The Detection of Soybean Bacterial Blight Based on Polarization Spectral Imaging Techniques
Abstract
:1. Introduction
2. Materials and Methods
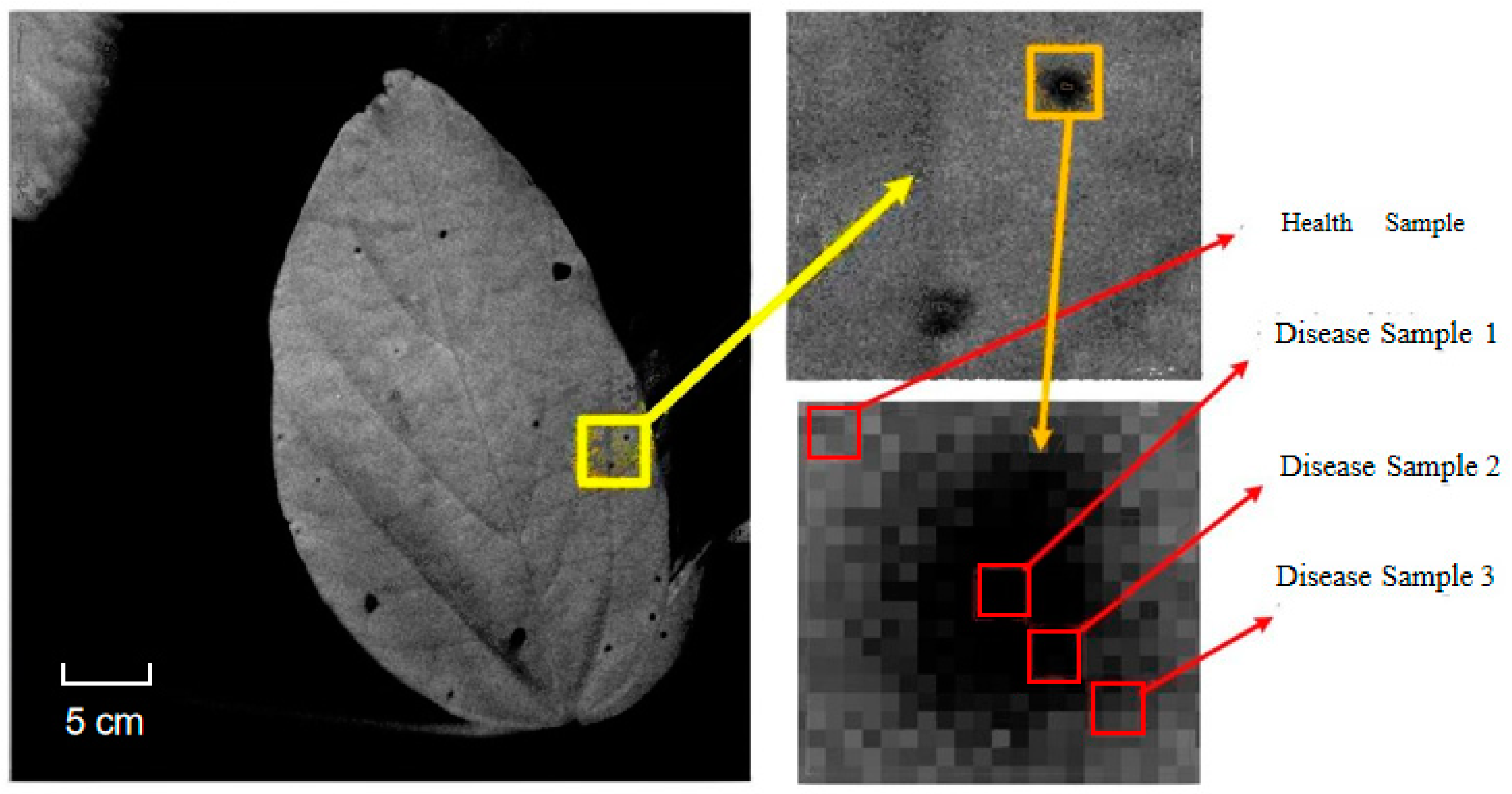
2.1. Source of Disease Object
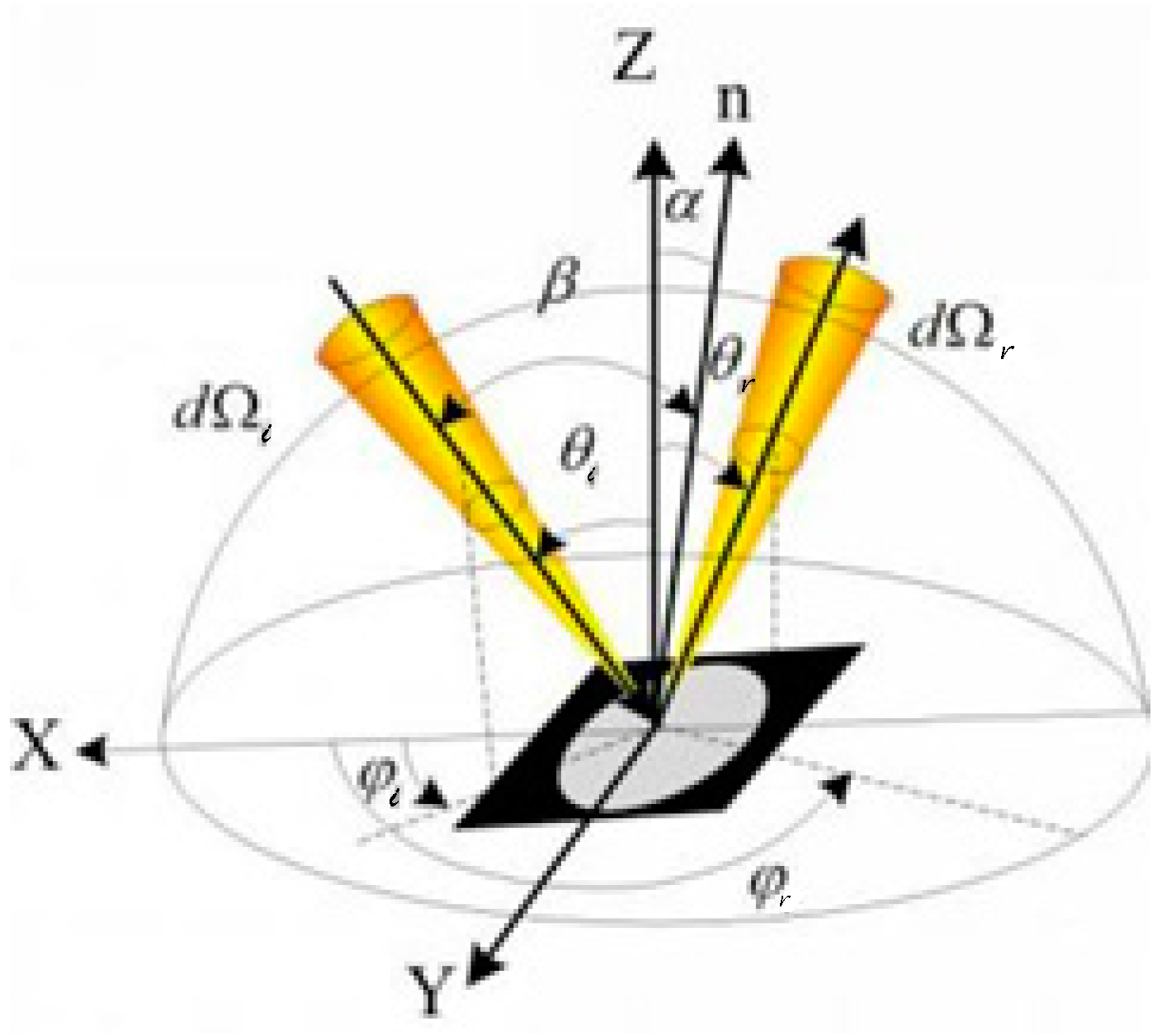
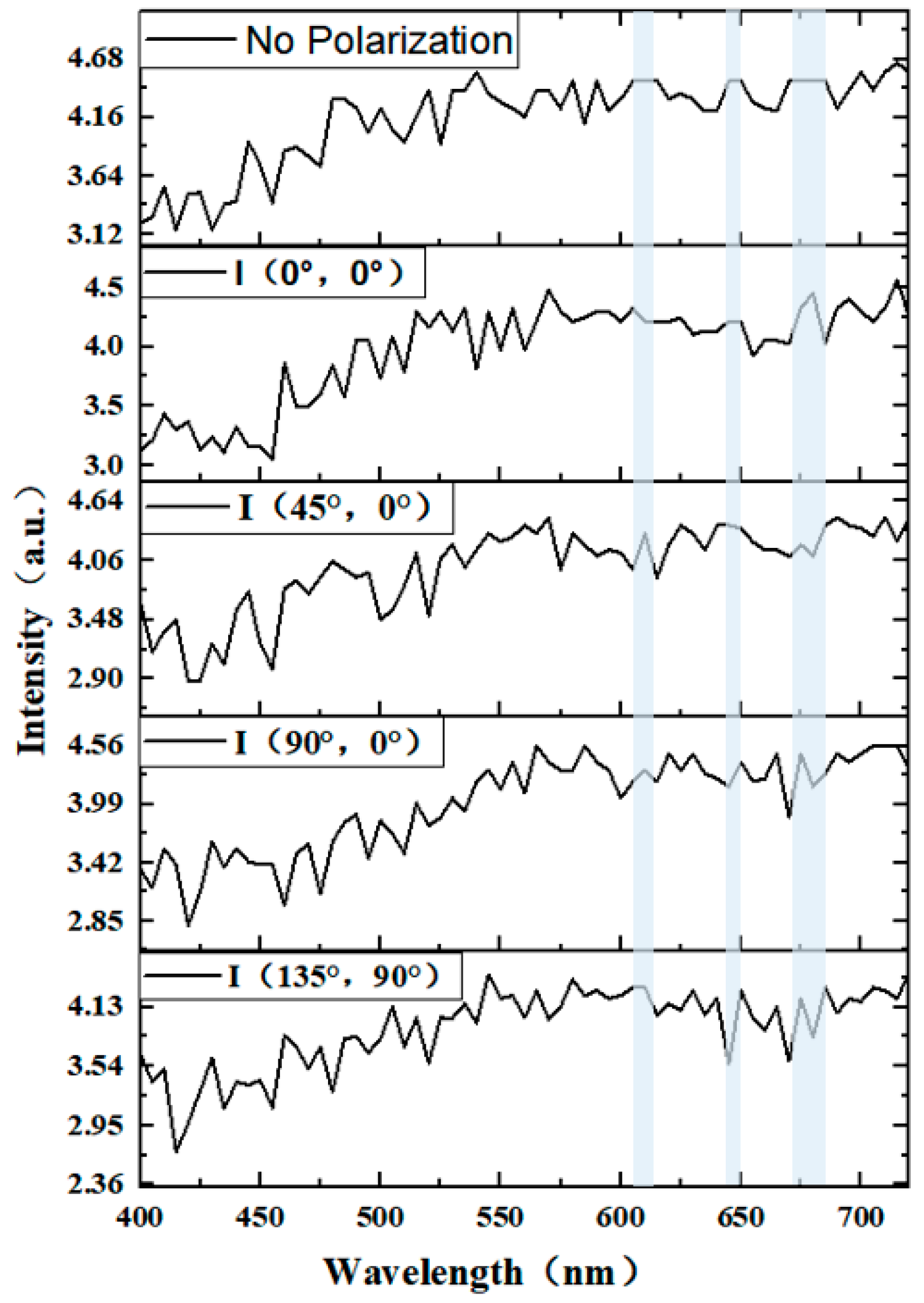
2.2. pBRDF Detection Method of Polarization Reflection Spectrum of Target Surface
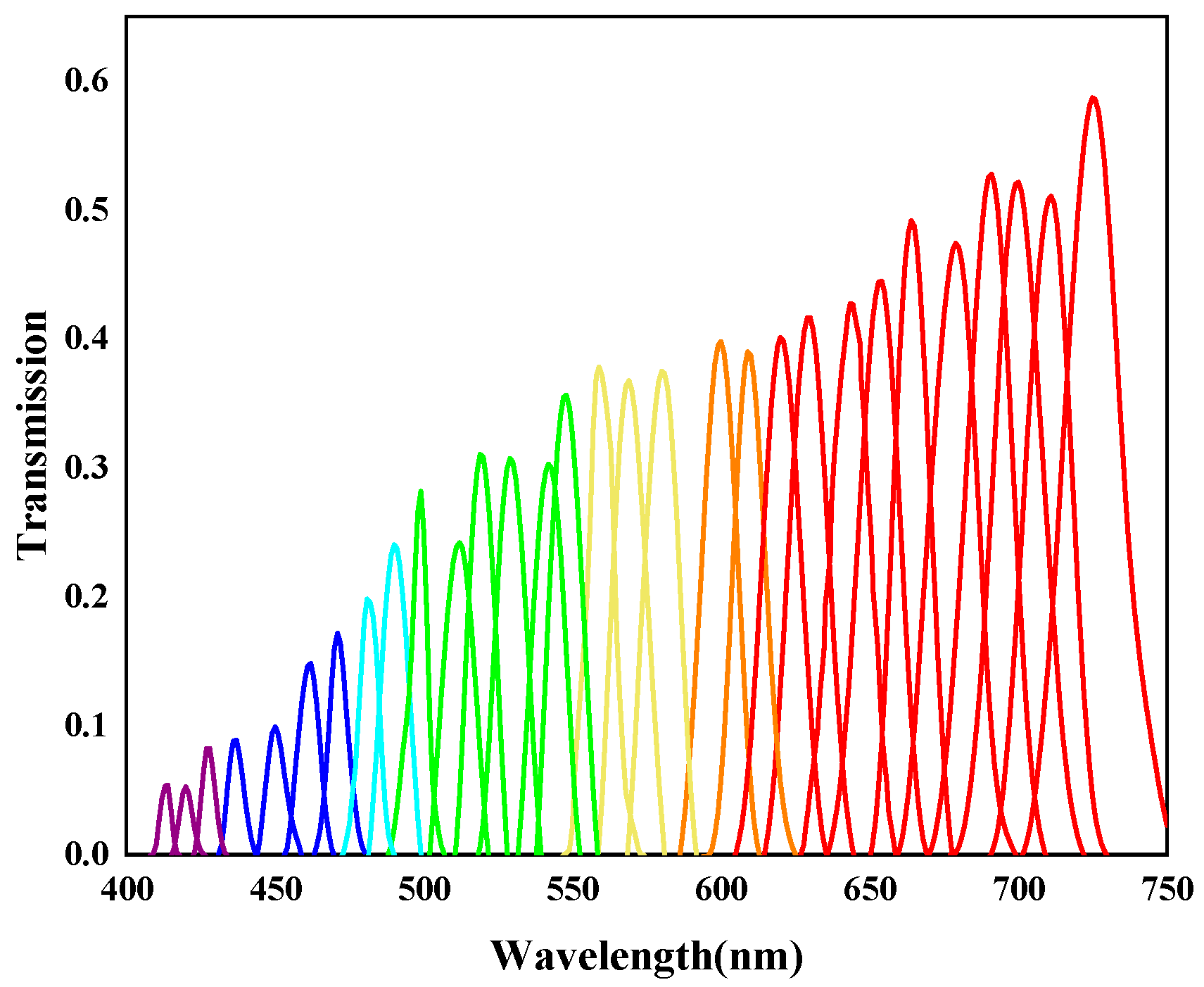
2.3. Multi-Dimensional Fusion Image Information Extraction Method
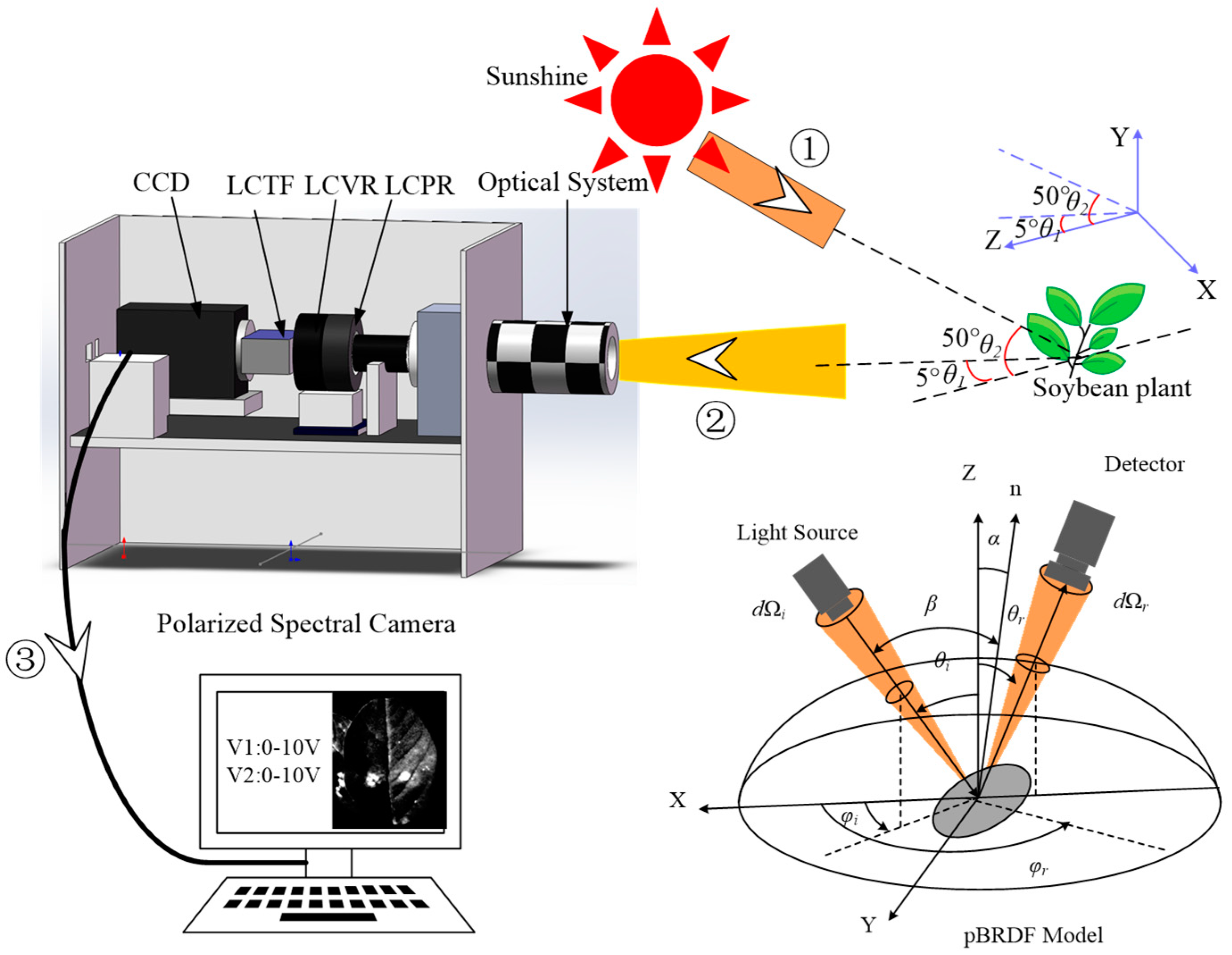
2.4. Experimental Device and Process
3. Results and Discussion
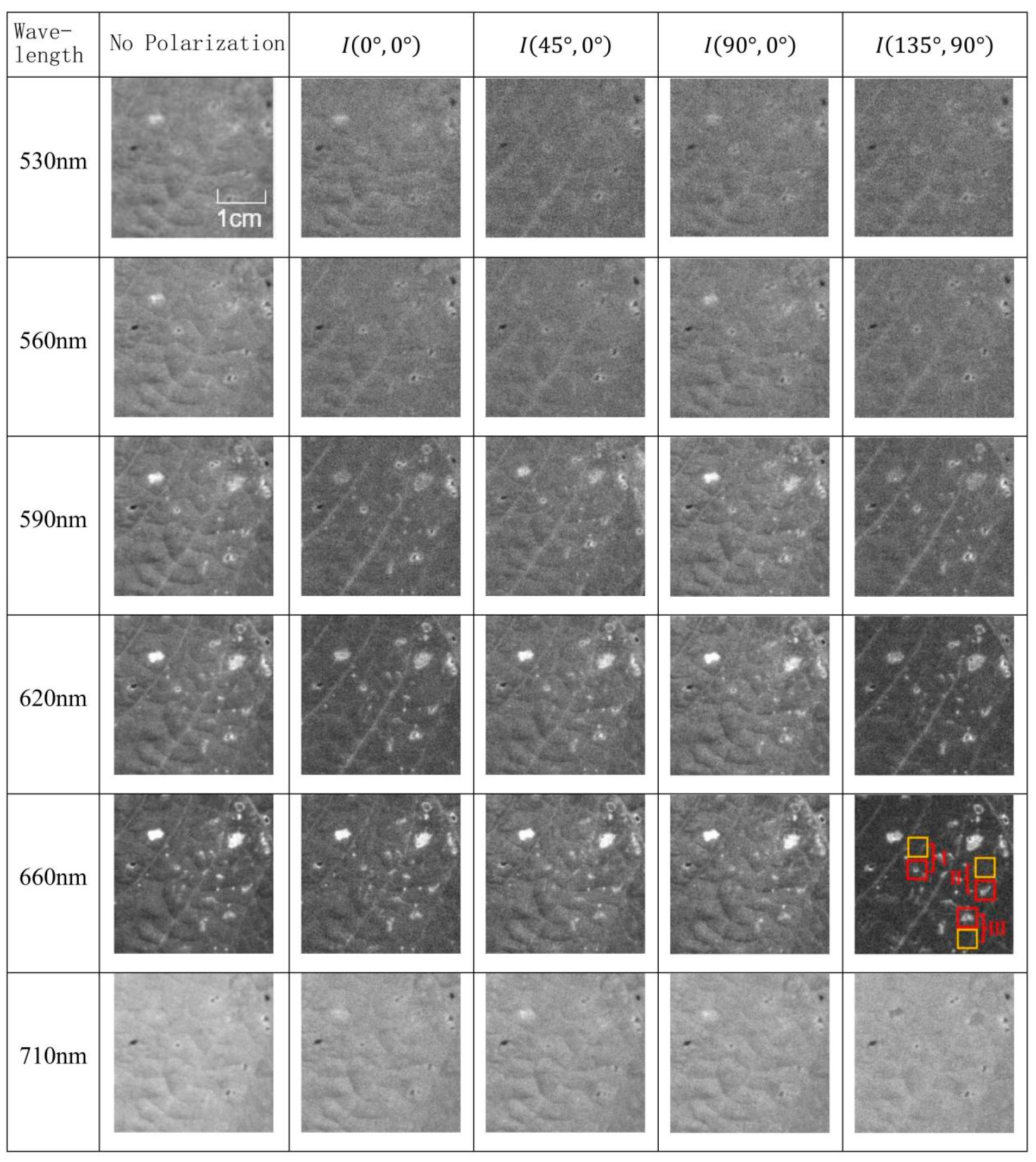
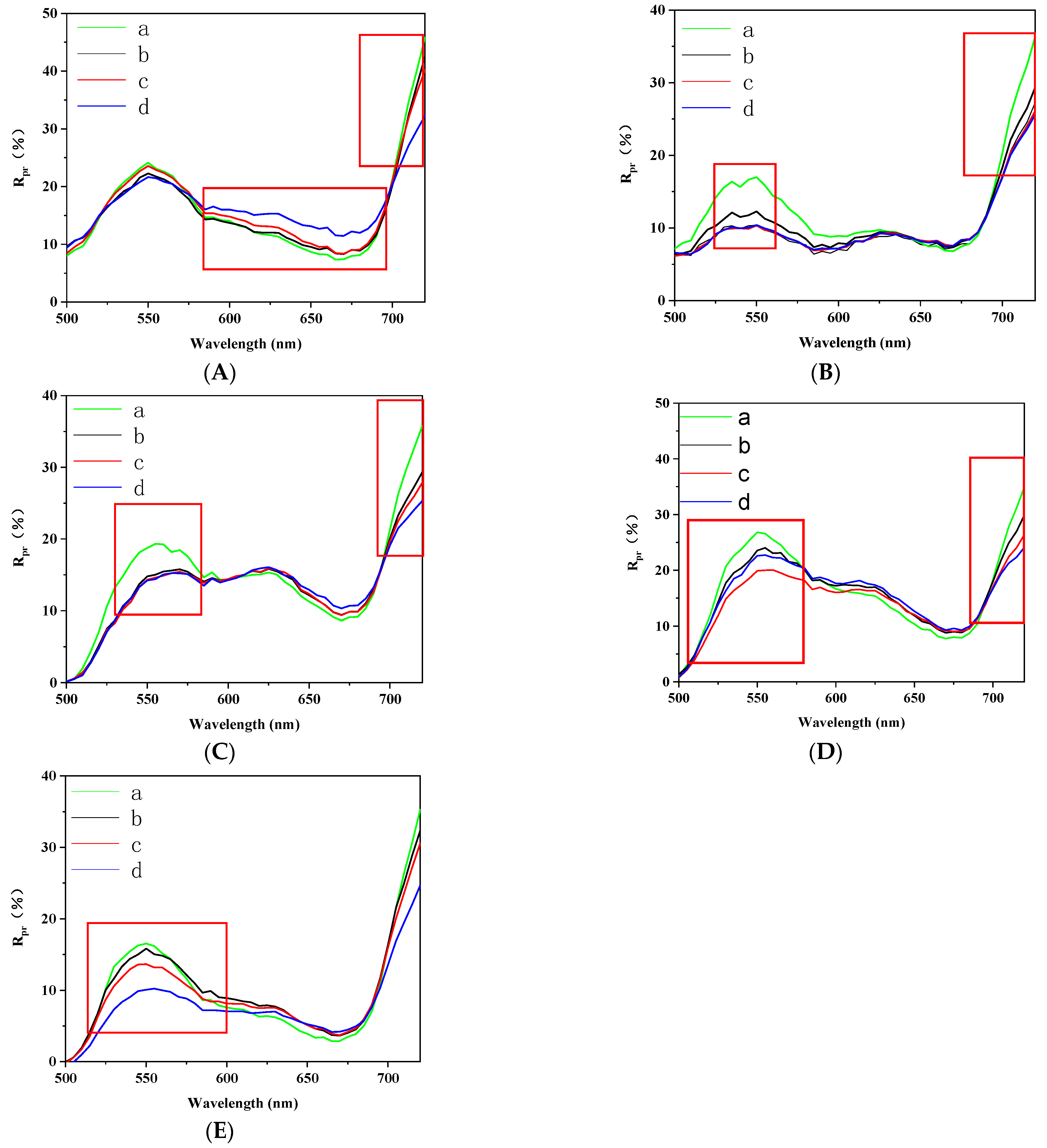
Analysis of Polarization Spectrum Characteristics of Diseased Leaves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Williams, D.J.; Nyvall, R.F. Leaf infection and yield losses caused by brown spot and bacterial blight diseases of soybean. Dis. Detect. Crop Losses 1980, 70, 900–902. [Google Scholar] [CrossRef]
- Sotelo, J.P.; Rovey, M.F.P.; María, E.C.; Moliva, M.V.; Oliva, M.D.L.M. Characterization of Pseudomonas syringae strains associated with soybean bacterial blight and in vitro inhibitory effect of oregano and thyme essential oils. Physiol. Mol. Plant Pathol. 2023, 128, 102133. [Google Scholar] [CrossRef]
- Zahra, A.; Qureshi, R.; Sajjad, M.; Sadak, F.; Nawaz, M.; Khan, H.A.; Uzair, M. Current advances in imaging spectroscopy and its state-of-the-art applications. Expert Syst. Appl. 2024, 238, 122172. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, Y.; Pan, T.; Zhang, Z.G.; Qu, J.Q.; Sun, Y.Z.; Zhao, H.J.; Miao, L.X.; Hu, Z.B.; Zhao, Z.M.; et al. Diagnosis of soybean bacterial blight progress stage based on deep learning in the context of data-deficient. Comput. Electron. Agric. 2023, 212, 108170. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Ali, H.M.; Hatamleh, A.A.; Al-Dosary, M.A.; El-Ballat, E.M. Multi-functional PGPR Serratia liquefaciens confers enhanced resistance to lead stress and bacterial blight in soybean (Glycine max L.). Curr. Plant Biol. 2024, 40, 100403. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Wang, Y.A.; Cheng, W.; Antwi-Boasiako, A.; Yan, W.K.; Zhang, C.T.; Gao, X.W.; Kong, J.J.; Liu, W.S.; Zhao, T.J. Genome-wide association study of bacterial blight resistance in soybean. Plant Dis. 2024. [Google Scholar] [CrossRef]
- Vanderbilt, V.C.; Grant, L.; Daughtry, C.S.T. Polarization of light scattered by vegetation. Proc. IEEE 1985, 73, 1012–1024. [Google Scholar] [CrossRef]
- Polder, G.; Van Der Heijden, G.W.A.M.; Waalwijk, C.; Young, I.T. Detection of Fusarium in single wheat kernels using spectral imaging. Seed Sci. Technol. 2005, 33, 655–668. [Google Scholar] [CrossRef]
- Khadabadi, G.C.; Rajpurohit, V.S.; Kumar, A.; Nargund, V.B. Disease detection in vegetables using image processing techniques: A review. Int. J. Emerg. Technol. Comput. Sci. Electron. 2015, 14, 954–960. [Google Scholar]
- Vannier, N.; Goudail, F.; Plassart, C.; Boffety, M.; Feneyrou, P.; Leviandier, L.; Bertaux, N. Active polarimetric imager with near-infrared laser illumination for adaptive contrast optimization. Appl. Opt. 2015, 54, 7622–7631. [Google Scholar] [CrossRef] [PubMed]
- Vannier, N.; Goudail, F.; Plassart, C.; Boffety, M.; Feneyrou, P.; Leviandier, L.; Bertaux, N. Comparison of diffez‘ent active polarimetric imaging modes for target detection in outdoor environment. Appl. Opt. 2016, 55, 2881–2891. [Google Scholar] [CrossRef]
- Vannier, N.; Goudail, F.; Plassart, C.; Boffety, M.; Feneyrou, P.; Leviandier, L.; Bertaux, N. Infrared active polarimetric imaging system controlled by image segmentation algorithms: Application to decamouflage. Spie Commer. Sci. Sens. Imaging 2016, 9853, 98530. [Google Scholar]
- Pourreza, A.; Lee, W.S.; Etxeberria, E.; Zhang, Y. Identification of citrus Huanglongbing disease at the pre-symptomatic stage using polarized imaging technique. IFAC-Pap. 2016, 49, 110–115. [Google Scholar] [CrossRef]
- Pourreza, A.; Lee, W.S.; Raveh, E.; Ehsani, R.; Etxeberria, E. Citrus Huanglongbing detection using narrow-band imaging and polarized illumination. Trans. ASABE 2014, 57, 259–272. [Google Scholar]
- Sarkar, S.K.; Das, J.; Ehsani, R.; Kumar, V. Towards autonomous phytopathology: Outcomes and challenges of citrus greening disease detection through close-range remote sensing. In Proceedings of the 2016 IEEE International Conference on Robotics and Automation (ICRA), Stockholm, Sweden, 16–21 May 2016; IEEE: New York, NY, USA, 2016; pp. 5143–5148. [Google Scholar]
- Xu, J.L.; Gobrecht, A.; Héran, D.; Gorretta, N.; Coque, M.; Gowen, A.A.; Sun, D.W. A polarized hyperspectral imaging system for in vivo detection: Multiple applications in sunflower leaf analysis. Comput. Electron. Agric. 2019, 158, 258–270. [Google Scholar] [CrossRef]
- Blake, A.J.; Go, M.C.; Hahn, G.S.; Grey, H.; Couture, S.; Gries, G. Polarization of foliar reflectance: Novel host plant cue for insect herbivores. Proc. R. Soc. B 2019, 286, 20192198. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Bonn, G.K.; Popp, M.; Huck, C.W. Principles and applications of vibrational spectroscopic imaging in plant science: A review. Front. Plant Sci. 2020, 11, 1226. [Google Scholar] [CrossRef] [PubMed]
- Knight, K. Cabbage whites have a unique take on polarized light. J. Exp. Biol. 2020, 223, jeb230441. [Google Scholar] [CrossRef]
- Zhu, W.; Li, J.; Li, L.; Wang, A.; Wei, X.; Mao, H. Nondestructive diagnostics of soluble sugar, total nitrogen and their ratio of tomato leaves in greenhouse by polarized spectra–hyperspectral data fusion. Int. J. Agric. Biol. Eng. 2020, 13, 189–197. [Google Scholar] [CrossRef]
- Peng, Y.; Dallas, M.M.; Ascencio-Ibáñez, J.T.; Hoyer, J.S.; Legg, J.; Hanley-Bowdoin, L.; Yin, H. Early detection of plant virus infection using multispectral imaging and spatial–spectral machine learning. Sci. Rep. 2022, 12, 3113. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiao, J.; Wang, C. Research on polarized multi-spectral system and fusion algorithm for remote sensing of vegetation status at night. Remote Sens. 2021, 13, 3510. [Google Scholar] [CrossRef]
- Xu, J.Y.; Luo, H.P.; Suo, Y.T.; Chen, C.; Li, W.; Wang, C.X. Application of Polarization Spectral in NDT of Jujube Leaves. Xinjiang Agric. Mech. 2021, 23–26. [Google Scholar] [CrossRef]
- Ye, Y.; Tan, Y.; Jin, G.Y. Accurate measurement for damage evolution of ceramics caused by nanosecond laser pulses with polarization spectrum imaging. Opt. Express 2019, 27, 16360–16376. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, J.; Jiang, H.; Tan, Y. The Detection of Soybean Bacterial Blight Based on Polarization Spectral Imaging Techniques. Agronomy 2025, 15, 50. https://doi.org/10.3390/agronomy15010050
Yi J, Jiang H, Tan Y. The Detection of Soybean Bacterial Blight Based on Polarization Spectral Imaging Techniques. Agronomy. 2025; 15(1):50. https://doi.org/10.3390/agronomy15010050
Chicago/Turabian StyleYi, Jia, Huilin Jiang, and Yong Tan. 2025. "The Detection of Soybean Bacterial Blight Based on Polarization Spectral Imaging Techniques" Agronomy 15, no. 1: 50. https://doi.org/10.3390/agronomy15010050
APA StyleYi, J., Jiang, H., & Tan, Y. (2025). The Detection of Soybean Bacterial Blight Based on Polarization Spectral Imaging Techniques. Agronomy, 15(1), 50. https://doi.org/10.3390/agronomy15010050





