Spray-Induced Gene Silencing (SIGS): Nanocarrier-Mediated dsRNA Delivery Improves RNAi Efficiency in the Management of Lettuce Gray Mold Caused by Botrytis cinerea
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Culture Conditions
2.2. Preparation and Characterization of Small MgAl-Layered Double Hydroxide (sLDH) Clay Nanosheets
2.3. Preparation of sLDH Clay Nanosheet–dsRNA Complexes and RNase A Protection Assay
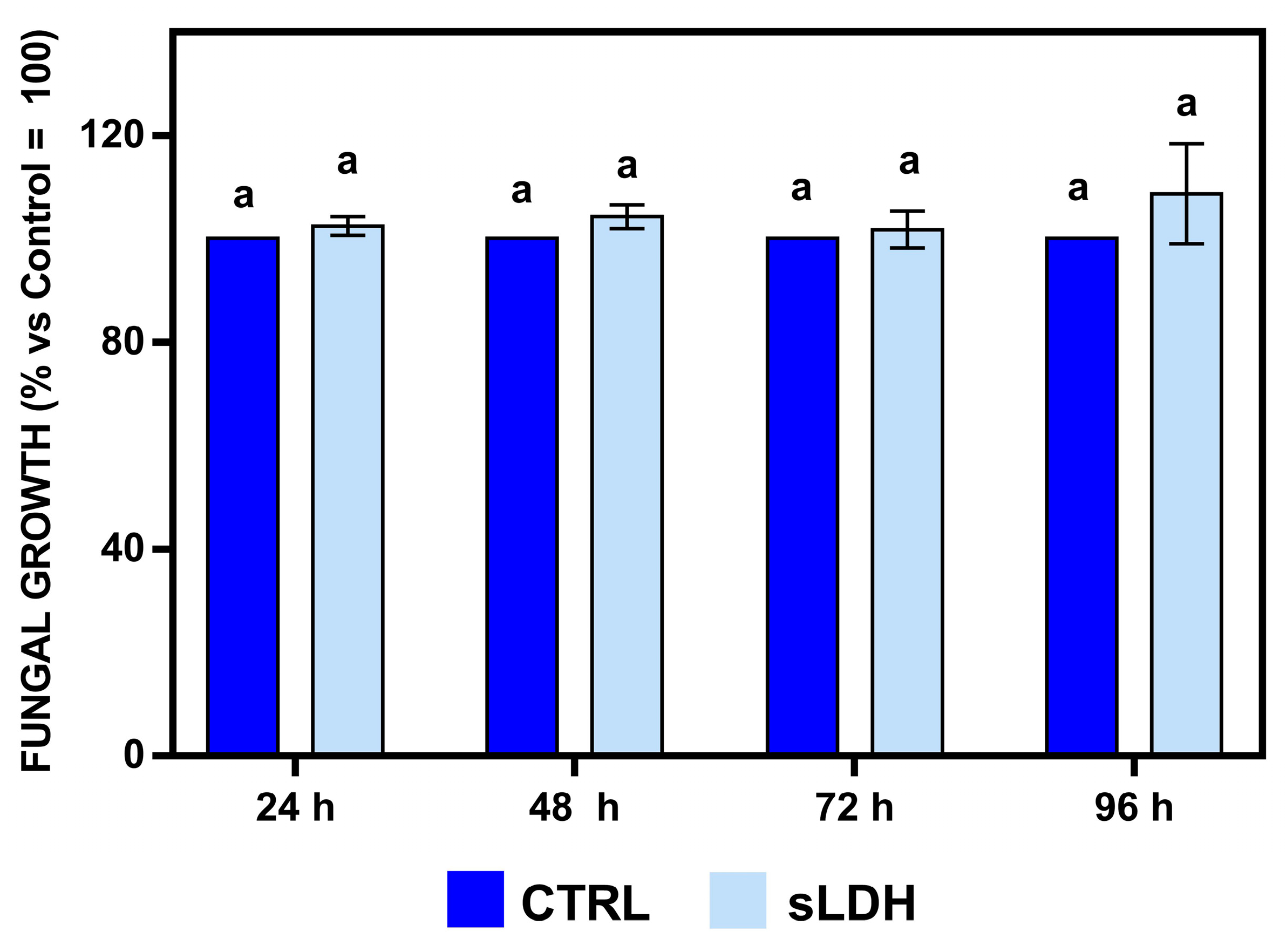
2.4. In Vitro Effects of sLDH Clay Nanosheets on the Growth of Botrytis cinerea
2.5. Spray-Induced Gene Silencing (SIGS): Application of Naked dsRNAs or sLDH Clay Nanosheet–dsRNA Complexes and Botrytis cinerea Conidia by Pressure Spraying
- Plants were treated with single dsRNAs (BcBmp1-, BcBmp3-, BcPls1-, and GFP-dsRNAs; 10 µg per plant) or sterile MilliQ water + TE buffer (CTRL);
- Plants were treated with all three dsRNAs mixed together (BcBmp1-dsRNA + BcBmp3-dsRNA + BcPls1-dsRNA; 10 µg each for a total of 30 µg per plant), or GFP-dsRNA (30 µg per plant), or sterile MilliQ water + TE buffer (CTRL);
- Plants were treated with single dsRNAs (BcBmp1-, BcBmp3-, BcPls1-, and GFP-dsRNAs; 10 µg per plant) complexed to sLDH clay nanosheets, or sterile MilliQ water + TE buffer (CTRL), or sLDH clay nanosheets.
- Plants were treated with the fungicide Switch® 62.5 WG (Syngenta, 37.5% cyprodinil and 25% fludioxonil, WG = water-dispersible granules) at the recommended dose (0.7 g/L) for lettuce.
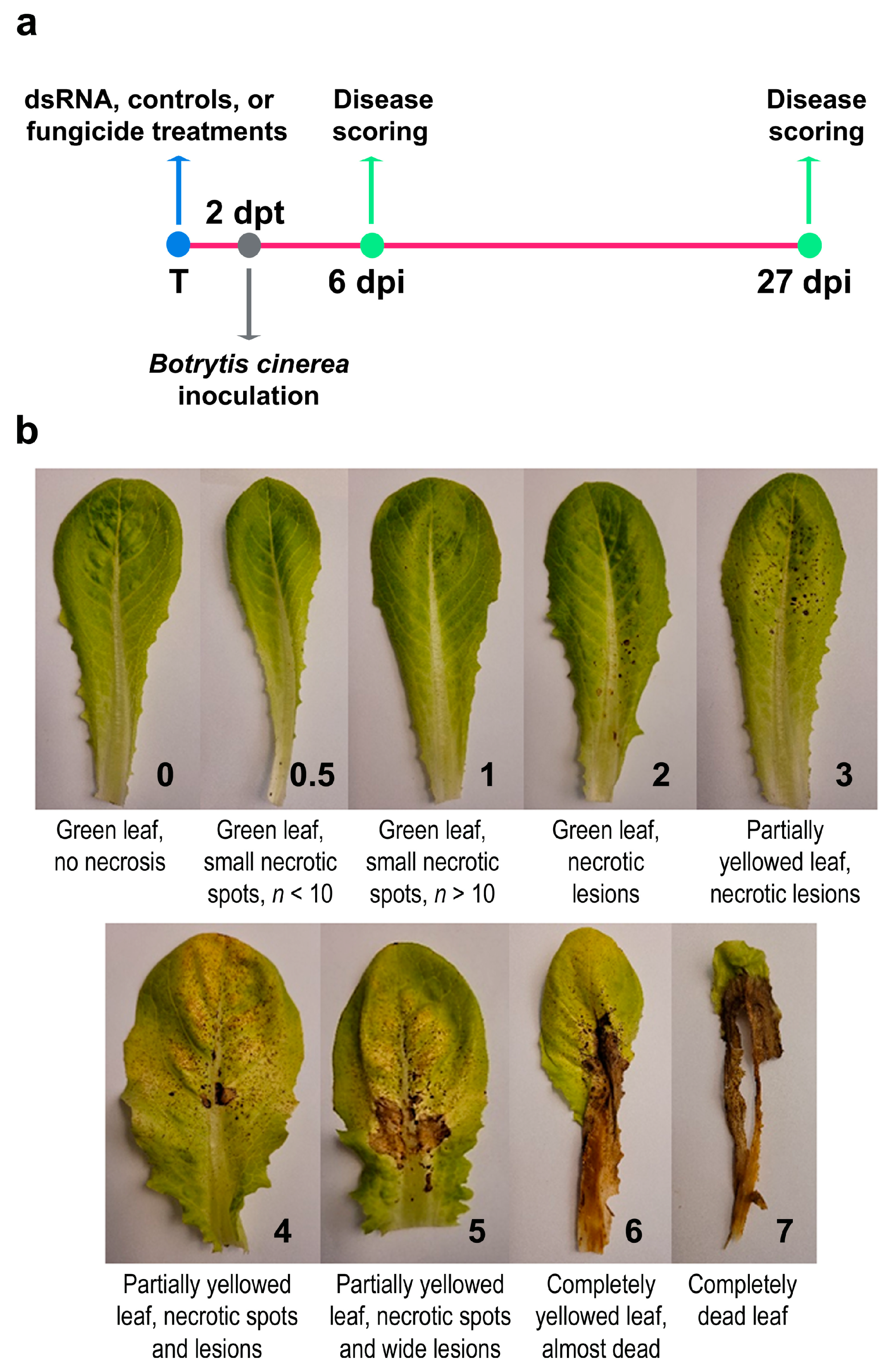
2.6. Disease Assessment
2.7. Statistical Analysis
3. Results
3.1. Spray-Induced Gene Silencing (SIGS): Application of Naked dsRNAs by Pressure Spraying Reduces Botrytis cinerea Symptoms on Lettuce Plants
3.2. Characterization of Small Layered Double Hydroxide (sLDH) Clay Nanosheets
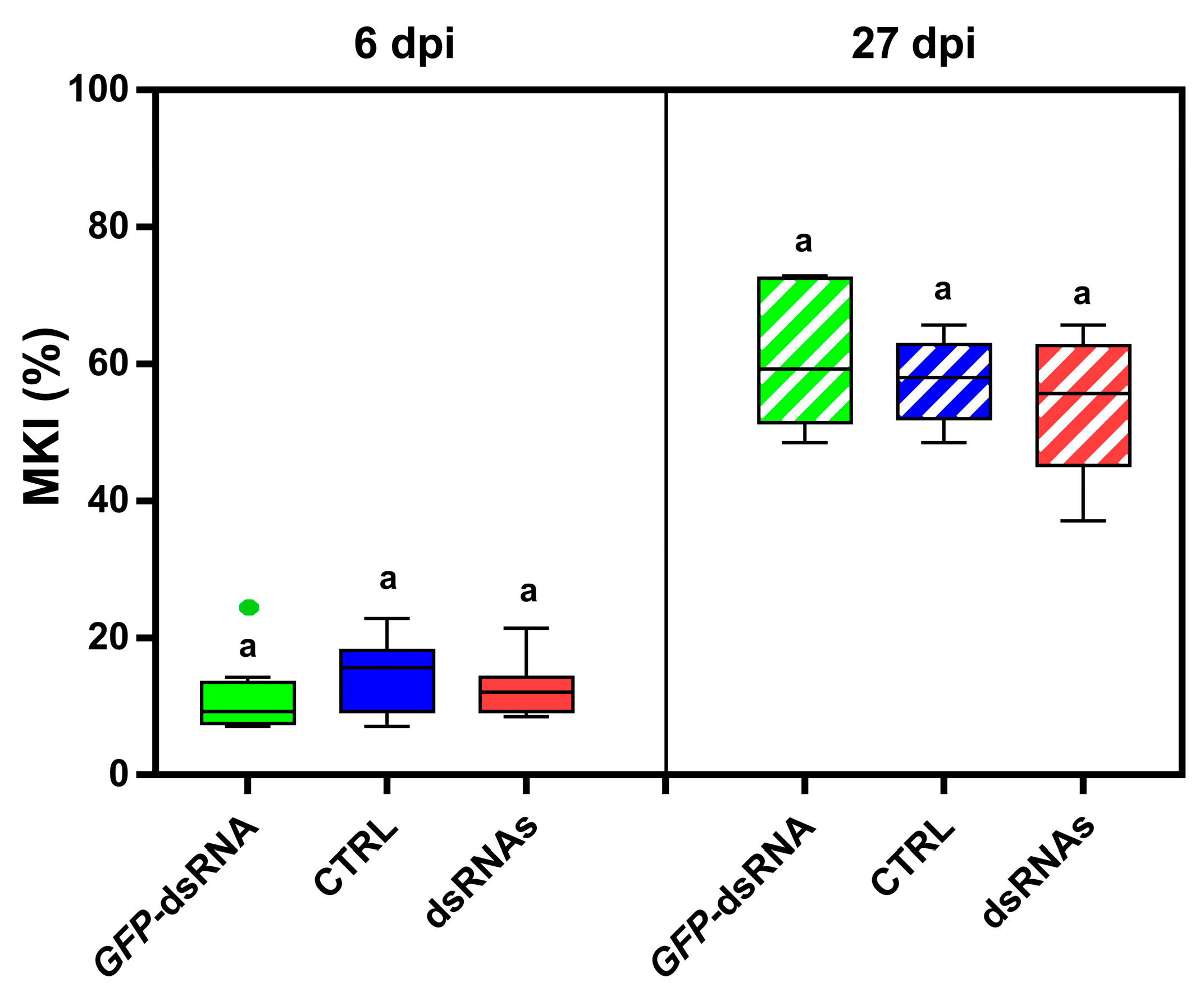
3.3. Spray-Induced Gene Silencing (SIGS): Application of sLDH Clay Nanosheet–dsRNA Complexes by Pressure Spraying Prolongs Protection Against Botrytis cinerea on Lettuce Plants
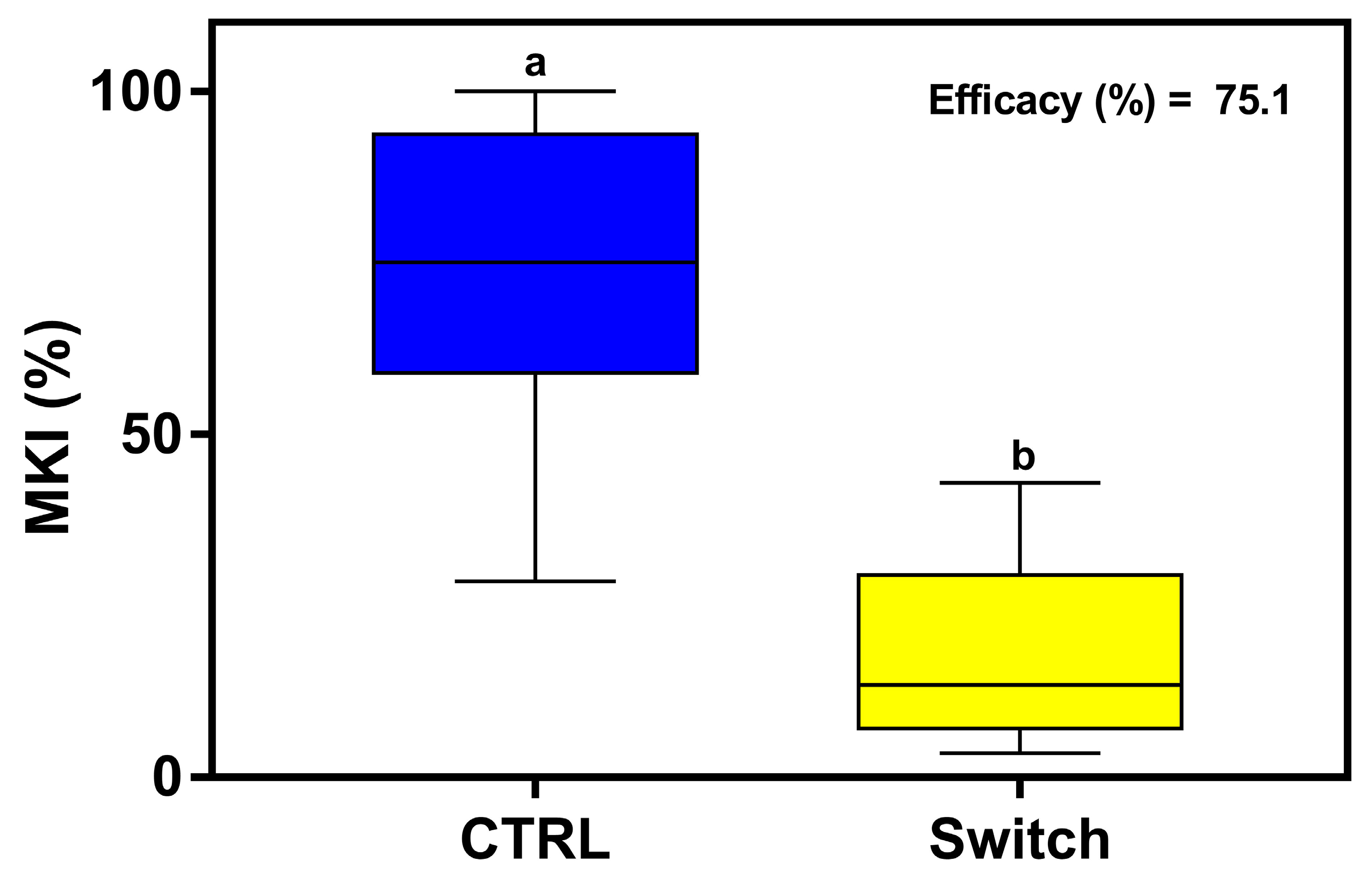
3.4. Effectiveness of Fungicide Treatment by Pressure Spraying Against Botrytis cinerea on Lettuce Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Romanazzi, G.; Feliziani, E. Botrytis cinerea. In Postharvest Decay: Control Strategies; Bautista-Baños, S., Ed.; Elsevier: London, UK, 2014; pp. 131–146. [Google Scholar]
- Fillinger, S.; Walker, A.S. Chemical control and resistance management of Botrytis diseases. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 189–216. [Google Scholar]
- Sofianos, G.; Samaras, A.; Karaoglanidis, G. Multiple and multidrug resistance in Botrytis cinerea: Molecular mechanisms of MLR/MDR strains in Greece and effects of co-existence of different resistance mechanisms on fungicide sensitivity. Front. Plant Sci. 2023, 14, 1273193. [Google Scholar] [CrossRef]
- Medina-Lozano, I.; Bertolín, J.R.; Díaz, A. Nutritional value of commercial and traditional lettuce (Lactuca sativa L.) and wild relatives: Vitamin C and anthocyanin content. Food Chem. 2021, 359, 129864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, W.; Tao, R.; Zhang, W.; Chen, J.; Wu, P.; Yan, C.; Jia, Y.; Larkin, R.M.; Lavelle, D.; et al. RNA sequencing provides insights into the evolution of lettuce and the regulation of flavonoid biosynthesis. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Raid, R.N. Lettuce diseases and their management. In Diseases of Fruits and Vegetables; Naqvi, S.A.M.H., Ed.; Kluwer Academic: Dordrecht, The Netherlands, 2004; Volume 2, pp. 121–148. [Google Scholar] [CrossRef]
- Horská, T.; Kocourek, F.; Stará, J.; Holý, K.; Mráz, P.; Krátký, F.; Kocourek, V.; Hajšlová, J. Evaluation of pesticide residue dynamics in lettuce, onion, leek, carrot and parsley. Foods 2020, 9, 680. [Google Scholar] [CrossRef]
- Shim, C.K.; Kim, M.J.; Kim, Y.K.; Jee, H.J. Evaluation of lettuce germplasm resistance to gray mold disease for organic cultivations. Plant Pathol. J. 2014, 30, 90–95. [Google Scholar] [CrossRef]
- Chakraborty, S.; Newton, A.C. Climate change, plant diseases and food security: An overview. Plant Pathol. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Koch, A.; Biedenkopf, D.; Furch, A.; Weber, L.; Rossbach, O.; Abdellatef, E.; Linicus, L.; Johannsmeler, J.; Jelonek, L.; Goesmann, A.; et al. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog. 2016, 12, e1005901. [Google Scholar] [CrossRef]
- Wang, M.; Weiberg, A.; Lin, F.-M.; Thomma, B.P.H.J.; Huang, H.-D.; Jin, H. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants 2016, 2, 1651. [Google Scholar] [CrossRef] [PubMed]
- Mezzetti, B.; Smagghe, G.; Arpaia, S.; Christiaens, O.; Dietz-Pfeilstetter, A.; Jones, H.; Kostov, K.; Sabbadini, S.; Opsahl-Sorteberg, H.G.; Ventura, V.; et al. RNAi: What is its position in agriculture? J. Pest Sci. 2020, 93, 1125–1130. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Petrick, J.S. Safety considerations for humans and other vertebrates regarding agricultural uses of externally applied RNA molecules. Front. Plant Sci. 2020, 11, 407. [Google Scholar] [CrossRef]
- Spada, M.; Pugliesi, C.; Fambrini, M.; Pecchia, S. Challenges and opportunities arising from host-Botrytis cinerea interactions to outline novel and sustainable control strategies: The key role of RNA interference. Int. J. Mol. Sci. 2024, 25, 6798. [Google Scholar] [CrossRef]
- Islam, M.T.; Sherif, S.M. RNAi-based biofungicides as a promising next-generation strategy for controlling devastating gray mold diseases. Int. J. Mol. Sci. 2020, 21, 2072. [Google Scholar] [CrossRef]
- Fletcher, S.J.; Reeves, P.T.; Hoang, B.T.; Mitter, N. A perspective on RNAi-based biopesticides. Front. Plant Sci. 2020, 11, 51. [Google Scholar] [CrossRef]
- Dalakouras, A.; Jarausch, W.; Buchholz, G.; Bassler, A.; Braun, M.; Manthey, T.; Krczal, G.; Wassenegger, M. Delivery of hairpin RNAs and small RNAs into woody and herbaceous plants by trunk injection and petiole absorption. Front. Plant Sci. 2018, 9, 1253. [Google Scholar] [CrossRef]
- Nerva, L.; Sandrini, M.; Gambino, G.; Chitarra, W. Double-stranded RNAs (dsRNAs) as a sustainable tool against gray mold (Botrytis cinerea) in grapevine: Effectiveness of different application methods in an open-air environment. Biomolecules 2020, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Duanis-Assaf, D.; Galsurker, O.; Davydov, O.; Maurer, D.; Feygenberg, O.; Sagi, M.; Poverenov, E.; Fluhr, R.; Alkan, N. Double-stranded RNA targeting fungal ergosterol biosynthesis pathway controls Botrytis cinerea and postharvest grey mould. Plant Biotechnol. J. 2022, 20, 226–237. [Google Scholar] [CrossRef]
- Hernández-Soto, A.; Chacón-Cerdas, R. RNAi crop protection advances. Int. J. Mol. Sci. 2021, 22, 12148. [Google Scholar] [CrossRef]
- Álvarez-Sánchez, A.R.; Romo-Quinones, C.; Rosas-Quijano, R.; Reyes, A.G.; Barraza, A.; Magallón-Barajas, F.; Angulo, C.; Mejía-Ruíz, C.H. Production of specific dsRNA against white spot syndrome virus in the yeast Yarrowia lipolytica. Aquacult. Res. 2018, 49, 480–491. [Google Scholar] [CrossRef]
- Taning, C.N.T.; Arpaia, S.; Christiaens, O.; Dietz-Pfeilstetter, A.; Jones, H.; Mezzetti, B.; Sabbadini, S.; Sorteberg, H.-G.; Sweet, J.; Ventura, V.; et al. RNA-based biocontrol compounds: Current status and perspectives to reach the market. Pest Manag. Sci. 2020, 76, 841–845. [Google Scholar] [CrossRef]
- Delgado-Martín, J.; Velasco, L. An efficient dsRNA constitutive expression system in Escherichia coli. Appl. Microbiol. Biotechnol. 2021, 105, 6381–6393. [Google Scholar] [CrossRef]
- Verdonckt, T.-W.; Vanden Broeck, J. Methods for the cost-effective production of bacteria-derived double-stranded RNA for in vitro knockdown studies. Front. Physiol. 2022, 13, 836106. [Google Scholar] [CrossRef] [PubMed]
- Scarpin, D.; Nerva, L.; Chitarra, W.; Moffa, L.; D’Este, F.; Vuerich, M.; Filippi, A.; Braidot, E.; Petrussa, E. Characterisation and functionalisation of chitosan nanoparticles as carriers for double-stranded RNA (dsRNA) molecules towards sustainable crop protection. Biosci. Rep. 2023, 43, BSR20230817. [Google Scholar] [CrossRef]
- Niño-Sánchez, J.; Chen, L.H.; de Souza, J.T.; Mosquera, S.; Stergiopoulos, I. Targeted delivery of gene silencing in fungi using genetically engineered bacteria. J. Fungi 2021, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef] [PubMed]
- Demirer, G.S.; Zhang, H.; Goh, N.S.; Pinals, R.L.; Chang, R.; Landry, M.P. Carbon nanocarriers deliver siRNA to intact plant cells for efficient gene knockdown. Sci. Adv. 2020, 6, eaaz0495. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Hendrix, B.; Hoffer, P.; Sanders, R.A.; Zheng, W. Carbon dots for efficient small interfering RNA delivery and gene silencing in plants. Plant Physiol. 2020, 184, 647–657. [Google Scholar] [CrossRef]
- Chen, A.; Halilovi, L.; Shay, J.-H.; Koch, A.; Mitter, N.; Jin, H. Improving RNA-based crop protection through nanotechnology and insights from cross-kingdom RNA trafficking. Curr. Opin. Plant Biol. 2023, 76, 102441. [Google Scholar] [CrossRef]
- Mann, C.W.G.; Sawyer, A.; Gardiner, D.M.; Mitter, N.; Carroll, B.J.; Eamens, A.L. RNA-based control of fungal pathogens in plants. Int. J. Mol. Sci. 2023, 24, 12391. [Google Scholar] [CrossRef]
- Christiaens, O.; Tardajos, M.G.; Martinez Reyna, Z.L.; Dash, M.; Dubruel, P.; Smagghe, G. Increased RNAi efficacy in Spodoptera exigua via the formulation of dsRNA with guanylated polymers. Front. Physiol. 2018, 9, 316. [Google Scholar] [CrossRef]
- Worrall, E.A.; Bravo-Cazar, A.; Nilon, A.T.; Fletcher, S.J.; Robinson, K.E.; Carr, J.P.; Mitter, N. Exogenous application of RNAi-inducing double-stranded RNA inhibits aphid-mediated transmission of a plant virus. Front. Plant Sci. 2019, 10, 265. [Google Scholar] [CrossRef]
- Niño-Sánchez, J.; Sambasivam, P.T.; Sawyer, A.; Hamby, R.; Chen, A.; Czislowski, E.; Li, P.; Manzie, N.; Gardiner, D.M.; Ford, R.; et al. BioClay™ prolongs RNA interference-mediated crop protection against Botrytis cinerea. J. Integr. Plant Biol. 2022, 64, 2187–2198. [Google Scholar] [CrossRef]
- Duanis-Assaf, D.; Shlar, I.; Galsurker, O.; Davydov, O.; Maurer, D.; Feygenberg, O.; Poverenov, E.; Fluhr, R.; Alkan, N. Nano-clay, layered-double hydroxide (LDH), improves the efficacy of double-stranded RNA in controlling postharvest decay. Postharvest Biol. Technol. 2022, 193, 112051. [Google Scholar] [CrossRef]
- Spada, M.; Pugliesi, C.; Fambrini, M.; Pecchia, S. Silencing of the Slt2-type MAP kinase Bmp3 in Botrytis cinerea by application of exogenous dsRNA affects fungal growth and virulence on Lactuca sativa. Int. J. Mol. Sci. 2021, 22, 5362. [Google Scholar] [CrossRef]
- Spada, M.; Pugliesi, C.; Fambrini, M.; Palpacelli, D.; Pecchia, S. Knockdown of Bmp1 and Pls1 virulence genes by exogenous application of RNAi-inducing dsRNA in Botrytis cinerea. Int. J. Mol. Sci. 2023, 24, 4869. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.H.; Pethybridge, S.J.; Barbedo, J.G.A.; Esker, P.D.; Mahlein, A.K.; Del Ponte, E.M. A phytopathometry glossary for the twenty-first century: Towards consistency and precision in intra- and inter-disciplinary dialogues. Trop. Plant Pathol. 2022, 47, 14–24. [Google Scholar] [CrossRef]
- Büttner, P.; Koch, F.; Voigt, K.; Quidde, T.; Risch, S.; Blaich, R.; Brückner, B.; Tudzynski, P. Variations in ploidy among isolates of Botrytis cinerea: Implications for genetic and molecular analyses. Curr. Genet. 1994, 25, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Quidde, T.; Osbourn, A.E.; Tudzynski, P. Detoxification of alpha-tomatine by Botrytis cinerea. Physiol. Mol. Plant Pathol. 1998, 52, 151–165. [Google Scholar] [CrossRef]
- Dong, H.; Chen, M.; Rahman, S.; Parekh, H.S.; Cooper, H.M.; Xu, Z.P. Engineering small MgAl-layered double hydroxide nanoparticles for enhanced gene delivery. Appl. Clay Sci. 2014, 100, 66–75. [Google Scholar] [CrossRef]
- McKinney, H.H. Influence of soil temperature and moisture on infection of wheat seedlings by Helminthosporium sativum. J. Agric. Res. 1923, 26, 195–218. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econom. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Marín, A.; Oliva, J.; Garcia, C.; Navarro, S.; Barba, A. Dissipation rates of cyprodinil and fludioxonil in lettuce and table grape in the field and under cold storage conditions. J. Agric. Food Chem. 2003, 51, 4708–4711. [Google Scholar] [CrossRef]
- Song, X.-S.; Gu, K.-X.; Duan, X.-X.; Xiao, X.-M.; Hou, Y.-P.; Duan, Y.-B.; Wang, J.-X.; Zhou, M.-G. A myosin5 dsRNA that reduces the fungicide resistance and pathogenicity of Fusarium asiaticum. Pestic. Biochem. Physiol. 2018, 150, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Roy-Barman, S. Spray-induced silencing of pathogenicity gene MoDES1 via exogenous double-stranded RNA can confer partial resistance against fungal blast in rice. Front. Plant Sci. 2021, 12, 733129. [Google Scholar] [CrossRef]
- McLoughlin, A.G.; Wytinck, N.; Walker, P.L.; Girard, I.J.; Rashid, K.Y.; de Kievit, T.; Fernando, W.G.D.; Whyard, S.; Belmonte, M.F. Identification and application of exogenous dsRNA confers plant protection against Sclerotinia sclerotiorum and Botrytis cinerea. Sci. Rep. 2018, 8, 7320. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Streetz, K.L.; Jopling, C.L.; Storm, T.A.; Pandey, K.; Davis, C.R.; Marion, P.; Salazar, F.; Kay, M.A. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 2006, 441, 537–541. [Google Scholar] [CrossRef]
- Bensoussan, N.; Dixit, S.; Tabara, M.; Letwin, D.; Milojevic, M.; Antonacci, M.; Pengyu, J.P.; Arai, Y.; Bruinsma, K.; Suzuki, T.; et al. Environmental RNA interference in two-spotted spider mite, Tetranychus urticae, reveals dsRNA processing requirements for efficient RNAi response. Sci. Rep. 2020, 10, 19126. [Google Scholar] [CrossRef] [PubMed]
- Parrish, S.; Fleenor, J.; Xu, S.; Mello, C.; Fire, A. Functional anatomy of a dsRNA trigger: Differential requirement for the two trigger strands in RNA interference. Mol. Cell 2000, 6, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Barik, S. RNAi in moderation. Nat. Biotechnol. 2006, 24, 796–797. [Google Scholar] [CrossRef]
- Sundaresha, S.; Sharma, S.; Bairwa, A.; Tomar, M.; Kumar, R.; Bhardwaj, V.; Jeevalatha, A.; Bakade, R.; Salaria, N.; Thakur, K.; et al. Spraying of dsRNA molecules derived from Phytophthora infestans, along with nanoclay carriers as a proof of concept for developing novel protection strategy for potato late blight. Pest Manag. Sci. 2022, 78, 3183–3192. [Google Scholar] [CrossRef]
- Qiao, L.; Niño-Sánchez, J.; Hamby, R.; Capriotti, L.; Chen, A.; Mezzetti, B.; Jin, H. Artificial nanovesicles for dsRNA delivery in spray-induced gene silencing for crop protection. Plant Biotech. J. 2023, 21, 854–865. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, Q.; Lan, C.; Tang, T.; Wang, K.; Shen, J.; Niu, D. Nanoparticle carriers enhance RNA stability and uptake efficiency and prolong the protection against Rhizoctonia solani. Phytopathol. Res. 2023, 5, 2. [Google Scholar] [CrossRef]
- Chen, M.; Cooper, H.M.; Zhou, J.Z.; Bartlett, P.F.; Xu, Z.P. Reduction in the size of layered double hydroxide nanoparticles enhances the efficiency of siRNA delivery. J. Colloid Interface Sci. 2013, 390, 275–281. [Google Scholar] [CrossRef]
- Zhi, H.; Chen, H.; Yu, M.; Wang, C.; Cui, B.; Zhao, X.; Wang, Y.; Cui, H.; Zhang, B.; Zeng, Z. Layered double hydroxide nanosheets improve the adhesion of fungicides to leaves and the antifungal performance. ACS Appl. Nano Mater. 2022, 5, 5316–5325. [Google Scholar] [CrossRef]
- Vuković, S.; Brzaković, N.; Lazić, S.; Šunjka, D.; Žunić, A.; Bošković, D. Control of gray rot (Botrytis cinerea Pers.: Fr.) on lettuce. Biljni Lekar (Plant Doctor) 2019, 47, 147–156. [Google Scholar]
- Qin, S.; Veloso, J.; Baak, M.; Boogmans, B.; Bosman, T.; Puccetti, G.; Shi-Kunne, X.; Smit, S.; Grant-Downton, R.; Leisen, T.; et al. Molecular characterization reveals no functional evidence for naturally occurring cross-kingdom RNA interference in the early stages of Botrytis cinerea–tomato interaction. Mol. Plant Pathol. 2022, 24, 3–15. [Google Scholar] [CrossRef]
- Weiberg, A.; Wang, M.; Lin, F.M.; Zhao, H.; Zhang, Z.; Kaloshian, I.; Huang, H.D.; Jin, H. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 2013, 342, 118–123. [Google Scholar] [CrossRef]
- De Schutter, K.; Taning, C.N.T.; Van Daele, L.; Van Damme, E.J.M.; Dubruel, P.; Smagghe, G. RNAi-based biocontrol products: Market status, regulatory aspects, and risk assessment. Front. Insect Sci. 2022, 1, 818037. [Google Scholar] [CrossRef] [PubMed]
- Halder, K.; Chaudhuri, A.; Abdin, M.Z.; Majee, M.; Datta, A. RNA interference for improving disease resistance in plants and its relevance in this clustered regularly interspaced short palindromic repeats-dominated era in terms of dsRNA-based biopesticides. Front. Plant Sci. 2022, 13, 885128. [Google Scholar] [CrossRef]


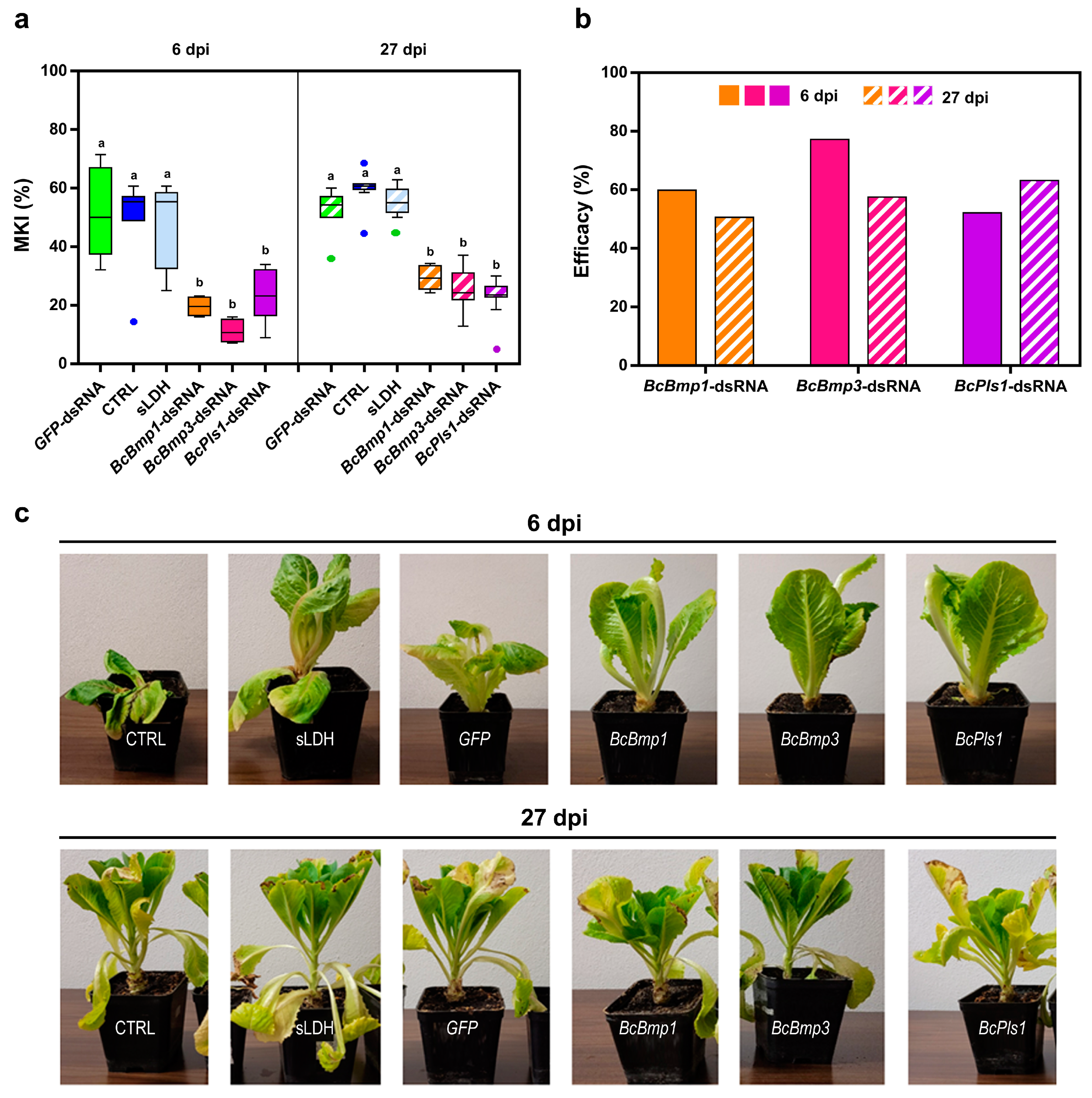
| Gene | 6 dpi | 27 dpi | ||
|---|---|---|---|---|
| dsRNA | dsRNA/sLDH | dsRNA | dsRNA/sLDH | |
| BcBmp1 | 52.1 (6.5) | 59.8 (2.2) | 43.0 (3.7) | 50.6 * (2.3) |
| BcBmp3 | 50.5 (4.9) | 77.2 *** (2.6) | 35.6 (3.2) | 57.5 *** (4.3) |
| BcPls1 | 51.1 (7.5) | 52.1 (6.5) | 30.8 (3.6) | 63.2 *** (4.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spada, M.; Pugliesi, C.; Fambrini, M.; Palpacelli, D.; Caneo, A.; Pecchia, S. Spray-Induced Gene Silencing (SIGS): Nanocarrier-Mediated dsRNA Delivery Improves RNAi Efficiency in the Management of Lettuce Gray Mold Caused by Botrytis cinerea. Agronomy 2025, 15, 194. https://doi.org/10.3390/agronomy15010194
Spada M, Pugliesi C, Fambrini M, Palpacelli D, Caneo A, Pecchia S. Spray-Induced Gene Silencing (SIGS): Nanocarrier-Mediated dsRNA Delivery Improves RNAi Efficiency in the Management of Lettuce Gray Mold Caused by Botrytis cinerea. Agronomy. 2025; 15(1):194. https://doi.org/10.3390/agronomy15010194
Chicago/Turabian StyleSpada, Maria, Claudio Pugliesi, Marco Fambrini, Diego Palpacelli, Andrea Caneo, and Susanna Pecchia. 2025. "Spray-Induced Gene Silencing (SIGS): Nanocarrier-Mediated dsRNA Delivery Improves RNAi Efficiency in the Management of Lettuce Gray Mold Caused by Botrytis cinerea" Agronomy 15, no. 1: 194. https://doi.org/10.3390/agronomy15010194
APA StyleSpada, M., Pugliesi, C., Fambrini, M., Palpacelli, D., Caneo, A., & Pecchia, S. (2025). Spray-Induced Gene Silencing (SIGS): Nanocarrier-Mediated dsRNA Delivery Improves RNAi Efficiency in the Management of Lettuce Gray Mold Caused by Botrytis cinerea. Agronomy, 15(1), 194. https://doi.org/10.3390/agronomy15010194







