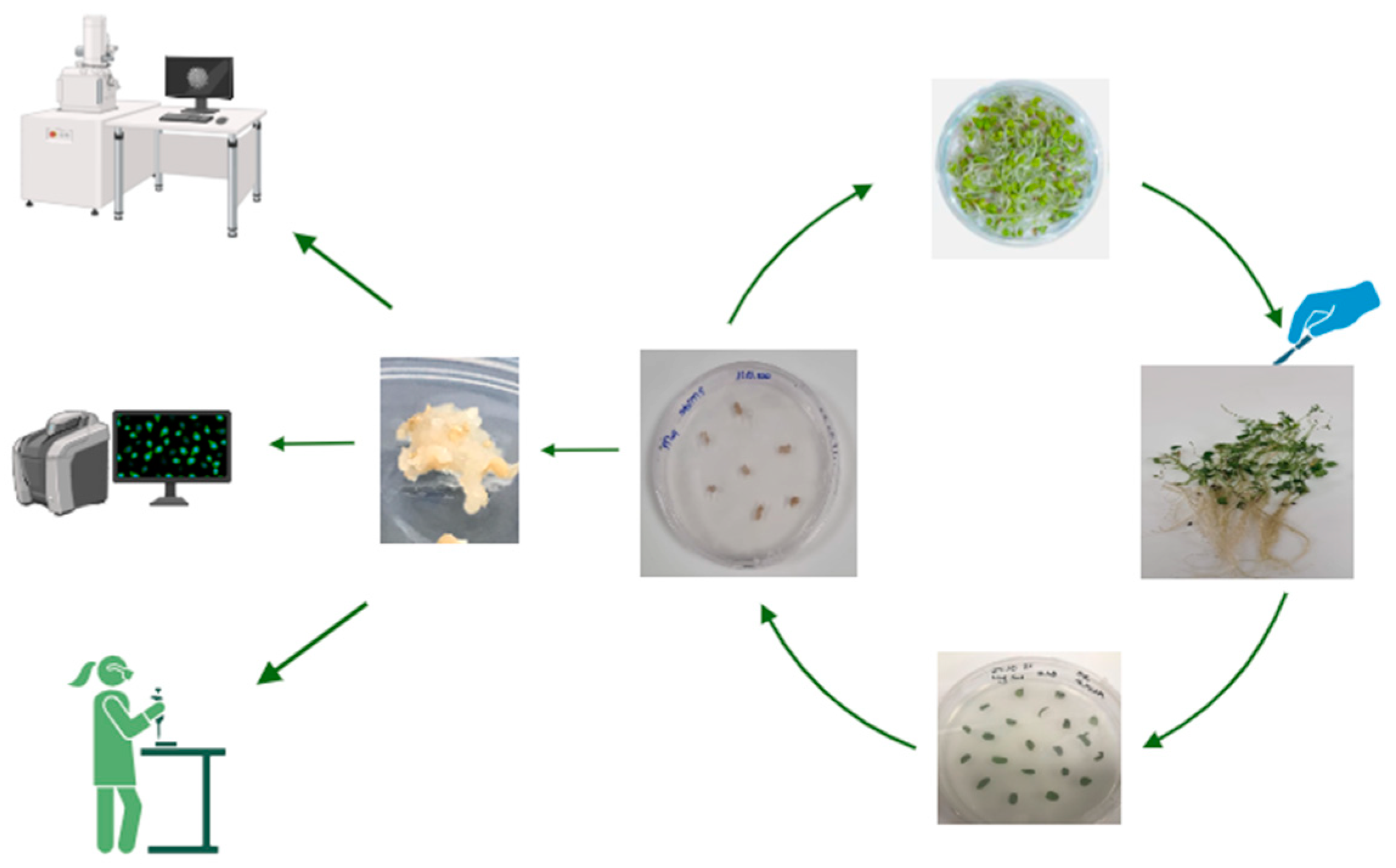
2.1. Plant Material and Callus Induction
2.2. Green Synthesis and Structural Characterization of the NPs
2.3. Characterization of CaO, CuO, and ZnO NPs
2.4. Salt Stress Treatment and NPs Applications
2.5. Determination of Lipid Peroxidation Level
2.6. Determination of Hydrogen Peroxide Content
2.7. Determination of Peroxidase Activity
2.8. Sectioning with Microtonal
2.9. Laser Scanning Confocal Microscope (CLSM)
2.10. Scanning Electron Microscopy
2.11. Statistical Analysis
3. Results
3.1. Result of Analysis of CaO, CuO, and ZnO NPs
3.2. X-Ray Diffraction Analysis
3.3. Lipid Peroxidation Level (As MDA)
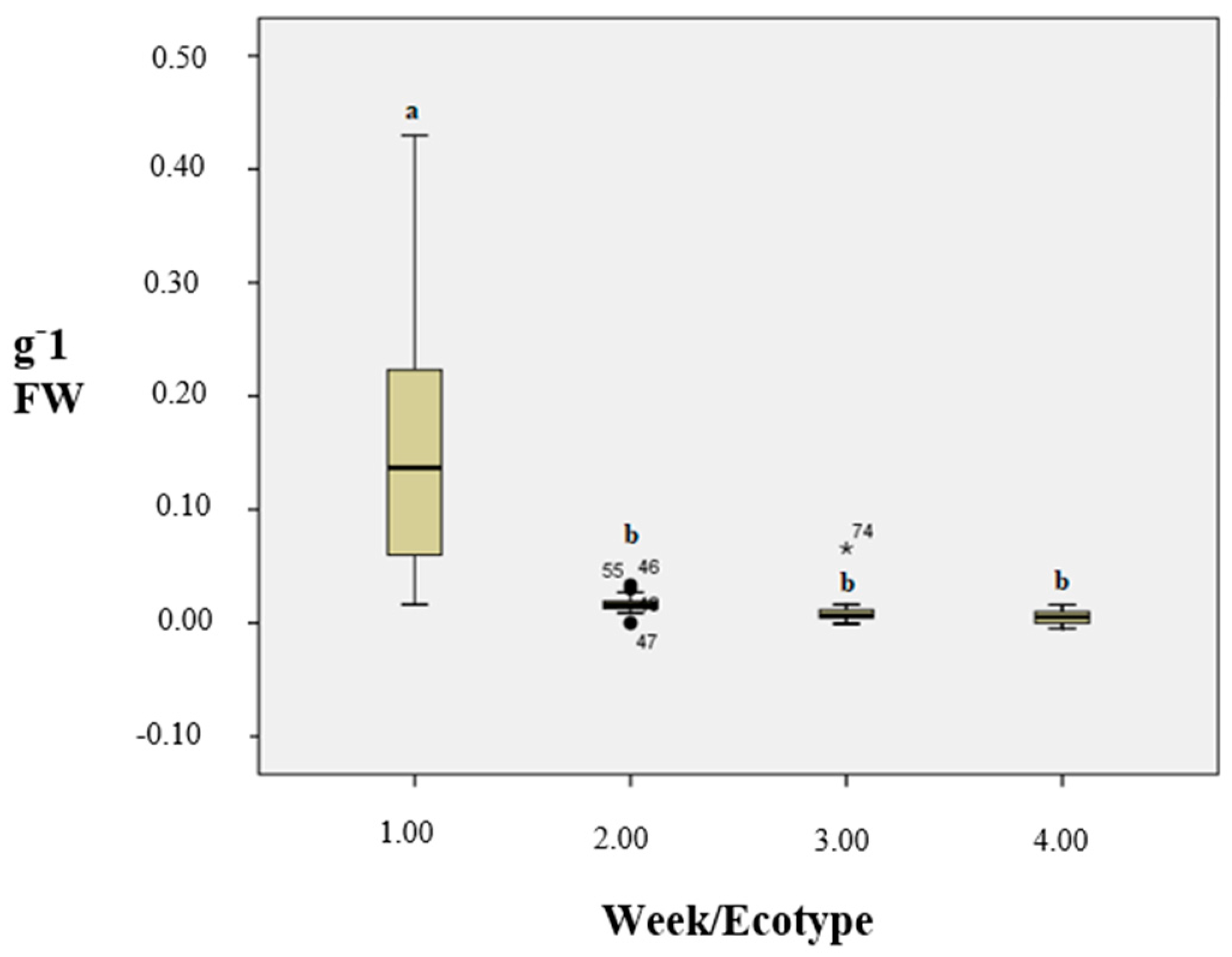
3.4. H2O2 Content
3.5. POX Activity
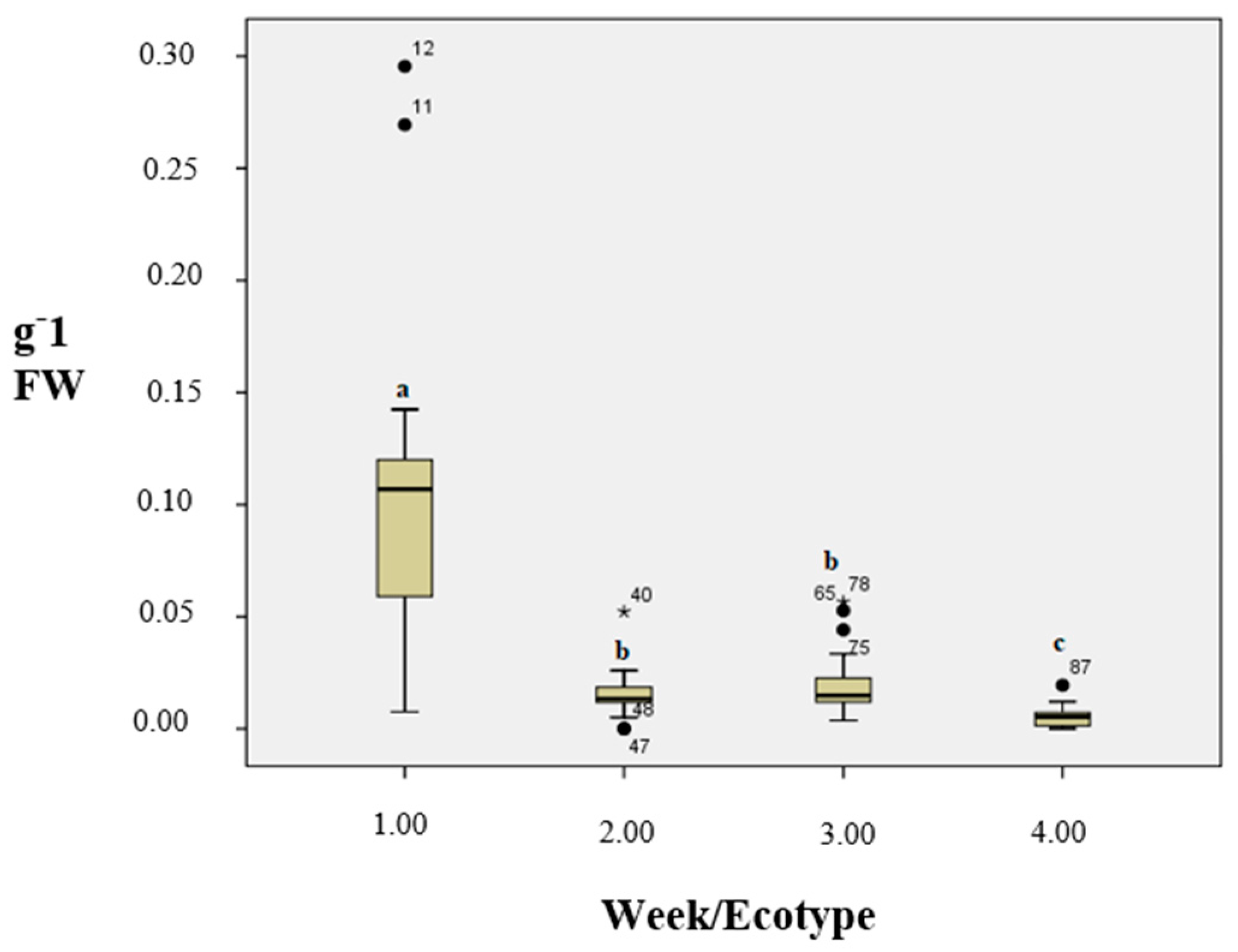
3.6. Soluble Protein Content
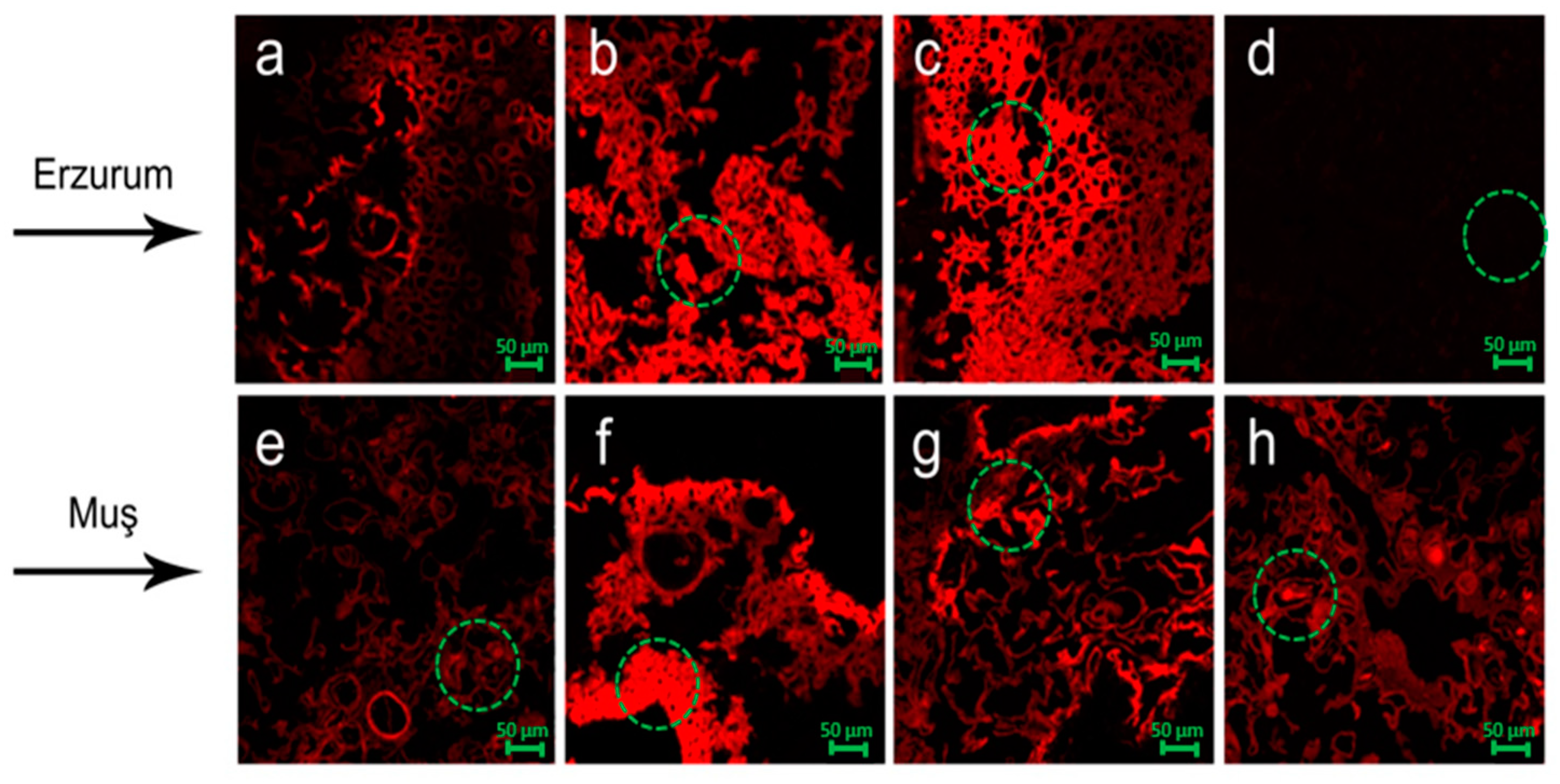
3.7. Laser Scanning Confocal Microscope (LSCM)
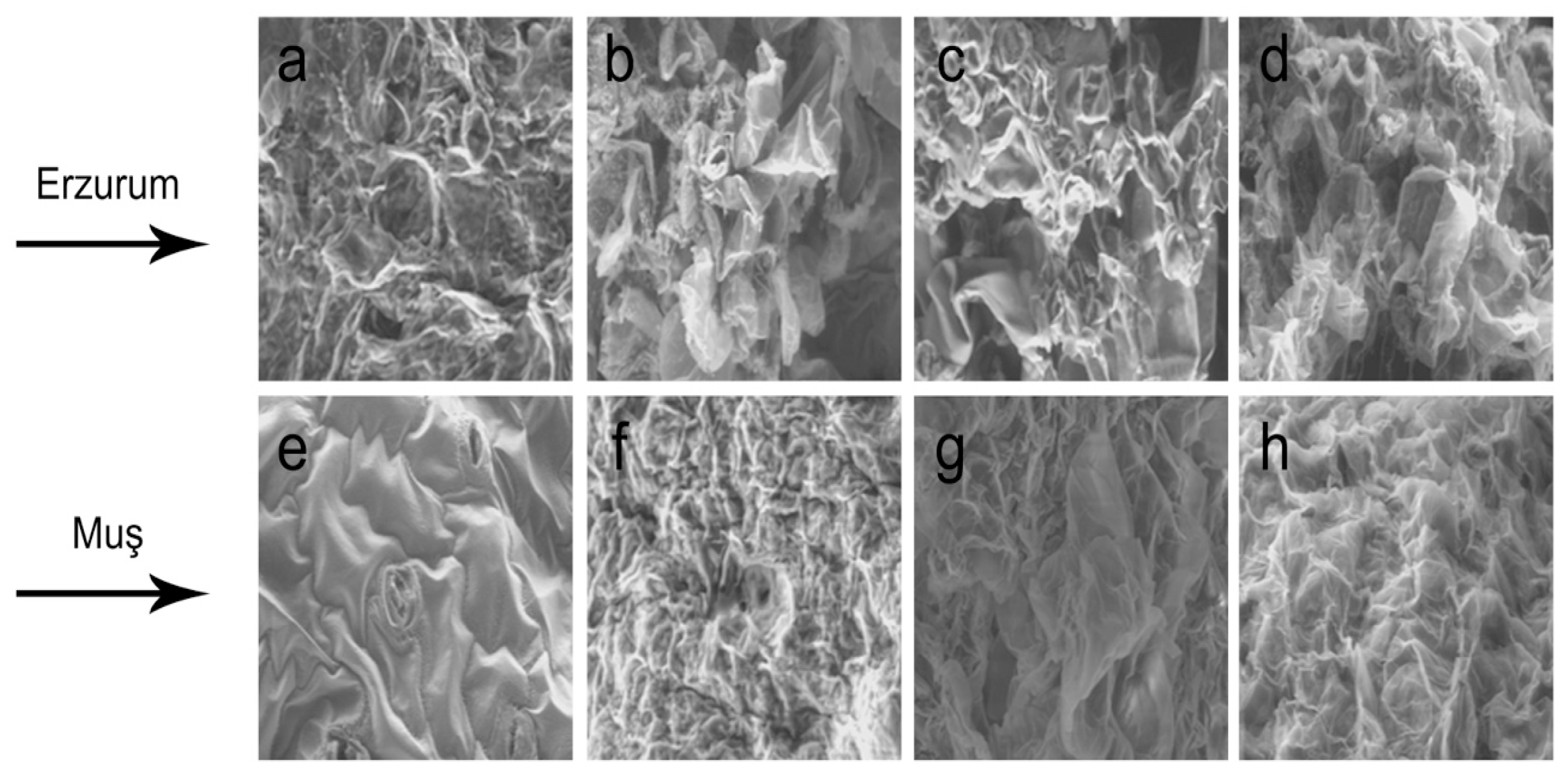
3.8. SEM Analysis of Callus Structures
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jalili, F.; Khavazi, K.; Pazira, E.; Nejati, A.; Rahmani, H.A.; Sadaghiani, H.R. Isolation and characterization of ACC deaminase-producing fluorescent Pseudomonads, to alleviate salinity stress on canola (Brassica napus L.) growth. J. Plant Physiol. 2009, 166, 667–674. [Google Scholar] [CrossRef]
- Zhu, T.; Deng, X.; Zhou, X.; Zhu, L.; Zou, L.; Li, P.; Zhang, D.; Lin, H. Ethylene and hydrogen peroxide are involved in brassinosteroid-induced salt tolerance in tomato. Sci. Rep. 2016, 6, 35392. [Google Scholar] [CrossRef]
- Jalili, B.; Bagheri, B.; Azadi, S.; Soltani, J. Identification and salt tolerance evaluation of endophyte fungi isolates from halophyte plants. Int. J. Environ. Sci. Technol. 2019, 17, 3459–3466. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Bezirganoglu, I. Response of five triticale genotypes to salt stress in in vitro culture. Turk. J. Agrıc. For. 2017, 41, 372–380. [Google Scholar] [CrossRef]
- Gao, S.; Yang, L.; Zeng, H.Q.; Zhou, Z.S.; Yang, Z.M.; Li, H.; Zhang, B. A cotton miRNA is involved in regulation of plant response to salt stress. Int. J. Sci. Rep. 2016, 6, 19736. [Google Scholar] [CrossRef]
- Dimpka, C.O.; McLean, J.E.; Latta, D.E.; Manangón, E.; Britt, D.W.; Johnson, W.P.; Maxim, I.B.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 2012, 14, 1125. [Google Scholar] [CrossRef]
- Castillo-González, J.; Ojeda-Barrios, D.; Hernández-Rodríguez, A.; González-Franco, A.C.; Robles-Hernández, L.; López-Ochoa, G.R. Zinc metalloenzymes in plants. Interciencia 2018, 43, 242–248. [Google Scholar]
- Lian, M.; Wang, J.; Sun, L.; Xu, Z.; Tang, J.; Yan, J.; Zeng, X. Profiles and potential health risks of heavy metals in soil and crops from the watershed of Xi River in Northeast China. Ecotoxicol. Environ. Saf. 2019, 169, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Thor, K. Calcium-nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef]
- Abeed, A.H.; Al-Huqail, A.A.; Albalawi, S.; Alghamdi, S.A.; Ali, B.; Alghanem, S.M.; El-Mahdy, M.T. Calcium nanoparticles mitigate severe salt stress in Solanum lycopersicon by instigating the antioxidant defense system and renovating the protein profile. S. Afr. J. Bot. 2023, 161, 36–52. [Google Scholar] [CrossRef]
- Mahawar, L.; Živčák, M.; Barboricova, M.; Kovár, M.; Filaček, A.; Ferencova, J.; Brestič, M. Effect of copper oxide and zinc oxide nanoparticles on photosynthesis and physiology of Raphanus sativus L. under salinity stress. Plant Physiol. Biochem. 2024, 206, 108281. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, P.S.; Abhilash, O.U.; Khan, B.M.; Prasad, B.L. Nanogold-Loaded Sharp-Edged Carbon Bullets as Plant-Gene Carriers. Adv. Funct. 2010, 20, 2416–2423. [Google Scholar] [CrossRef]
- Kim, D.H.; Gopal, J.; Sivanesan, I. Nanomaterials in plant tissue culture: The disclosed and undisclosed. RSC Adv. 2017, 7, 36492–36505. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Singh, A.K.; Singh, S.P.; Ansari, M.I. Nanoparticles and plant interaction with respect to stress response. Nanomater. Environ. Biotechnol. 2020, 1–15. [Google Scholar] [CrossRef]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef] [PubMed]
- Ruttkay-Nedecky, B.; Krystofova, O.; Nejdl, L.; Adam, V. Nanoparticles based on essential metals and their phytotoxicity. J. Nanobiotechnol. 2017, 15, 33. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Zhao, F.J.; Kopittke, P.M. Nanotechnology: A new opportunity in plant sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Alabdallah, N.M.; Alzahrani, H.S. The potential mitigation effect of ZnO nanoparticles on [Abelmoschus esculentus L. Moench] metabolism under salt stress conditions. Saudi J. Biol. Sci. 2020, 27, 3132–3137. [Google Scholar] [CrossRef]
- Vijai Anand, K.; Reshma, M.; Kannan, M.; Muthamil Selvan, S.; Chaturvedi, S.; Shalan, A.E.; Govindaraju, K. Preparation and characterization of calcium oxide nanoparticles from marine molluscan shell waste as nutrient source for plant growth. J. Nanostruct. Chem. 2021, 11, 409–422. [Google Scholar] [CrossRef]
- Yazıcılar, B.; Böke, F.; Alaylı, A.; Nadaroglu, H.; Gedikli, S.; Bezirganoglu, I. In vitro effects of CaO nanoparticles on Triticale callus exposed to short and long-term salt stress. Plant Cell Rep. 2021, 40, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Sidhic, J.; Sarbadhikary, P.; Narayanankutty, A.; George, S.; George, B.P.; Abrahamse, H. Role of metal nanoparticles in organogenesis, secondary metabolite production and genetic transformation of plants under in vitro condition: A comprehensive review. Plant Cell Tissue Organ Cult. 2024, 158, 33. [Google Scholar] [CrossRef]
- Yazıcılar, B.; Bezirganoglu, I. Characterization of the SOS1, SERK1, and WEE1 Conferring a Defense Response to Salt Stress in alfalfa (Medicago sativa L.) Callus. J. Plant Growth Regul. 2023, 42, 7257–7265. [Google Scholar] [CrossRef]
- Latif, A.; Sun, Y.; Noman, A. Herbaceous Alfalfa plant as a multipurpose crop and predominant forage species in Pakistan. Front. Sustain. Food Syst. 2023, 7, 1126151. [Google Scholar] [CrossRef]
- Sakiroglu, M.; Brummer, E.C. Identification of loci controlling forage yield and nutritive value in diploid alfalfa using GBS-GWAS. Theor. Appl. Genet. 2017, 130, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Parajulee, D.; Dhir, S.; Sangra, A.; Dhir, S.K. Improved Protocol for Efficient Agrobacterium-mediated Transformation in Medicago sativa L. Plants 2024, 13, 2992. [Google Scholar] [CrossRef] [PubMed]
- Nadaroglu, H.; Alaylı Güngör, A.; Ince, S. Synthesis of nanoparticles by green synthesis method. Int. J. Innov. Res. Rev. 2017, 1, 6–9. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Sankar, B.; Sridharan, R.; Panneerselvam, R. Soil salinity alters growth, chlorophyll content, and secondary metabolite accumulation in Catharanthus roseus. Turk. J. Biol. 2007, 32, 79–83. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Yee, Y.; Tam, N.F.Y.; Wong, Y.S.; Lu, C.Y. Growth and physiological responses of two mangrove species (Bruguiera gymnorrhiza and Kandelia candel) to water logging. Environ. Exp. Bot. 2003, 49, 209–221. [Google Scholar] [CrossRef]
- Rolls, G.O.; Farmer, N.J.; Hall, J.B. Artefacts in Histological and Cytological Preparations; Leica Biosystems Pty Ltd.: Melbourne, Australia, 2012; p. 106. [Google Scholar]
- Minta, A.; Kao, J.P.; Tsien, R.Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. Biol. Chem. 1989, 15, 8171–8178. [Google Scholar] [CrossRef]
- Anantharaman, A.; George, M. Green Synthesis of Calcium Oxide Nanoparticles and Its Applications. Int. J. Eng. Res. Appl. 2016, 6, 27–31. [Google Scholar]
- Meshkatalsadat, M.H.; Zahedifar, M.; Pouramiri, B. Facile Green Synthesis of CaO NPs Using the Crataegus Pontica C.Koch Extract for Photo-Degradation of MB Dye. Environ. Sci. Pollut. Res. 2022, 29, 54688–54697. [Google Scholar] [CrossRef]
- Sahu, G.; Saha, S.; Datta, S.; Chavan, P.; Naik, S. Methanolysis of Jatropha curcas oil using K2CO3/CaO as a solid base catalyst. Turk. J. Chem. 2017, 41, 845–861. [Google Scholar] [CrossRef]
- Nadaroglu, H.; Alaylı Gungor, A. Highly sensitive glucose sensor based on ZnO NPs as a biomimetic enzyme. Biosci. Res. 2020, 17, 775–785. [Google Scholar]
- Gultekin, D.D.; Alaylı, A.; Nadaroglu, H. Eco-friendly synthesis of nano copper and its use in Fenton-like reactions for methylene blue degradation. Int. J. Chem. Tech. 2020, 4, 71–78. [Google Scholar] [CrossRef]
- Grodetskaya, T.A.; Evlakov, P.M.; Fedorova, O.A.; Mikhin, V.I.; Zakharova, O.V.; Kolesnikov, E.A.; Gusev, A.A. Influence of copper oxide nanoparticles on gene expression of birch clones in vitro under stress caused by phytopathogens. Nanomaterials 2022, 12, 864. [Google Scholar] [CrossRef]
- Gunasena, M.D.K.M.; Alahakoon, A.M.P.D.; Polwaththa, K.P.G.D.M.; Galpaya, G.D.C.P.; Priyanjani, H.A.S.A.; Koswattage, K.R.; Senarath, W.T.P.S.K. Transforming Plant Tissue Culture with Nanoparticles: A Review of Current Applications. Plant Nano Biol. 2024, 10, 100102. [Google Scholar] [CrossRef]
- Singh, Y.; Kumar, U.; Panigrahi, S.; Balyan, P.; Mehla, S.; Sihag, P.; Dhankher, O.P. Nanoparticles as novel elicitors in plant tissue culture applications: Current status and future outlook. Plant Physiol. Biochem. 2023, 203, 108004. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Li, X.; Xin, C.; Li, S.; Li, Y.; Zheng, X.; Li, H.; Wei, X.; Zhang, Z.; et al. Naomo-ZnO priming induces salt tolerance by promoting photosynthetic carbon assimilation in wheat. Arch. Agron. Soil. Sci. 2019, 66, 1259–1273. [Google Scholar] [CrossRef]
- Javed, R.; Usman, M.; Yücesan, B.; Zia, M.; Gürel, E. Effect of zinc oxide (ZnO) nanoparticles on physiology and steviol glycosides production in micro propagated shoots of Stevia rebaudiana Bertoni. Plant Physiol. Biochem. 2017, 110, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Shoukat, A.; Pitann, B.; Zafar, M.M.; Farooq, M.A.; Haroon, M.; Nawaz, A.; Saqib, Z.A. Nanotechnology for climate change mitigation: Enhancing plant resilience under stress environments. J. Plant Nutr. Soil. Sci. 2024, 187, 604–620. [Google Scholar] [CrossRef]
- Ditta, A.; Arshad, M. Applications and perspectives of using nanomaterials for sustainable plant nutrition. Nanotechnol. Rev. 2016, 5, 209–229. [Google Scholar] [CrossRef]
- Liu, R.H.; Kang, Y.H.; Pei, L.; Wan, S.Q.; Liu, S.P.; Liu, S.H. Use of a new controlled-loss-fertilizer to reduce nitrogen losses during winter wheat cultivation in the Danjiangkou Reservoir Area of China. Commun. Soil. Sci. Plant. Anal. 2016, 47, 1137–1147. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Rashmi, R.; Surya Ulhas, R.; Sudheer, W.N.; Banadka, A.; Nagella, P.; Almaghasla, M.I. The role of nanoparticles in response of plants to abiotic stress at physiological, biochemical, and molecular levels. Plants 2023, 12, 292. [Google Scholar] [CrossRef]
- Zari, H.; Babak, P.; Asad, R. The Effect of Priming with Nano-sliver on Agronomic Traits of Safflower Cultivars. J. Essent. Oil-Bear. Plants 2015, 18, 1148–1156. [Google Scholar] [CrossRef]
- Aazami, M.A.; Rasouli, F.; Ebrahimzadeh, A. Oxidative damage, antioxidant mechanism, and gene expression in tomato responding to salinity stress under in vitro conditions and application of iron and zinc oxide nanoparticles on callus induction and plant regeneration. BMC Plant Biol. 2021, 21, 597. [Google Scholar] [CrossRef]
- Soleymanzadeh, R.; Iranbakhsh, A.; Habıbı, G.; Ardebılı, Z.O. Selenıum nanopartıcle protected strawberry against salt stress through modifications in salicylic acid, ion homeostasis, antioxidant machinery, and photosynthesis performance. Acta Biol. Crac. Ser. Bot. 2020, 62, 33–42. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Jadczak, P.; Kulpa, D.; Drozd, R.; Przewodowski, W.; Przewodowska, A. Effect of AuNPs and AgNPs on the antioxidant system and antioxidant activity of lavender (Lavandula angustifolia Mill.) from in vitro cultures. Molecules 2020, 25, 5511. [Google Scholar] [CrossRef]
- Sousa, V.S.; Teixeira, M.R. Aggregation kinetics and surface charge of CuO nanoparticles: The influence of pH, ionic strength and humic acids. Environ. Chem. 2013, 10, 313–322. [Google Scholar] [CrossRef]
- Conway, J.R.; Adeleye, A.S.; Gardea-Torresdey, J.; Keller, A.A. Aggregation, Dissolution, and Transformation of Copper Nanoparticles in Natural Waters. Environ. Sci. Technol. 2015, 49, 2749–2756. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, T.N.; Savassa, S.M.; Montanha, G.S.; Ishida, J.K.; de Almeida, E.; Tsai, S.M.; Pereira de Carvalho, H.W. A new glance on root-to-shoot in vivo zinc transport and time-dependent physiological effects of ZnSO4 and ZnO nanoparticles on plants. Sci. Rep. 2019, 9, 10416. [Google Scholar] [CrossRef]




| Treatments | MDA (ng g−1 FW) | POX Activity (EU mg−1 Protein) | Soluble Protein (mg g−1 FW) | H2O2 (ng g−1 FW) |
|---|---|---|---|---|
| 0.8 ppm ZnO (+) | 0.0210 ± 0.005 e | 1.4727 ± 0.186 ab | 2.0439 ± 0.260 a | 0.0386 ± 0.014 bc |
| 0.8 ppm CuO (+) | 0.0411 ± 0.005 bc | 1.2792 ± 0.186 ab | 1.5331 ± 0.260 a | 0.0979 ± 0.014 a |
| 0.8 ppm CaO (+) | 0.0194 ± 0.005 e | 0.9267 ± 0.186 b | 1.7457 ± 0.260 a | 0.0319 ± 0.014 bc |
| 0.8 ppm ZnO 50 mM NaCI (+) | 0.0329 ± 0.005 bcde | 1.1658 ± 0.186 ab | 2.0548 ± 0.260 a | 0.0481 ± 0.014 bc |
| 0.8 ppm CuO 50 mM NaCI (+) | 0.0466 ± 0.005 b | 1.5335 ± 0.186 ab | 2.4061 ± 0.260 a | 0.0377 ± 0.014 bc |
| 0.8 ppm CaO 50 mM NaCI (+) | 0.0831 ± 0.005 a | 1.2862 ± 0.186 ab | 2.4005 ± 0.260 a | 0.0645 ± 0.014 ab |
| Control | 0.0410 ± 0.005 bc | 0.9985 ± 0.186 b | 1.1589 ± 0.260 a | 0.0545 ± 0.014 bc |
| 50 Mm NaCI | 0.0250 ± 0.005 cde | 1.6668 ± 0.186 a | 1.7398 ± 0.260 a | 0.0388 ± 0.014 bc |
| 0.8 ppm ZnO (−) | 0.0236 ± 0.005 de | 0.9699 ± 0.186 b | 1.9880 ± 0.260 a | 0.0293 ± 0.014 bc |
| 0.8 ppm CuO (−) | 0.0323 ± 0.005 bcde | 0.1035 ± 0.186 c | 0.6741 ± 0.260 b | 0.0134 ± 0.014 c |
| 0.8 ppm CaO (−) | 0.0445 ± 0.005 b | 1.0015 ± 0.186 b | 1.6147 ± 0.260 a | 0.0389 ± 0.014 bc |
| 0.8 ppm ZnO 50 mM NaCI (−) | 0.0312 ± 0.005 bcde | 0.9742 ± 0.186 b | 1.6574 ± 0.260 a | 0.0289 ± 0.014 bc |
| 0.8 ppm CuO 50 mM NaCI (−) | 0.0384 ± 0.005 bcd | 1.0663 ± 0.186 ab | 2.3167 ± 0.260 a | 0.0450 ± 0.014 bc |
| 0.8 ppm CaO 50 mM NaCI (−) | 0.0168 ± 0.005 d | 1.4176 ± 0.186 ab | 1.9674 ± 0.260 a | 0.0445 ± 0.014 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akçay, M.; Geyik, M.S.; Yazicilar, B.; Boke, F.; Nadaroglu, H.; Atıcı, O.; Bezirganoğlu, İ. Assessment of the Effects of Newly Fabricated CaO, CuO, ZnO Nanoparticles on Callus Formation Maintenance of Alfalfa (Medicago sativa L.) Under In Vitro Salt Stress. Agronomy 2025, 15, 180. https://doi.org/10.3390/agronomy15010180
Akçay M, Geyik MS, Yazicilar B, Boke F, Nadaroglu H, Atıcı O, Bezirganoğlu İ. Assessment of the Effects of Newly Fabricated CaO, CuO, ZnO Nanoparticles on Callus Formation Maintenance of Alfalfa (Medicago sativa L.) Under In Vitro Salt Stress. Agronomy. 2025; 15(1):180. https://doi.org/10.3390/agronomy15010180
Chicago/Turabian StyleAkçay, Mustafa, Merve Simsek Geyik, Busra Yazicilar, Fatma Boke, Hayrunnisa Nadaroglu, Okkes Atıcı, and İsmail Bezirganoğlu. 2025. "Assessment of the Effects of Newly Fabricated CaO, CuO, ZnO Nanoparticles on Callus Formation Maintenance of Alfalfa (Medicago sativa L.) Under In Vitro Salt Stress" Agronomy 15, no. 1: 180. https://doi.org/10.3390/agronomy15010180
APA StyleAkçay, M., Geyik, M. S., Yazicilar, B., Boke, F., Nadaroglu, H., Atıcı, O., & Bezirganoğlu, İ. (2025). Assessment of the Effects of Newly Fabricated CaO, CuO, ZnO Nanoparticles on Callus Formation Maintenance of Alfalfa (Medicago sativa L.) Under In Vitro Salt Stress. Agronomy, 15(1), 180. https://doi.org/10.3390/agronomy15010180






