The Impact of Daily Light Integral from Artificial Lighting on Tomato Seedling Cultivation in Plant Factory
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions
2.2. Light Treatments
2.3. Biometric Measurements
2.4. Phytochemical Determination
2.4.1. Chlorophyll (Chl) and Carotenoid Contents
2.4.2. Soluble Sugar Content
2.4.3. Soluble Protein Content
2.4.4. Root Vitalitymin
2.4.5. Enzyme Activities
2.5. Chlorophyll Fluorescence
2.6. Flowering in Tomato Plant After Transplant
2.7. Statistical Analysis
3. Results
3.1. Experiment 1 and Experiment 2
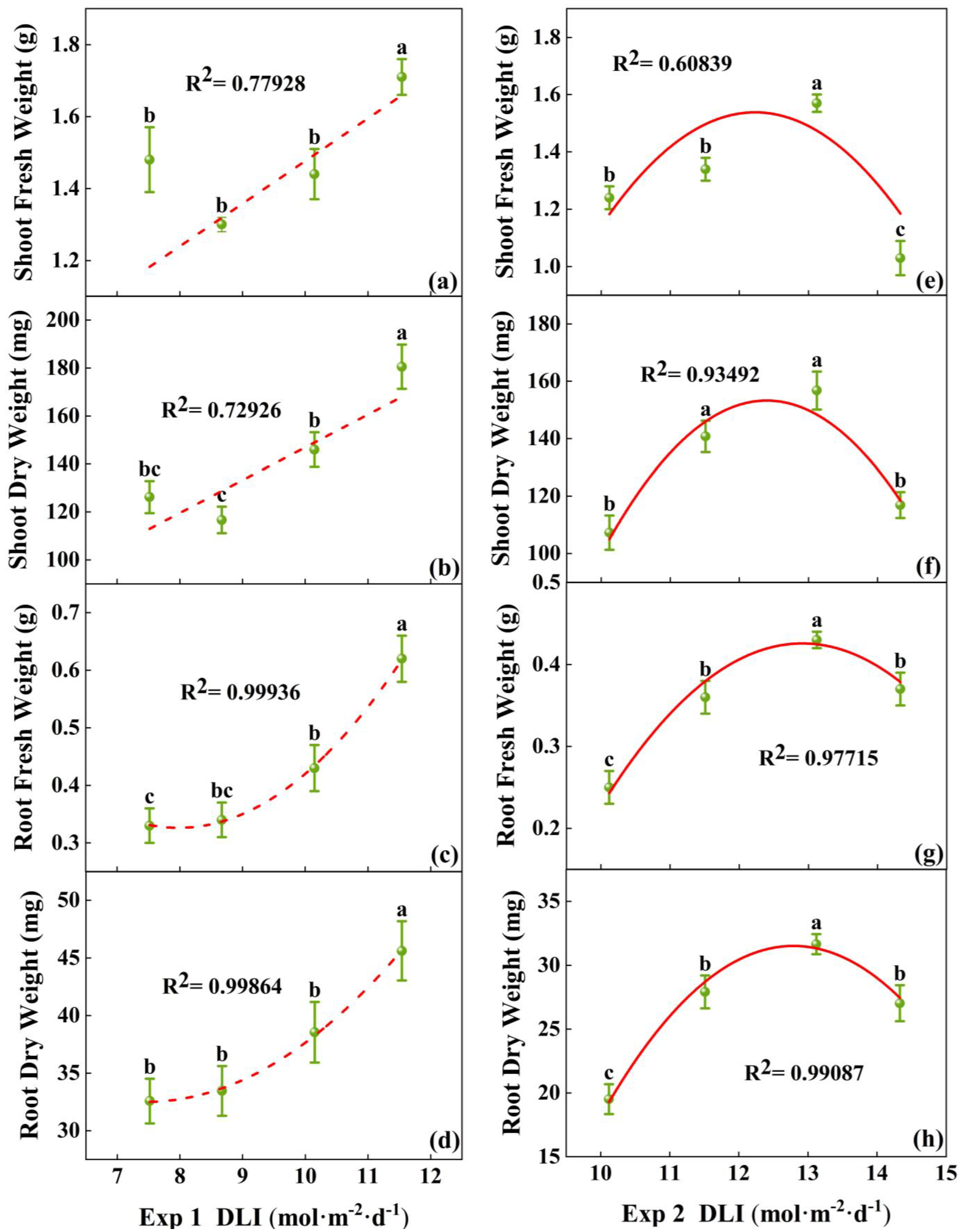
3.1.1. Morphology and Growth of Tomato Plug Seedlings
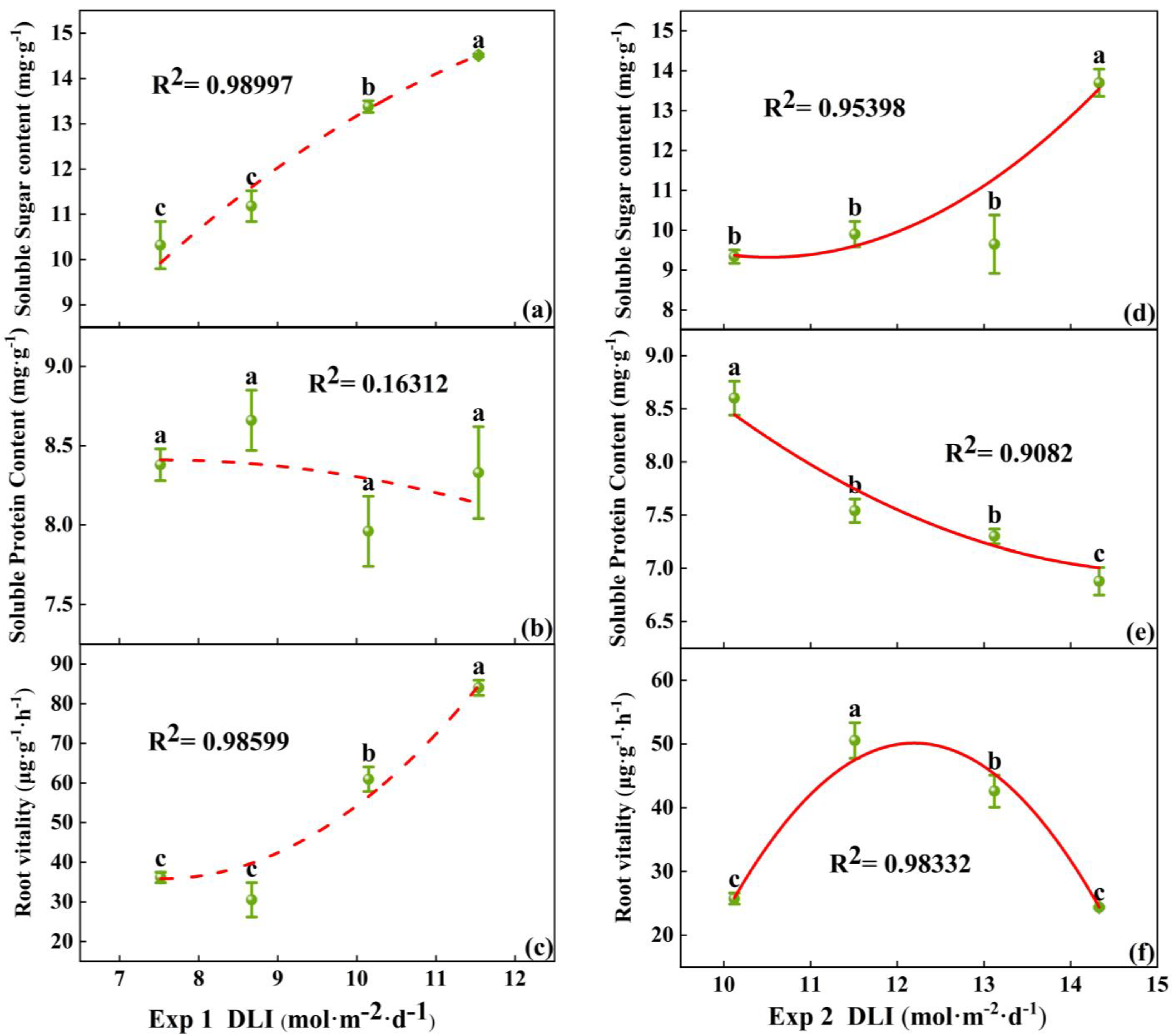
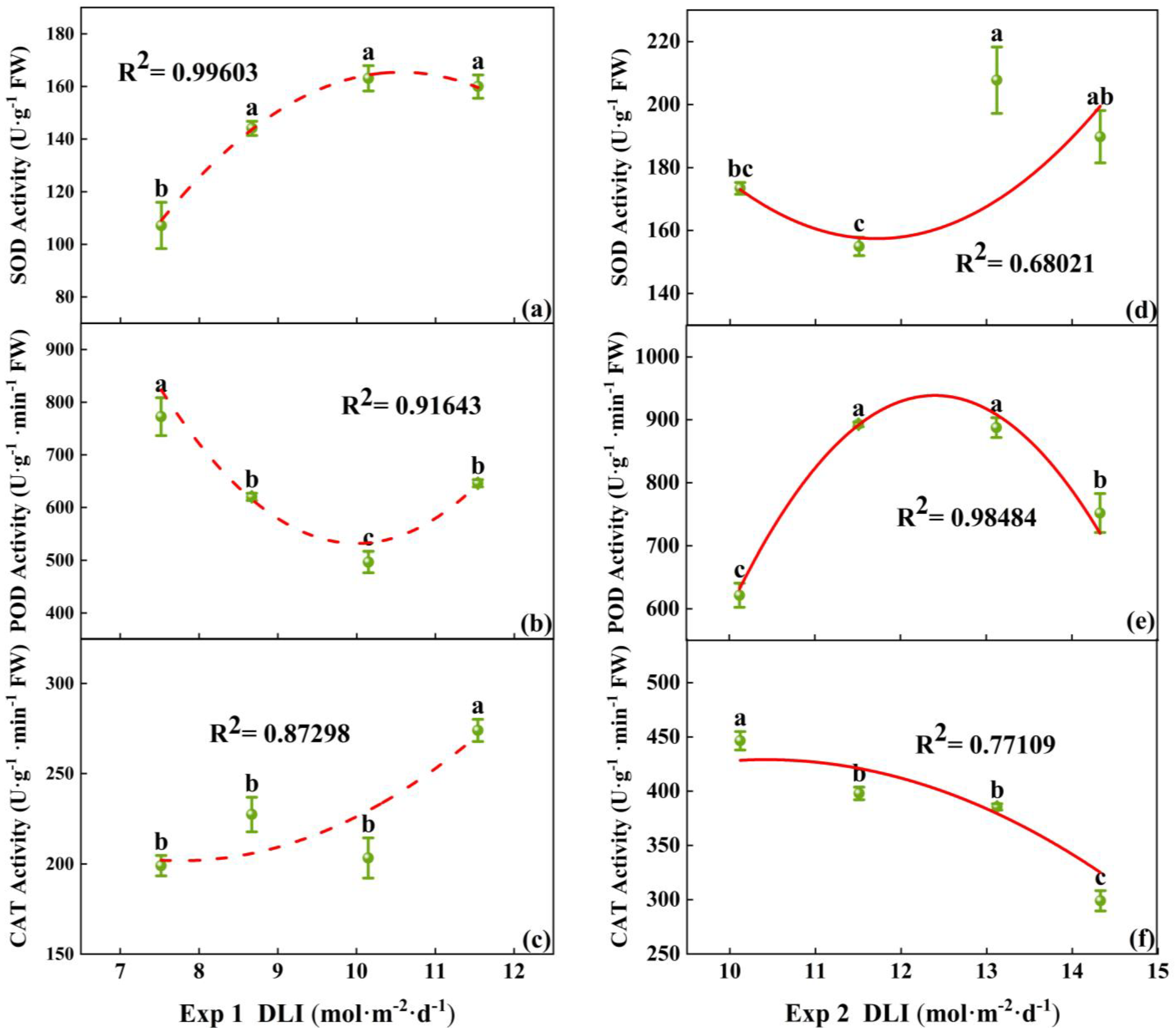
3.1.2. Physiological Characteristics, Enzyme Activities, and Photosynthesis Pigment Content of Tomato Plug Seedlings
3.1.3. Flowering in Tomato Plant After Transplant
3.2. Experiment 3
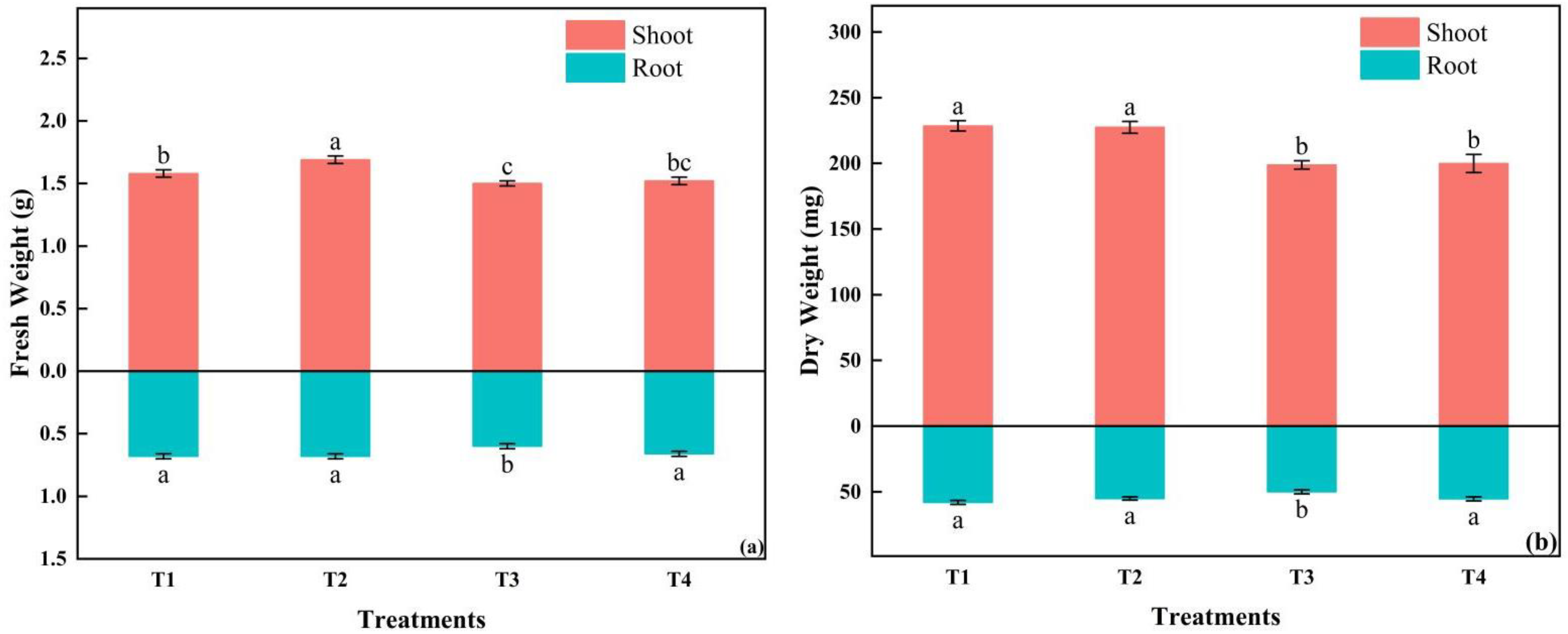
3.2.1. Morphology and Growth of Tomato Plug Seedlings
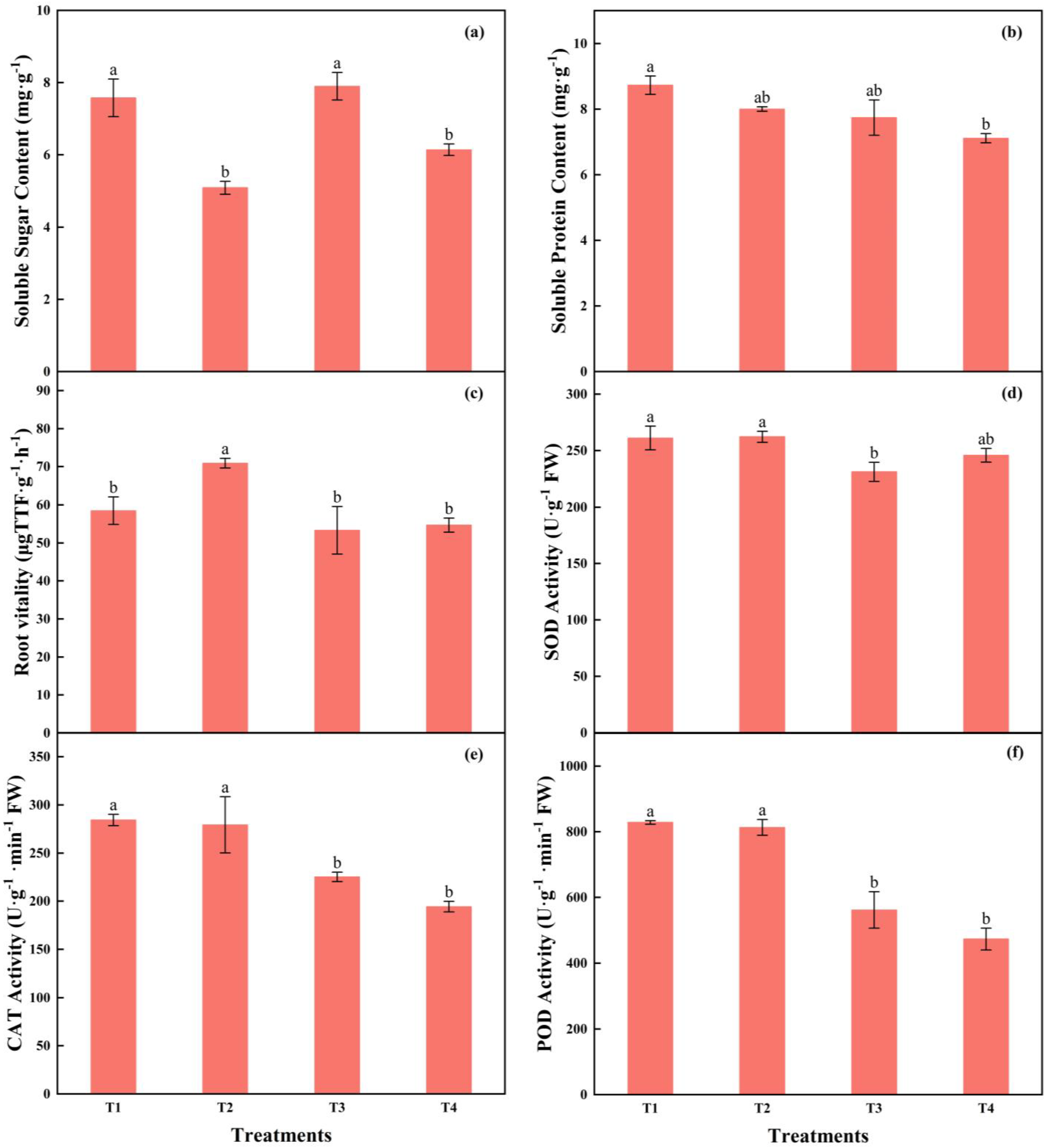
3.2.2. Physiological Characteristics and Related Enzyme Activities of Tomato Plug Seedlings
3.2.3. Photosynthesis Pigment Content and Chlorophyll Fluorescence of Tomato Plug Seedlings
4. Discussion
4.1. The Impact of DLI on Tomato Seedling Growth and Development
4.2. The Impact of DLI on the Physiological Indices of Tomato Seedlings
4.3. The Impact of DLI on the Related Enzyme Activities of Tomato Seedlings
4.4. The Impact of DLI on Photosynthetic Pigments and Chlorophyll Fluorescence of Tomato Seedlings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mandriota, L.; Blanco, I.; Scarascia-Mugnozza, G. Plant Factory with Artificial Lighting: Innovation Technology for Sustainable Agriculture Production. In Proceedings of the AIIA 2022: Biosystems Engineering Towards the Green Deal; Ferro, V., Giordano, G., Orlando, S., Vallone, M., Cascone, G., Porto, S.M.C., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1163–1172. [Google Scholar]
- Yan, W.; Zhang, Y.; Zhang, Y.; Cheng, R.; Zhang, Y.; Yang, Q.; Li, T. Effects of Supplementary Artificial Light on Growth of the Tomato (Solanum lycopersicum) in a Chinese Solar Greenhouse. Hortic. J. 2018, 87, 516–523. [Google Scholar] [CrossRef]
- Palmitessa, O.D.; Pantaleo, M.A.; Santamaria, P. Applications and Development of LEDs as Supplementary Lighting for Tomato at Different Latitudes. Agronomy 2021, 11, 835. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Agarwal, A. Artificial Lighting System for Plant Growth and Development: Chronological Advancement, Working Principles, and Comparative Assessment. In Light Emitting Diodes for Agriculture: Smart Lighting; Dutta Gupta, S., Ed.; Springer: Singapore, 2017; pp. 1–25. ISBN 978-981-10-5807-3. [Google Scholar]
- Jin, W.; Formiga Lopez, D.; Heuvelink, E.; Marcelis, L.F.M. Light Use Efficiency of Lettuce Cultivation in Vertical Farms Compared with Greenhouse and Field. Food Energy Secur. 2023, 12, e391. [Google Scholar] [CrossRef]
- Engler, N.; Krarti, M. Review of Energy Efficiency in Controlled Environment Agriculture. Renew. Sustain. Energy Rev. 2021, 141, 110786. [Google Scholar] [CrossRef]
- Gruda, N.; Bisbis, M.; Tanny, J. Influence of Climate Change on Protected Cultivation: Impacts and Sustainable Adaptation Strategies—A Review. J. Clean. Prod. 2019, 225, 481–495. [Google Scholar] [CrossRef]
- Gallegos-Cedillo, V.M. An In-Depth Analysis of Sustainable Practices in Vegetable Seedlings Nurseries: A Review. Sci. Hortic. 2024, 334, 113342. [Google Scholar] [CrossRef]
- Vatistas, C.; Avgoustaki, D.D.; Bartzanas, T. A Systematic Literature Review on Controlled-Environment Agriculture: How Vertical Farms and Greenhouses Can Influence the Sustainability and Footprint of Urban Microclimate with Local Food Production. Atmosphere 2022, 13, 1258. [Google Scholar] [CrossRef]
- Dsouza, A.; Newman, L.; Graham, T.; Fraser, E.D.G. Exploring the Landscape of Controlled Environment Agriculture Research: A Systematic Scoping Review of Trends and Topics. Agric. Syst. 2023, 209, 103673. [Google Scholar] [CrossRef]
- van Delden, S.H.; SharathKumar, M.; Butturini, M.; Graamans, L.J.A.; Heuvelink, E.; Kacira, M.; Kaiser, E.; Klamer, R.S.; Klerkx, L.; Kootstra, G.; et al. Current Status and Future Challenges in Implementing and Upscaling Vertical Farming Systems. Nat. Food 2021, 2, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.; Wu, B.-S.; MacPherson, S.; Lefsrud, M. How the Distribution of Photon Delivery Impacts Crops in Indoor Plant Environments: A Review. Sustainability 2023, 15, 4645. [Google Scholar] [CrossRef]
- Park, B.G.; Lee, J.H.; Shin, E.J.; Kim, E.A.; Nam, S.Y. Light Quality Influence on Growth Performance and Physiological Activity of Coleus Cultivars. Int. J. Plant Biol. 2024, 15, 807–826. [Google Scholar] [CrossRef]
- Kusuma, P.; Pattison, P.M.; Bugbee, B. From Physics to Fixtures to Food: Current and Potential LED Efficacy. Hortic. Res. 2020, 7, 56. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, A.; Cheng, Z.-M. Effects of Light Emitting Diode Lights on Plant Growth, Development and Traits a Meta-Analysis. Hortic. Plant J. 2021, 7, 552–564. [Google Scholar] [CrossRef]
- Shin, E.J.; Lee, J.H.; Nam, S.Y. Changes in Growth, Visual Qualities, and Photosynthetic Parameters in Peperomia Species and Cultivars Under Different Color Temperatures of White Lighting Conditions. J. Agric. Life Environ. Sci. 2023, 35, 307–321. [Google Scholar] [CrossRef]
- Faust, J.E.; Logan, J. Daily Light Integral: A Research Review and High-Resolution Maps of the United States. HortScience 2018, 53, 1250–1257. [Google Scholar] [CrossRef]
- Torres, A.P.; Lopez, R.; Horticulture, P.; Architecture, L. Measuring Daily Light Integral in a Greenhouse. Available online: https://www.extension.purdue.edu/extmedia/HO/HO-238-W.pdf (accessed on 26 December 2024).
- Pramuk, L.A.; Runkle, E.S. Photosynthetic Daily Light Integral During the Seedling Stage Influences Subsequent Growth and Flowering of Celosia, Impatiens, Salvia, Tagetes, and Viola. HortScience 2005, 40, 1336–1339. [Google Scholar] [CrossRef]
- Lim, S.H.; Im, N.H.; An, S.K.; Lee, H.B.; Kim, K.S. Daily Light Integral Affects Photosynthesis, Growth, and Flowering of Korean Native Veronica rotunda and V. longifolia. Hortic. Environ. Biotechnol. 2022, 63, 13–22. [Google Scholar] [CrossRef]
- Whitman, C.; Padhye, S.; Runkle, E.S. A High Daily Light Integral Can Influence Photoperiodic Flowering Responses in Long Day Herbaceous Ornamentals. Sci. Hortic. 2022, 295, 110897. [Google Scholar] [CrossRef]
- Lee, H.B.; Lee, J.H.; An, S.K.; Park, J.H.; Kim, K.S. Growth Characteristics and Flowering Initiation of Phalaenopsis Queen Beer ‘Mantefon’ as Affected by the Daily Light Integral. Hortic. Environ. Biotechnol. 2019, 60, 637–645. [Google Scholar] [CrossRef]
- Kelly, N.; Choe, D.; Meng, Q.; Runkle, E.S. Promotion of Lettuce Growth under an Increasing Daily Light Integral Depends on the Combination of the Photosynthetic Photon Flux Density and Photoperiod. Sci. Hortic. 2020, 272, 109565. [Google Scholar] [CrossRef]
- Gavhane, K.P.; Hasan, M.; Singh, D.K.; Kumar, S.N.; Sahoo, R.N.; Alam, W. Determination of Optimal Daily Light Integral (DLI) for Indoor Cultivation of Iceberg Lettuce in an Indigenous Vertical Hydroponic System. Sci. Rep. 2023, 13, 10923. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Severin, S.N. The Alternating Light Pattern and Daily Light Integral Interactively Determine Crop-Specific Growth Responses Indoors. In Proceedings of the 2023 Annual Conference, Orlando, FL, USA, 31 July–4 August 2023; ASHS: Alexandria, VA, USA, 2023. [Google Scholar]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Responses of Sweet Basil to Different Daily Light Integrals in Photosynthesis, Morphology, Yield, and Nutritional Quality. HortScience 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Huber, B.M.; Louws, F.J.; Hernández, R. Impact of Different Daily Light Integrals and Carbon Dioxide Concentrations on the Growth, Morphology, and Production Efficiency of Tomato Seedlings. Front. Plant Sci. 2021, 12, 615853. [Google Scholar] [CrossRef]
- Song, J.; Fan, Y.; Li, X.; Li, Y.; Mao, H.; Zuo, Z.; Zou, Z. Effects of Daily Light Integral on Tomato (Solanum lycopersicon L.) Grafting and Quality in a Controlled Environment. Int. J. Agric. Biol. Eng. 2022, 15, 44–50. [Google Scholar] [CrossRef]
- Cui, J.; Song, S.; Yu, J.; Liu, H. Effect of Daily Light Integral on Cucumber Plug Seedlings in Artificial Light Plant Factory. Horticulturae 2021, 7, 139. [Google Scholar] [CrossRef]
- Mao, H.; Hang, T.; Zhang, X.; Lu, N. Both Multi-Segment Light Intensity and Extended Photoperiod Lighting Strategies, with the Same Daily Light Integral, Promoted Lactuca sativa L. Growth and Photosynthesis. Agronomy 2019, 9, 857. [Google Scholar] [CrossRef]
- Palmer, S.; van Iersel, M.W. Increasing Growth of Lettuce and Mizuna under Sole-Source LED Lighting Using Longer Photoperiods with the Same Daily Light Integral. Agronomy 2020, 10, 1659. [Google Scholar] [CrossRef]
- Kang, Y.; Wu, Q.; Pan, G.; Yang, H.; Li, J.; Yang, X.; Zhong, M. High Daily Light Integral Positively Regulate Photosynthetic Capacity Through Mediating Nitrogen Partitioning and Leaf Anatomical Characteristic in Flowering Chinese Cabbage. Sci. Hortic. 2024, 326, 112715. [Google Scholar] [CrossRef]
- Cruz, S.; Gómez, C. Effects of Daily Light Integral on Compact Tomato Plants Grown for Indoor Gardening. Agronomy 2022, 12, 1704. [Google Scholar] [CrossRef]
- Slattery, R.A.; Ort, D.R. Photosynthesis|Photosynthetic Efficiency Improvement. In Encyclopedia of Biological Chemistry III, 3rd ed.; Jez, J., Ed.; Elsevier: Oxford, UK, 2021; pp. 256–267. ISBN 978-0-12-822040-5. [Google Scholar]
- Wu, A.; Hammer, G.L.; Doherty, A.; von Caemmerer, S.; Farquhar, G.D. Quantifying Impacts of Enhancing Photosynthesis on Crop Yield. Nat. Plants 2019, 5, 380–388. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpää, M.; Matsubara, S.; Pons, T.L. A Meta-Analysis of Plant Responses to Light Intensity for 70 Traits Ranging from Molecules to Whole Plant Performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef]
- Shomali, A.; Lastochkina, O.V.; Mohammadian, M.; Rastogi, A.; Bosacchi, M.; Li, T.; Aliniaeifard, S. Photoinhibition in Horticultural Crops: An Overview of the Effect of Light Quality and Signaling in the Underlying Photoprotection Mechanisms. Int. J. Hortic. Sci. Technol. 2023, 10, 39–50. [Google Scholar] [CrossRef]
- Xu, D.-Q.; Shen, Y.-K. Light Stress: Photoinhibition of Photosynthesis in Plants Under Natural Conditions; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 1999; Volume 19990540, pp. 483–497. [Google Scholar]
- Zheng, Y.; Zou, J.; Lin, S.; Jin, C.; Shi, M.; Yang, B.; Yang, Y.; Jin, D.; Li, R.; Li, Y. Effects of Different Light Intensity on the Growth of Tomato Seedlings in a Plant Factory. PLoS ONE 2023, 18, e0294876. [Google Scholar] [CrossRef] [PubMed]
- Trivellini, A.; Toscano, S.; Romano, D.; Ferrante, A. The Role of Blue and Red Light in the Orchestration of Secondary Metabolites, Nutrient Transport and Plant Quality. Plants 2023, 12, 2026. [Google Scholar] [CrossRef]
- He, W.; Chai, Q.; Zhao, C.; Yin, W.; Fan, H.; Yu, A.; Fan, Z.; Hu, F.; Sun, Y.; Wang, F. Response of Blue Light in Different Proportions on the Growth & Flowering in Sunflower. Sci. Hortic. 2024, 338, 113689. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.; Liu, C.; Shi, Q.; Yang, F.; Wei, M. Effects of Red and Blue Light on Leaf Anatomy, CO2 Assimilation and the Photosynthetic Electron Transport Capacity of Sweet Pepper (Capsicum annuum L.) Seedlings. BMC Plant Biol. 2020, 20, 318. [Google Scholar] [CrossRef]
- Gao, W.; He, D.; Ji, F.; Zhang, S.; Zheng, J. Effects of Daily Light Integral and LED Spectrum on Growth and Nutritional Quality of Hydroponic Spinach. Agronomy 2020, 10, 1082. [Google Scholar] [CrossRef]
- Wang, Y.; Folta, K.M. Contributions of Green Light to Plant Growth and Development. Am. J. Bot. 2013, 100, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bian, Z.; Marcelis, L.F.M.; Heuvelink, E.; Yang, Q.; Kaiser, E. Green Light Is Similarly Effective in Promoting Plant Biomass as Red/Blue Light: A Meta-Analysis. J. Exp. Bot. 2024, 75, 5655–5666. [Google Scholar] [CrossRef]
- Kaiser, E.; Weerheim, K.; Schipper, R.; Dieleman, J.A. Partial Replacement of Red and Blue by Green Light Increases Biomass and Yield in Tomato. Sci. Hortic. 2019, 249, 271–279. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, Y.; Zhang, X.; Grundy, S.; Hardy, K.; Yang, Q.; Lu, C. A Transcriptome Analysis Revealing the New Insight of Green Light on Tomato Plant Growth and Drought Stress Tolerance. Front. Plant Sci. 2021, 12, 649283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ju, J.; Hu, Y.; He, R.; Song, J.; Liu, H. Meta-Analysis of the Impact of Far-Red Light on Vegetable Crop Growth and Quality. Plants 2024, 13, 2508. [Google Scholar] [CrossRef]
- Ma, L.; Upadhyaya, M.K. Effects of Red/Far-Red Light Ratio on Common Lamb’s-Quarters, Redroot Pigweed, and Tomato Plants. Can. J. Plant Sci. 2016, 97, 494–500. [Google Scholar] [CrossRef]
- Meijer, D.; van Doesburg, F.; Jungerling, L.; Weldegergis, B.T.; Kappers, I.F.; Van Oystaeyen, A.; Loon, J.J.A.V.; Dicke, M. Supplemental Far-Red Light Influences Flowering Traits and Interactions with a Pollinator in Tomato Crops. Environ. Exp. Bot. 2023, 213, 105438. [Google Scholar] [CrossRef]
- Kong, J.; Zhao, Y.; Fan, P.; Wang, Y.; Xu, X.; Wang, L.; Li, S.; Duan, W.; Liang, Z.; Dai, Z. Far-Red Light Modulates Grapevine Growth by Increasing Leaf Photosynthesis Efficiency and Triggering Organ-Specific Transcriptome Remodelling. BMC Plant Biol. 2024, 24, 189. [Google Scholar] [CrossRef]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. UV-A and FR Irradiation Improves Growth and Nutritional Properties of Lettuce Grown in an Artificial Light Plant Factory. Food Chem. 2021, 345, 128727. [Google Scholar] [CrossRef] [PubMed]
- Vanhaelewyn, L.; Van Der Straeten, D.; De Coninck, B.; Vandenbussche, F. Ultraviolet Radiation From a Plant Perspective: The Plant-Microorganism Context. Front. Plant Sci. 2020, 11, 597642. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Zhang, Y.; Zhang, Y.; Zou, J.; Yang, Q.; Li, T. Ultraviolet-A Radiation Stimulates Growth of Indoor Cultivated Tomato (Solanum Lycopersicum) Seedlings. HortScience 2018, 53, 1429–1433. [Google Scholar] [CrossRef]
- Xu, X.; Yang, F.; Song, J.; Zhang, R.; Cai, W. Does the Daily Light Integral Influence the Sowing Density of Tomato Plug Seedlings in a Controlled Environment? Horticulturae 2024, 10, 730. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, W.-H.; Xing, X.-J.; Wang, Y.; Jin, Y.-R.; Zhang, L.; Song, Y.-F.; Dong, L.-H.; Liu, H.-B. Study on Tobacco Vigorous Seedling Indexes Model. Sci. Agric. Sin. 2014, 47, 1086–1098. [Google Scholar] [CrossRef]
- Currey, C.J.; Lopez, R.G. Biomass Accumulation and Allocation, Photosynthesis, and Carbohydrate Status of New Guinea Impatiens, Geranium, and Petunia Cuttings Are Affected by Photosynthetic Daily Light Integral during Root Development. J. Am. Soc. Hortic. Sci. 2015, 140, 542–549. [Google Scholar] [CrossRef]
- Faust, J.E.; Holcombe, V.; Rajapakse, N.C.; Layne, D.R. The Effect of Daily Light Integral on Bedding Plant Growth and Flowering. HortScience 2005, 40, 645–649. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Li, H.; Sun, Q.; Zhao, S.; Zhang, W. Principles and Techniques of Plant Physiological Biochemical Experiment; Higher Education: Beijing, China, 2000; pp. 195–197. [Google Scholar]
- Flohé, L.; Ötting, F. Superoxide Dismutase Assays. In Methods in Enzymology; Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 93–104. [Google Scholar]
- Amako, K.; Chen, G.-X.; Asada, K. Separate Assays Specific for Ascorbate Peroxidase and Guaiacol Peroxidase and for the Chloroplastic and Cytosolic Isozymes of Ascorbate Peroxidase in Plants. Plant Cell Physiol. 1994, 35, 497–504. [Google Scholar]
- Patra, H.K.; Kar, M.; Mishra, D. Catalase Activity in Leaves and Cotyledons During Plant Development and Senescence. Biochem. Physiol. Pflanz. 1978, 172, 385–390. [Google Scholar] [CrossRef]
- Randall, W.C.; Lopez, R.G. Comparison of Supplemental Lighting from High-Pressure Sodium Lamps and Light-Emitting Diodes during Bedding Plant Seedling Production. HortScience 2014, 49, 589–595. [Google Scholar] [CrossRef]
- Currey, C.J.; Hutchinson, V.A.; Lopez, R.G. Growth, Morphology, and Quality of Rooted Cuttings of Several Herbaceous Annual Bedding Plants Are Influenced by Photosynthetic Daily Light Integral During Root Development. HortScience 2012, 47, 25–30. [Google Scholar] [CrossRef]
- Ji, F.; Wei, S.; Liu, N.; Xu, L.; Yang, P. Growth of Cucumber Seedlings in Different Varieties as Affected by Light Environment. Int. J. Agric. Biol. Eng. 2020, 13, 73–78. [Google Scholar] [CrossRef]
- Wang, J.L.; Evers, J.B.; Anten, N.P.R.; Li, Y.; Yang, X.; Douma, J.C.; Schneider, H.M. Far-Red Light Perception by the Shoot Influences Root Growth and Development in Cereal-Legume Crop Mixtures. Plant Soil 2024, 1–18. [Google Scholar] [CrossRef]
- Kou, X.; Han, W.; Kang, J. Responses of Root System Architecture to Water Stress at Multiple Levels: A Meta-Analysis of Trials under Controlled Conditions. Front. Plant Sci. 2022, 13, 1085409. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Lyu, J.; Chen, Y.; Zhang, J.; Ye, N. Effects of Stress-Induced ABA on Root Architecture Development: Positive and Negative Actions. Crop J. 2023, 11, 1072–1079. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Xu, L.; Chen, Z.; He, N. Variation in Leaf Morphological, Stomatal, and Anatomical Traits and Their Relationships in Temperate and Subtropical Forests. Sci. Rep. 2019, 9, 5803. [Google Scholar] [CrossRef]
- Casal, J.J. Phytochromes, Cryptochromes, Phototropin: Photoreceptor Interactions in Plants. Photochem. Photobiol. 2000, 71, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Hempton, A.K.; Imaizumi, T. Photoperiodic Flowering in Arabidopsis: Multilayered Regulatory Mechanisms of CONSTANS and the Florigen FLOWERING LOCUS T. Plant Comm. 2023, 4, 100552. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, W.; Leung, C.C.; Tarté, D.A.; Gendron, J.M. Plants Distinguish Different Photoperiods to Independently Control Seasonal Flowering and Growth. Science 2024, 383, eadg9196. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, C. Mechanisms of Cryptochrome-Mediated Photoresponses in Plants. Annu. Rev. Plant Biol. 2020, 71, 103–129. [Google Scholar] [CrossRef]
- Cerdán, P.D.; Chory, J. Regulation of Flowering Time by Light Quality. Nature 2003, 423, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Vaid, T.M. Improving the Scheduling and Profitability of Annual Bedding Plant Production by Manipulating Temperature, Daily Light Integral, Photoperiod, and Transplant Size. Master’s Thesis, Michigan State University, East Lansing, MI, USA, 2012. ISBN 1-267-58936-1. [Google Scholar]
- Chen, L.-Q.; Cheung, L.S.; Feng, L.; Tanner, W.; Frommer, W.B. Transport of Sugars. Annu. Rev. Biochem. 2015, 84, 865–894. [Google Scholar] [CrossRef] [PubMed]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of Sugars under Abiotic Stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Eghbal, E.; Aliniaeifard, S.; Mehrjerdi, M.Z.; Abdi, S.; Hassani, S.B.; Rassaie, T.; Gruda, N.S. Growth, Phytochemical, and Phytohormonal Responses of Basil to Different Light Durations and Intensities under Constant Daily Light Integral. BMC Plant Biol. 2024, 24, 935. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.-Q.; Jia, Z.-X.; Zhang, X.; Shao, H.-B. Effects of Soil Rhizosphere Aeration on the Root Growth and Water Absorption of Tomato. CLEAN Soil Air Water 2012, 40, 1364–1371. [Google Scholar] [CrossRef]
- Shahriari, Y. Plant Drought Stress: Fromimpact of Drought Stress on Plant Morphological, Biochemical and Physiological Features to Role of Nutrients in Drought Stress Alleviation. NVEO Nat. Volatiles Essent. Oils J. NVEO 2021, 8, 5344–5369. [Google Scholar]
- Didaran, F.; Kordrostami, M.; Ghasemi-Soloklui, A.A.; Pashkovskiy, P.; Kreslavski, V.; Kuznetsov, V.; Allakhverdiev, S.I. The Mechanisms of Photoinhibition and Repair in Plants under High Light Conditions and Interplay with Abiotic Stressors. J. Photochem. Photobiol. B Biol. 2024, 259, 113004. [Google Scholar] [CrossRef] [PubMed]
- Fayezizadeh, M.R.; Ansari, N.A.; Sourestani, M.M.; Hasanuzzaman, M. Variations in Photoperiods and Their Impact on Yield, Photosynthesis and Secondary Metabolite Production in Basil Microgreens. BMC Plant Biol. 2024, 24, 712. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Ali, M.B.; Hahn, E.-J.; Paek, K.-Y. Effects of Light Intensities on Antioxidant Enzymes and Malondialdehyde Content during Short-Term Acclimatization on Micropropagated Phalaenopsis Plantlet. Environ. Exp. Bot. 2005, 54, 109–120. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Yu, F.; Hu, B.; Jia, Y.; Sha, H.; Zhao, H. Differential Activity of the Antioxidant Defence System and Alterations in the Accumulation of Osmolyte and Reactive Oxygen Species Under Drought Stress and Recovery in Rice (Oryza sativa L.) Tillering. Sci. Rep. 2019, 9, 8543. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for Measuring Reactive Oxygen Species and Oxidative Damage in Cells and In Vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Björn, L.O.; Papageorgiou, G.C.; Blankenship, R.E.; Govindjee, R.E. A Viewpoint: Why Chlorophyll a? Photosynth. Res. 2009, 99, 85–98. [Google Scholar] [CrossRef]
- Song, J.; Chen, Z.; Zhang, A.; Wang, M.; Jahan, M.S.; Wen, Y.; Liu, X. The Positive Effects of Increased Light Intensity on Growth and Photosynthetic Performance of Tomato Seedlings in Relation to Night Temperature Level. Agronomy 2022, 12, 343. [Google Scholar] [CrossRef]
- Kume, A.; Akitsu, T.; Nasahara, K.N. Why Is Chlorophyll b Only Used in Light-Harvesting Systems? J. Plant Res. 2018, 131, 961–972. [Google Scholar] [CrossRef]
- Fan, X.-X.; Xu, Z.-G.; Liu, X.-Y.; Tang, C.-M.; Wang, L.-W.; Han, X. Effects of Light Intensity on the Growth and Leaf Development of Young Tomato Plants Grown under a Combination of Red and Blue Light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Leister, D. Enhancing the Light Reactions of Photosynthesis: Strategies, Controversies, and Perspectives. Mol. Plant 2023, 16, 4–22. [Google Scholar] [CrossRef]
- Slattery, R.A.; Ort, D.R. Perspectives on Improving Light Distribution and Light Use Efficiency in Crop Canopies. Plant Physiol. 2021, 185, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Gao, M.; Jiang, H.; Ou, S.; Li, X.; He, R.; Li, Y.; Liu, H. Light Intensity and Photoperiod Affect Growth and Nutritional Quality of Brassica Microgreens. Molecules 2022, 27, 883. [Google Scholar] [CrossRef] [PubMed]
- Éva, C.; Oszvald, M.; Tamás, L. Current and Possible Approaches for Improving Photosynthetic Efficiency. Plant Sci. 2019, 280, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, Z.; Jiang, X.; Ahammed, G.J.; Zhou, Y. Light Regulation of Horticultural Crop Nutrient Uptake and Utilization. Hortic. Plant J. 2021, 7, 367–379. [Google Scholar] [CrossRef]
- Lacour, T.; Robert, E.; Lavaud, J. Sustained Xanthophyll Pigments-Related Photoprotective NPQ Is Involved in Photoinhibition in the Haptophyte Tisochrysis Lutea. Sci. Rep. 2023, 13, 14694. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, M.; Kagawa, T.; Oikawa, K.; Suetsugu, N.; Miyao, M.; Wada, M. Chloroplast Avoidance Movement Reduces Photodamage in Plants. Nature 2002, 420, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Barbato, R.; Tadini, L.; Cannata, R.; Peracchio, C.; Jeran, N.; Alboresi, A.; Morosinotto, T.; Bajwa, A.A.; Paakkarinen, V.; Suorsa, M.; et al. Higher Order Photoprotection Mutants Reveal the Importance of ΔpH-Dependent Photosynthesis-Control in Preventing Light Induced Damage to Both Photosystem II and Photosystem I. Sci. Rep. 2020, 10, 6770. [Google Scholar] [CrossRef] [PubMed]
- Elkins, C.; van Iersel, M.W. Longer Photoperiods with the Same Daily Light Integral Increase Daily Electron Transport Through Photosystem II in Lettuce. Plants 2020, 9, 1172. [Google Scholar] [CrossRef] [PubMed]
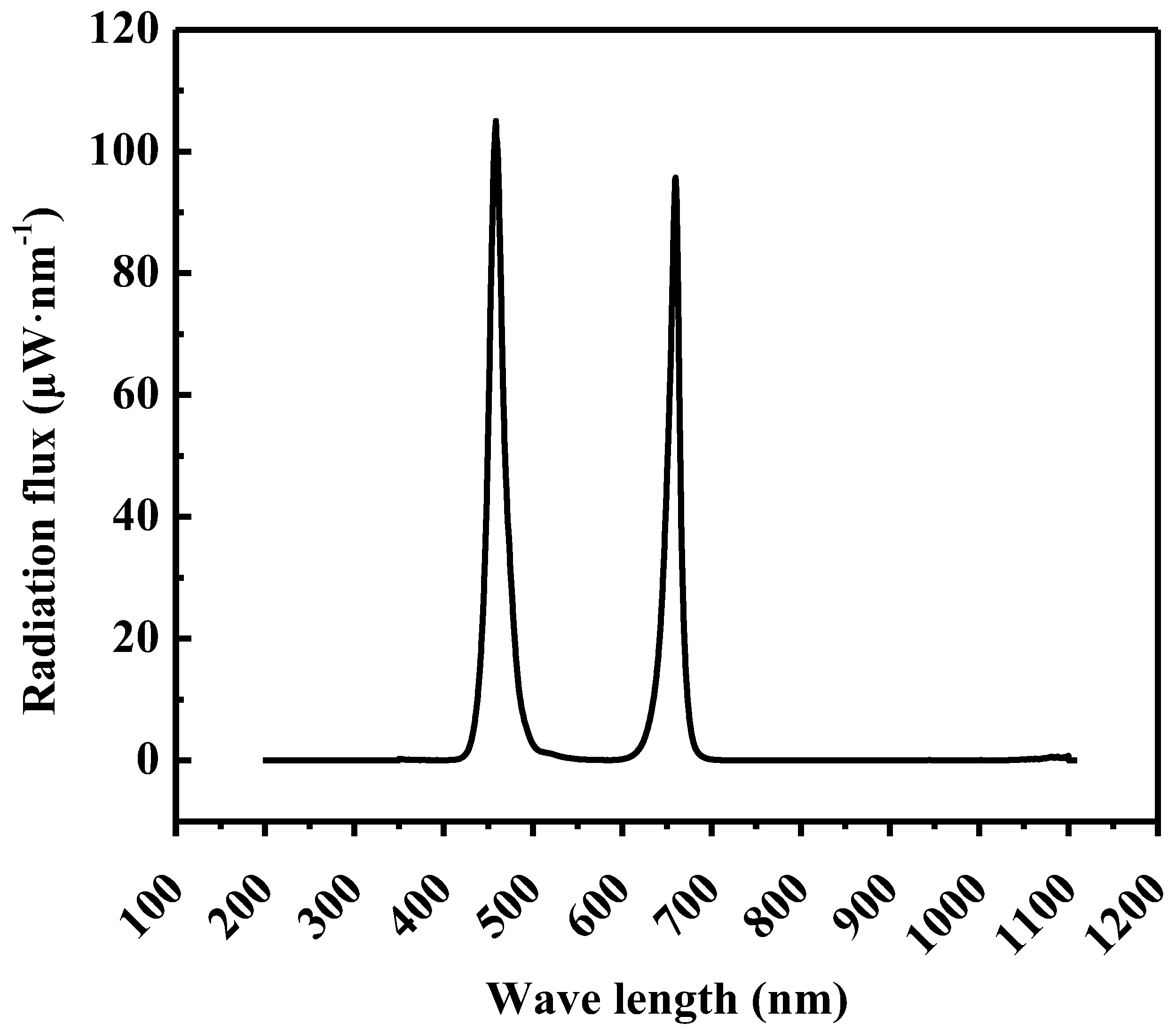




| Light Intensity 1 (μmol·m−2·s−1) | Photoperiod (h) | Average DLI 1 (mol·m−2·d−1) | |
|---|---|---|---|
| Exp 1 | 149.17 ± 1.54 | 14 | 7.52 ± 0.08 |
| 150.50 ± 1.12 | 16 | 8.67 ± 0.06 | |
| 201.33 ± 0.42 | 14 | 10.15 ± 0.02 | |
| 200.33 ± 0.96 | 16 | 11.54 ± 0.06 | |
| Exp 2 | 200.83 ± 1.68 | 14 | 10.12 ± 0.09 |
| 199.83 ± 1.25 | 16 | 11.51 ± 0.07 | |
| 251.33 ± 0.99 | 14.5 | 13.12 ± 0.05 | |
| 248.83 ± 1.33 | 16 | 14.33 ± 0.08 |
| Treatments | Light Intensity 1 (μmol·m−2·s−1) | Photoperiod (h) | Average DLI 1 (mol·m−2·d−1) |
|---|---|---|---|
| T1 | 203.17 ± 1.14 | 18 | 13.17 ± 0.07 |
| T2 | 227.67 ± 0.88 | 16 | 13.11 ± 0.05 |
| T3 | 258.67 ± 1.75 | 14 | 13.04 ± 0.09 |
| T4 | 300.67 ± 1.75 | 12 | 12.99 ± 0.08 |
| Average DLI 2 (mol·m−2·d−1) | Seeding Index | Root-To-Shoot Ratio | Shoot Dry Matter Rate | Total Plant Dry Matter Rate | Specific Leaf Area (SLA) | |
|---|---|---|---|---|---|---|
| Exp 1 | 7.52 ± 0.08 | 0.4667 ± 0.0286 b | 0.2586 ± 0.0086 a | 0.0859 ± 0.0038 c | 0.0883 ± 0.0036 a | 266.27 ± 10.69 a |
| 8.67 ± 0.06 | 0.4972 ± 0.0345 b | 0.2859 ± 0.0101 a | 0.0900 ± 0.0036 bc | 0.0917 ± 0.0036 a | 251.60 ± 14.97 ab | |
| 10.15 ± 0.02 | 0.5641 ± 0.0408 b | 0.2637 ± 0.0113 a | 0.1017 ± 0.0036 ab | 0.0993 ± 0.0040 a | 232.89 ± 10.74 ab | |
| 11.54 ± 0.06 | 0.6717 ± 0.0381 a | 0.2539 ± 0.0134 a | 0.1061 ± 0.0053 a | 0.0970 ± 0.0041 a | 216.15 ± 12.25 b | |
| Exp 2 | 10.12 ± 0.09 | 0.2658 ± 0.0162 c | 0.1843 ± 0.0112 b | 0.0864 ± 0.0030 c | 0.0847 ± 0.0022 b | 222.45 ± 6.13 a |
| 11.51 ± 0.07 | 0.3839 ± 0.0187 b | 0.2001 ± 0.0109 b | 0.1050 ± 0.0022 b | 0.0994 ± 0.0018 a | 187.27 ± 6.71 bc | |
| 13.12 ± 0.05 | 0.4403 ± 0.0105 a | 0.2036 ± 0.0057 ab | 0.0999 ± 0.0037 b | 0.0944 ± 0.0031 a | 209.72 ± 10.50 ab | |
| 14.33 ± 0.08 | 0.3791 ± 0.0213 b | 0.2335 ± 0.0136 a | 0.1152 ± 0.0063 a | 0.1030 ± 0.0042 a | 177.47 ± 7.69 c |
| Average DLI 2 (mol·m−2·d−1) | Days to First Flower from Transplanting | First Flower Cluster Node Position | Second Flower Cluster Node Position | Number of Lateral Branches | Total Flower at 33 rd DAT | Total Flower at 40 th DAT |
|---|---|---|---|---|---|---|
| 10.12 ± 0.09 | 25.20 ± 0.37 a | 14.00 ± 0.32 a | 16.20 ± 0.37 a | 0.60 ± 0.24 a | 3.80 ± 0.58 a | 8.60 ± 1.33 b |
| 11.51 ± 0.07 | 24.00 ± 0.32 b | 13.20 ± 0.20 b | 16.00 ± 0.00 a | 2.00 ± 0.63 a | 4.40 ± 0.81 a | 14.80 ± 2.27 a |
| 13.12 ± 0.05 | 24.6 ± 0.24 ab | 13.20 ± 0.20 b | 15.40 ± 0.24 a | 1.80 ± 0.86 a | 5.00 ± 0.32 a | 11.80 ± 1.53 ab |
| 14.33 ± 0.08 | 24.6 ± 0.24 ab | 13.20 ± 0.20 b | 15.60 ± 0.24 a | 1.80 ± 0.37 a | 4.20 ± 0.20 a | 13.40 ± 2.04 ab |
| Treatment 2 | Plant Height (cm) | Stem Diameter (mm) | Leaf Area (cm2) |
|---|---|---|---|
| T1 | 8.13 ± 0.09 b | 2.61 ± 0.03 a | 35.83 ± 0.78 a |
| T2 | 8.96 ± 0.14 a | 2.55 ± 0.04 a | 36.20 ± 0.65 a |
| T3 | 7.59 ± 0.17 c | 2.56 ± 0.03 a | 34.22 ± 0.57 a |
| T4 | 7.35 ± 0.10 c | 2.56 ± 0.03 a | 34.42 ± 0.77 a |
| Treatment 2 | Seeding Index | Root-To-Shoot Ratio | Shoot Dry Matter Rate | Total Plant Dry Matter Rate | Specific Leaf Area (SLA) |
|---|---|---|---|---|---|
| T1 | 0.6312 ± 0.0364 a | 0.1969 ± 0.0073 a | 0.0850 ± 0.0025 a | 0.0735 ± 0.0019 a | 289.53 ± 12.70 c |
| T2 | 0.6511 ± 0.0304 a | 0.2104 ± 0.0050 a | 0.0856 ± 0.0028 a | 0.0719 ± 0.0018 ab | 285.02 ± 11.57 c |
| T3 | 0.5784 ± 0.0332 a | 0.2088 ± 0.0087 a | 0.0767 ± 0.0030 b | 0.0670 ± 0.0024 bc | 343.98 ± 19.16 b |
| T4 | 0.4155 ± 0.0311 b | 0.1673 ± 0.0072 b | 0.0685 ± 0.0027 c | 0.0623 ± 0.0021 c | 424.99 ± 23.79 a |
| Treatment 2 | Chlorophyll a (mg·g−1) | Chlorophyll b (mg·g−1) | Total Chlorophyll (mg·g−1) | Carotenoid (mg·g−1) | Chlorophyll a/b |
|---|---|---|---|---|---|
| T1 | 0.6312 ± 0.0364 a | 0.1969 ± 0.0073 a | 0.0850 ± 0.0025 a | 0.0735 ± 0.0019 a | 1.44 ± 0.01 a |
| T2 | 0.6511 ± 0.0304 a | 0.2104 ± 0.0050 a | 0.0856 ± 0.0028 a | 0.0719 ± 0.0018 ab | 1.44 ± 0.01 a |
| T3 | 0.5784 ± 0.0332 a | 0.2088 ± 0.0087 a | 0.0767 ± 0.0030 b | 0.0670 ± 0.0024 bc | 1.36 ± 0.02 b |
| T4 | 0.4155 ± 0.0311 b | 0.1673 ± 0.0072 b | 0.0685 ± 0.0027 c | 0.0623 ± 0.0021 c | 1.36 ± 0.01 b |
| Treatment 2 | Photochemical Quenching | Non-Photochemical Quenching | Y (NO) | Y (NPQ) | ΦPSII | Fv/Fm | ||
|---|---|---|---|---|---|---|---|---|
| qP | qL | qN | NPQ | |||||
| T1 | 0.68 ± 0.02 a | 0.63 ± 0.02 b | 0.44 ± 0.02 a | 1.20 ± 0.07 b | 0.26 ± 0.01 a | 0.31 ± 0.02 b | 0.42 ± 0.01 a | 0.78 ± 0.01 ab |
| T2 | 0.62 ± 0.01 b | 0.71 ± 0.01 a | 0.41 ± 0.01 a | 1.55 ± 0.06 a | 0.25 ± 0.00 ab | 0.39 ± 0.01 a | 0.36 ± 0.01 b | 0.78 ± 0.00 b |
| T3 | 0.65 ± 0.02 b | 0.70 ± 0.01 a | 0.43 ± 0.02 a | 1.52 ± 0.07 a | 0.24 ± 0.00 b | 0.37 ± 0.02 a | 0.38 ± 0.02 ab | 0.79 ± 0.00 ab |
| T4 | 0.61 ± 0.03 b | 0.72 ± 0.01 a | 0.40 ± 0.03 a | 1.67 ± 0.10 a | 0.24 ± 0.01 b | 0.40 ± 0.02 a | 0.36 ± 0.02 b | 0.79 ± 0.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Cui, J.; Ju, J.; Hu, Y.; Liu, X.; He, R.; Song, J.; Huang, Y.; Liu, H. The Impact of Daily Light Integral from Artificial Lighting on Tomato Seedling Cultivation in Plant Factory. Agronomy 2025, 15, 70. https://doi.org/10.3390/agronomy15010070
Zhang M, Cui J, Ju J, Hu Y, Liu X, He R, Song J, Huang Y, Liu H. The Impact of Daily Light Integral from Artificial Lighting on Tomato Seedling Cultivation in Plant Factory. Agronomy. 2025; 15(1):70. https://doi.org/10.3390/agronomy15010070
Chicago/Turabian StyleZhang, Minggui, Jiawei Cui, Jun Ju, Youzhi Hu, Xiaojuan Liu, Rui He, Jiali Song, Yanwu Huang, and Houcheng Liu. 2025. "The Impact of Daily Light Integral from Artificial Lighting on Tomato Seedling Cultivation in Plant Factory" Agronomy 15, no. 1: 70. https://doi.org/10.3390/agronomy15010070
APA StyleZhang, M., Cui, J., Ju, J., Hu, Y., Liu, X., He, R., Song, J., Huang, Y., & Liu, H. (2025). The Impact of Daily Light Integral from Artificial Lighting on Tomato Seedling Cultivation in Plant Factory. Agronomy, 15(1), 70. https://doi.org/10.3390/agronomy15010070







