Removal of Persistent Bacterial Contaminants from In Vitro Shoot Cultures of Raspberry (Rubus idaeus L.) Using Vacuum Infiltration and Its Effect on Multiplication Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Identification and Characteristics of Bacteria
2.3. Antibiograms
2.4. Elimination of Bacteria Using Biocides
2.5. The Effect of Freeing Shoots from Bacteria on Shoot Multiplication, Rooting, and Surviving Storage Conditions
2.6. Statistical Analysis
3. Results
3.1. Effect of Biocides on Bacterial Culture (Antibiograms)
3.2. Survival of Bacteria in Biocide Solutions After Completion of Vacuum Treatment
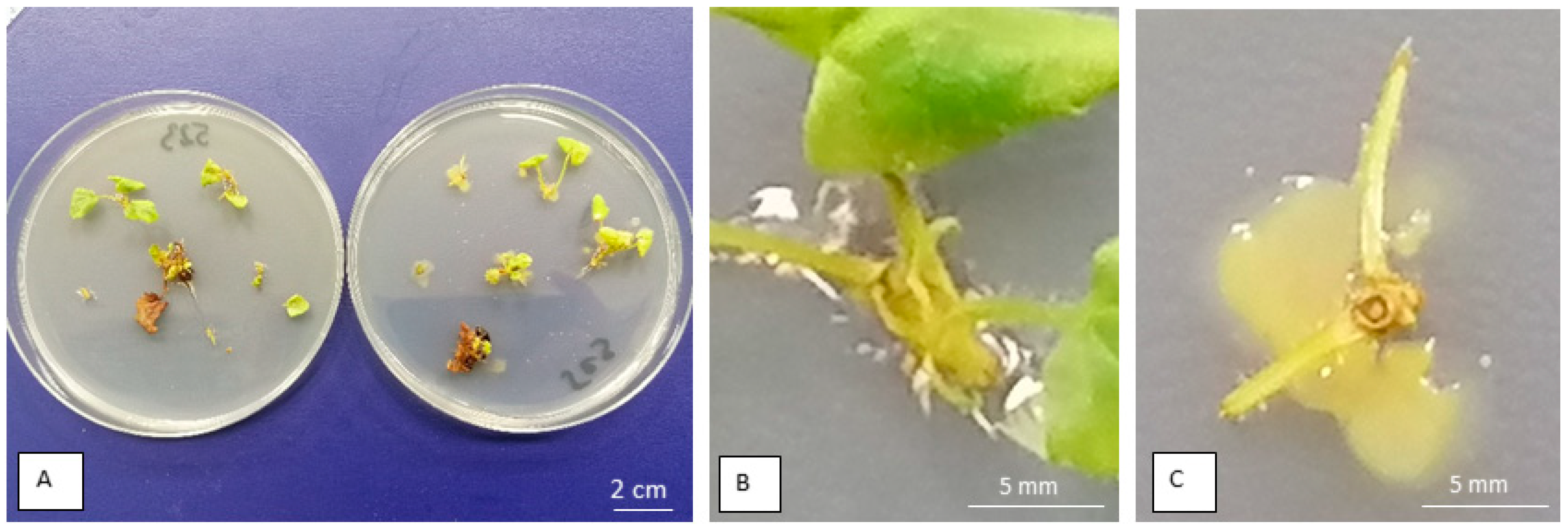
3.3. Effect of Biocide Infiltration on Shoot Necrosis and Bacterial Survival in Raspberry Shoots
3.4. Effect of Freeing from Bacteria on Shoot Multiplication, Rooting, and Survival Storage Conditions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volk, G.M.; Bonnart, R.; Araújo de Oliveira, A.C.; Henk, A.D. Minimizing the deleterious effects of endophytes in plant shoot tip cryopreservation. Appl. Plant Sci. 2022, 10, e11489. [Google Scholar] [CrossRef]
- Thomas, P.; Sekhar, A.C. Live cell imaging reveals extensive intracellular cytoplasmic colonization of banana by normally non-cultivable endophytic bacteria. AoB Plants 2014, 6, plu002. [Google Scholar] [CrossRef] [PubMed]
- Pirttilä, A.M.; Laukkanen, H.; Pospiech, H.; Myllylä, R.; Hohtola, A. Detection of intracellular bacteria in the buds of Scotch pine (Pinus sylvestris L.) by in situ hybridization. Appl. Environ. Microbiol. 2000, 66, 3073–3077. [Google Scholar] [CrossRef]
- Partida-Martinez, L.P.; Heil, M. The microbe-free plant: Fact or artifact? Front. Plant Sci. 2011, 2, 100. [Google Scholar] [CrossRef]
- De Almeida, D.F.; Andreote, F.D.; Yara, R.; Tanaka, F.A.O.; Azevedo, J.L.; de Almeida, M. Bacteriosomes in axenic plants: Endophytes as stable endosymbionts. World J. Microbiol. Biotechnol. 2009, 25, 1757–1764. [Google Scholar] [CrossRef]
- Orlikowska, T.; Ogórek, L.; Trzewik, A.; Nowak, K.; Maciorowski, R. Micropropagation of raspberry (Rubus idaeus L.) using biocides for retarding bacterial contamination. Zesz. Nauk. Inst. Ogrod. 2018, 26, 77–83, (In Polish with English Abstract). [Google Scholar]
- Leifert, C.; Waites, W.M.; Camotta, H.; Nicholas, J.R. Lactobacillus plantarum: A deleterious contaminant of plant tissue culture. J. Appl. Bacteriol. 1989, 67, 363–370. [Google Scholar] [CrossRef]
- Marino, G.; Altan, A.D.; Biavati, B. The effect of bacterial contamination on the growth and gas evolution of in vitro cultured apricot shoots. In Vitro Cell. Dev. Biol. Plant 1996, 32, 51–56. [Google Scholar] [CrossRef]
- Thomas, P. In vitro decline in plant cultures: Detection of a legion of covert bacteria as the cause for degeneration of long-term micropropagated triploid watermelon cultures. Plant Cell Tissue Organ Cult. 2004, 77, 173–179. [Google Scholar] [CrossRef]
- Wojtania, A.; Puławska, J.; Gabryszewska, E. Identification and elimination of bacterial contaminants from Pelargonium tissue cultures. J. Fruit. Ornam. Plant Res. 2005, 13, 101–108. [Google Scholar]
- Reed, B.M.; Buckley, P.M.; DeWilde, T.N. Detection and eradication of endophytic bacteria from micropropagated mint plants. In Vitro Cell. Dev. Biol. Plant 1995, 31P, 53–57. [Google Scholar] [CrossRef]
- Thomas, P.; Prabhakara, B.S.; Pitchaimuthu, M. Cleansing the long term micropropagated triploid watermelon cultures from covert bacteria and field testing the plants for clonal fidelity and fertility during the 7–10 year period in vitro. Plant Cell Tissue Organ Cult. 2006, 85, 317–329. [Google Scholar] [CrossRef]
- Sarasan, V.A.; Cripps, R.; Ramsay, M.M.; Atherton, C.; McMichen, M.; Prendergast, G.; Rowntree, J.K. Conservation in vitro of threatened plants—Progress in the past decade. In Vitro Cell. Dev. Biol. Plant 2006, 42, 206–214. [Google Scholar] [CrossRef]
- Philips, R.; Arnott, S.M.; Kaplan, S.E. Antibiotics in plant tissue culture: Rifampicin effectively controls bacterial contaminants without affecting the growth of short-term explant cultures of Helianthus tuberosus. Plant Sci. Lett. 1981, 21, 235–240. [Google Scholar] [CrossRef]
- Leifert, C.; Camotta, H.; Waites, W.M. Effect of combinations of antibiotics on micropropagated Clematis, Delphinium, Hosta, Iris and Photinia. Plant Cell Tissue Organ Cult. 1992, 29, 153–160. [Google Scholar] [CrossRef]
- Mbah, E.I.; Wakil, S.M. Elimination of bacteria from in vitro yam tissue cultures using antibiotics. J. Plant Pathol. 2012, 94, 53–58. [Google Scholar]
- Plant Preservative Mixture. Available online: https://www.plantcelltechnology.com/plant-preservative-mixture-ppm-1/ (accessed on 7 June 2022).
- Bent, A.F. Arabidopsis in planta transformation. Uses, mechanisms, and prospects for transformation of other species. Plant Physiol. 2000, 124, 1540–1547. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 156–163. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laural, Kalmia latifolia, by use of shoot tip culture. Comb. Proc. Int. Plant Prop. Soc. 1980, 30, 421–427. [Google Scholar]
- Viss, P.R.; Brooks, E.M.; Driver, J.A. A simplified method for the control of bacterial contamination in woody plant tissue culture. In Vitro Cell. Dev. Biol. Plant 1991, 27, 42. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Cardoso, E.J.B.N. Isolation, selection and characterization of root-associated growth promoting bacteria In Brazil Pine (Araucaria angustifolia). Microbiol. Res. 2012, 167, 69–78. [Google Scholar] [CrossRef]
- Bric, J.M.; Bostock, R.M.; Silverstone, S.E. Rapid In Situ Assay for Indoleacetic Acid Production by Bacteria Immobilized on a Nitrocellulose Membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef]
- Figueiredo de Vasconcellos, R.F.; Pinheiro da Silva, M.C.; Ribeiro, C.M.; Bran Nogueira Cardoso, E.J. Isolation and screening for plant growth-promoting (PGP) actinobacteria from Araucaria angustifolia rhizosphere soil. Sci. Agric. 2010, 67, 743–746. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An effcient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.B.; Zuberer, D.A. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol. Fert. Soils 1991, 12, 39–45. [Google Scholar] [CrossRef]
- Thomas, P.; Kumari, S.; Swarna, G.K.; Prakash, D.P.; Dinesh, M.R. Ubiquitous presence of fastidious endophytic bacteria in field shoots and index-negative apparently clean shoot-tip cultures of papaya. Plant Cell. Rep. 2007, 26, 1491–1499. [Google Scholar] [CrossRef]
- Cassells, A.C. Pathogen and microbial contamination management in micropropagation—An overview. In Pathogen and Microbial Contamination Management in Micropropagation; Cassells, A.C., Ed.; Kluwer Academic Publishers: Alphen aan den Rhein, The Netherlands, 1997; pp. 1–13. [Google Scholar]
- Abdalla, N.; El-Ramady, H.; Seliem, M.K.; El-Mahrouk, M.E.; Taha, N.; Bayoumi, Y.; Shalaby, T.A.; Dobranszki, J. An Academic and Technical Overview on Plant Micropropagation Challenges. Hortuculturae 2022, 8, 677. [Google Scholar] [CrossRef]
- Orlikowska, T.; Zawadzka, M.; Zenkteler, E.; Sobiczewski, P. Influence of the biocides PPM tm and vitrofural on bacteria isolated from contaminated plant tissue cultures and on plant microshoots grown on various media. J. Hortic. Sci. Biotech. 2015, 87, 223–230. [Google Scholar] [CrossRef]
- Reed, B.M.; Tanprasert, P. Detection and control of bacterial contaminants of plant tissue cultures. A review of recent literature. Plant Tissue Cult. Biotechnol. 1995, 3, 137–142. [Google Scholar]
- Liang, C.; Wu, R.; Han, Y.; Wan, T.; Cai, Y. Optimizing Suitable Antibiotics for Bacterium Control in Micropropagation of Cherry Rootstock Using a Modified Leaf Disk Diffusion Method and E Test. Plants 2019, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Romadanova, N.V.; Tolegen, A.B.; Kushnarenko, S.V.; Zholdybayeva, E.V.; Bettoni, J.C. Effect of Plant Preservative MixtureTM on Endophytic Bacteria Eradication from In Vitro-Grown Apple Shoots. Plants 2022, 11, 2624. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.; Tan, B.H.; Errington, S.G. Eradication of endophytic bacteria via treatment for axillary buds of Petunia hybrida using Plant Preservative Mixture (PPMTM). Plant Cell Tissue Organ Cult. 2010, 102, 365–372. [Google Scholar] [CrossRef]
- Bunn, E.; Tan, B. Microbial contaminants in plant tissue culture propagation. In Microorganisms in Plant Conservation and Biodiversity; Sivasithamparam, K., Dixon, K.W., Barrett, R.L., Eds.; Kluwer Academic Publishers: Alphen aan den Rhein, The Netherlands, 2002; pp. 307–335. [Google Scholar]



| Cultivar/ Bacteria | Days After Inoculation | Biocides | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HgCL2 0.05% | HgCL2 0.1% | PPMTM 0.2% | PPMTM 0.4% | Rif * 100 mg L−1 | Rif. 200 mg L−1 | NaOCl 1% | NaOCl 10% | NaOCl 20% | ||
| ‘Norna’/ Curtobacterium sp. | 2 | 8 | 10 | 0 | 0 | 17 | 19 | 0 | 0 | 1 |
| 4 | 0 | 0 | 0 | 0 | 17 | 19 | 0 | 0 | 0 | |
| 10 | 0 | 0 | 0 | 0 | 17 | 19 | 0 | 0 | 0 | |
| ‘Polka’/ Pseudomonas sp. | 2 | 12 | 16 | 0 | 0 | 16 | 17 | 0 | 0 | 0 |
| 4 | 12 | 16 | 0 | 0 | 16 | 17 | 0 | 0 | 0 | |
| 10 | 12 | 16 | 0 | 0 | 16 | 17 | 0 | 0 | 0 | |
| ‘Polana’/ Luteibacter sp. | 2 | 6 | 8 | 0 | 0 | 6 | 9 | 0 | 0 | 0 |
| 4 | 6 | 8 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | |
| 10 | 6 | 8 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | |
| Treatment Biocide Concentration | Bacteria/Cultivar | ||
|---|---|---|---|
| Curtobacterium sp./‘Norna’ | Pseudomonas sp./‘Polka’ | Luteibacter sp./‘Polana’ | |
| Water | 2nd *—>20 ** | 4th—10–20 | 1st—>20 |
| HgCl2 0.05% | No bacteria | No bacteria | No bacteria |
| HgCl2 0.1% | No bacteria | No bacteria | No bacteria |
| Rifampicin 100 mg/L | No bacteria | No bacteria | 5th—10–20 |
| Rifampicin 200 mg/L | No bacteria | No bacteria | 5th—15–10 |
| PPM 0.2% | 4th—>20 | 8th—5–10 | 3rd—10–20 |
| PPM 0.4% | 4th—>20 | 8th—<5 | 3rd—>20 |
| NaOCl 1% | 2nd—>20 | No bacteria | 1st—>20 |
| NaOCl 10% | 2nd—>20 | No bacteria | 1st—>20 |
| NaOCl 20% | 2nd—>20 | No bacteria | 3rd—10–20 |
| Exp. No No. of Shoots | Biocide | Vacuum | No. of Necrotic Shoots After 4 Weeks | No. of Contaminated Shoots on MS Medium After 4 Weeks | No. of Contaminated Shoots on NA Medium After 4 Weeks | No. of Bacteria-Free Shoots After 4 Weeks |
|---|---|---|---|---|---|---|
| I n = 10 | Water | No vacuum | 0 | 10 | - | - |
| Water | 20 min | 0 | 10 | - | - | |
| Rifamp.50 mg/L | No vacuum | 0 | 5 | 5 | - | |
| Rifamp.50 mg/L | 20 min | 0 | 2 | 8 | - | |
| Rifamp.100 mg/L | No vacuum | 0 | 2 | 8 | - | |
| Rifamp.100 mg/L | 20 min | 0 | 2 | 8 | - | |
| PPM 0.2% | No vacuum | 0 | 0 | 10 | - | |
| PPM 0.2% | 20 min. | 0 | 0 | 10 | - | |
| PPM 0.4% | No vacuum | 0 | 0 | 10 | - | |
| PPM 0.4% | 20 min | 0 | 0 | 10 | - | |
| II n = 10 | Water | No vacuum | 0 | 10 | - | - |
| Water | 20 min | 0 | 10 | - | - | |
| Rifamp.150 mg/L | No vacuum | 0 | 2 | 8 | - | |
| Rifamp.150 mg/L | 20 min | 0 | 2 | 8 | - | |
| Rifamp.200 mg/L | No vacuum | 0 | 2 | 8 | - | |
| Rifamp.200 mg/L | 20 min | 0 | 2 | 8 | - | |
| PPM 2% | No vacuum | 0 | 0 | 10 | - | |
| PPM 2% | 20 min | 0 | 0 | 10 | - | |
| PPM 4% | No vacuum | 0 | 0 | 10 | - | |
| PPM 4% | 20 min | 0 | 0 | 10 | - | |
| III n = 10 | Water | 20 min | 0 | 10 | - | - |
| Water | 90 min | 0 | 10 | - | - | |
| Rifamp.50 mg/L | 20 min | 0 | 5 | 5 | - | |
| Rifamp.50 mg/L | 90 min | 0 | 3 | 7 | - | |
| Rifamp.100 mg/L | 20 min | 0 | 1 | 9 | - | |
| Rifamp.100 mg/L | 90 min | 0 | 2 | 8 | - | |
| PPM 0.2% | 20 min | 0 | 0 | 10 | - | |
| PPM 0.2% | 90 min | 0 | 0 | 10 | - | |
| PPM 0.4% | 20 min | 0 | 0 | 10 | - | |
| PPM 0.4% | 90 min | 0 | 0 | 10 | - |
| Exp. No No. of Shoots | Biocide | Vacuum | No. of Necrotic Shoots After 4 Weeks | No. of Contaminated Shoots on MS Medium After 4 Weeks | No. of Contaminated Shoots on NA Medium After 4 Weeks | No. of Bacteria-Free Shoots After 4 Weeks |
|---|---|---|---|---|---|---|
| V n = 10 | Water | 2 × 15 min | 0 | 6 | - | - |
| HgCl2 0.05% | 2 × 15 min | 8 | 0 | 0 | 2 | |
| HgCl2 0.1% | 2 × 15 min | 9 | 0 | 0 | 1 | |
| Rifamp.100 mg/L | 2 × 15 min | 0 | 5 | 5 | - | |
| Rifamp.200 mg/L | 2 × 15 min | 0 | 5 | 5 | - | |
| PPM 0.2% | 2 × 15 min | 0 | 0 | 10 | - | |
| PPM 0.4% | 2 × 15 min | 0 | 0 | 10 | - | |
| NaOCl 1% | 2 × 15 min | 0 | 8 | 2 | - | |
| NaOCl 10% | 2 × 15 min | 0 | 5 | 5 | - | |
| NaOCl 20% | 2 × 15 min | 7 | 3 | - | - | |
| VI n = 30 | Water | 2 × 15 min | 0 | 30 | - | - |
| Water | 1 × 30 min | 0 | 30 | - | - | |
| HgCl2 0.05% | 2 × 15 min | 17 | 0 | 0 | 13 | |
| HgCl2 0.05% | 1 × 30 min | 10 | 0 | 0 | 20 | |
| HgCl2 0.1% | 2 × 15 min | 12 | 0 | 0 | 18 | |
| HgCl2 0.1% | 1 × 30 min | 12 | 0 | 0 | 18 | |
| NaOCl 0.1% | 2 × 15 min | 0 | 8 | 22 | - | |
| NaOCl 0.1% | 1 × 30 min | 0 | 8 | 22 | - | |
| NaOCl 0.5% | 2 × 15 min | 0 | 15 | 15 | - | |
| NaOCl 0.5% | 1 × 30 min | 0 | 16 | 14 | - | |
| VI n = 10 | Water | 2 × 15 min | 0 | 6 | 4 | - |
| HgCl2 0.05% | 2 × 15 min | 8 | 0 | 0 | 2 | |
| HgCl2 0.1% | 2 × 15 min | 9 | 0 | 0 | 1 | |
| Rifamp.100 mg/L | 2 × 15 min | 0 | 5 | 5 | - | |
| Rifamp.200 mg/L | 2 × 15 min | 0 | 5 | 5 | - | |
| PPM 0.2% | 2 × 15 min | 0 | 0 | 10 | - | |
| PPM 0.4% | 2 × 15 min | 0 | 0 | 10 | - | |
| NaOCl 1% | 2 × 15 min | 0 | 8 | 2 | - | |
| NaOCl 10% | 2 × 15 min | 0 | 5 | 5 | - | |
| NaOCl 20% | 2 × 15 min | 7 | 3 | - | - |
| Exp. No No. of Shoots | Biocide | Vacuum | No. of Necrotic Shoots After 4 Weeks | No. of Contaminated Shoots on MS Medium After 4 Weeks | No. of Contaminated Shoots on NA Medium After 4 Weeks | No. of Bacteria-Free Shoots After 4 Weeks |
|---|---|---|---|---|---|---|
| V n = 10 | Water | 2 × 15 min | 0 | 7 | 3 | - |
| HgCl2 0.05% | 2 × 15 min | 1 | 0 | 4 | ||
| HgCl2 0.1% | 2 × 15 min | 6 | 0 | 2 | 5 | |
| Rifamp.100 mg/L | 2 × 15 min | 0 | 0 | 10 | 2 | |
| Rifamp.200 mg/L | 2 × 15 min | 0 | 0 | 10 | - | |
| PPM 0.2% | 2 × 15 min | 0 | 0 | 10 | - | |
| PPM 0.4% | 2 × 15 min | 0 | 0 | 10 | - | |
| NaOCl 1% | 2 × 15 min | 1 | 7 | 3 | - | |
| NaOCl 10% | 2 × 15 min | 0 | 7 | 3 | - | |
| NaOCl 20% | 2 × 15 min | 5 | 5 | - | - | |
| VI n = 20 | Water | 2 × 15 min | 0 | 20 | - | - |
| Water | 1 × 30 min | 0 | 20 | - | - | |
| HgCl2 0.05% | 2 × 15 min | 15 | 0 | 0 | 5 | |
| HgCl2 0.05% | 1 × 30 min | 14 | 0 | 0 | 6 | |
| HgCl2 0.1% | 2 × 15 min | 14 | 0 | 0 | 6 | |
| HgCl2 0.1% | 1 × 30 min | 14 | 0 | 0 | 6 | |
| NaOCl 0.1% | 2 × 15 min | 0 | 16 | 4 | - | |
| NaOCl 0.1% | 1 × 30 min | 0 | 18 | 2 | - | |
| NaOCl 0.5% | 2 × 15 min | 0 | 16 | 4 | - | |
| NaOCl 0.5% | 1 × 30 min | 0 | 19 | 1 | - | |
| VII n = 10 | Water | 2 × 15 min | 0 | 7 | 3 | - |
| HgCl2 0.05% | 2 × 15 min | 1 | 0 | 6 | 3 | |
| HgCl2 0.1% | 2 × 15 min | 8 | 0 | 2 | - | |
| Rifamp.100 mg/L | 2 × 15 min | 0 | 0 | 10 | - | |
| Rifamp.200 mg/L | 2 × 15 min | 0 | 0 | 10 | - | |
| PPM 0.2% | 2 × 15 min | 0 | 0 | 10 | - | |
| PPM 0.4% | 2 × 15 min | 0 | 0 | 10 | - | |
| NaOCl 1% | 2 × 15 min | 1 | 7 | 2 | - | |
| NaOCl 10% | 2 × 15 min | 0 | 7 | 3 | - | |
| NaOCl 20% | 2 × 15 min | 5 | 5 | - | - |
| Exp. No No. of Shoots | Biocide | Vacuum | No. of Necrotic Shoots After 4 Weeks | No. of Contaminated Shoots on MS Medium After 4 Weeks | No. of Contaminated Shoots on NA Medium After 4 Weeks | No. of Bacteria-Free Shoots After 4 Weeks |
|---|---|---|---|---|---|---|
| IV n = 10 | Water | 30 min | 0 | 10 | - | - |
| HgCl2 0.05% | 30 min | 1 | 0 | 0 | 9 | |
| NaOCl 4% | 30 min | 0 | 10 | - | - | |
| NaOCl 7% | 30 min | 0 | 10 | - | - | |
| NaOCl 10% | 30 min | 0 | 8 | 2 | - | |
| NaOCl 20% | 30 min | 2 | 8 | 2 | - | |
| NaOCl 30% | 30 min | 5 | 5 | - | - | |
| NaOCl 40% | 30 min | 5 | 5 | - | - | |
| NaOCl 50% | 30 min | 7 | 3 | - | - | |
| NaOCl 60% | 30 min | 10 | 9 | - | - | |
| V n = 10 | Water | 2 × 15 min | 0 | 7 | 3 | - |
| HgCl2 0.05% | 2 × 15 min | 2 | 0 | 6 | 2 | |
| HgCl2 0.1% | 2 × 15 min | 4 | 0 | 0 | 6 | |
| Rifamp.100 mg/L | 2 × 15 min | 0 | 0 | 10 | - | |
| Rifamp.200 mg/L | 2 × 15 min | 0 | 0 | 10 | - | |
| PPM 0.2% | 2 × 15 min | 0 | 0 | 10 | - | |
| PPM 0.4% | 2 × 15 min | 0 | 0 | 10 | - | |
| NaOCl 1% | 2 × 15 min | 0 | 5 | 5 | - | |
| NaOCl 10% | 2 × 15 min | 0 | 5 | 5 | - | |
| NaOCl 20% | 2 × 15 min | 4 | 6 | - | - | |
| VI n = 10 | Water | 2 × 15 min | 0 | 10 | - | - |
| Water | 1 × 30 min | 0 | 10 | - | - | |
| HgCl2 0.05% | 2 × 15 min | 7 | 0 | 0 | 3 | |
| HgCl2 0.05% | 1 × 30 min | 6 | 0 | 0 | 4 | |
| HgCl2 0.1% | 2 × 15 min | 6 | 0 | 0 | 4 | |
| HgCl2 0.1% | 1 × 30 min | 5 | 0 | 0 | 5 | |
| NaOCl 0.1% | 2 × 15 min | 0 | 8 | 2 | - | |
| NaOCl 0.1% | 1 × 30 min | 0 | 8 | 2 | - | |
| NaOCl 0.5% | 2 × 15 min | 0 | 8 | 2 | - | |
| NaOCl 0.5% | 1 × 30 min | 0 | 9 | 1 | - | |
| VII n = 10 | Water | 2 × 15 min | 0 | 7 | 3 | - |
| HgCl2 0.05% | 2 × 15 min | 4 | 0 | 6 | - | |
| HgCl2 0.1% | 2 × 15 min | 6 | 0 | 0 | 4 | |
| Rifamp.100 mg/L | 2 × 15 min | 0 | 0 | 10 | - | |
| Rifamp.200 mg/L | 2 × 15 min | 0 | 0 | 10 | - | |
| PPM 0.2% | 2 × 15 min | 0 | 0 | 10 | - | |
| PPM 0.4% | 2 × 15 min | 0 | 0 | 10 | - | |
| NaOCl 1% | 2 × 15 min | 0 | 5 | 5 | - | |
| NaOCl 10% | 2 × 15 min | 0 | 5 | 5 | - | |
| NaOCl 20% | 2 × 15 min | 4 | 6 | - | - |
| Repetition in Subsequent Passages | Total Shoots | Shoots > 1 cm | ||
|---|---|---|---|---|
| Bacteria − | Bacteria + | Bacteria − | Bacteria + | |
| 1 | 5.2 a * | 3.2 b | 3.9 a | 2.4 b |
| 2 | 4.9 a | 2.9 b | 4.4 a | 2.5 b |
| 3 | 3.5 a | 3.4 a | 3.1 a | 2.6 a |
| 4 | 3.0 a | 2.7 a | 2.8 a | 2.3 a |
| Mean | 4.2 a | 3.1 b | 3.6 a | 2.5 b |
| Repetition in Subsequent Passages | Total Shoots | Shoot > 1 cm | ||
|---|---|---|---|---|
| Bacteria − | Bacteria + | Bacteria − | Bacteria + | |
| 1 | 3.1 b * | 4.0 a | 2.4 a | 2.1 a |
| 2 | 2.8 b | 3.3 a | 2.6 a | 1.7 b |
| 3 | 4.3 a | 3.5 b | 3.8 a | 1.4 b |
| 4 | 3.3 b | 4.0 a | 2.5 a | 1.7 b |
| Mean | 3.4 a | 3.7 a | 2.8 a | 1.7 b |
| Repetition in Subsequent Passages | Total Shoots | Shoots >1 cm | ||
|---|---|---|---|---|
| Bacteria − | Bacteria + | Bacteria − | Bacteria + | |
| 1 | 3.1 b * | 4.0 a | 2.4 a | 2.1 a |
| 2 | 2.8 b | 3.3 a | 2.6 a | 1.7 b |
| 3 | 4.3 a | 3.5 b | 3.8 a | 1.4 b |
| 4 | 3.3 b | 4.0 a | 2.5 a | 1.7 b |
| Mean | 3.4 a | 3.7 a | 2.8 a | 1.7 b |
| Repetition | ‘Norna’/ Curtobacterium sp | ‘Polka’/Pseudomonas sp. | ‘Polana’/Luteibacter sp. | |||
|---|---|---|---|---|---|---|
| − | + | − | + | − | + | |
| 1 | 37 b * | 48 a | 74 a | 86 a | 87 a | 84 a |
| 2 | 38 b | 47 a | 22 b | 57 a | 22 b | 34 a |
| 3 | - | - | - | - | 42 b | 81 a |
| Mean | 38 b | 48 a | 48 b | 72 a | 50 b | 66 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trzewik, A.; Malinowski, T.; Niewiadomska-Wnuk, A.; Mynett, K.; Orlikowska, T. Removal of Persistent Bacterial Contaminants from In Vitro Shoot Cultures of Raspberry (Rubus idaeus L.) Using Vacuum Infiltration and Its Effect on Multiplication Efficiency. Agronomy 2025, 15, 2452. https://doi.org/10.3390/agronomy15112452
Trzewik A, Malinowski T, Niewiadomska-Wnuk A, Mynett K, Orlikowska T. Removal of Persistent Bacterial Contaminants from In Vitro Shoot Cultures of Raspberry (Rubus idaeus L.) Using Vacuum Infiltration and Its Effect on Multiplication Efficiency. Agronomy. 2025; 15(11):2452. https://doi.org/10.3390/agronomy15112452
Chicago/Turabian StyleTrzewik, Aleksandra, Tadeusz Malinowski, Angelika Niewiadomska-Wnuk, Katarzyna Mynett, and Teresa Orlikowska. 2025. "Removal of Persistent Bacterial Contaminants from In Vitro Shoot Cultures of Raspberry (Rubus idaeus L.) Using Vacuum Infiltration and Its Effect on Multiplication Efficiency" Agronomy 15, no. 11: 2452. https://doi.org/10.3390/agronomy15112452
APA StyleTrzewik, A., Malinowski, T., Niewiadomska-Wnuk, A., Mynett, K., & Orlikowska, T. (2025). Removal of Persistent Bacterial Contaminants from In Vitro Shoot Cultures of Raspberry (Rubus idaeus L.) Using Vacuum Infiltration and Its Effect on Multiplication Efficiency. Agronomy, 15(11), 2452. https://doi.org/10.3390/agronomy15112452






