Population Dynamics of Cypripedium macranthos Sw. and Its Interactions with Environmental Factors in the Changbai Mountains
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Materials
2.3. Determination of Soil Physicochemical Properties
2.4. Morphological Index Determination
2.4.1. Determination of Plant Growth Index
2.4.2. Microstructure of Root
2.5. Physiological Index Determination
2.5.1. Determination of Chlorophyll Concentration, Antioxidant Enzyme Activities, and Osmoregulation Substance Concentration
2.5.2. Determination of Photosynthetic Parameters
2.5.3. Determination of Chlorophyll-Fluorescence Parameters
2.5.4. Determination of δ13C, δ15N and C and N Concentrations of Leaves
2.6. Statistical Analyses
3. Results
3.1. Habitat Characteristics
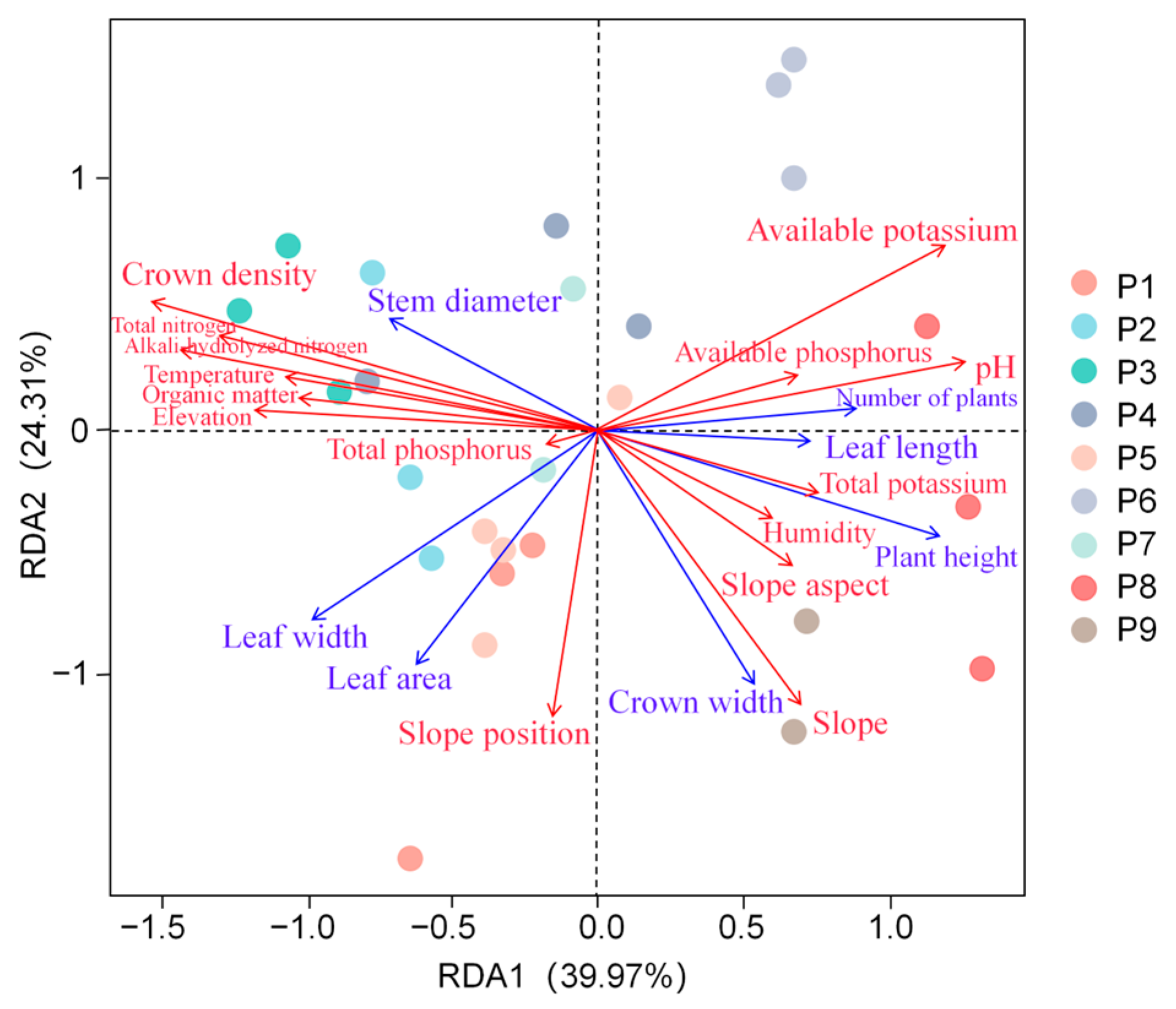
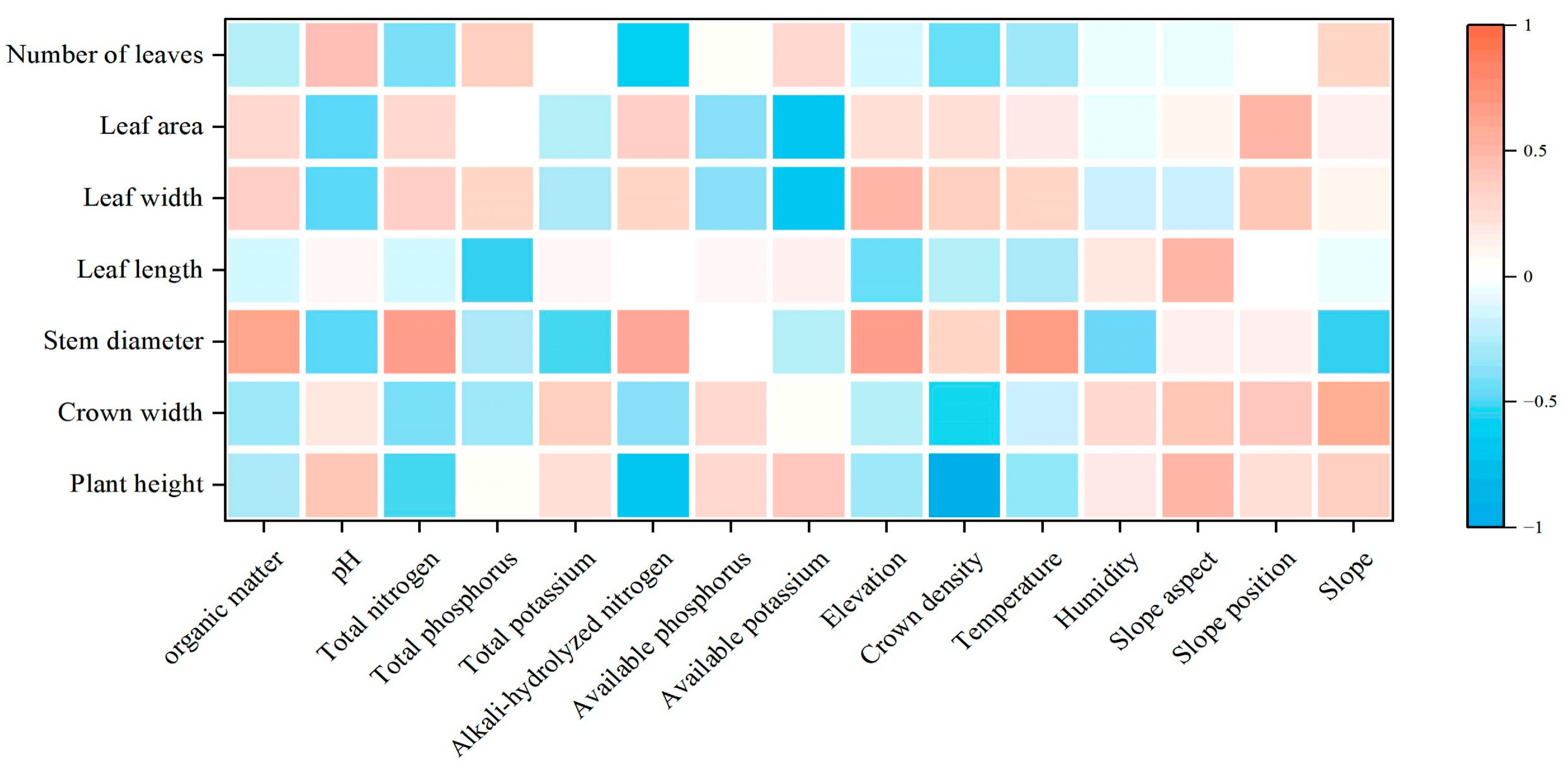
3.2. Morphological Characters of C. macranthos and Their Correlation with Environmental Factors
3.2.1. Plant Growth Metrics of C. macranthos and Their Correlation with Environmental Factors
3.2.2. Root Microstructure and Fungal Infestation
3.3. Physiological Characteristics of C. macranthos and Their Correlation with Environmental Factors
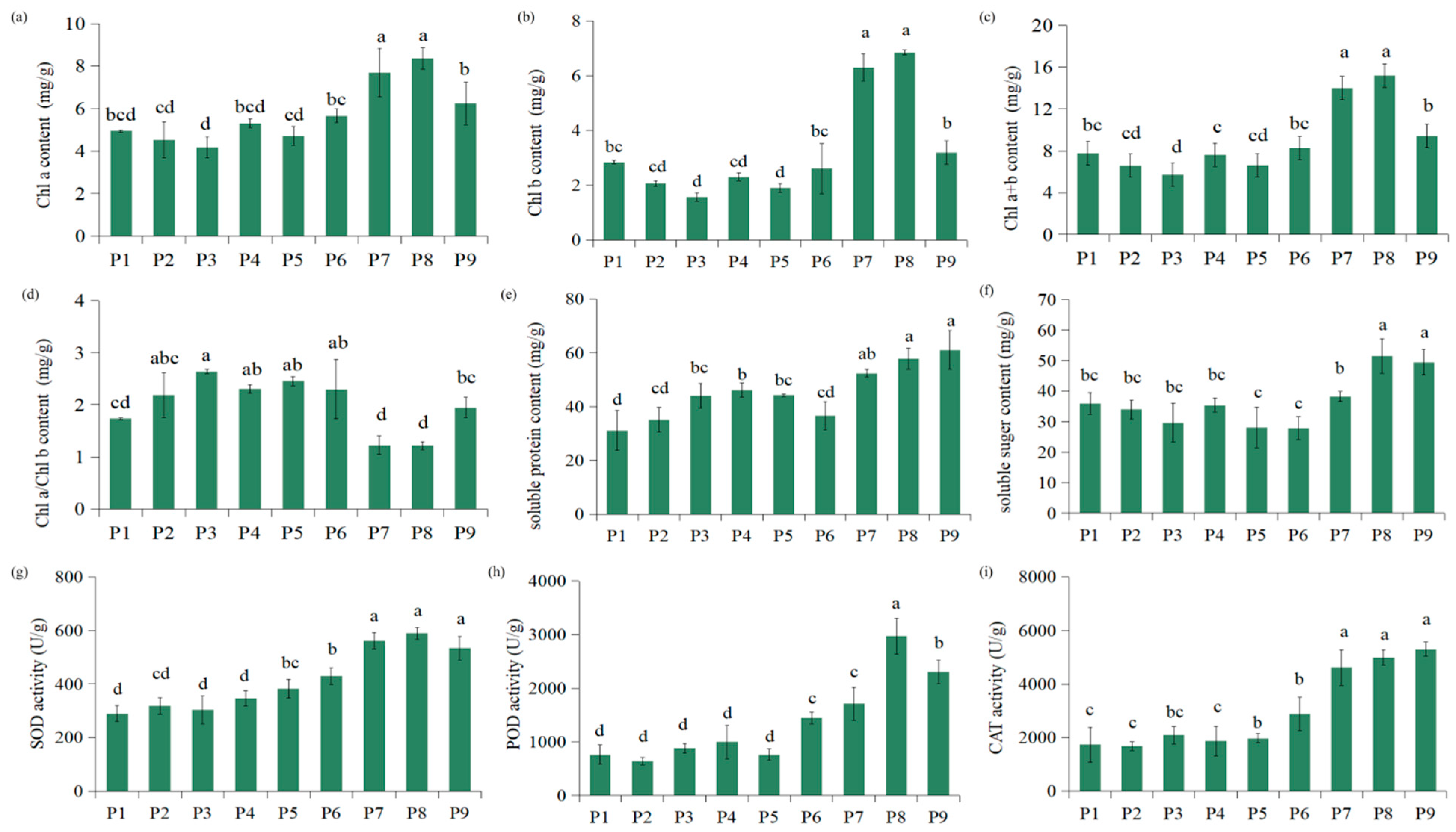
3.3.1. Effects of Different Environments on the Chlorophyll Concentration, Antioxidant Enzyme Activities, and Osmoregulation Substance Concentration of C. macranthos
3.3.2. Effects of Different Environments on the Photosynthetic Properties of C. macranthos
3.3.3. Effects of Different Environments on δ13C, δ15N, C and N Concentrations of C. macranthos
3.3.4. Correlation Analysis of Photosynthetic Indices with δ13C, δ15N and C and N Concentrations
4. Discussion
4.1. Distribution Population Characteristics of C. macranthos in Changbai Mountains
4.2. Morphological Characteristics of Different Populations of C. macranthos and Their Relationship with Environmental Factors
4.2.1. Relationship Between Plant Growth and Environmental Factors in C. macranthos
4.2.2. Relationships Between Root Microstructure and Fungal Infestation in C. macranthos and Environmental Factors
4.3. Physiological Characteristics of Different Populations of C. macranthos and Their Relationship with Environmental Factors
4.3.1. Relationships Between Chlorophyll Concentration, Antioxidant Enzyme Activities, and Osmoregulation Substance Concentration in C. macranthos and Environmental Factors
4.3.2. Relationships Between Photosynthetic Properties of C. macranthos and Environmental Factors
4.3.3. Relationships Between δ13C, δ15N and C and N Concentrations of C. macranthos Leaves and Environmental Factors
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Christenhusz, M.J.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Moreno-Camarena, M.; Ortega-Larrocea, M.P. Mesoamerican Cypripedium: Mycorrhizal contributions to promote their conservation as critically endangered species. Plants 2022, 11, 1554. [Google Scholar] [CrossRef]
- Chang, J.T.; Zhang, Y.; Andronova, E.; Averyanov, L.V.; Filippov, E.G.; Kawahara, T.; Shefferson, R.; Kim, J.H.; Hsu, R.C.C.; Cho, Y.H.; et al. Middle pleistocene climate change influenced northward divergence and cold adaptation in an Asian lady’s slipper orchid, Cypripedium macranthos Sw.(Orchidaceae). Bot. J. Linn. Soc. 2024, 205, 150–160. [Google Scholar] [CrossRef]
- Liu, H.; Jacquemyn, H.; He, X.; Chen, W.; Huang, Y.; Yu, S.; Lu, Y.; Zhang, Y. The impact of human pressure and climate change on the habitat availability and protection of Cypripedium (Orchidaceae) in Northeast China. Plants 2021, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lee, Y.I.; Deng, L.; Zhao, S. Asymbiotic germination of immature seeds and the seedling development of Cypripedium macranthos Sw., an endangered lady’s slipper orchid. Sci. Hortic. 2013, 164, 130–136. [Google Scholar] [CrossRef]
- Fay, M.F. Orchid conservation: Further links. Ann. Bot. 2016, 118, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Shi, R.; Zhang, Y.; Xing, X.; Jin, X. Orchid conservation in China from 2000 to 2020: Achievements and perspectives. Plant Divers. 2021, 43, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sotomayor, A.; Weiss, A.; Paparozzi, E.T.; Arkebauer, T.J. Stability of leaf anatomy and light response curves of field grown maize as a function of age and nitrogen status. J. Plant Physiol. 2002, 159, 819–826. [Google Scholar] [CrossRef]
- Sim-Sim, M.; Lopes, T.; Ruas, S.; Stech, M. Does altitude shape molecular diversity and richness of bryophytes in Madeira’s natural forest? A case study with four bryophyte species at two altitudinal levels. Plant Ecol. Evol. 2015, 148, 171–180. [Google Scholar] [CrossRef]
- Sundqvist, M.K.; Sanders, N.J.; Wardle, D.A. Community and ecosystem responses to elevational gradients: Processes, mechanisms, and insights for global change. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 261–280. [Google Scholar] [CrossRef]
- Yang, J.; El-Kassaby, Y.A.; Guan, W. The effect of slope aspect on vegetation attributes in a mountainous dry valley, Southwest China. Sci. Rep. 2020, 10, 16465. [Google Scholar] [CrossRef]
- Auslander, M.; Nevo, E.; Inbar, M. The effects of slope orientation on plant growth, developmental instability and susceptibility to herbivores. J. Arid Environ. 2003, 55, 405–416. [Google Scholar] [CrossRef]
- Khan, R.; Ma, X.; Hussain, Q.; Chen, K.; Farooq, S.; Asim, M.; Ren, X.; Shah, S.; Shi, Y. Transcriptome and anatomical studies reveal alterations in leaf thickness under long-term drought stress in tobacco. J. Plant Physiol. 2023, 281, 153920. [Google Scholar] [CrossRef]
- Terashima, I.; Hanba, Y.T.; Tholen, D.; Niinemets, Ü. Leaf functional anatomy in relation to photosynthesis. Plant Physiol. 2011, 155, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Fu, X.; Kou, L.; Wang, H.; Shock, C.C. C: N: P stoichiometry of rhizosphere soils differed significantly among overstory trees and understory shrubs in plantations in subtropical China. Can. J. For. Res. 2018, 48, 1398–1405. [Google Scholar] [CrossRef]
- in’t Zandt, D.; van den Brink, A.; de Kroon, H.; Visser, E.J. Plant-soil feedback is shut down when nutrients come to town. Plant Soil. 2019, 439, 541–551. [Google Scholar] [CrossRef]
- Reich, P.B.; Tjoelker, M.G.; Machado, J.L.; Oleksyn, J. Universal scaling of respiratory metabolism, size and nitrogen in plants. Nature 2006, 439, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Shimura, H.; Koda, Y. Micropropagation of Cypripedium macranthos var. rebunense through protocorm-like bodies derived from mature seeds. Plant Cell, Tissue Organ Cult. 2004, 78, 273–276. [Google Scholar] [CrossRef]
- Shimura, H.; Koda, Y. Enhanced symbiotic seed germination of Cypripedium macranthos var. rebunense following inoculation after cold treatment. Physiol. Plant. 2005, 123, 281–287. [Google Scholar] [CrossRef]
- Taniguchi, H.; Katsumi, M.; Yamamoto, Y.; Tatsumi, Y.; Sano, C.M.; Choi, Y.E.; Sano, H. In vitro proliferation and genetic diversity of Cypripedium macranthos var. rebunense. Plant Biotechnol. 2008, 25, 341–346. [Google Scholar] [CrossRef]
- Chung, J.M.; Park, K.W.; Park, C.S.; Lee, S.H.; Chung, M.G.; Chung, M.Y. Contrasting levels of genetic diversity between the historically rare orchid Cypripedium japonicum and the historically common orchid Cypripedium macranthos in South Korea. Bot. J. Linn. Soc. 2009, 160, 119–129. [Google Scholar] [CrossRef]
- Izawa, T.; Kawahara, T.; Takahashi, H. Genetic diversity of an endangered plant, Cypripedium macranthos var. rebunense (Orchidaceae): Background genetic research for future conservation. Conserv. Genet. 2007, 8, 1369–1376. [Google Scholar] [CrossRef]
- Wu, Q.; Dong, S.; Zhao, Y.; Yang, L.; Qi, X.; Ren, Z.; Dong, S.; Cheng, J. Genetic diversity, population genetic structure and gene flow in the rare and endangered wild plant Cypripedium macranthos revealed by genotyping-by-sequencing. BMC Plant Biol. 2023, 23, 254. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, P.; Edens-Meier, R. What we think we know vs. what we need to know about orchid pollination and conservation: Cypripedium L. as a model lineage. Bot. Rev. 2010, 76, 204–219. [Google Scholar] [CrossRef]
- Li, P.; Luo, Y.B.; Bernhardt, P.; Yang, X.Q.; Kou, Y. Deceptive pollination of the Lady’s Slipper Cypripedium tibeticum (Orchidaceae). Plant Syst. Evol. 2006, 262, 53–63. [Google Scholar] [CrossRef]
- Sugiura, N.; Fujie, T.; Inoue, K.; Kitamura, K. Flowering phenology, pollination, and fruit set of Cypripedium macranthos var. rebunense, a threatened lady’s slipper (Orchidaceae). J. Plant Res. 2001, 114, 171–178. [Google Scholar] [CrossRef]
- Sugiura, N. Consistent pollination services to Cypripedium macranthos var. rebunense (Orchidaceae) by Bombus pseudobaicalensis. Plant Species Biol. 2019, 34, 38–42. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Lu, X.; Li, S.; Li, Y.; Shan, Y.; Wang, S.; Zhou, Y.; Chen, L. Effects of different light conditions on morphological, anatomical, photosynthetic and biochemical parameters of Cypripedium macranthos Sw. Photosynth. Res. 2024, 160, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Zhao, F.; Sun, J. An integrated multi-scale approach to restoring a degraded secondary forest ecosystem: A case study in the Changbai Mountains, northeastern China. Ecol. Eng. 2018, 125, 98–105. [Google Scholar] [CrossRef]
- Dai, L.; Qi, L.; Wang, Q.; Su, D.; Yu, D.; Wang, Y.; Ye, Y.; Jiang, S.; Zhao, W. Changes in forest structure and composition on Changbai Mountain in Northeast China. Ann. For. Sci. 2011, 68, 889–897. [Google Scholar] [CrossRef]
- Fiala, A.C.; Garman, S.L.; Gray, A.N. Comparison of five canopy cover estimation techniques in the western Oregon Cascades. For. Ecol. Manage. 2006, 232, 188–197. [Google Scholar] [CrossRef]
- Zhang, Z.; Ai, N.; Liu, G.; Liu, C.; Qiang, F. Soil quality evaluation of various microtopography types at different restoration modes in the loess area of Northern Shaanxi. Catena 2021, 207, 105633. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Chen, L.; Li, S.; Li, Y.; Zhang, Y.; Bai, Y.; Cong, H.; Liu, W.; Zhou, Y. Comparative study of Cypripedium plant photosynthetic characteristics from Changbai Mountain. Horticulturae 2023, 9, 358. [Google Scholar] [CrossRef]
- Schrader, J.; Shi, P.; Royer, D.L.; Peppe, D.J.; Gallagher, R.V.; Li, Y.; Wang, R.; Wright, I.J. Leaf size estimation based on leaf length, width and shape. Ann. Bot. 2021, 128, 395–406. [Google Scholar] [CrossRef]
- Shi, P.; Liu, M.; Yu, X.; Gielis, J.; Ratkowsky, D.A. Proportional relationship between leaf area and the product of leaf length and width of four types of special leaf shapes. Forests 2019, 10, 178. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Jin, Z.; Yang, Z.; Li, Y. Leaf anatomy, photosynthesis, and chloroplast ultrastructure of Heptacodium miconioides seedlings reveal adaptation to light environment. Environ. Exp. Bot. 2022, 195, 104780. [Google Scholar] [CrossRef]
- Wang, L.; Jia, X.; Zhao, Y.; Zhang, C.; Zhao, J. Effect of arbuscular mycorrhizal fungi in roots on antioxidant enzyme activity in leaves of Robinia pseudoacacia L. seedlings under elevated CO2 and Cd exposure. Environ. Pollut. 2022, 294, 118652. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, L.; Qin, Y.; Lu, B.; Xu, D.; Zhuang, W.; Shu, X.; Zhang, F.; Wang, N.; Wang, Z. Effects of seasonal changes on chlorophyll fluorescence and physiological characteristics in the two Taxus species. Plants 2023, 12, 2636. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yu, J.; Lu, X.; Wang, Q.; Wang, S.; Shan, Y.; Liu, Y.; Meng, Y.; Zhou, Y. Iris typhifolia responses to saline–alkali stress: Germination, antioxidant activity, hormones, and photosynthetic performance. Horticulturae 2024, 10, 588. [Google Scholar] [CrossRef]
- Song, W.; Zhou, Y. Linking leaf δ15N and δ13C with soil fungal biodiversity, ectomycorrhizal and plant pathogenic abundance in forest ecosystems of China. Catena 2021, 200, 105176. [Google Scholar] [CrossRef]
- Djordjević, V.; Tsiftsis, S.; Kindlmann, P.; Stevanović, V. Orchid diversity along an altitudinal gradient in the central Balkans. Front. Ecol. Evol. 2022, 10, 929266. [Google Scholar] [CrossRef]
- Swarts, N.D.; Dixon, K.W. Terrestrial orchid conservation in the age of extinction. Ann. Bot. 2009, 104, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.; Dar, M.E.U.I.; Khan, A.M.; Iqbal, T.; Mehmood, A.; Habib, T.; Moussa, I.M.; Casini, F.; Elansary, H.O. Temperature, topography, woody vegetation cover and anthropogenic disturbance shape the orchids distribution in the western Himalaya. S. Afr. J. Bot. 2024, 166, 344–359. [Google Scholar] [CrossRef]
- Wang, H.; Wang, R.; Harrison, S.P.; Prentice, I.C. Leaf morphological traits as adaptations to multiple climate gradients. J. Ecol. 2022, 110, 1344–1355. [Google Scholar] [CrossRef]
- Brelsford, C.C.; Nybakken, L.; Kotilainen, T.K.; Robson, T.M. The influence of spectral composition on spring and autumn phenology in trees. Tree Physiol. 2019, 39, 925–950. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Gao, L.; Wang, W.; Zhang, W.; Wei, J.; Wang, J.; Li, L.; Zhou, Q.; Liang, H.; Liu, Y. Modelling alteration of leaf coloration peak date in cotinus coggygria in a high-elevation karst region. Agric. For. Meteorol. 2022, 323, 109044. [Google Scholar] [CrossRef]
- Chapin, F.S.; Bloom, A.J.; Field, C.B.; Waring, R.H. Plant responses to multiple environmental factors. BioScience 1987, 37, 49–57. [Google Scholar] [CrossRef]
- Perez, J.; Kliewer, W.M. Effect of shading on bud necrosis and bud fruitfulness of Thompson Seedless grapevines. Am. J. Enol. Vitic. 1990, 41, 168–175. [Google Scholar] [CrossRef]
- Mahanta, D.; Rai, R.K.; Dhar, S.; Varghese, E.; Raja, A.; Purakayastha, T.J. Modification of root properties with phosphate solubilizing bacteria and arbuscular mycorrhiza to reduce rock phosphate application in soybean-wheat cropping system. Ecol. Eng. 2018, 111, 31–43. [Google Scholar] [CrossRef]
- Jin, Y.; Liao, M.; Hou, Y.; Wang, H.; Xia, H.; Xia, J.; Wu, K.; Zhou, B.; Li, K.; Ni, J. Spatial patterns and variations in leaf traits of alpine plants on the interior Tibetan Plateau. Glob. Ecol. Conserv. 2024, 53, e03037. [Google Scholar] [CrossRef]
- McCormick, M.K.; Whigham, D.F.; Canchani-Viruet, A. Mycorrhizal fungi affect orchid distribution and population dynamics. New Phytol. 2018, 219, 1207–1215. [Google Scholar] [CrossRef]
- Mujica, M.I.; Saez, N.; Cisternas, M.; Manzano, M.; Armesto, J.J.; Pérez, F. Relationship between soil nutrients and mycorrhizal associations of two Bipinnula species (Orchidaceae) from central Chile. Ann. Bot. 2016, 118, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Shimura, H.; Matsuura, M.; Takada, N.; Koda, Y. An antifungal compound involved in symbiotic germination of Cypripedium macranthos var. rebunense (Orchidaceae). Phytochemistry 2007, 68, 1442–1447. [Google Scholar] [CrossRef]
- Varela-Stasinopoulou, D.S.; Nektarios, P.A.; Tsanakas, G.F.; Ntoulas, N.; Roukounakis, G.I.; Economou, A.S. Impact of substrate depth and irrigation regime on growth, flowering and physiological indices of Greek sage (Salvia fruticosa Mill.) grown on urban extensive green roof systems. Ecol. Eng. 2023, 186, 106816. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Babani, F.; Langsdorf, G. Chlorophyll fluorescence imaging of photosynthetic activity in sun and shade leaves of trees. Photosynth Res. 2007, 93, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.Z.; Cui, H.Y.; Camberato, J.J.; Dong, S.T.; Liu, P.; Zhao, B.; Zhang, J.W. Effects of shading on the photosynthetic characteristics and mesophyll cell ultrastructure of summer maize. Sci. Nat. 2016, 103, 67. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, D.; Wang, J.; Jian, S. Morphological, biochemical and physiological responses of a tropical coastal plant Guettarda speciosa to salt stress. Glob. Ecol. Conserv. 2021, 32, e01887. [Google Scholar] [CrossRef]
- Pociecha, E.; Dziurka, M. Trichoderma interferes with cold acclimation by lowering soluble sugars accumulation resulting in reduced pink snow mould (Microdochium nivale) resistance of winter rye. Environ. Exp. Bot. 2015, 109, 193–200. [Google Scholar] [CrossRef]
- Yadav, S.K. Cold stress tolerance mechanisms in plants. A review. Agron. Sustainable Dev. 2010, 30, 515–527. [Google Scholar] [CrossRef]
- Sadeghpour, N.; Asadi-Gharneh, H.A.; Nasr-Esfahani, M.; Khankahdani, H.H.; Golabadi, M. Antioxidant enzymes associated with resistance to Fusarium oxysporum f. sp. melonis race 1.2 in melon. Physiol. Mol. Plant Pathol. 2022, 121, 101880. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 165102. [Google Scholar] [CrossRef]
- Huang, W.; Reddy, G.V.; Shi, P.; Huang, J.; Hu, H.; Hu, T. Allelopathic effects of Cinnamomum septentrionale leaf litter on Eucalyptus grandis saplings. Glob. Ecol. Conserv. 2020, 21, e00872. [Google Scholar] [CrossRef]
- Pyngrope, S.; Bhoomika, K.; Dubey, R. Reactive oxygen species, ascorbate–glutathione pool, and enzymes of their metabolism in drought-sensitive and tolerant indica rice (Oryza sativa L.) seedlings subjected to progressing levels of water deficit. Protoplasma 2013, 250, 585–600. [Google Scholar] [CrossRef]
- Meravi, N.; Kumar Prajapati, S. Effect street light pollution on the photosynthetic efficiency of different plants. Biol. Rhythm Res. 2020, 51, 67–75. [Google Scholar] [CrossRef]
- Lu, C.; Cao, J.; Guo, S.; Yao, C.; Wang, Z.; Tong, X.; Wu, F. Water use efficiency and its influencing factors following plantations restoration with Caragana korshinskii and Robinia pseudoacacia in the loess hilly region. Ecol. Eng. 2024, 202, 107229. [Google Scholar] [CrossRef]
- da Silva Branco, M.C.; de Almeida, A.A.F.; Dalmolin, Â.C.; Ahnert, D.; Baligar, V.C. Influence of low light intensity and soil flooding on cacao physiology. Sci. Hortic. 2017, 217, 243–257. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Pires, M.V.; Almeida, A.A.F.; Figueiredo, A.L.; Gomes, F.P.; Souza, M.M. Photosynthetic characteristics of ornamental passion flowers grown under different light intensities. Photosynthetica 2011, 49, 593–602. [Google Scholar] [CrossRef]
- Zou, T.; Zhang, J. A new fluorescence quantum yield efficiency retrieval method to simulate chlorophyll fluorescence under natural conditions. Remote Sens. 2020, 12, 4053. [Google Scholar] [CrossRef]
- Liu, Y.C.; Tseng, K.M.; Chen, C.C.; Tsai, Y.T.; Liu, C.H.; Chen, W.H.; Wang, H.L. Warm-night temperature delays spike emergence and alters carbon pool metabolism in the stem and leaves of Phalaenopsis aphroide. Sci. Hortic. 2013, 161, 198–203. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Rascher, U.; Liebig, M.; Lüttge, U. Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ. 2010, 23, 1397–1405. [Google Scholar] [CrossRef]
- Hu, L.L.; Liao, W.B.; Dawuda, M.M.; Yu, J.H.; Lv, J. Appropriate NH4+: NO3− ratio improves low light tolerance of mini Chinese cabbage seedlings. BMC Plant Biol. 2017, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.L.; Mao, S.L.; Wang, L.H.; Zhang, B.X.; Zhang, Z.X. Effect of low light on the characteristics of photosynthesis and chlorophyll a fluorescence during leaf development of sweet pepper. J. Integr. Agric. 2012, 11, 1633–1643. [Google Scholar] [CrossRef]
- Sun, J.L.; Sui, X.L.; Huang, H.Y.; Wang, S.X.; Wei, Y.X.; Zhang, Z.X. Low light stress down-regulated Rubisco gene expression and photosynthetic capacity during cucumber (Cucumis sativus L.) leaf development. J. Integr. Agric. 2014, 13, 997–1007. [Google Scholar] [CrossRef]
- Smallwood, P.A.; Trapnell, D.W. Species distribution modeling reveals recent shifts in suitable habitat for six North American Cypripedium spp.(Orchidaceae). Diversity 2022, 14, 694. [Google Scholar] [CrossRef]
- Yang, Z.H.; Huang, W.; Yang, Q.Y.; Chang, W.; Zhang, S.B. Anatomical and diffusional determinants inside leaves explain the difference in photosynthetic capacity between Cypripedium and Paphiopedilum, Orchidaceae. Photosynth. Res. 2018, 136, 315–328. [Google Scholar] [CrossRef]
- Adams, M.A.; Grierson, P.F. Stable isotopes at natural abundance in terrestrial plant ecology and ecophysiology: An update. Plant Biol. 2001, 3, 299–310. [Google Scholar] [CrossRef]
- Dawson, T.E.; Mambelli, S.; Plamboeck, A.H.; Templer, P.H.; Tu, K.P. Stable isotopes in plant ecology. Annu. Rev. Ecol. Syst. 2002, 33, 507–559. [Google Scholar] [CrossRef]
- Chen, L.; Flynn, D.F.; Zhang, X.; Gao, X.; Lin, L.; Luo, J.; Zhao, C. Divergent patterns of foliar δ13C and δ15N in Quercus aquifolioides with an altitudinal transect on the Tibetan Plateau: An integrated study based on multiple key leaf functional traits. J. Plant Ecol. 2015, 8, 303–312. [Google Scholar] [CrossRef]
- Dearnaley, J.D.W.; Bougoure, J.J. Isotopic and molecular evidence for saprotrophic Marasmiaceae mycobionts in rhizomes of Gastrodia sesamoides. Fungal Ecol. 2010, 3, 288–294. [Google Scholar] [CrossRef]
- Sun, L.; Liu, W.; Liu, G.; Chen, T.; Zhang, W.; Wu, X.; Zhang, G.; Zhang, Y.; Li, L.; Zhang, B.; et al. Temporal and spatial variations in the stable carbon isotope composition and carbon and nitrogen concentrations in current-season twigs of Tamarix chinensis Lour. and their relationships to environmental factors in the Laizhou Bay wetland in China. Ecol. Eng. 2016, 90, 417–426. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, J.; Cheng, Y.; Raza, M.A.; Wu, X.; Wang, Z.; Liu, Q.; Wang, R.; Wang, X.; Yong, T.; et al. Effect of shading and light recovery on the growth, leaf structure, and photosynthetic performance of soybean in a maize-soybean relay-strip intercropping system. PloS ONE 2018, 13, e0198159. [Google Scholar] [CrossRef]
- Chen, H.; Li, Q.P.; Zeng, Y.L.; Deng, F.; Ren, W.J. Effect of different shading materials on grain yield and quality of rice. Sci. Rep. 2019, 9, 9992. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.B.; Huang, R.D.; Zhou, Y.F. Effects of shading stress during the reproductive stages on photosynthetic physiology and yield characteristics of peanut (Arachis hypogaea Linn.). J. Integr. Agric. 2021; 20, 1250–1265. [Google Scholar] [CrossRef]
- Wang, X.; Wu, X.; Ding, G.; Yang, F.; Yong, T.; Wang, X.; Yang, W. Analysis of grain yield differences among soybean cultivars under maize–Soybean intercropping. Agronomy 2020, 10, 110. [Google Scholar] [CrossRef]
- Wang, J.; Shi, K.; Lu, W.; Lu, D. Post-silking shading stress affects leaf nitrogen metabolism of spring maize in southern China. Plants 2020, 9, 210. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Liang, Y.; Han, Y.; Han, Y.; Tan, J. High potassium application rate increased grain yield of shading-stressed winter wheat by improving photosynthesis and photosynthate translocation. Front. Plant Sci. 2020, 11, 134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.B.; Hu, H.; Xu, K.; Li, Z.R. Gas exchanges of three co-occurring species of Cypripedium in a scrubland in the Hengduan Mountains. Photosynthetica 2006, 44, 241–247. [Google Scholar] [CrossRef]
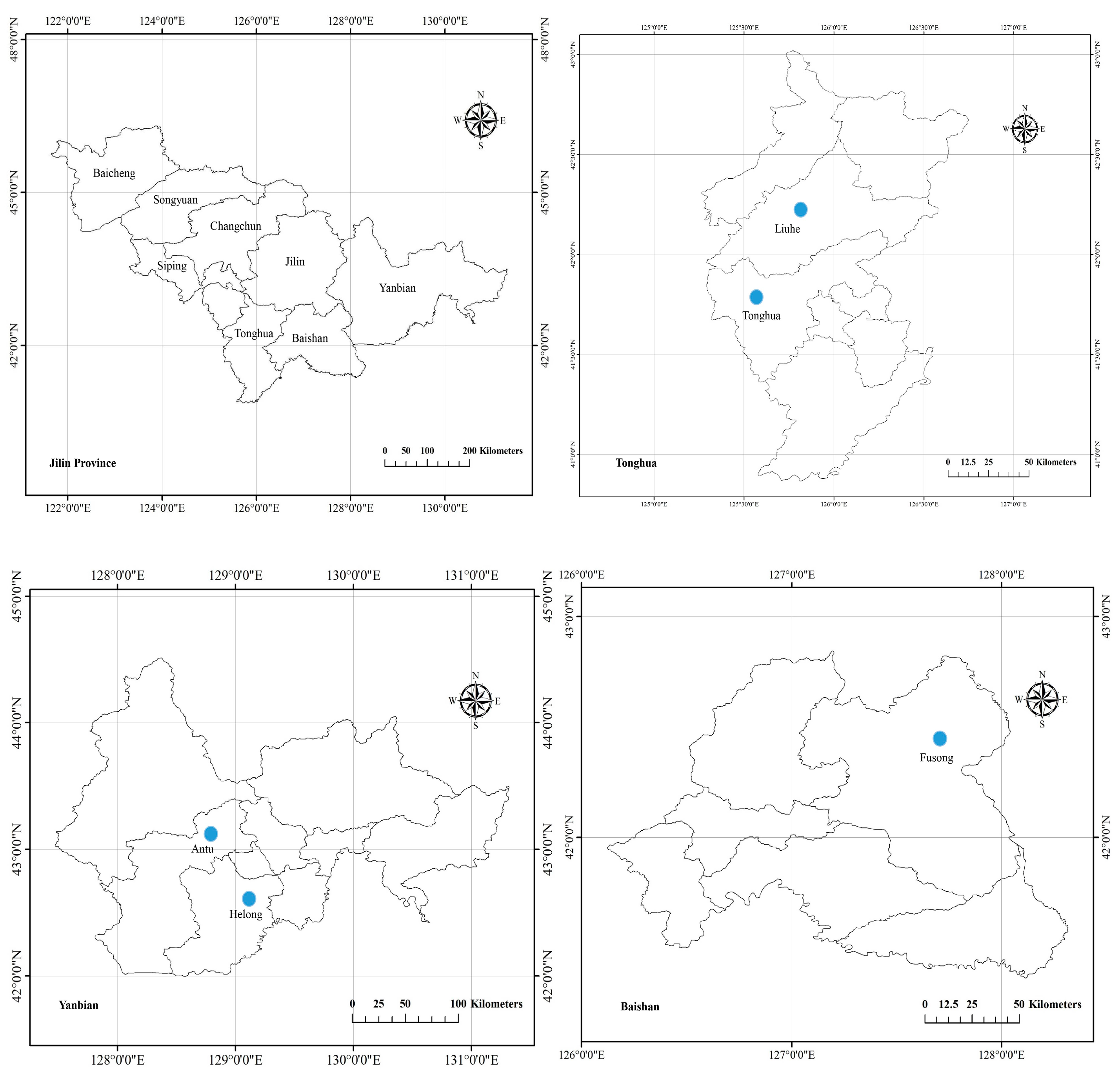



| No.Plot | Distribution Place | Number of Plants (Piece) | Elevation (m) | Crown Density (%) | Temperature (°C) | Humidity (%) | Slope (°) | Slope Aspect | Slope Position | Longitude | Latitude |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | Tonghua County, Tonghua City | 7 | 586 | 91 | 17.5 | 65 | 35 | west | top | E125°50′ | N41°36′ |
| P2 | Fusong County, Baishan City | 8 | 1421 | 91 | 27.5 | 60.8 | 15 | west | top | E127°55′ | N41°57′ |
| P3 | Fusong County, Baishan City | 7 | 1418 | 90 | 26.2 | 56.5 | 15 | west | top | E127°55′ | N41°57′ |
| P4 | Antu County, Yanbian Prefecture | 12 | 679 | 90 | 16.7 | 68.7 | 45 | northwest | bottom | E128°55′ | N43°07′ |
| P5 | Liuhe County, Tonghua City | 6 | 588 | 89 | 20 | 66.6 | 45 | southwest | middle | E125°20′ | N41°51′ |
| P6 | Fusong County, Baishan City | 9 | 435 | 82 | 18.5 | 65.6 | 10 | west | bottom | E127°47′ | N42°48′ |
| P7 | Fusong County, Baishan City | 8 | 435 | 70 | 18.5 | 65.6 | 10 | west | bottom | E127°47′ | N42°48′ |
| P8 | Helong City, Yanbian Prefecture | 17 | 691 | 61 | 18.8 | 62 | 42 | northwest | top | E128°48′ | N42°20′ |
| P9 | Helong City, Yanbian Prefecture | 9 | 685 | 50 | 18.7 | 69.2 | 51 | northwest | top | E128°48′ | N42°20′ |
| No. | pH | Organic Matter (g/kg) | Total Nitrogen (g/kg) | Total Phosphorus (g/kg) | Total Potassium (g/kg) | Alkali-Hydrolyzed Nitrogen (mg/kg) | Available Phosphorus (mg/kg) | Available Potassium (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| P1 | 4.40 ± 0.05 ef | 154.63 ± 10.09 b | 3.11 ± 0.12 b | 0.88 ± 0.03 ac | 27.92 ± 0.29 f | 407.44 ± 8.17 b | 10.03 ± 0.50 de | 287.84 ± 15.6 d |
| P2 | 4.43 ± 0.02 e | 135.56 ± 1.52 c | 2.93 ± 0.05 c | 0.85 ± 0.02 c | 26.00 ± 1.13 f | 386.76 ± 2.29 b | 7.07 ± 1.27 e | 273.97 ± 0.58 d |
| P3 | 4.25 ± 0.04 f | 193.73 ± 14.28 a | 3.53 ± 0.02 a | 0.85 ± 0.02 c | 23.48 ± 0.29 f | 476.79 ± 20.41 a | 15.48 ± 1.86 b | 353.97 ± 2.89 c |
| P4 | 5.94 ± 0.05 ab | 116.92 ± 2.68 de | 2.67 ± 0.02 d | 1.31 ± 0.50 a | 32.19 ± 0.72 cd | 167.17 ± 2.14 e | 14.85 ± 1.41 bc | 426.20 ± 4.36 b |
| P5 | 5.72 ± 0.18 c | 108.57 ± 2.55 e | 2.67 ± 0.01 d | 0.40 ± 0.07 d | 34.81 ± 2.23 b | 268.72 ± 1.04 d | 15.18 ± 0.91 bc | 405.53 ± 2.52 c |
| P6 | 5.56 ± 0.05 d | 134.70 ± 1.90 c | 2.92 ± 0.01 c | 0.41 ± 0.06 d | 30.71 ± 0.53 de | 332.97 ± 1.79 c | 15.54 ± 1.28 b | 473.2 ± 2.00 a |
| P7 | 5.99 ± 0.09 a | 133.19 ± 2.73 c | 2.85 ± 0.02 c | 1.16 ± 0.04 ac | 38.08 ± 0.64 a | 246.98 ± 2.04 d | 12.00 ± 0.69 cd | 502.87 ± 2.52 a |
| P8 | 5.82 ± 0.05 bc | 127.14 ± 1.92 cd | 2.68 ± 0.02 d | 0.97 ± 0.05 ac | 29.66 ± 0.72 ef | 155.57 ± 0.63 e | 13.35 ± 3.37 bc | 432.53 ± 1.53 b |
| P9 | 5.51 ± 0.06 d | 126.18 ± 0.76 cd | 2.60 ± 0.00 d | 0.77 ± 0.02 bc | 33.06 ± 0.52 bc | 155.06 ± 3.59 e | 18.96 ± 0.87 a | 435.20 ± 1.73 b |
| No. | Plant Height (cm) | Crown Width (cm) | Stem Diameter (cm) | Leaf Length (cm) | Leaf Width (cm) | Leaf Area (cm2) | Number of Leaves (Piece) |
|---|---|---|---|---|---|---|---|
| P1 | 35.33 ± 0.58 cd | 24.07 ± 0.98 cd | 0.32 ± 0.03 c | 16.40 ± 0.35 b | 6.93 ± 0.49 a | 89.35 ± 8.31 a | 5.00 ± 0.00 b |
| P2 | 34.50 ± 2.18 cde | 21.33 ± 2.02 de | 0.47 ± 0.06 ab | 15.63 ± 0.06 bcd | 6.80 ± 0.26 a | 83.46 ± 3.46 abc | 5.33 ± 0.58 ab |
| P3 | 31.90 ± 1.82 e | 20.50 ± 2.29 de | 0.57 ± 0.06 a | 14.97 ± 0.25 d | 6.80 ± 0.10 a | 79.90 ± 2.24 abc | 5.33 ± 0.58 ab |
| P4 | 33.73 ± 1.14 de | 19.50 ± 2.29 e | 0.34 ± 0.13 c | 14.80 ± 0.26 d | 6.57 ± 0.38 ab | 76.30 ± 4.88 bc | 6.00 ± 0.00 a |
| P5 | 33.03 ± 0.61 de | 27.17 ± 0.29 ab | 0.35 ± 0.05 bc | 15.83 ± 0.61 bc | 6.40 ± 0.36 ab | 79.65 ± 7.37 abc | 5.00 ± 0.00 b |
| P6 | 37.27 ± 0.96 bc | 20.00 ± 1.00 de | 0.43 ± 0.02 bc | 17.40 ± 0.10 a | 5.17 ± 0.12 c | 70.58 ± 1.94 c | 5.33 ± 0.58 ab |
| P7 | 38.40 ± 0.92 b | 22.83 ± 2.36 cde | 0.37 ± 0.02 bc | 15.47 ± 0.25 cd | 6.33 ± 0.32 ab | 76.86 ± 2.89 bc | 5.00 ± 0.00 b |
| P8 | 48.70 ± 1.41 a | 25.67 ± 3.01 ab | 0.34 ± 0.02 c | 16.30 ± 0.72 bc | 6.03 ± 0.15 b | 77.25 ± 5.25 bc | 5.00 ± 0.00 b |
| P9 | 46.83 ± 1.76 a | 28.73 ± 2.54 a | 0.33 ± 0.06 c | 16.17 ± 0.61 bc | 6.13 ± 0.31 b | 77.91 ± 6.37 bc | 5.33 ± 0.58 ab |
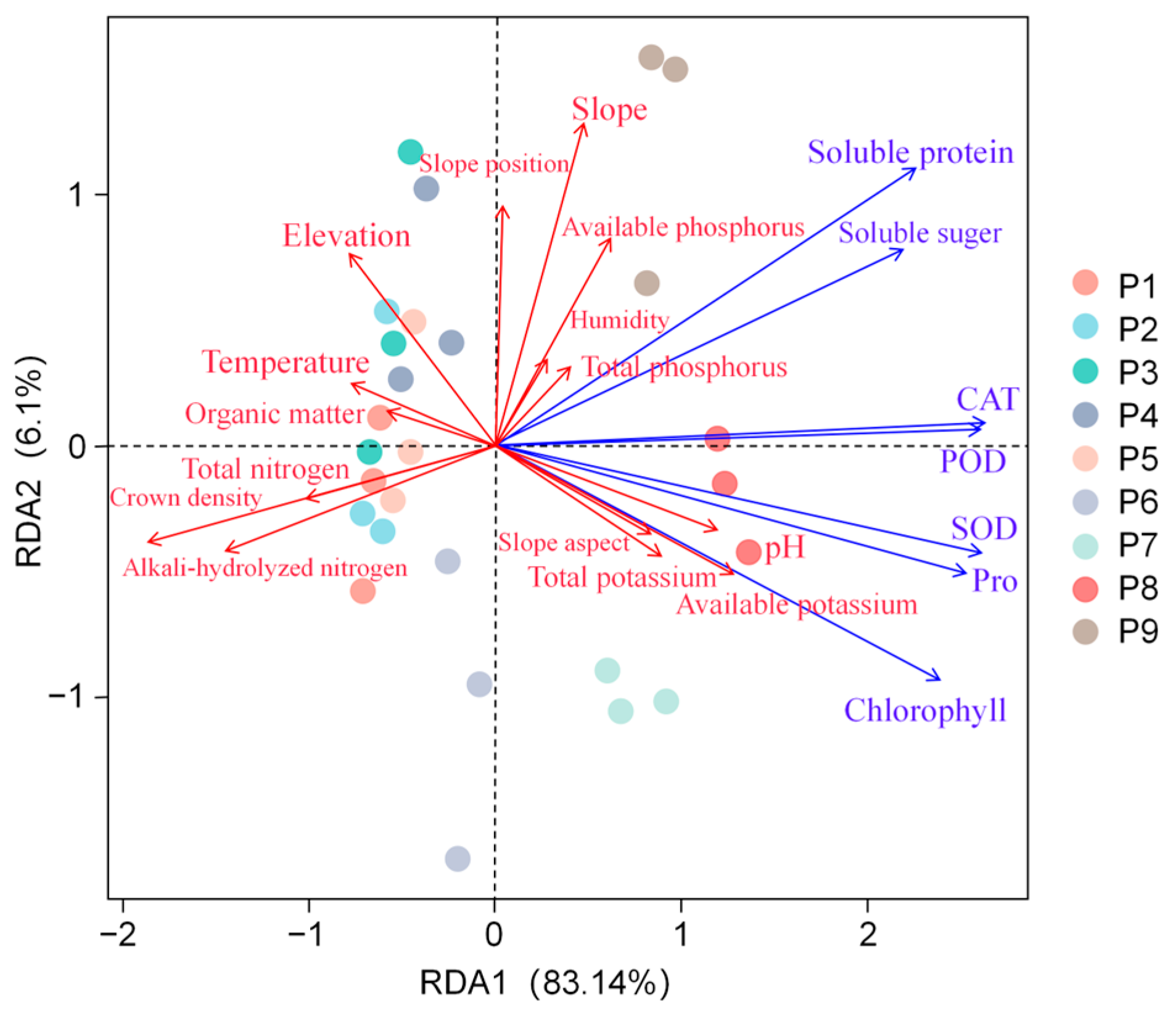
| Environmental Factor | p-Value | r2 |
|---|---|---|
| organic matter | 0.034 | 0.057 |
| pH | 0.006 | 0.087 |
| total nitrogen | 0.002 | 0.096 |
| total phosphorus | 0.917 | 0.002 |
| total potassium | 0.152 | 0.034 |
| alkali-hydrolyzed nitrogen | 0.002 | 0.113 |
| available phosphorus | 0.213 | 0.027 |
| available potassium | 0.001 | 0.100 |
| elevation | 0.006 | 0.074 |
| crown density | 0.001 | 0.137 |
| temperature | 0.017 | 0.063 |
| humidity | 0.236 | 0.025 |
| slope aspect | 0.116 | 0.037 |
| slope position | 0.019 | 0.065 |
| slope | 0.004 | 0.083 |
| No. | Root Diameter (mm) | Root Cover Thickness (μm) | Cortical Thickness (μm) | Mycelial Pellets (Piece) | Infection Rate (%) |
|---|---|---|---|---|---|
| P1 | 1.19 ± 0.12 a | 47.78 ± 8.54 a | 450.15 ± 58.63 a | 60 ± 9 a | 28.78 ± 1.51 a |
| P2 | 1.15 ± 0.18 a | 46.90 ± 5.51 a | 455.96 ± 35.32 a | 56 ± 7 a | 33.81 ± 1.57 a |
| P3 | 1.19 ± 0.17 a | 47.97 ± 7.55 a | 449.42 ± 30.96 a | 60 ± 9 a | 29.02 ± 1.67 a |
| P4 | 1.01 ± 0.13 a | 35.64 ± 6.16 a | 440.00 ± 15.90 a | 55 ± 5 a | 11.80 ± 0.25 b |
| P5 | 1.10 ± 0.16 a | 35.36 ± 5.80 a | 415.99 ± 30.21 a | 50 ± 6 a | 7.30 ± 1.10 b |
| P6 | 1.13 ± 0.18 a | 32.54 ± 8.76 a | 436.25 ± 42.99 a | 18 ± 3 b | 2.83 ± 0.56 c |
| P7 | 1.08 ± 0.18 a | 33.13 ± 7.43 a | 408.13 ± 36.68 a | 17 ± 3 b | 2.77 ± 0.56 c |
| P8 | 0.99 ± 0.13 a | 33.80 ± 7.47 a | 345.59 ± 28.24 a | 10 ± 4 bc | 2.06 ± 0.17 c |
| P9 | 0.95 ± 0.17 a | 32.86 ± 8.13 a | 344.21 ± 33.86 a | 9 ± 1 c | 2.37 ± 0.09 c |
| Environmental Factor | p-Value | r2 |
|---|---|---|
| organic matter | 0.362 | 0.019 |
| pH | 0.007 | 0.086 |
| total nitrogen | 0.029 | 0.059 |
| total phosphorus | 0.456 | 0.014 |
| total potassium | 0.047 | 0.054 |
| alkali-hydrolyzed nitrogen | 0.001 | 0.125 |
| available phosphorus | 0.035 | 0.054 |
| available potassium | 0.001 | 0.104 |
| elevation | 0.021 | 0.061 |
| crown density | 0.001 | 0.199 |
| temperature | 0.105 | 0.036 |
| humidity | 0.58 | 0.01 |
| slope aspect | 0.069 | 0.044 |
| slope position | 0.083 | 0.043 |
| slope | 0.006 | 0.091 |
| No. | Crown Density | Pn | E | Ci | Gs | WUE |
|---|---|---|---|---|---|---|
| P4 | 90% | 1.70 ± 0.22 b | 1.54 ± 0.22 b | 384.71 ± 1.09 a | 0.02 ± 0.01 b | 1.11 ± 0.1 b |
| P6 | 82% | 2.08 ± 0.19 b | 1.87 ± 0.17 b | 329.31 ± 7.61 b | 0.03 ± 0.01 b | 1.12 ± 0.16 b |
| P8 | 61% | 3.39 ± 0.51 a | 2.38 ± 0.17 a | 314.45 ± 8.53 c | 0.05 ± 0.01 a | 1.42 ± 0.12 a |
| No. | Crown Density | Fv/Fm | Fv/Fo | ΦPSII | qP | qN | ETR |
|---|---|---|---|---|---|---|---|
| P4 | 90% | 0.76 ± 0.08 a | 2.01 ± 0.16 a | 0.19 ± 0.07 b | 0.86 ± 0.08 ab | 0.37 ± 0.06 b | 94.80 ± 10.15 a |
| P6 | 82% | 0.78 ± 0.11 a | 2.16 ± 0.19 a | 0.26 ± 0.05 b | 0.88 ± 0.01 a | 0.50 ± 0.04 a | 108.86 ± 23.18 a |
| P8 | 61% | 0.81 ± 0.04 a | 2.49 ± 0.45 a | 0.39 ± 0.04 a | 0.65 ± 0.15 b | 0.50 ± 0.04 a | 120.54 ± 35.38 a |
| No. | Crown Density | δ13C (‰) | δ15N (‰) | C (%) | N (%) |
|---|---|---|---|---|---|
| P4 | 90% | −30.83 ± 0.47 a | −0.97 ± 2.01 a | 41.44 ± 2.06 ab | 2.33 ± 0.27 b |
| P6 | 82% | −33.12 ± 0.80 b | 0.09 ± 0.81 a | 38.68 ± 0.70 b | 3.09 ± 0.18 a |
| P8 | 61% | −30.23 ± 0.30 a | −1.33 ± 0.42 a | 43.64 ± 0.31 a | 3.48 ± 0.05 a |
| Index | E (mmol m−2 s−1) | Ci (µmol mol−1) | Gs (mmol m−2 s−1) | WUE (µmol mmol−1) | Fv/Fm | Fv/Fo | ΦPSII | qP | qN | ETR (umol.mol−1) | δ13C (‰) | δ15N (‰) | C (%) | N (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pn (µmol m−2 s−1) | 0.925 ** | −0.696 | −0.706 | 0.885 ** | 0.460 | 0.439 | 0.871 ** | −0.602 | −0.666 | 0.527 | 0.446 | −0.248 | 0.625 | 0.743 * |
| E (mmol m−2 s−1) | 1.000 | −0.774 | −0.714 * | 0.648 | 0.719 * | 0.447 | 0.749 * | −0.505 | −0.630 | 0.675 * | 0.257 | 0.015 | 0.489 | 0.761 * |
| Ci (µmol mol−1) | 1.000 | 0.947 ** | −0.493 | −0.726 * | −0.604 | −0.761 * | 0.485 | 0.567 | −0.397 | 0.149 | −0.098 | −0.141 | −0.944 ** | |
| Gs (mmol m−2 s−1) | 1.000 | −0.593 | −0.563 | −0.625 | −0.866 ** | 0.488 | 0.558 | −0.371 | −0.017 | 0.107 | −0.132 | −0.920 ** | ||
| WUE (µmol mmol−1) | 1.000 | 0.041 | 0.402 | 0.872 ** | −0.619 | −0.569 | 0.215 | 0.550 | −0.501 | 0.652 | 0.580 | |||
| Fv/Fm | 1.000 | 0.407 | 0.301 | −0.340 | −0.553 | 0.498 | −0.117 | 0.335 | 0.084 | 0.672 * | ||||
| Fv/Fo | 1.000 | 0.498 | −0.709 * | −0.578 | −0.160 | 0.195 | 0.045 | 0.358 | 0.444 | |||||
| ΦPSII | 1.000 | −0.616 | −0.648 | 0.411 | 0.378 | −0.445 | 0.479 | 0.828 ** | ||||||
| qP | 1.000 | 0.932 ** | 0.157 | −0.497 | 0.487 | −0.635 | −0.542 | |||||||
| qN | 1.000 | −0.093 | −0.515 | 0.494 | −0.577 | −0.680 * | ||||||||
| ETR (umol.mol−1) | 1.000 | 0.114 | 0.142 | 0.208 | 0.432 | |||||||||
| δ13C (‰) | 1.000 | −0.669 * | 0.728 * | 0.009 | ||||||||||
| δ15N (‰) | 1.000 | −0.331 | −0.166 | |||||||||||
| C(%) | 1.000 | 0.211 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Liu, W.; Jiang, N.; Xiao, Y.; Shan, Y.; Wang, S.; Wu, S.; Wang, Q.; Yu, J.; Zhang, Y.; et al. Population Dynamics of Cypripedium macranthos Sw. and Its Interactions with Environmental Factors in the Changbai Mountains. Agronomy 2025, 15, 68. https://doi.org/10.3390/agronomy15010068
Chen L, Liu W, Jiang N, Xiao Y, Shan Y, Wang S, Wu S, Wang Q, Yu J, Zhang Y, et al. Population Dynamics of Cypripedium macranthos Sw. and Its Interactions with Environmental Factors in the Changbai Mountains. Agronomy. 2025; 15(1):68. https://doi.org/10.3390/agronomy15010068
Chicago/Turabian StyleChen, Lifei, Wei Liu, Nan Jiang, Yiting Xiao, Yuze Shan, Shizhuo Wang, Sulei Wu, Qi Wang, Jiahui Yu, Yuqing Zhang, and et al. 2025. "Population Dynamics of Cypripedium macranthos Sw. and Its Interactions with Environmental Factors in the Changbai Mountains" Agronomy 15, no. 1: 68. https://doi.org/10.3390/agronomy15010068
APA StyleChen, L., Liu, W., Jiang, N., Xiao, Y., Shan, Y., Wang, S., Wu, S., Wang, Q., Yu, J., Zhang, Y., Lu, X., & Qiao, H. (2025). Population Dynamics of Cypripedium macranthos Sw. and Its Interactions with Environmental Factors in the Changbai Mountains. Agronomy, 15(1), 68. https://doi.org/10.3390/agronomy15010068







