Analysis of the Control Effect of Bacillus amyloliquefaciens C4 Wettable Powder on Potato Bacterial Wilt Caused by Ralstonia solanacearum
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Reagents and Microbial Inocula
2.2. Determination of Antibacterial Effect and Growth
2.3. Analysis of Antibacterial Agents
2.4. Optimization of Fermentation Conditions for Surfactins
2.5. Screening Methods for Carriers and Additives of Biocontrol Agents
2.6. Selection of Single-Factor Experiments and the Box–Behnken Design
2.7. RSM Analysis and Model Validation
2.8. Preparation, Quality Testing, and Analysis of the Biocontrol Effect of Wettable Powder
2.9. Determination of Physiological Indices Related to the Disease Resistance of Bacillus amyloliquefaciens C4 Wettable Powder
2.10. Statistical Analysis
3. Results
3.1. Studies on the Antibacterial Activity and Growth of Bacillus amyloliquefaciens C4
3.2. Study on Antibacterial Active Ingredients
3.3. Optimization of the Culture Conditions for Surfactin
3.4. Screening of Carriers and Adjuvants for C4 Wettable Powders
3.5. Selection of Carrier and Additive Amounts of Wettable Powder
3.6. The Influencing Factors of RSM Were Optimized
3.7. Wettable Powder Quality Test and Biocontrol Effect of Potato Leaves
3.8. The Measurement of Physiological Indicators
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Factor | Level (%) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| KA | 0 | 10 | 15 | 20 | 25 | 30 |
| SDBS | 0 | 4 | 6 | 8 | 10 | 12 |
| SL | 0 | 3 | 5 | 7 | 9 | 11 |
| HA | 0 | 2 | 4 | 6 | 8 | 10 |
| Gene Name | Gene Sequences |
|---|---|
| >iturinC (CP029466.1) | TTATTCCAGTTTGCTATGGGTGAAGACTTGATTGACATAAAGTT ATGTTTTAATGAACAAGTCTATGATCGTCAGTATATGATGCAGG TGCTCGGACATTTAAACCGGCTATTTTCTGTCATATTATTTCAGC CTGAGCTCCCCCTCGGTCAAGTGAATATTTTGCCAGAATCGGAG ACACATTCACTTCTCGTTGACAATCAAACTGCGAAAACTGAATA TCCGCGGGATAAGACGGTTTATCAGTTATTCGAAGAACAGATG AAACGAACACCGGATCAAGCAGCCGTTATTTACGGAGAAAAGC AATTCACATATCGTCAGCTCAATGAACGTGCCAATCAATTAGCC CGAACGTTAAGGAAAAAGGGGGTAAAGACGGATCGGCTCA |
| >SrfAA (MK570509.1) | ACAGGAAGACATCATCGTGGGAACACCGTCAGCGGGAAGAAA TCACTCCGATACCGAGGGGCTTATCGGGATGTTTGTCAACACG CTTGCGCTGCGAAGCTCCGTGAAGCAGGATCAGACATTTGCCG GCTTGTTAGGTCATGTGCGCAAGCAGGTGCTGGATGCGTTTTCT CATCAGGATTATCCGTTTGAGTGG |
| >fenD (CP044132.1) | AATCCATGTTTCTCTGAAGCTCTGCGACGCGGCGTTTTACAAAA CGTTCCTTCTCATGCTCATCGCCTTCCATTTCTAATATGGTCAGG CTGTAAAGCTGCTCATCTGCGAGGTCAGCCGGTCTGTTGAAGA GGAGAAGACCTTTCTCTTCGTCTTTTTTGCAGACAATGCGCAAG GCATCATGATGAACGGTAATGGCTTTTAACGTTTTCCTCAGAGC CTCTTCATCTATTGAATTTGCTCTCG |
| Ordinal Numbers | Test Factors | Bacterial Count (×108 CFU.mL−1) | |||
|---|---|---|---|---|---|
| pH | Temperature (°C) | Speed (r.min−1) | Inoculum (%) | ||
| 1 | 5 | 23 | 170 | 3 | 51.67 ± 3.86 |
| 2 | 5 | 28 | 200 | 5 | 49 ± 4.32 |
| 3 | 5 | 33 | 230 | 7 | 39.33 ± 0.94 |
| 4 | 5 | 23 | 170 | 5 | 48.37 ± 2.98 |
| 5 | 5 | 28 | 200 | 7 | 50.63 ± 3.07 |
| 6 | 5 | 33 | 230 | 3 | 37.29 ± 1.03 |
| 7 | 7 | 23 | 200 | 7 | 59.33 ± 2.49 |
| 8 | 7 | 28 | 230 | 3 | 55.33 ± 7.58 |
| 9 | 7 | 33 | 170 | 5 | 35 ± 1.41 |
| 10 | 7 | 23 | 230 | 3 | 51.09 ± 1.32 |
| 11 | 7 | 28 | 170 | 5 | 48.31 ± 0.36 |
| 12 | 7 | 33 | 200 | 7 | 40.07 ± 2.09 |
| 13 | 9 | 23 | 230 | 5 | 38.67 ± 1.25 |
| 14 | 9 | 28 | 170 | 7 | 46 ± 1.63 |
| 15 | 9 | 33 | 200 | 3 | 57.67 ± 4.50 |
| 16 | 9 | 23 | 230 | 7 | 40.87 ± 0.92 |
| 17 | 9 | 28 | 200 | 5 | 44.31 ± 1.83 |
| 18 | 9 | 33 | 170 | 3 | 51.27 ± 3.33 |
| k1 | 46.04 | 48.33 | 46.77 | 50.72 | |
| k2 | 48.19 | 48.93 | 50.17 | 43.92 | |
| k3 | 46.47 | 43.44 | 43.76 | 46.04 | |
| R | 2.15 | 5.49 | 6.41 | 6.80 | |
| Sum of Squares | Degree of Freedom | Mean Square | F | P | |
|---|---|---|---|---|---|
| pH (A) | 15.446 | 2 | 7.723 | 0.290 | 0.755 |
| Temperature (B) | 246.970 | 2 | 123.485 | 4.643 | 0.041 |
| Speed (C) | 252.482 | 2 | 126.241 | 4.747 | 0.039 |
| Inoculum (D) | 379.063 | 2 | 189.532 | 7.126 | 0.014 |
| Test Factors | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A (Sodium dodecyl benzene sulfonate) | 5 | 6 | 7 |
| B (Sodium lignosulfonate) | 4 | 5 | 6 |
| C (Humic acid) | 5 | 6 | 7 |
| Ordinal Numbers | A (Sodium Dodecyl Benzene Sulfonate) | B (Sodium Lignosulfonate) | C (Humic Acid) | Bacterial Count (×108 CFU.g−1) |
|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 55.33 |
| 2 | 0 | 0 | 0 | 66.67 |
| 3 | 0 | 0 | 0 | 65.33 |
| 4 | 1 | 1 | 0 | 52.33 |
| 5 | 1 | −1 | 0 | 57.67 |
| 6 | 0 | 0 | 0 | 66.33 |
| 7 | −1 | 1 | 0 | 52 |
| 8 | 0 | 1 | −1 | 56 |
| 9 | 0 | 0 | 0 | 68 |
| 10 | 1 | 0 | 1 | 50 |
| 11 | 0 | −1 | −1 | 60 |
| 12 | 0 | 0 | 0 | 67.33 |
| 13 | −1 | 0 | 1 | 51.67 |
| 14 | −1 | 0 | −1 | 52.67 |
| 15 | 0 | −1 | 1 | 58.33 |
| 16 | 0 | 1 | 1 | 51.33 |
| 17 | 1 | 0 | −1 | 58 |
| Source | Sum of Variance | Degree of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 639.49 | 9 | 71.05 | 86.02 | <0.0001 | ** |
| A-Sodium dodecyl benzene sulfonate | 5.01 | 1 | 5.01 | 6.06 | 0.0433 | * |
| B-Sodium lignosulfonate | 48.36 | 1 | 48.36 | 58.55 | 0.0001 | ** |
| C-Humic acid | 29.41 | 1 | 29.41 | 35.61 | 0.0006 | ** |
| AB | 1.01 | 1 | 1.01 | 1.22 | 0.3054 | |
| AC | 12.25 | 1 | 12.25 | 14.83 | 0.0063 | ** |
| BC | 2.25 | 1 | 2.25 | 2.72 | 0.1428 | |
| A2 | 260.44 | 1 | 260.44 | 315.30 | <0.0001 | ** |
| B2 | 86.59 | 1 | 86.59 | 104.82 | <0.0001 | ** |
| C2 | 140.78 | 1 | 140.78 | 170.43 | <0.0001 | ** |
| Residual | 5.78 | 7 | 0.83 | |||
| Lack of Fit | 1.69 | 3 | 0.56 | 0.55 | 0.6753 | |
| Pure Error | 4.10 | 4 | 1.02 | |||
| Total | 645.27 | 16 |

References
- Kaur, G.; Jain, S.; Bhushan, S.; Das, N.; Sharma, N.; Sharma, D. Role of microRNAs and their putative mechanism in regulating potato (Solanum tuberosum L.) life cycle and response to various environmental stresses. Plant Physiol. Biochem. 2024, 207, 108–334. [Google Scholar] [CrossRef]
- Karow, M.F.; Santos, F.N.D.; Biduski, B.; Krolow, A.C.R.; Silva, F.T.D.; Halal, S.L.M.E.; Macagnan, K.L.; Zavareze, E.D.R.Z.; Dias, A.R.G.; Diaz, P.S. Natural fermentation of potato (Solanum tuberosum L.) starch: Effect of cultivar, amylose content, and drying method on expansion, chemical and morphological properties. Int. J. Biol. Macromol. 2024, 261, 129608. [Google Scholar] [CrossRef] [PubMed]
- Odamea, A.A.A.; Calogero, S.; Aldo, L. Automatic blight disease detection in potato (Solanum tuberosum L.) and tomato (Solanum lycopersicum L. 1753) plants using deep learning. Smart Agric. Technol. 2023, 4, 100178. [Google Scholar] [CrossRef]
- Genesis, T.Y.; Jonas, A. Crop yield gaps in Cameroon. Ambio 2014, 43, 175–190. [Google Scholar] [CrossRef]
- Jiang, G.F.; Wei, Z.; Xu, J.; Chen, H.L.; Zhang, Y.; She, X.M.; Macho, A.P.; Ding, W.; Liao, B.S. Bacterial Wilt in China: History Current Status and Future Perspectives. Front. Plant Sci. 2017, 8, 1549. [Google Scholar] [CrossRef]
- Wicker, E.; Grassart, L.; Beaudu, R.C.; Mian, D.; Guilbaud, C.; Fegan, M.; Prior, P. Ralstonia solanacearum Strains from Martinique (French West Indies) Exhibiting a New Pathogenic Potential. Appl. Environ. Microbiol. 2009, 75, 558. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Cai, X.C.; Liu, C.H.; Wang, B.T.; Xue, Y.R. Genomic and metabolic traits endow Bacillus velezensis CC09 with a potential biocontrol agent in control of wheat powdery mildew disease. Microbiol. Res. 2017, 196, 89–94. [Google Scholar] [CrossRef]
- Thakore, H.; McMahon, T. An interactive e-tutorial in pathology. Med Educ. 2006, 40, 1135. [Google Scholar] [CrossRef]
- Wraight, S.P.; Ramos, M.E. Effects of inoculation method on efficacy of wettable powder and oil dispersion formulations of Beauveria bassiana against Colorado potato beetle larvae under low-humidity conditions. Biocontrol Sci. Technol. 2017, 27, 348–363. [Google Scholar] [CrossRef]
- Chen, J.; Lv, Z.; Cheng, Z.; Wang, T.; Li, P.; Wu, A.; Nepovimova, E.; Long, M.; Wu, W.; Kuca, K. Bacillus amyloliquefaciens B10 inhibits aflatoxin B1-induced cecal inflammation in mice by regulating their intestinal flora. Food Chem. Toxicol. 2021, 156, 112438. [Google Scholar] [CrossRef]
- Jin, W.Y.; Zhang, F.J.; Zhang, X.M.; Yu, J.J.; Zou, L.; Sun, B.S.; Yan, Y.Z.; Xue, J. Antibacterial and antiseptic effects of Bacillus amyloliquefaciens B15 wettable powder. J. Jiangsu Agric. Sci. 2021, 49, 169–172. (In Chinese) [Google Scholar] [CrossRef]
- Nian, X.G.; He, Y.R.; Lu, L.H.; Zhao, R. Evaluation of alternative Plutella xylostella control by two Isaria fumosorosea conidial formulations—Oil-based formulation and wettable powder—Combined with Bacillus thuringiensis. Pest Manag. Sci. 2015, 71, 1675–1684. [Google Scholar] [CrossRef]
- Honeycutt, E.W.; Benson, D.M. Formulation of binucleate Rhizoctonia spp. and biocontrol of Rhizoctonia solani on impatiens. Plant Dis. 2001, 85, 1241–1248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leggett, M.; Leland, J.; Kellar, K.; Epp, B. Formulation of microbial biocontrol agents—An industrial perspective. Can. J. Plant Pathol. 2011, 33, 101–107. [Google Scholar] [CrossRef]
- Couch, T.L. Book Review: Formulation of Microbial Pesticides: Beneficial Microorganisms, Nematodes and Seed Treatment. Entomol. Exp. Appl. 2002, 102, 211–212. [Google Scholar] [CrossRef]
- Zanotto, A.W.; Valério, A.; de Andrade, C.J.; Pastore, G.M. New sustainable alternatives to reduce the production costs for surfactin 50 years after the discovery. Appl. Microbiol. Biotechnol. 2019, 103, 8647–8656. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Liu, W.; He, X.; Du, C. A review on surfactin: Molecular regulation of biosynthesis. Arch. Microbiol. 2023, 205, 313. [Google Scholar] [CrossRef]
- Aliye, N.; Fininsa, C.; Hiskias, Y. Evaluation of rhizosphere bacterial antagonists for their potential to bioprotect potato (Solanum tuberosum) against bacterial wilt (Ralstonia solanacearum). Biol. Control. 2008, 47, 282–288. [Google Scholar] [CrossRef]
- Mora, I.; Cabrefiga, J.; Montesinos, E. Antimicrobial peptide genes in Bacillus strains from plant environments. Int. Microbiol. 2011, 14, 213–223. [Google Scholar] [CrossRef]
- Feng, R.Y.; Chen, Y.H.; Lin, C.; Tsai, C.H.; Yang, Y.L.; Chen, Y.L. Surfactin secreted by Bacillus amyloliquefaciens Ba01 is required to combat Streptomyces scabies causing potato common scab. Front. Plant Sci. 2022, 13, 998707. [Google Scholar] [CrossRef]
- Vignesh, M.; Shankar, S.R.M.; MubarakAli, D.; Hari, B.N.V. A Novel Rhizospheric Bacterium: Bacillus velezensis NKMV-3 as a Biocontrol Agent Against Alternaria Leaf Blight in Tomato. Appl. Biochem. Biotechnol. 2022, 194, 1–17. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, R.; Liu, Y.; Liu, Y. Evaluation of the Biocontrol Efficiency of Bacillus subtilis Wettable Powder on Pepper Root Rot Caused by Fusarium solani. Pathogens 2023, 12, 225. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, H.; Zhu, T.; Han, S.; Li, S. Preparation of Nanomaterial Wettable Powder Formulations of Antagonistic Bacteria from Phellodendron chinense and the Biological Control of Brown Leaf Spot Disease. Plant Pathol. J. 2021, 37, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, A.; Davidson, C.E. Estimation method for serial dilution experiments. J. Microbiol. Methods 2014, 107, 214–221. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Z.Q.; Qin, G.Z.; Tian, S.P. Effects of brassinosteroids on postharvest disease and senescence of jujube fruit in storage. Postharvest Biol. Technol. 2009, 56, 50–55. [Google Scholar] [CrossRef]
- Vater, J.; Gao, X.; Hitzeroth, G.; Wilde, C.; Franke, P. “Whole cell”—matrix-assisted laser desorption ionization-time of flight-mass spectrometry, an emerging technique for efficient screening of biocombinatorial libraries of natural compounds-present state of research. Comb. Chem. High Throughput Screen. 2003, 6, 557–567. [Google Scholar] [CrossRef]
- Luo, L.; Zhao, C.; Wang, E.; Raza, A.; Yin, C. Bacillus amyloliquefaciens as an excellent agent for biofertilizer and biocontrol in agriculture: An overview for its mechanisms. Microbiol. Res. 2022, 259, 127016. [Google Scholar] [CrossRef]
- Zhou, K.; Wei, D.L.; Zhang, F.F.; Zhao, Q.; Zhao, Y.J.; Xie, F.X. Inhibitory effects of Bacillus amyloliquefaciens HN on the fungus pathogens of tomato and cucumber. J. Tianjin Norm. Univ. 2019, 39, 51–57. (In Chinese) [Google Scholar] [CrossRef]
- An, Y.J.; Zhu, W.J.; Liu, Y.; Zhang, X.M.; Sun, L.J.; Hong, P.Z.; Wang, Y.L.; Xu, C.H.; Xu, D.F.; Liu, H.M. Purification and characterization of a novel bacteriocin CAMT2 produced by Bacillus amyloliquefaciens isolated from marine fish Epinephelus areolatus. Food Control. 2015, 51, 278–282. [Google Scholar] [CrossRef]
- You, W.J.; Ge, C.H.; Jiang, Z.C.; Chen, M.M.; Li, W.; Shao, Y.Z. Screening of a broad-spectrum antagonist-Bacillus siamensis, and its possible mechanisms to control postharvest disease in tropical fruits. Biol. Control. 2021, 157, 104584. [Google Scholar] [CrossRef]
- Ahmad, T.; Xing, F.G.; Nie, C.G.; Cao, C.Y.; Xiao, Y.; Yu, X.; Moosa, A.; Liu, Y. Biocontrol potential of lipopeptides produced by the novel Bacillus subtilis strain Y17B against postharvest Alternaria fruit rot of cherry. Front. Microbiol. 2023, 14, 1150217. [Google Scholar] [CrossRef]
- Sarwar, A.; Hassan, M.N.; Imran, M.; Iqbal, M.; Majeed, S.; Brader, G.; Sessitsch, A.; Hafeez, F.Y. Biocontrol activity of surfactin A purified from Bacillus NH-100 and NH-217 against rice bakanae disease. Microbiol. Res. 2018, 209, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.S.; Wang, F.G.; Qi, Z.Q.; Qiao, J.Q.; Du, Y.; Yu, J.J.; Yu, M.N.; Liang, D.; Song, T.Q.; Yan, P.X.; et al. Iturins produced by Bacillus velezensis Jt84 play a key role in the biocontrol of rice blast disease. Biol. Control. 2022, 174, 105001. [Google Scholar] [CrossRef]
- Yang, L.; Ying, T.; Luo, X.Q.; Li, Z.G. Development of wettable powder of Trichoderma reesei FS10-C and its plant growth-promoting effects. Biotechnol. Bull. 2016, 32, 194–199. (In Chinese) [Google Scholar] [CrossRef]
- Chen, R.; Cao, X.M.; Wu, H.X.; Li, H.; Chen, X.Y.; Bao, Z.H.; Ma, G.Z.; Wang, J.Q. Preparation of Paenibacillus polymyxa wettable powder. Plant Prot. 2020, 46, 62–69. (In Chinese) [Google Scholar] [CrossRef]
- Du, D.C.; Lu, L.M.; Hu, X.R.; Pu, Z.X.; Huang, Z.D.; Chen, G.Q.; Liu, S.M. Virulence of; strain ZJPL08 and efficacy of a wettable powder formulation against the Asian citrus psyllid. Biotechnol. Biotechnol. Equip. 2020, 34, 1104–1113. [Google Scholar] [CrossRef]
- Qiu, D.W. Research progress and prospect of bio-pesticide. Plant Prot. 2013, 39, 81–89. [Google Scholar] [CrossRef]
- Ranjbar, Z.; Salehi, M.; Safaie, N. An endophytic Trichoderma-based wettable powder formulation for biocontrol of apple stem cankers. J. Phytopathol. 2024, 172, e13266. [Google Scholar] [CrossRef]
- Arunsiri, A.T.; Suphantharika, M.; Ketunuti, U. Preparation of spray-dried wettable powder formulations of Bacillus thuringiensis-based biopesticides. J. Econ. Entomol. 2003, 96, 292–299. [Google Scholar] [CrossRef]
- Chen, W.; Juang, R.; Wei, Y. Applications of a lipopeptide biosurfactant, surfactin, produced by microorganisms. Biochem. Eng. J. 2015, 103, 158–169. [Google Scholar] [CrossRef]
- Loomans, M.J.A. Every generalist biological control agent requires a special risk assessment. BioControl 2020, 66, 23–35. [Google Scholar] [CrossRef]
- Amaresan, N.; Jayakumar, V.; Kumar, K.; Thajuddin, N. Biocontrol and plant growth-promoting ability of plant-associated bacteria from tomato (Lycopersicum esculentum) under field condition. Microb. Pathog. 2019, 136, 103713. [Google Scholar] [CrossRef]
- Li, S.M.; Hua, G.G.; Liu, H.X.; Guo, J.H. Analysis of defence enzymes induced by antagonistic bacterium Bacillus subtilis strain AR12 towards Ralstonia solanacearum in tomato. Ann. Microbiol. 2008, 58, 573–578. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yue, Q.R.; Xin, Y.; Ngea, G.L.N.; Dhanasekaran, S.; Luo, R.J.; Li, J.; Zhao, L.N.; Zhang, H.Y. The biocontrol potentiality of Bacillus amyloliquefaciens against postharvest soft rot of tomatoes and insights into the underlying mechanisms. Postharvest Biol. Technol. 2024, 214, 112983. [Google Scholar] [CrossRef]
- Marc, O.; Emmanuel, J.; Akram, A.; Michel, P.; Alain, B.; Bernard, J.; Louis, A.J.; Philippe, T. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ. Microbiol. 2007, 9, 1084–1090. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Cong, R.; Zhao, W.S.; Qu, Y.H.; Su, Z.H.; Guo, Q.G.; Lu, X.Y.; Li, S.Z.; Ma, P. Development of 3 billion CFU/g Bacillus wettable powder and its control efficacy on potato Verticillium wilt and scab. Chin. J. Pestic. Sci. 2023, 25, 140–149. (In Chinese) [Google Scholar] [CrossRef]

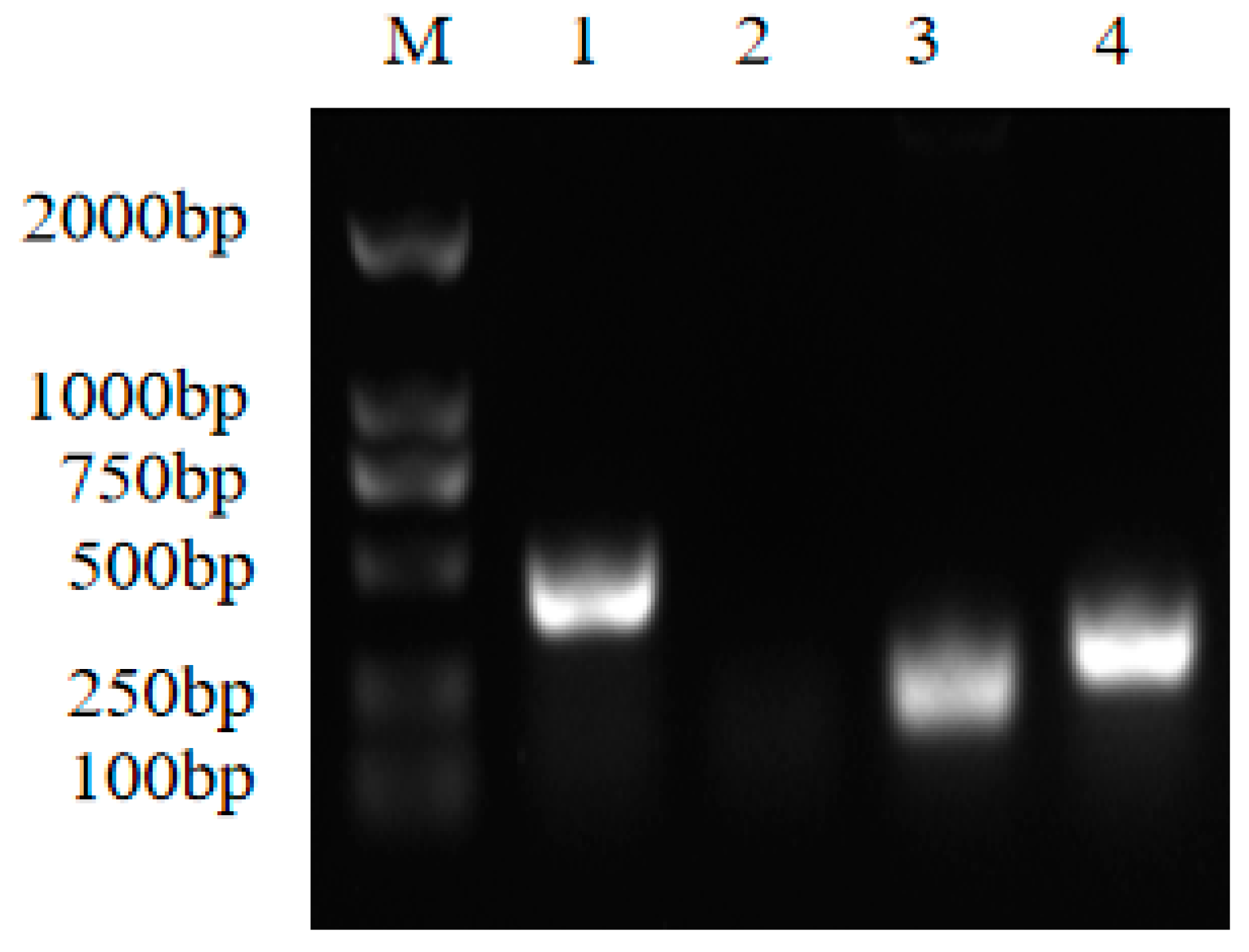
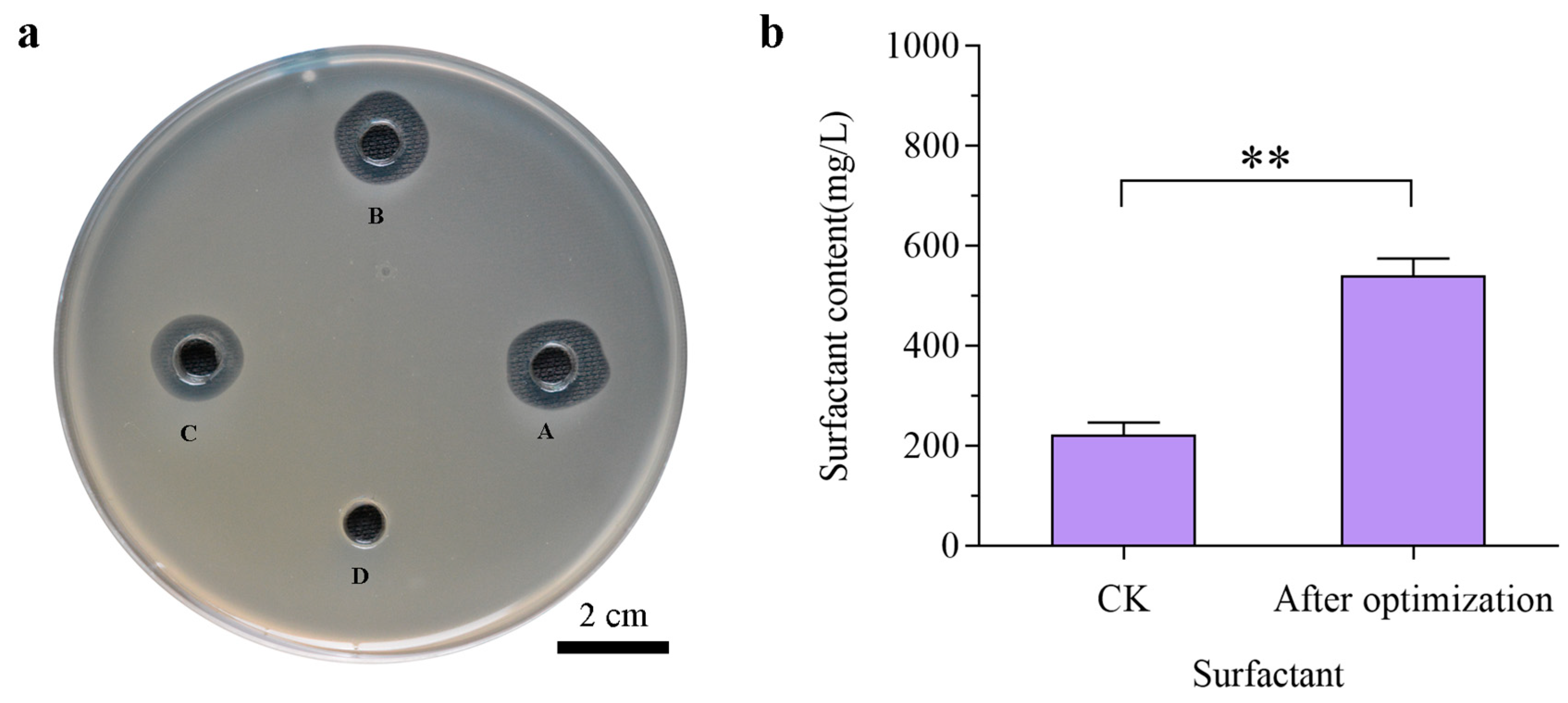


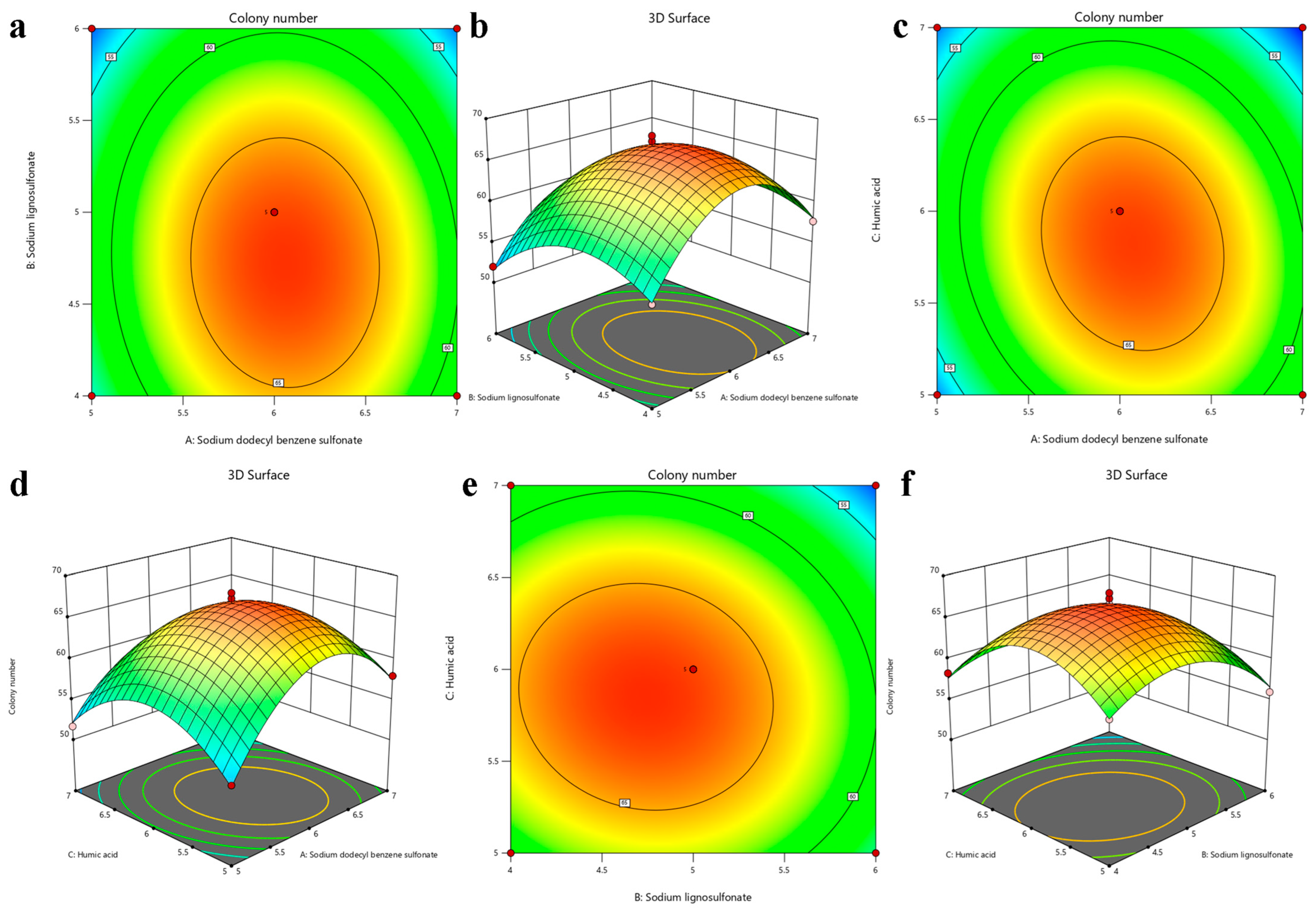
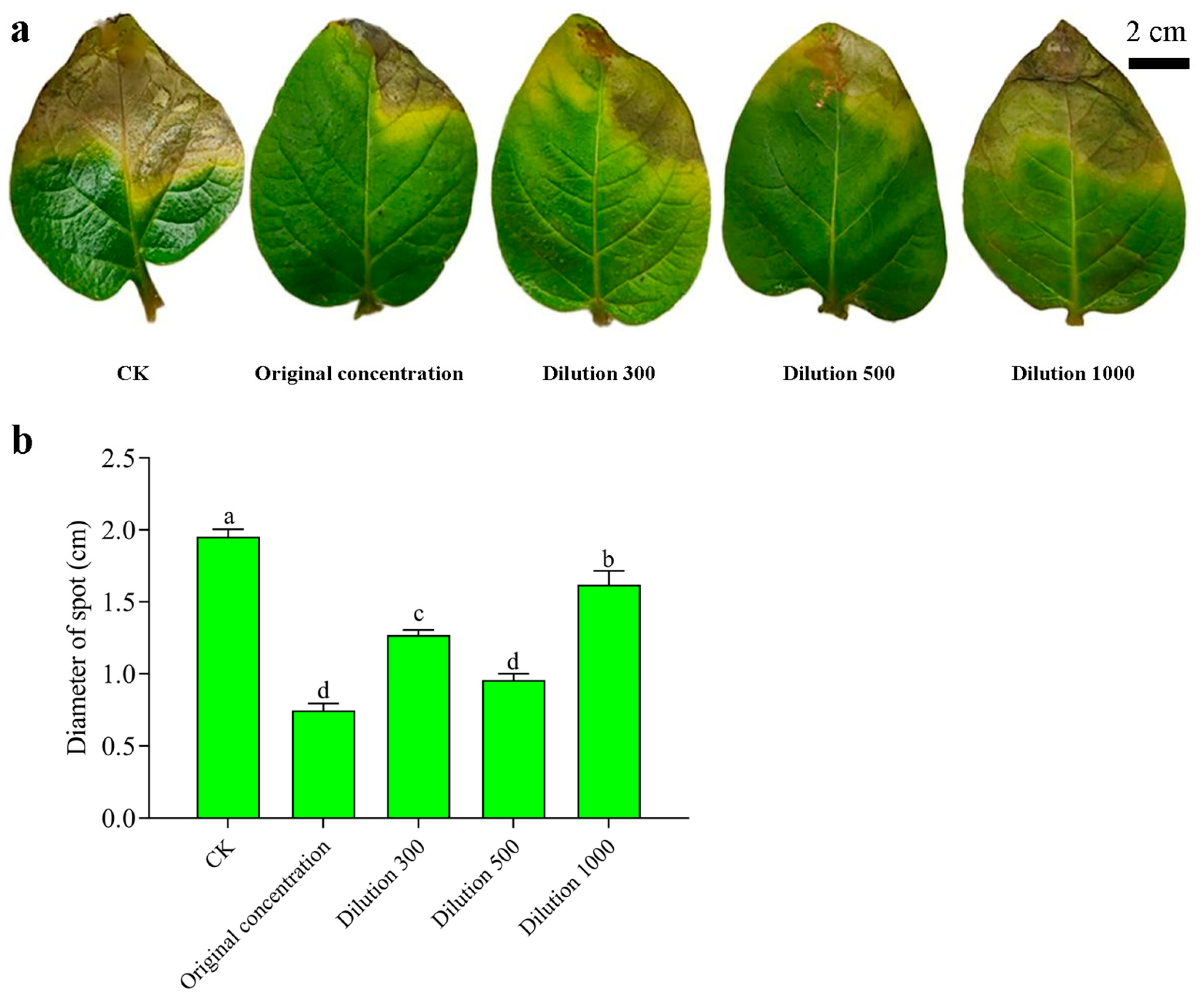

| Primer Name | Primer Sequence (5′-3′) | Target Gene |
|---|---|---|
| IturinC Forward Primer | GGCTGCTGCAGATGC | iturinC |
| IturinC Reverse Primer | TCGCAGATAATCGCA | |
| spaS Forward Primer | GGTTTGTTGGATGGA | spaS |
| spaS Reverse Primer | GCAAGGAGTCAGAGC | |
| srfAA Forward Primer | TCGGGACAGGAAGAC | srfAA |
| srfAA Reverse Primer | CCACTCAAACGGATA | |
| fenD Forward Primer | GGCCCGTTCTCTAAAT | fenD |
| fenD Reverse Primer | GTCATGCTGACGAGAGCAAA |
| Name | Materials to Be Selected | Mixing Ratio |
|---|---|---|
| Carrier | Calcium carbonate, talc powder, kaolinite, diatomite, and precipitated silica | 5% (w/v) |
| Wetting agent | Sodium dodecyl benzene sulfonate, saponin powder, and sodium diisobutyl naphthalenesulfonate | 250 μg/mL |
| Dispersant | Sodium lignosulfonate, sodium tripolyphosphate, sodium carboxymethyl cellulose, and polyvinyl alcohol | 1500 μg/mL |
| Protectants | Humic acid, methylcellulose, and xanthan gum | 50 μg/mL |
| Treatment | Diameter of Inhibition Zone (mm) |
|---|---|
| Fermentation broth (A) | 11.6 ± 0.37 |
| Supernatant fluid (B) | 11.58 ± 0.09 |
| Crude extract of lipopeptide (C) | 11.7 ± 0.33 |
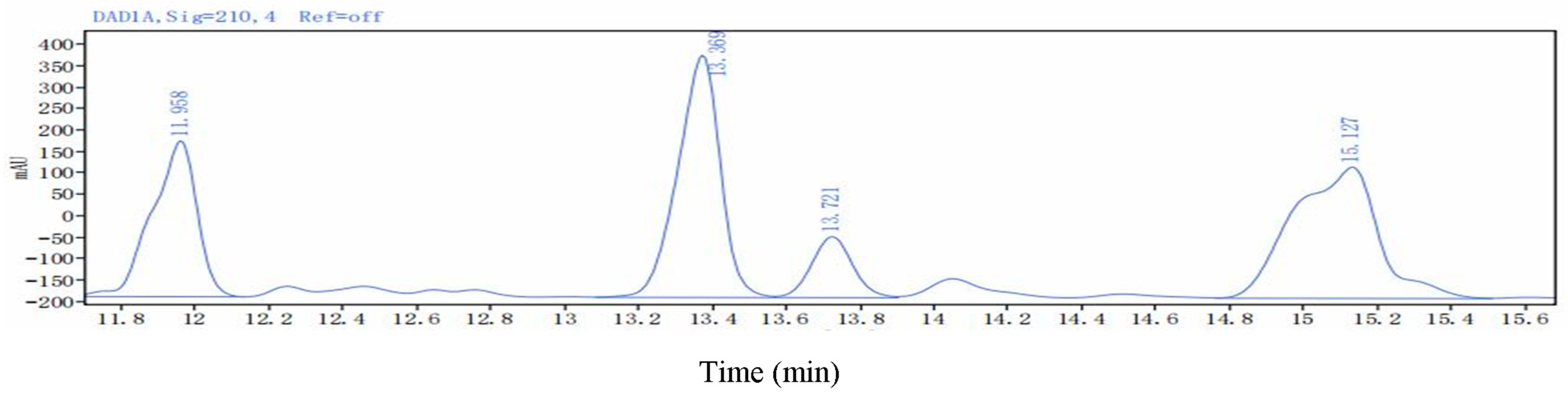
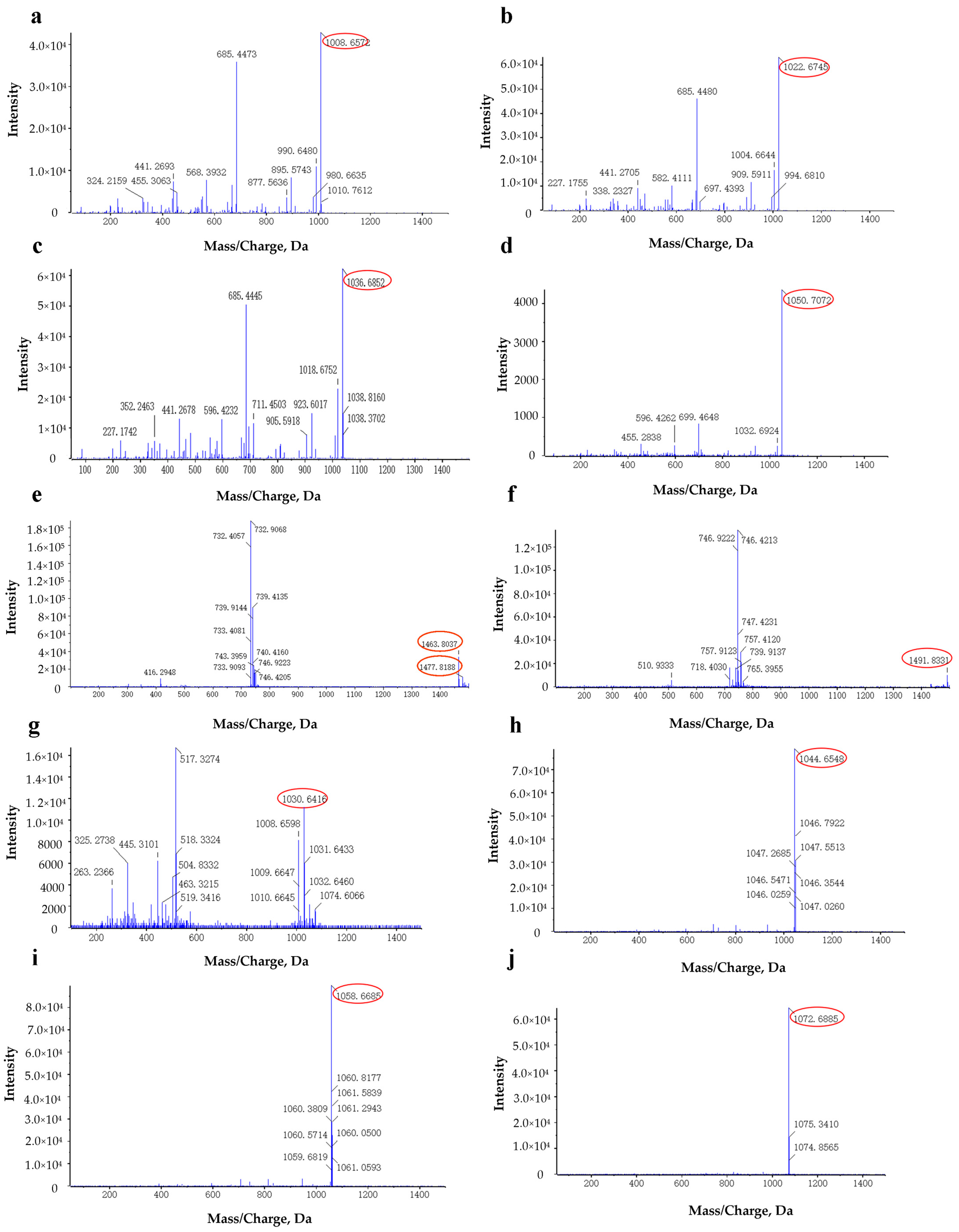
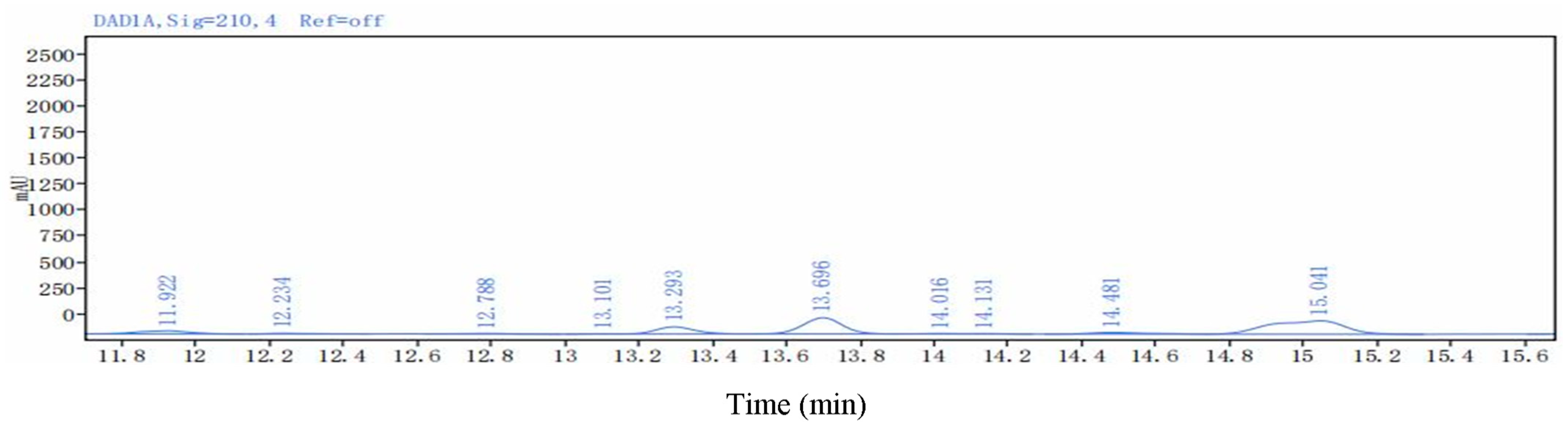
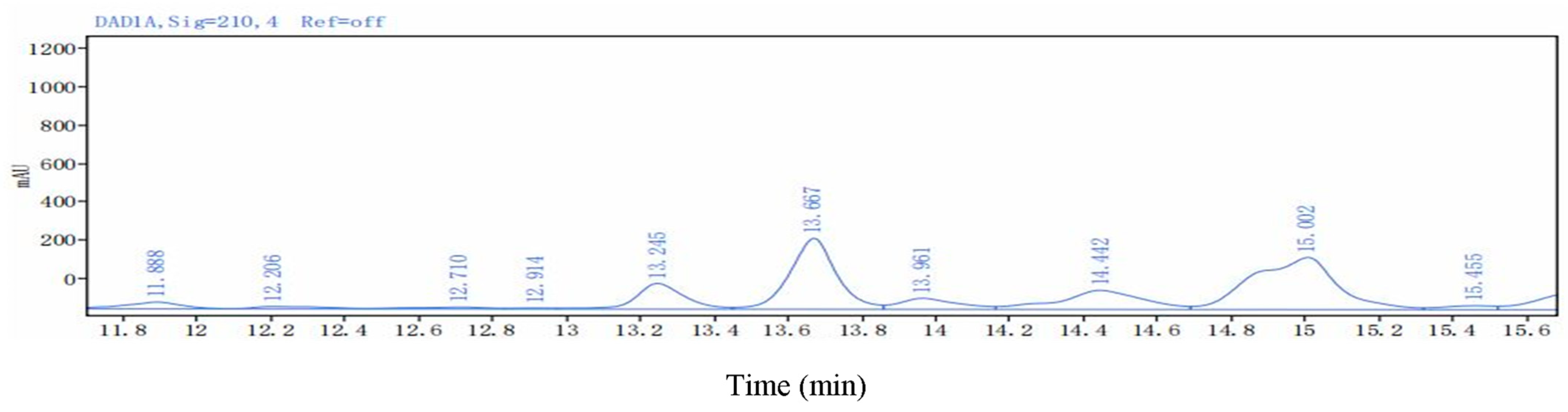
| Lipopeptides | Retention Time (min) | Mass Value (M + H+) | |
|---|---|---|---|
| Surfactins | Surfactin A (C13) | 15.755 | 1008.6572 |
| Surfactin B (C14) | 15.861 | 1022.6754 | |
| Surfactin C (C15) | 16.325 | 1036.6852 | |
| Surfactin C (C16) | 17.230 | 1050.7072 | |
| Fengycins | Fengycin B (C16) | 11.180 | 1463.8037 |
| Fengycin C (C17) | 11.262 | 1477.8188 | |
| Fengycin D (C18) | 11.730 | 1491.8331 | |
| Iturin A | IturinA (C13) | 15.848 | 1030.6416 |
| IturinA (C14) | 16.732 | 1044.6548 | |
| IturinA (C15) | 17.549 | 1058.6685 | |
| IturinA (C16) | 18.005 | 1072.6885 | |
| Lipopeptide Antibiotics | Diameter of Inhibition Zone (mm) |
|---|---|
| surfactins | 13.95 ± 0.23 |
| fengycins | 12.87 ± 0.05 |
| iturins | 11.75 ± 0.16 |
| Project | Standard | Actual Value |
|---|---|---|
| Frequency of microbial contamination (%) | ≤3 | 0.16 |
| PH | 5.5–8.5 | 7.72 |
| Fineness (%) | ≥80 | 91.79 |
| Moisture content (%) | ≤4 | 2.54 |
| Suspension rate (%) | ≥70 | 75.17 |
| Wetting time (s) | ≤180 | 95.19 |
| Drying loss (%) | ≤6 | 1.09 |
| Storage stability (%) | ≥80 | 82.57 |
| Treatment | Incidence Rate (%) | Disease Index | Control Efficacy (%) |
|---|---|---|---|
| Control (Water treatment) | 83.05 ± 7.15 a | 28.89 ± 33.63 a | |
| C4 | 17.69 ± 12.09 b | 7.78 ± 8.27 b | 73.08 ± 28.61 a |
| C4 wettable powder (1:300) | 8.82 ± 10.25 b | 5 ± 6.45 b | 82.7 ± 22.31 a |
| C4 wettable powder (1:500) | 12.13 ± 10.85 b | 6.12 ± 7.29 b | 79.05 ± 24.79 a |
| C4 wettable powder (1:1000) | 13.89 ± 10.99 b | 6.67 ± 8.36 b | 76.9 ± 28.95 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Z.; Liu, D.; Luo, M.; Yang, Z.; Pang, W.; Feng, Y.; Yan, J.; He, F.; Feng, X.; Yuan, Q.; et al. Analysis of the Control Effect of Bacillus amyloliquefaciens C4 Wettable Powder on Potato Bacterial Wilt Caused by Ralstonia solanacearum. Agronomy 2025, 15, 206. https://doi.org/10.3390/agronomy15010206
Xing Z, Liu D, Luo M, Yang Z, Pang W, Feng Y, Yan J, He F, Feng X, Yuan Q, et al. Analysis of the Control Effect of Bacillus amyloliquefaciens C4 Wettable Powder on Potato Bacterial Wilt Caused by Ralstonia solanacearum. Agronomy. 2025; 15(1):206. https://doi.org/10.3390/agronomy15010206
Chicago/Turabian StyleXing, Zhixiang, Dan Liu, Meng Luo, Zelin Yang, Wenyuan Pang, Yexing Feng, Jiani Yan, Fumeng He, Xu Feng, Qiang Yuan, and et al. 2025. "Analysis of the Control Effect of Bacillus amyloliquefaciens C4 Wettable Powder on Potato Bacterial Wilt Caused by Ralstonia solanacearum" Agronomy 15, no. 1: 206. https://doi.org/10.3390/agronomy15010206
APA StyleXing, Z., Liu, D., Luo, M., Yang, Z., Pang, W., Feng, Y., Yan, J., He, F., Feng, X., Yuan, Q., Wang, Y., & Li, F. (2025). Analysis of the Control Effect of Bacillus amyloliquefaciens C4 Wettable Powder on Potato Bacterial Wilt Caused by Ralstonia solanacearum. Agronomy, 15(1), 206. https://doi.org/10.3390/agronomy15010206






