Optimization Study on the Freshwater Production Ratio from the Freezing and Thawing Process of Saline Water with Varied Qualities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Experimental Design
2.3. Index Measurement
2.4. Data Processing
3. Results
3.1. The Primary Factors Affecting the Freshwater Production Ratio After Saline Water Freezing and Thawing
3.2. The Impact of Individual Factors on the Freshwater Production Ratio of Saline Ice
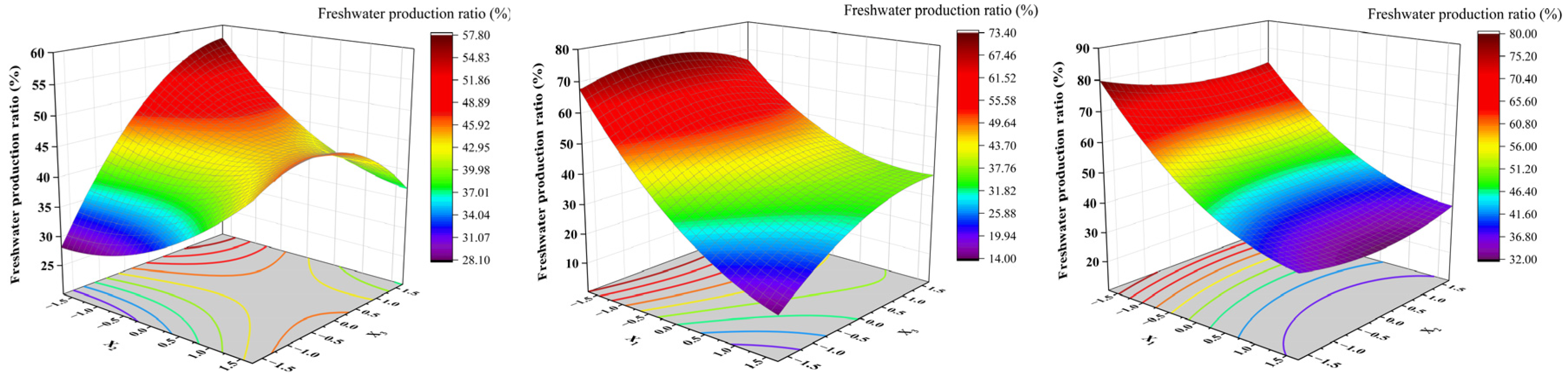
3.3. The Influence of Factor Interactions on the Freshwater Production Ratio from Saline Ice
3.4. Characteristics of Water Quantity and Quality Changes During the Melting Process
4. Discussion
4.1. The Influence of Saline Water’s Salinity, SAR, and Freezing Temperature on the Freshwater Production Ratio
4.2. Characteristics of Water Quantity and Quality Changes in the Melting Process
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, A. Soil Salinization Management for Sustainable Development: A Review. J. Environ. Manag. 2021, 277, 111383. [Google Scholar] [CrossRef]
- FAO. Global Symposium on Salt-Affected Soils: Outcome Document; FAO: Rome, Italy, 2022; ISBN 978-92-5-136142-9. [Google Scholar]
- Montgomery, D.R. Soil Security and Global Food Security. Front. Agric. Sci. Eng. 2024, 11, 297–302. [Google Scholar] [CrossRef]
- FAO. The State of the World’s Land and Water Resources for Food and Agriculture—Systems at Breaking Point (SOLAW 2021); FAO: Rome, Italy, 2021; ISBN 978-92-5-135327-1. [Google Scholar]
- Okur, B.; Örçen, N. Soil Salinization and Climate Change. In Climate Change and Soil Interactions; Elsevier: Amsterdam, The Netherlands, 2020; pp. 331–350. ISBN 978-0-12-818032-7. [Google Scholar]
- Yuan, C.; Feng, S.; Wang, J.; Huo, Z.; Ji, Q. Effects of Irrigation Water Salinity on Soil Salt Content Distribution, Soil Physical Properties and Water Use Efficiency of Maize for Seed Production in Arid Northwest China. Int. J. Agric. Biol. Eng. 2018, 11, 137–145. [Google Scholar] [CrossRef]
- Xia, J.; Ning, L.; Wang, Q.; Chen, J.; Wan, L.; Hong, S. Vulnerability of and Risk to Water Resources in Arid and Semi-Arid Regions of West China under a Scenario of Climate Change. Clim. Change 2017, 144, 549–563. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for 550 Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Zaman, M., Shahid, S.A., Heng, L., Eds.; 551 Springer International Publishing: Cham, Switzerland, 2018; pp. 43–53. ISBN 978-3-319-96190-3. [Google Scholar]
- Zhang, L. Analysis and Countermeasure Research on Saline-Alkali Land Change in Xinjiang Irrigation Area in Recent 20 Years. Water Resour. Dev. Manag. 2020, 6, 72–76. [Google Scholar] [CrossRef]
- Wu, H.; Xi, W.; Tang, H.; Tang, M. The Current Status and Countermeasures for the Improvement and Utilization of Saline-Alkali Cultivated Land in Xinjiang. Agric. Compr. Dev. China 2023, 8, 6–8. [Google Scholar]
- Chen, W.; Zheng, Z.; Xie, J.; Zhao, Y.G.; Hu, J. Study on the Unconventional Water Sources: Bitter-Salty Water Resources and Its Distribution Characteristics in China. J. China Hydrol. 2021, 41, 1–6. [Google Scholar] [CrossRef]
- Duong, H.C.; Tran, T.L.; Ansari, A.; Cao, H.T.; Vu, T.D.; Do, K.-U. Advances in Membrane Materials and Processes for Desalination of Brackish Water. Curr. Pollut. Rep. 2019, 5, 319–336. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, J.; Shi, S.Q.; Li, J.; Kim, K.-H. Advances in Desalination Technology and Its Environmental and Economic Assessment. J. Clean. Prod. 2023, 397, 136498. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse Osmosis Desalination: Water Sources, Technology, and Today’s Challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Kurihara, M.; Wang, R. Seawater Desalination by Reverse Osmosis: Current Development and Future Challenges in Membrane Fabrication—A Review. J. Membr. Sci. 2021, 629, 119292. [Google Scholar] [CrossRef]
- Wang, L.; He, J.; Heiranian, M.; Fan, H.; Song, L.; Li, Y.; Elimelech, M. Water Transport in Reverse Osmosis Membranes Is Governed by Pore Flow, Not a Solution-Diffusion Mechanism. Sci. Adv. 2023, 9, eadf8488. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, X.; Li, Z.; Dong, X. Adaptability of Reverse Osmosis Technology for Salt Water Desalination in Southern Xinjiang. Technol. Water Treat. 2019, 45, 121–124. [Google Scholar] [CrossRef]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef]
- Galama, A.H.; Saakes, M.; Bruning, H.; Rijnaarts, H.H.M.; Post, J.W. Seawater Predesalination with Electrodialysis. Desalination 2014, 342, 61–69. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, H. Research Status of High Salinity Wastewater Desalination Treatment Technology. Liaoning Chem. Ind. 2023, 52, 907–910. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Khalil, A.; Hilal, N. Emerging Desalination Technologies: Current Status, Challenges and Future Trends. Desalination 2021, 517, 115183. [Google Scholar] [CrossRef]
- Gohil, P.P.; Desai, H.; Kumar, A.; Kumar, R. Current Status and Advancement in Thermal and Membrane-Based Hybrid Seawater Desalination Technologies. Water 2023, 15, 2274. [Google Scholar] [CrossRef]
- Ali, E.; Orfi, J.; AlAnsary, H.; Lee, J.-G.; Alpatova, A.; Ghaffour, N. Integration of Multi Effect Evaporation and Membrane Distillation Desalination Processes for Enhanced Performance and Recovery Ratios. Desalination 2020, 493, 114619. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Leaper, S.; Skuse, C.; Zaragoza, G.; Gryta, M.; Gorgojo, P. Membrane Cleaning and Pretreatments in Membrane Distillation—A Review. Chem. Eng. J. 2021, 422, 129696. [Google Scholar] [CrossRef]
- Nebbia, G.; Menozzi, G.N. Early Experiments on Water Desalination by Freezing. Desalination 1968, 5, 49–54. [Google Scholar] [CrossRef]
- Rahman, M.S.; Al-Khusaibi, M. Freezing-Melting Desalination Process. In Desalination; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 473–501. ISBN 978-1-118-90485-5. [Google Scholar]
- Williams, P.M.; Ahmad, M.; Connolly, B.S.; Oatley-Radcliffe, D.L. Technology for Freeze Concentration in the Desalination Industry. Desalination 2015, 356, 314–327. [Google Scholar] [CrossRef]
- Rahman, M.S.; Ahmed, M.; Chen, X.D. Freezing Melting Process and Desalination: Review of Present Status and Future Prospects. Int. J. Nucl. Desalination 2007, 2, 253. [Google Scholar] [CrossRef]
- Najim, A. A Review of Advances in Freeze Desalination and Future Prospects. NPJ Clean Water 2022, 5, 15. [Google Scholar] [CrossRef]
- Kalista, B.; Shin, H.; Cho, J.; Jang, A. Current Development and Future Prospect Review of Freeze Desalination. Desalination 2018, 447, 167–181. [Google Scholar] [CrossRef]
- Gu, W.; Lin, Y.; Xu, Y.; Chen, W.; Tao, J.; Yuan, S. Gravity-Induced Sea Ice Desalination under Low Temperature. Cold Reg. Sci. Technol. 2013, 86, 133–141. [Google Scholar] [CrossRef]
- Guo, K.; Liu, X. Reclamation Effect of Freezing Saline Water Irrigation on Heavy Saline-Alkali Soil in the Hetao Irrigation 603 District of North China. Catena 2021, 204, 105420. [Google Scholar] [CrossRef]
- Guo, K.; Ju, Z.; Feng, X.; LI, X.; Liu, X. Advances and Expectations of Researches on Saline Soil Reclamation by Freezing Saline Water Irrigation. Chin. J. Eco-Agric. 2016, 24, 1016–1024. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, F.; Wang, Z. Research Advances of Saline Soil Reclamation by Freezing Saline Water Irrigation and Meltwater Leaching. Soils Crops 2021, 10, 202–212. [Google Scholar] [CrossRef]
- Ju, Z.; Du, Z.; Guo, K.; Liu, X. Irrigation with Freezing Saline Water for 6 Years Alters Salt Ion Distribution within Soil Aggregates. J. Soils Sediments 2019, 19, 97–105. [Google Scholar] [CrossRef]
- Li, X.; Li, Y. Effects of Freezing Irrigation with Saline Water on Coastal Saline Land Soil under Different Salinities. Sci. Soil Water Conserv. 2015, 13, 64–68. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Y.; Mao, X. Influences of salt adsorption ratio and salt concentration on the physical properties of typical sandy loam in xinjiang. Trans. Chin. Soc. Agric. Eng. 2022, 38, 86–95. [Google Scholar] [CrossRef]
- Guo, K.; Liu, X. Dynamics of Meltwater Quality and Quantity during Saline Ice Melting and Its Effects on the Infiltration and Desalinization of Coastal Saline Soils. Agric. Water Manag. 2014, 139, 1–6. [Google Scholar] [CrossRef]
- Beier, N.; Sego, D.; Donahue, R.; Biggar, K. Laboratory Investigation on Freeze Separation of Saline Mine Waste Water. Cold Reg. Sci. Technol. 2007, 48, 239–247. [Google Scholar] [CrossRef]
- Bing, H.; Ma, W. Laboratory Investigation of the Freezing Point of Saline Soil. Cold Reg. Sci. Technol. 2011, 67, 79–88. [Google Scholar] [CrossRef]
- Rich, A.; Mandri, Y.; Bendaoud, N.; Mangin, D.; Abderafi, S.; Bebon, C.; Semlali, N.; Klein, J.-P.; Bounahmidi, T.; Bouhaouss, A.; et al. Freezing Desalination of Sea Water in a Static Layer Crystallizer. Desalination Water Treat. 2010, 13, 120–127. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Li, Z. Desalination and Application of Saline Water in Southern XinJiang Based on Unidirectional Freezing. Environ. Eng. 2019, 37, 126–131, 136. [Google Scholar] [CrossRef]
- Wang, R.; Hu, Y.; Yuan, X.; Chen, J.; Jiang, S.; Li, X. Unsynchronized Migrations of Different Salt Ions and Ice Microstructure Development during Unidirectional Freeze-Thaw. Desalination 2023, 549, 116326. [Google Scholar] [CrossRef]
- Chu, F.; Li, S.; Zhao, C.; Feng, Y.; Lin, Y.; Wu, X.; Yan, X.; Miljkovic, N. Interfacial Ice Sprouting during Salty Water Droplet Freezing. Nat. Commun. 2024, 15, 2249. [Google Scholar] [CrossRef] [PubMed]
- Vrbka, L.; Jungwirth, P. Brine Rejection from Freezing Salt Solutions: A Molecular Dynamics Study. Phys. Rev. Lett. 2005, 95, 148501. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, P.; Osterkamp, T.E.; Weeks, W.F. The Migration of Liquid Inclusions in Single Ice Crystals. J. Geophys. Res. 1965, 70, 5035–5041. [Google Scholar] [CrossRef]
- Cole, D.M.; Shapiro, L.H. Observations of Brine Drainage Networks and Microstructure of First-year Sea Ice. J. Geophys. Res. Ocean. 1998, 103, 21739–21750. [Google Scholar] [CrossRef]
- Notz, D.; Worster, M.G. Desalination Processes of Sea Ice Revisited. J. Geophys. Res. Ocean. 2009, 114, C05006. [Google Scholar] [CrossRef]
- Barma, M.C.; Peng, Z.; Moghtaderi, B.; Doroodchi, E. Freeze Desalination of Drops of Saline Solutions. Desalination 2021, 517, 115265. [Google Scholar] [CrossRef]
- Yuan, H.; Sun, K.; Wang, K.; Zhang, J.; Zhang, Z.; Zhang, L.; Li, S.; Li, Y. Ice Crystal Growth in the Freezing Desalination Process of Binary Water-NaCl System. Desalination 2020, 496, 114737. [Google Scholar] [CrossRef]
- Lofgren, G.; Weeks, W.F. Effect of Growth Parameters on Substructure Spacing in NaCl Ice Crystals. J. Glaciol. 1969, 8, 153–164. [Google Scholar] [CrossRef]
- Luo, C.; Chen, W.; Han, W. Experimental Study on Factors Affecting the Quality of Ice Crystal during the Freezing Concentration for the Brackish Water. Desalination 2010, 260, 231–238. [Google Scholar] [CrossRef]
- Guo, K.; Liu, X. The Primary Research on the Variation of Melted Water Quality and Quantity during Saline Ice Melting. J. Irrig. Drain. 2013, 32, 56–60. [Google Scholar]
- Yang, Y.; Wang, H.; Huang, W.; Gao, Y.; Li, Z.; Wang, X. Ion Migration during Freeze-Thaw Process: A Cryo-Desalination Experiment of Saltwater from Southern Xinjiang, China. Desalination 2022, 544, 116118. [Google Scholar] [CrossRef]
- Lv, H.; Li, C.; Shi, X.; Zhao, S.; Yang, F.; Yong, W.; Shuang, S. Pollutant Distribution under Different Conditions in Lake Ulansuhai Ice-Water System. J. Lake Sci. 2015, 27, 1151–1158. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T.; Tang, Y.; Ren, F.; Zhao, T.; Liu, Y. The Migration Law of Magnesium Ions during Freezing and Melting Processes. Environ. Sci. Pollut. Res. 2022, 29, 26675–26687. [Google Scholar] [CrossRef]
- Cottier, F.; Eicken, H.; Wadhams, P. Linkages between Salinity and Brine Channel Distribution in Young Sea Ice. J. Geophys. Res. Ocean. 1999, 104, 15859–15871. [Google Scholar] [CrossRef]
- Xu, H.; Spitler, J.D. The Relative Importance of Moisture Transfer, Soil Freezing and Snow Cover on Ground Temperature Predictions. Renew. Energy 2014, 72, 1–11. [Google Scholar] [CrossRef]
- Teubner, I.E.; Haimberger, L.; Hantel, M. Estimating Snow Cover Duration from Ground Temperature. J. Appl. Meteorol. Climatol. 2015, 54, 959–965. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; He, X.; Lin, E.; Yang, G. Winter Irrigation Effects on Soil Moisture, Temperature and Salinity, and on Cotton Growth in Salinized Fields in Northern Xinjiang, China. Sustainability 2020, 12, 7573. [Google Scholar] [CrossRef]
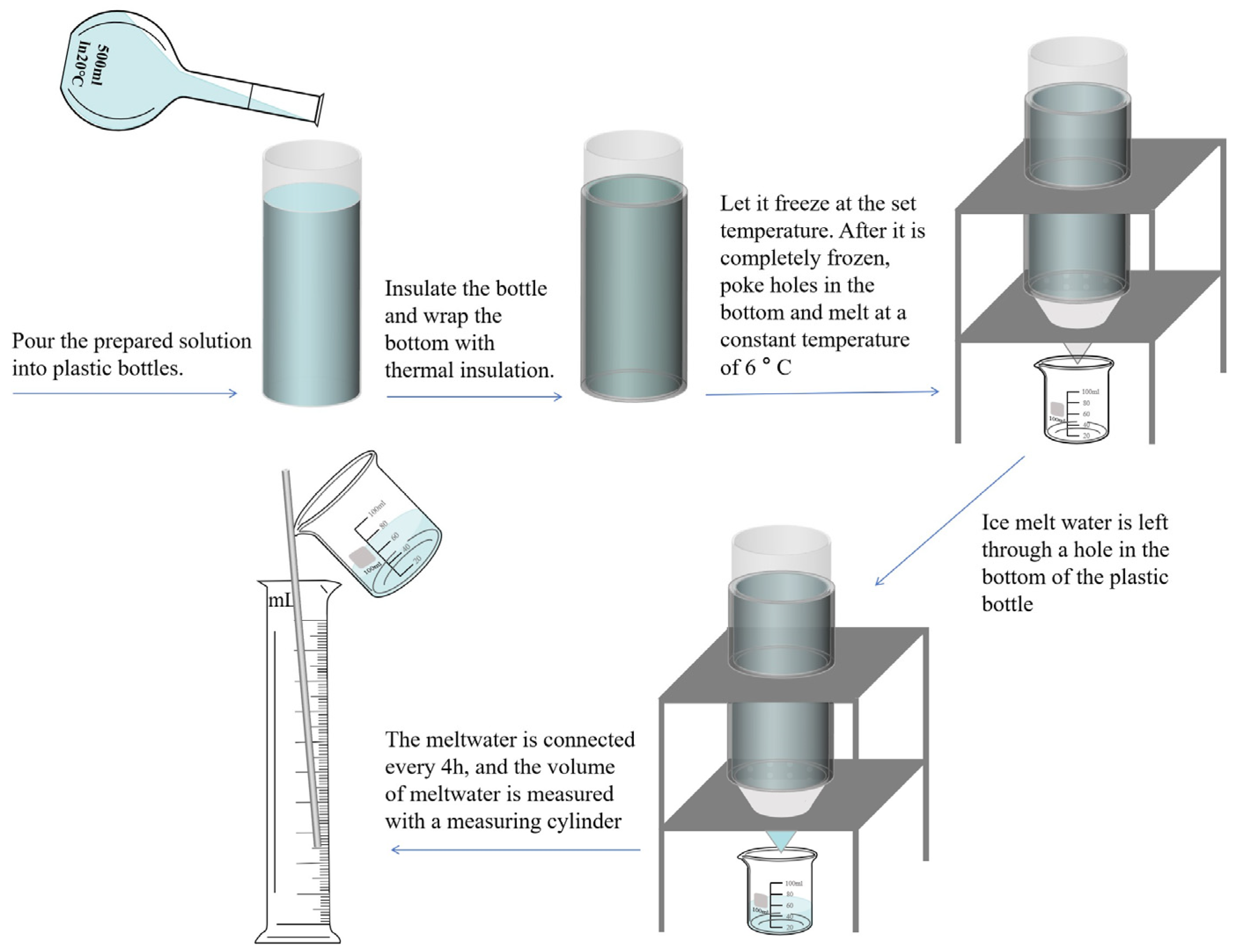


| Experimental Factor Encoding Value | Actual Value of Test Factor | ||
|---|---|---|---|
| Salinity (g/L) | SAR | Freezing Temperatures (°C) | |
| 1.682 | 23 | 52 | −6 |
| 1 | 19 | 42 | −9 |
| 0 | 13 | 27 | −14 |
| −1 | 7 | 12 | −19 |
| −1.682 | 3 | 2 | −22 |
| Processing Number | Coded Value | Actual Value | ||||
|---|---|---|---|---|---|---|
| Salinity | SAR | Freezing Temperatures | Salinity (g/L) | SAR | Freezing Temperatures (°C) | |
| 1 | 1 | 1 | 1 | 19 | 42 | −9 |
| 2 | 1 | 1 | −1 | 19 | 42 | −19 |
| 3 | 1 | −1 | 1 | 19 | 12 | −9 |
| 4 | 1 | −1 | −1 | 19 | 12 | −19 |
| 5 | −1 | 1 | 1 | 7 | 42 | −9 |
| 6 | −1 | 1 | −1 | 7 | 42 | −19 |
| 7 | −1 | −1 | 1 | 7 | 12 | −9 |
| 8 | −1 | −1 | −1 | 7 | 12 | −19 |
| 9 | 1.682 | 0 | 0 | 23 | 27 | −14 |
| 10 | −1.682 | 0 | 0 | 3 | 27 | −14 |
| 11 | 0 | 1.682 | 0 | 13 | 52 | −14 |
| 12 | 0 | −1.682 | 0 | 13 | 2 | −14 |
| 13 | 0 | 0 | 1.682 | 13 | 27 | −6 |
| 14 | 0 | 0 | −1.682 | 13 | 27 | −22 |
| 15 | 0 | 0 | 0 | 13 | 27 | −14 |
| 16 | 0 | 0 | 0 | 13 | 27 | −14 |
| 17 | 0 | 0 | 0 | 13 | 27 | −14 |
| 18 | 0 | 0 | 0 | 13 | 27 | −14 |
| 19 | 0 | 0 | 0 | 13 | 27 | −14 |
| 20 | 0 | 0 | 0 | 13 | 27 | −14 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 2578.32 | 9 | 286.48 | 33.75 | <0.0001 ** |
| 2040.27 | 1 | 2040.27 | 240.35 | <0.0001 ** | |
| 7.05 | 1 | 7.05 | 0.8309 | 0.3834 | |
| 147.81 | 1 | 147.81 | 17.41 | 0.0019 ** | |
| 1.63 | 1 | 1.63 | 0.1921 | 0.6705 | |
| 37.27 | 1 | 37.27 | 4.39 | 0.0626 | |
| 85.36 | 1 | 85.36 | 10.06 | 0.0100 ** | |
| 134.10 | 1 | 134.10 | 15.80 | 0.0026 ** | |
| 30.11 | 1 | 30.11 | 3.55 | 0.0890 | |
| 74.85 | 1 | 74.85 | 8.82 | 0.0141 * | |
| Residual | 84.89 | 10 | 8.49 | ||
| Lack of Fit | 62.21 | 5 | 12.44 | 2.74 | 0.1462 |
| Pure Error | 22.68 | 5 | 4.54 | ||
| Cor Total | 2663.21 | 19 | |||
| R2 | 0.9681 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Ding, X.; Yuan, J.; Xu, W.; Liu, J. Optimization Study on the Freshwater Production Ratio from the Freezing and Thawing Process of Saline Water with Varied Qualities. Agronomy 2025, 15, 33. https://doi.org/10.3390/agronomy15010033
Feng X, Ding X, Yuan J, Xu W, Liu J. Optimization Study on the Freshwater Production Ratio from the Freezing and Thawing Process of Saline Water with Varied Qualities. Agronomy. 2025; 15(1):33. https://doi.org/10.3390/agronomy15010033
Chicago/Turabian StyleFeng, Xinyu, Xue Ding, Jiale Yuan, Wanli Xu, and Jiao Liu. 2025. "Optimization Study on the Freshwater Production Ratio from the Freezing and Thawing Process of Saline Water with Varied Qualities" Agronomy 15, no. 1: 33. https://doi.org/10.3390/agronomy15010033
APA StyleFeng, X., Ding, X., Yuan, J., Xu, W., & Liu, J. (2025). Optimization Study on the Freshwater Production Ratio from the Freezing and Thawing Process of Saline Water with Varied Qualities. Agronomy, 15(1), 33. https://doi.org/10.3390/agronomy15010033





