Biochar and Compost as Sustainable Alternatives to Peat
Abstract
1. Introduction
2. Materials and Methods
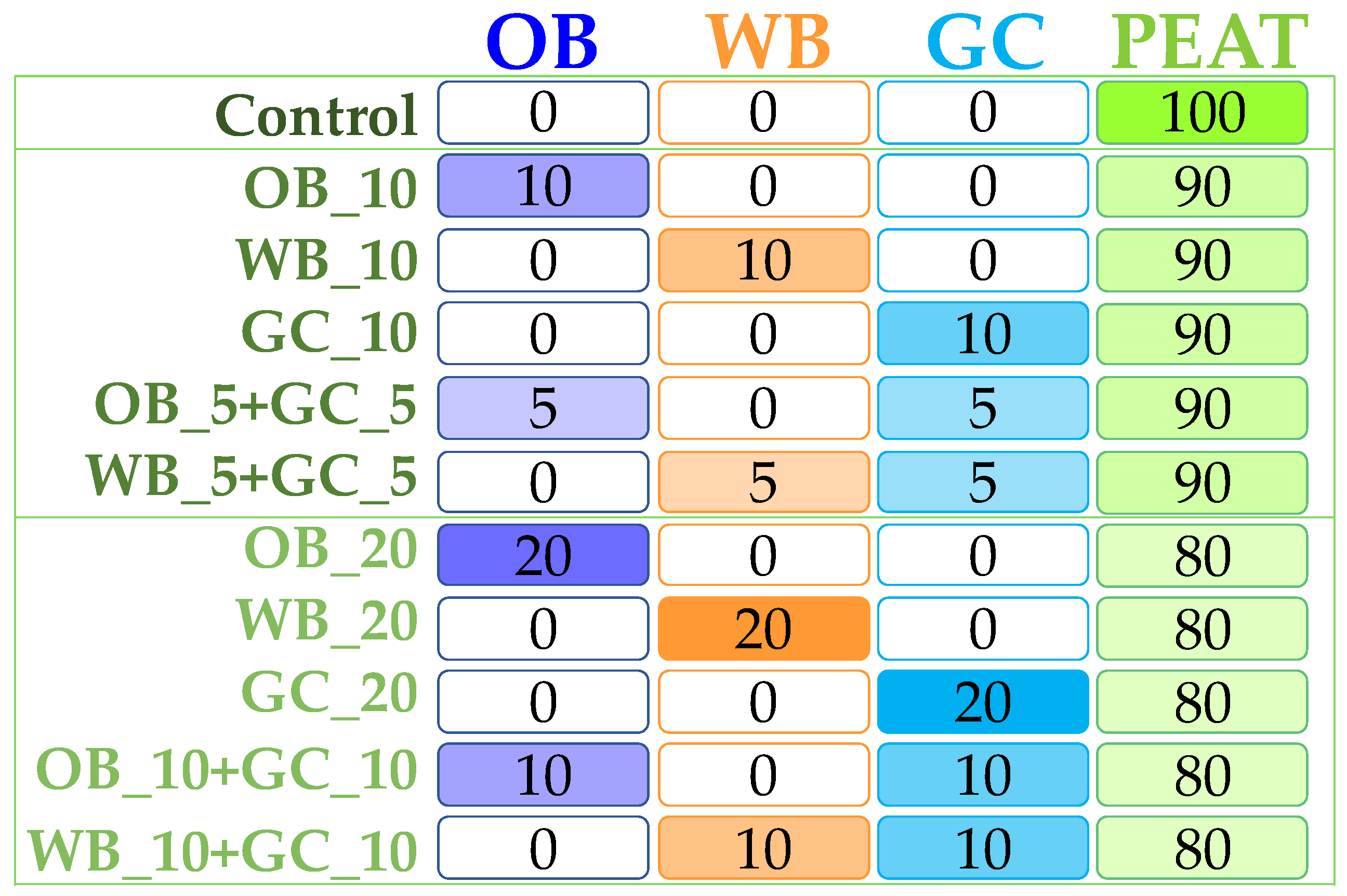
2.1. Substrates (Biochars, Compost and Commercial Substrate)
2.2. Germination Tests
2.3. Pot Experiments
2.3.1. Analysis of Substrates Properties
2.3.2. Pot Experiment I
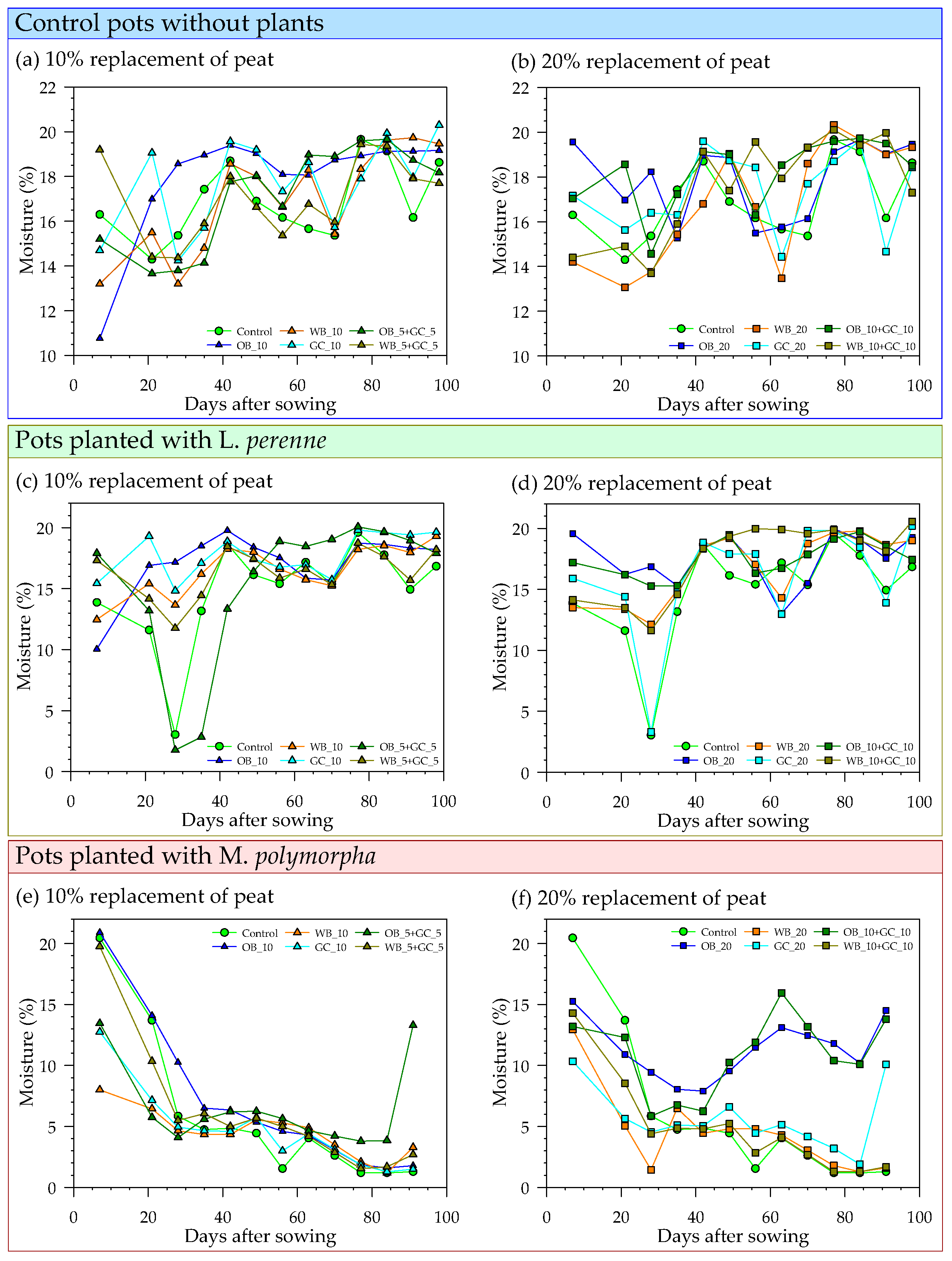
2.3.3. Pot Experiment II
2.4. Statistical Analyses
3. Results and Discussion
3.1. Germination Performance Under Peat-Replaced Substrates
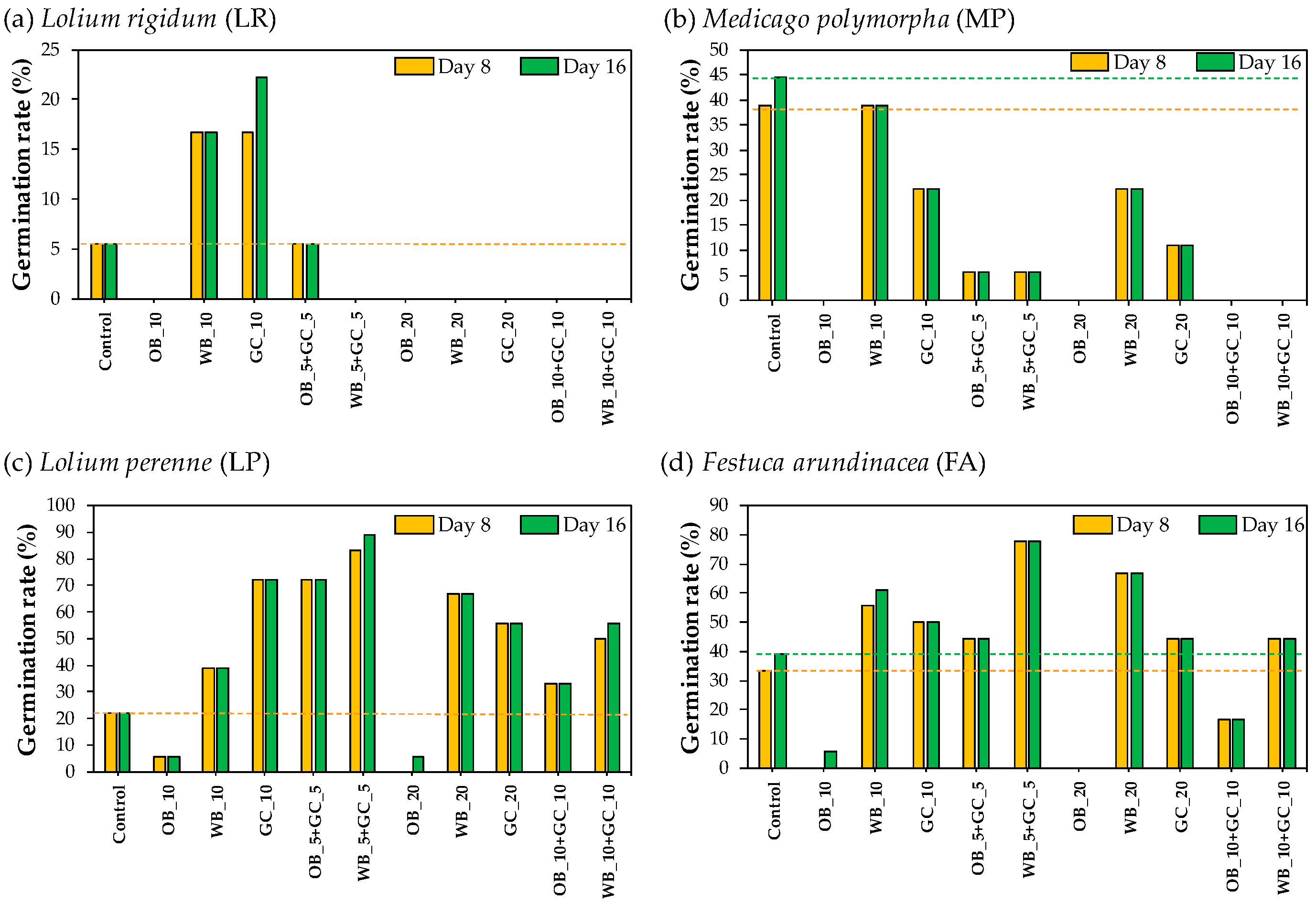
3.1.1. Germination Rates in Petri Dishes
3.1.2. Evaluation of Lolium perenne and Medicago polymorpha Germination in Container-Grown Substrates
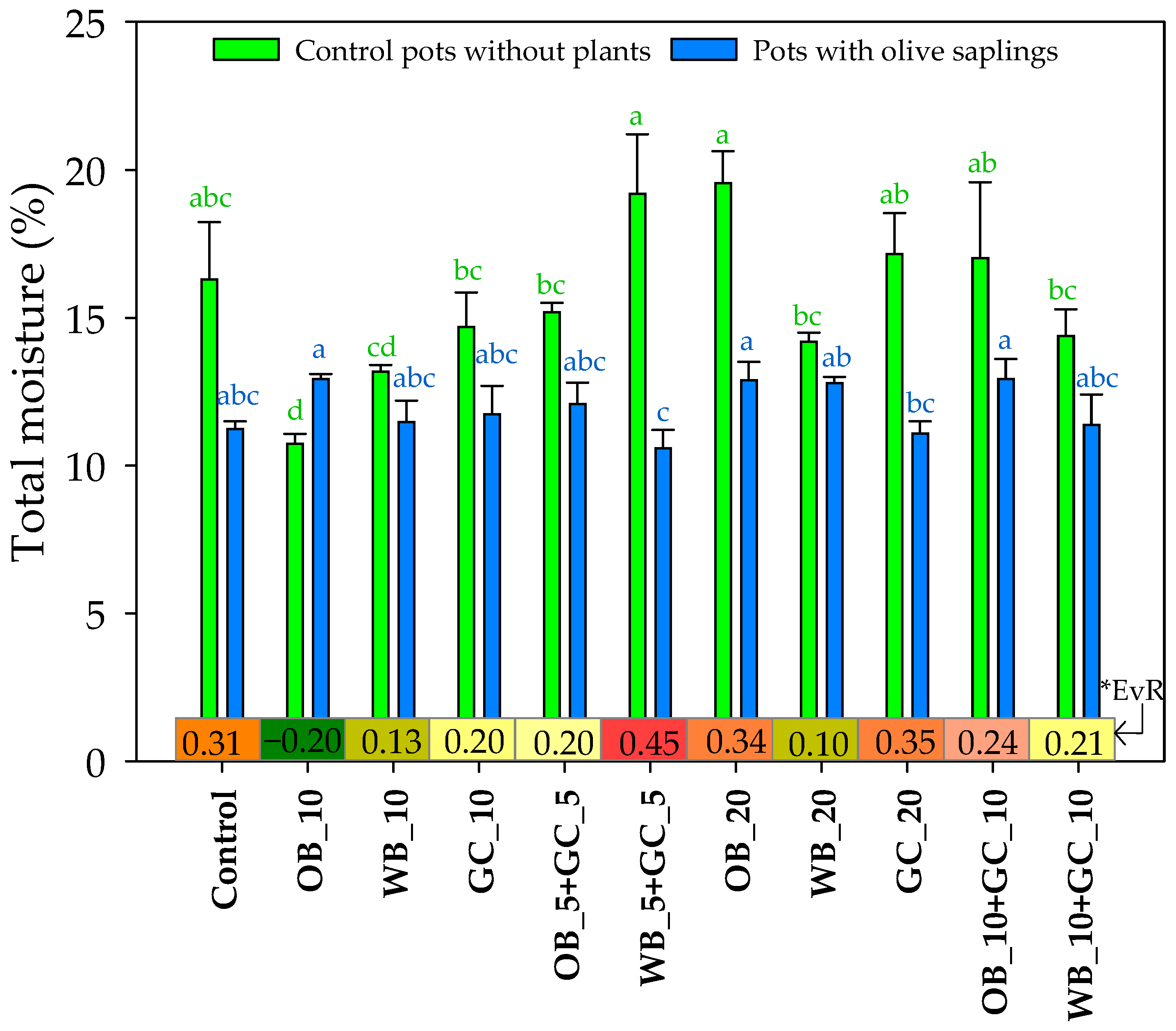
3.2. Physical and Chemical Changes in Substrate Mixtures Following Peat Substitution
3.3. Plant Yield, Physiological Performance and Nutrient Uptake Under Peat-Reduced Substrates
3.3.1. Experiment I: Biomass and Carbon Uptake in Forage Species
3.3.2. Experiment II: Olive Trees Performance and Nutrient Status
3.3.3. Nutrient Uptake and Elemental Composition of Olive Plants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LP | Lolium perenne |
| MP | Medicago polymorpha |
| LR | Lolium rigidum |
| FA | Festuca arundinacea |
| EC | Electrical Conductivity |
| SPAD | Soil–Plant Analysis Development |
| Ms | Middle section |
| WHC | Water holding capacity |
| OB | Olive pomace Biochar |
| WB | Wood Biochar |
| GC | Green Compost |
| DAS | Days After Sowing |
References
- Tanneberger, F.; Tegetmeyer, C.; Busse, S.; Barthelmes, A.; Shumka, S.; Mariné, A.M.; Jenderedjian, K.; Steiner, G.M.; Essl, F.; Etzold, J.; et al. The peatland map of Europe. Mires Peat 2017, 19, 22. [Google Scholar] [CrossRef]
- European Commission. The European Green Deal (COM/2019/640 Final); Publications Office of the European Union: Brussels, Belgium, 2019; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52019DC0640 (accessed on 24 July 2025).
- European Commission. Directorate-General for Communication, Circular Economy Action Plan—For a Cleaner and More Competitive Europe; Publications Office of the European Union: Luxembourg, 2020; Available online: https://data.europa.eu/doi/10.2779/05068 (accessed on 24 July 2025).
- European Commission. Commission Delegated Regulation (EU) 2021/2088 of 7 July 2021 Amending Annexes II, III and IV to Regulation (EU) 2019/1009 of the European Parliament and of the Council for the Purpose of Adding Pyrolysis and Gasification Materials as a Component Material Category in EU Fertilising Products. 2021. Available online: http://data.europa.eu/eli/reg_del/2021/2088/oj (accessed on 24 July 2025).
- Nguyen, C.N.; Chau, H.-W.; Kumar, A.; Chakraborty, A.; Muttil, N. Biochar Amendment in Green Roof Substrate: A Comprehensive Review of the Benefits, Performance, and Challenges. Appl. Sci. 2024, 14, 7421. [Google Scholar] [CrossRef]
- Santos, M.G.B.d.; Paiva, A.B.; Viana, R.d.S.R.; Jindo, K.; Figueiredo, C.C.d. Biochar as a Feedstock for Sustainable Fertilizers: Recent Advances and Perspectives. Agriculture 2025, 15, 894. [Google Scholar] [CrossRef]
- Campos, P.; Miller, A.Z.; Knicker, H.; Costa-Pereira, M.F.; Merino, A.; De la Rosa, J.M. Chemical, physical and morphological properties of biochars produced from agricultural residues: Implications for their use as soil amendment. Waste Manag. 2020, 105, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, A.; Drosos, M. The essential role of humified organic matter in preserving soil health. Chem. Biol. Technol. Agric. 2025, 12, 21. [Google Scholar] [CrossRef]
- Chafik, Y.; Sena-Velez, M.; Henaut, H.; Missbah El Idrissi, M.; Carpin, S.; Bourgerie, S.; Morabito, D. Synergistic Effects of Compost and Biochar on Soil Health and Heavy Metal Stabilization in Contaminated Mine Soils. Agronomy 2025, 15, 1295. [Google Scholar] [CrossRef]
- Méndez, A.; Paz-Ferreiro, J.; Gil, E.; Gascó, G. The effect of paper sludge and biochar addition on brown peat and coir based growing media properties. Sci. Hortic. 2015, 193, 225–230. [Google Scholar] [CrossRef]
- García-Rodríguez, Á.F.; Moreno-Racero, F.J.; García de Castro Barragán, J.M.; Colmenero-Flores, J.M.; Greggio, N.; Knicker, H.; Rosales, M.A. Influence of Biochar Mixed into Peat Substrate on Lettuce Growth and Nutrient Supply. Horticulturae 2022, 8, 1214. [Google Scholar] [CrossRef]
- Nocentini, M.; Panettieri, M.; García de Castro Barragán, J.M.; Mastrolonardo, G.; Knicker, H. Recycling pyrolyzed organic waste from plant nurseries, rice production and shrimp industry as peat substitute in potting substrates. J. Environ. Manag. 2021, 277, 111436. [Google Scholar] [CrossRef]
- Margenot, A.J.; Griffin, D.E.; Alves, B.S.Q.; Rippner, D.A.; Li, C.; Parikh, S.J. Substitution of peat moss with softwood biochar for soil-free marigold growth. Ind. Crops Prod. 2018, 112, 160–169. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Prasad, M.; Kavanagh, A.; Tzortzakis, N. Biochar Type and Ratio as a Peat Additive/Partial Peat Replacement in Growing Media for Cabbage Seedling Production. Agronomy 2019, 9, 693. [Google Scholar] [CrossRef]
- Fornes, F.; Liu-Xu, L.; Lidón, A.; Sánchez-García, M.; Cayuela, M.L.; Sánchez-Monedero, M.A.; Belda, R.M. Biochar Improves the Properties of Poultry Manure Compost as Growing Media for Rosemary Production. Agronomy 2020, 10, 261. [Google Scholar] [CrossRef]
- Lazcano, C.; Arnold, J.; Tato, A.; Zaller, J.; Domínguez, J. Compost and vermicompost as nursery pot components: Effects on tomato plant growth and morphology. Span. J. Agric. Res. 2009, 7, 944–951. [Google Scholar] [CrossRef]
- Erdal, İ.; Aktaş, H.; Yaylacı, C.; Türkan, Ş.A.; Aydın, G.; Hor, Y. Effects of peat based substrate combinations on mineral nutrition, growth and yield of tomato. J. Plant Nutr. 2023, 47, 30–48. [Google Scholar] [CrossRef]
- Bignami, C.; Melegari, F.; Zaccardelli, M.; Pane, C.; Ronga, D. Composted Solid Digestate and Vineyard Winter Prunings Partially Replace Peat in Growing Substrates for Micropropagated Highbush Blueberry in the Nursery. Agronomy 2022, 12, 337. [Google Scholar] [CrossRef]
- Miler, N.; Wojewódzki, P.; Woźny, A.; Rymarz, D.; Gołębiewska, A. Exploring Coffee Silverskin as a Sustainable Peat Additive in the Plant Nursery Production. Agronomy 2025, 15, 1633. [Google Scholar] [CrossRef]
- Iacomino, G.; Cozzolino, A.; Idbella, M.; Amoroso, G.; Bertoli, T.; Bonanomi, G.; Motti, R. Potential of Biochar as a Peat Substitute in Growth Media for Lavandula angustifolia, Salvia rosmarinus and Fragaria x ananassa. Plants 2023, 12, 3689. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Agronomic and remedial benefits and risks of applying biochar to soil: Current knowledge and future research directions. Environ. Int. 2016, 87, 1–12. [Google Scholar] [CrossRef]
- Hirschler, O.; Osterburg, B.; Weimar, H.; Glasenapp, S.; Ohmes, M.-F. Peat replacement in horticultural growing media: Availability of bio-based alternative materials. Thünen Work. Paper 2022, 190, 56. [Google Scholar] [CrossRef]
- MINERVA. Datos Abiertos del Ministerio. Available online: https://sedeaplicaciones.minetur.gob.es/Minerva/Index.aspx (accessed on 24 July 2025).
- Bu, X.; Ji, H.; Ma, W.; Mu, C.; Xian, T.; Zhou, Z.; Wang, F.; Xue, J. Effects of biochar as a peat-based substrate component on morphological, photosynthetic and biochemical characteristics of Rhododendron delavayi Franch. Sci. Hortic. 2022, 302, 111148. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Laird, D.A.; Fleming, P.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.; Hilhorst, H.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Schrieke, D.; Farrell, C. Trait-based green roof plant selection: Water use and drought response of nine common spontaneous plants. Urban For. Urban Green. 2021, 65, 127368. [Google Scholar] [CrossRef]
- JunJun, C.; Shuai, H.; Qin, D.; LiJiao, L.; ZhaoLong, W. Green roof cooling contributed by plant species with different photosynthetic strategies. Energy Build. 2019, 195, 45–50. [Google Scholar] [CrossRef]
- Tous, J.; Romero, A.; Hermoso, J.F. Variability and selection of ‘Arbequina’ clones for super-high-density olive orchard planting. HortScience 2010, 45, 1296–1299. [Google Scholar] [CrossRef]
- De la Rosa, J.M.; Campos, P.; Diaz-Espejo, A. Soil Biochar Application: Assessment of the Effects on Soil Water Properties, Plant Physiological Status, and Yield of Super-Intensive Olive Groves under Controlled Irrigation Conditions. Agronomy 2022, 12, 2321. [Google Scholar] [CrossRef]
- López, R.; Giráldez, I.; Palma, A.; Díaz, M.J. Assessment of compost maturity by using an electronic nose. Waste Manag. 2016, 4, 174–180. [Google Scholar] [CrossRef]
- Maris, S.C.; Teira-Esmatges, M.R.; Arbonés, A.; Rufat, J. Effect of irrigation, nitrogen application, and a nitrification inhibitor on nitrous oxide, carbon dioxide and methane emissions from an olive (Olea europaea L.) orchard. Sci. Total Environ. 2015, 538, 966–978. [Google Scholar] [CrossRef]
- Gascó, G.; Álvarez, M.L.; Paz-Ferreiro, J.; Miguel, G.S.; Méndez, A. Valorization of biochars from pinewood gasification and municipal solid waste torrefaction as peat substitutes. Environ. Sci. Pollut. Res. Int. 2018, 25, 26461–26469. [Google Scholar] [CrossRef]
- Jung, J.Y.; Ha, S.Y.; Yang, J.K. Steam Treated Sawdust as Soilless Growing Media for Germination and Growth of Horticulture Plant. J. Korean Wood Sci. Technol. 2017, 45, 857–871. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Gill, G.; Preston, C. Influence of environmental factors on seed germination and seedling emergence of rigid ryegrass (Lolium rigidum). Weed Sci. 2006, 54, 1004–1012. [Google Scholar] [CrossRef]
- Ellery, A.J.; Gallagher, R.S.; Dudley, S.V. Dormancy and germination ecology of annual ryegrass (Lolium rigidum Gaud.). In The Biology of Seeds: Recent Research Advances, Proceedings of the Seventh International Workshop on Seeds, Salamanca, Spain, 15–20 September 2002; Côme, D., Corbineau, F., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 389–396. [Google Scholar] [CrossRef]
- Beckie, H.J.; Jasieniuk, M. Lolium rigidum and Lolium multiflorum. In Biology and Management of Problematic Crop Weed Species; Nandula, V.K., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 261–283. [Google Scholar] [CrossRef]
- Jimenez-Lopez, J.C. (Ed.) Seed Dormancy and Germination; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Alane, F.; Chabaca, R.; Ouafi, L.; Abdelguerfi-laouar, M.; Abdelguerfi, A. Break dormancy, germination capacity of medics after different techniques of scarification (Physical, Chemical and Mechanical). Afr. J. Agric. Res. 2016, 11, 340–351. [Google Scholar] [CrossRef]
- Lin, J.; Hua, X.; Peng, X.; Dong, B.; Yan, X. Germination Responses of Ryegrass (Annual vs. Perennial) Seed to the Interactive Effects of Temperature and Salt-Alkali Stress. Front. Plant Sci. 2018, 9, 1458. [Google Scholar] [CrossRef]
- Nichols, P.G.H.; Malik, A.I.; Stockdale, M.; Colmer, T.D. Salt tolerance and avoidance mechanisms at germination of annual pasture legumes: Importance for adaptation to saline environments. Plant Soil 2009, 315, 241–255. [Google Scholar] [CrossRef]
- Agarwal, P.; Saha, S.; Hariprasad, P. Agro-industrial-residues as potting media: Physicochemical and biological characters and their influence on plant growth. Biomass Conv. Bioref. 2023, 13, 9601–9624. [Google Scholar] [CrossRef] [PubMed]
- El Moussaoui, H.; Idardare, Z.; Bouqbis, L. Comprehensive assessment of phytotoxic effects, morphology, chemical compositions, and water retention capacities of biochars. RSC Sustain. 2025, 3, 3459–3472. [Google Scholar] [CrossRef]
- Campos, P.; Knicker, H.; Velasco-Molina, M.; De la Rosa, J.M. Assessment of the biochemical degradability of crop derived biochars in trace elements polluted soils. J. Anal. Appl. Pyrolysis 2021, 157, 105186. [Google Scholar] [CrossRef]
- Araújo, M.D.M.; Feitosa, M.M.; Primo, A.A.; Taniguchi, C.A.K.; Souza, H.A. Mineralization of nitrogen and carbon from organic compost from animal production waste. Rev. Caatinga 2020, 33, 310–320. [Google Scholar] [CrossRef]
- Picca, G.; Goñi-Urtiaga, A.; Lozano de Sosa, L.; Colombo Rodriguez, M.V.; Fernández Navarro, I.; Plaza, C.; Panettieri, M. Sustainability in urban agriculture: The role of biochar in enhancing the productive capacity of compost-based growing media for rooftop farming. Urban For. Urban Green. 2025, 107, 128774. [Google Scholar] [CrossRef]
- Charman, N.; Ballard, R.A. Burr medic (Medicago polymorpha L.) selections for improved N2 fixation with naturalised soil rhizobia. Soil Biol. Biochem. 2004, 36, 1331–1337. [Google Scholar] [CrossRef]
- Denton, M.D.; Hill, C.R.; Bellotti, W.D.; Coventry, D.R. Nodulation of Medicago truncatula and Medicago polymorpha in two pastures of contrasting soil pH and rhizobial populations. Appl. Soil Ecol. 2007, 35, 441–448. [Google Scholar] [CrossRef]
- Tosca, A.; Valagussa, M.; Martinetti, L.; Frangi, P. Biochar and green compost as peat alternatives in the cultivation of photinia and olive tree. Acta Hortic. 2021, 1305, 257–262. [Google Scholar] [CrossRef]
- Modica, G.; Arcidiacono, F.; Puglisi, I.; Baglieri, A.; La Malfa, S.; Gentile, A.; Arbona, V.; Continella, A. Response to Water Stress of Eight Novel and Widely Spread Citrus Rootstocks. Plants 2025, 14, 773. [Google Scholar] [CrossRef] [PubMed]
- Intrigliolo, F.; Giuffrida, A.; Rapisarda, P.; Calabretta, M.; Roccuzzo, G. SPAD as an indicator of nitrogen status in Citrus. In Proceedings of the IXth International Citrus Congress, Orlando, FL, USA, 3–7 December 2000; pp. 665–667. [Google Scholar]
- Boussadia, O.; Steppe, K.; Zgallai, H.; Ben El Hadj, S.; Braham, M.; Lemeur, R.; Van Labeke, M.C. Effects of nitrogen deficiency on leaf photosynthesis, carbohydrate status and biomass production in two olive cultivars ‘Meski’ and ‘Koroneiki’. Sci. Hortic. 2010, 123, 336–342. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; Benlloch, M.; Herrera, E.; García-Novelo, J.M. Effect of traditional and slow-release N fertilizers on growth of olive nursery plants and N losses by leaching. Sci. Hortic. 2004, 101, 39–49. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; Beltrán, G.; Sánchez-Zamora, M.A.; García-Novelo, J.; Aguilera, M.P.; Uceda, M. Olive Oil Quality Decreases with Nitrogen Over-fertilization. HortScience 2006, 41, 215–219. [Google Scholar] [CrossRef]
- Radulov, I.; Berbecea, A. Nutrient Management for Sustainable Soil Fertility; IntechOpen: London, UK, 2024; pp. 1–29. [Google Scholar] [CrossRef]
- Fornes, F.; Lidón, A.; Belda, R.M.; Macan, G.P.F.; Cayuela, M.L.; Sánchez-García, M.; Sánchez-Monedero, M.A. Soil fertility and plant nutrition in an organic olive orchard after 5 years of amendment with compost, biochar or their blend. Sci. Rep. 2024, 14, 16606. [Google Scholar] [CrossRef]
- Rodrigues, M.Â.; Ferreira, I.Q.; Claro, A.M.; Arrobas, M. Fertilizer recommendations for olive based upon nutrients removed in crop and pruning. Sci. Hortic. 2012, 142, 205–211. [Google Scholar] [CrossRef]
- Fernández-Escobar, R. Trends in olive nutrition (a review). Acta Hortic. 2018, 1199, 215–223. [Google Scholar] [CrossRef]
- Ran, E.; Uri, Y.; Hagai, Y.; Alon, B.-G.; Isaac, Z.; Arnon, D. Elevated fruit nitrogen impairs oil biosynthesis in olive (Olea europaea L.). Front. Plant Sci. 2023, 14, 1180391. [Google Scholar] [CrossRef]

| TN (%) | TC (%) | Density (g ml−1) | WHC (%) | EC (µS cm−1) | pH (H2O) | |
|---|---|---|---|---|---|---|
| OB | 1.13 ± 0.03 | 56.3 ± 1.7 | 0.57 | 78 ± 15 | 13,700 ± 389 | 9.9 ± 0.1 |
| WB | 0.22 ± 0.01 | 83.4 ± 0.2 | 0.50 | 159 ± 19 | 232 ± 48 | 9.5 ± 0.2 |
| GC | 0.68 ± 0.02 | 14.9 ± 0.2 | 0.75 | 114 ± 11 | 1184 ± 178 | 8.3 ± 0.2 |
| PEAT | 0.90 ± 0.02 | 41.3 ± 0.5 | 0.23 | 421 ± 38 | 1678 ± 190 | 4.9 ± 0.1 |
| Control Pots Without Plants | Experiment I | Experiment II | ||||
|---|---|---|---|---|---|---|
| (0 DAS 1) | (100 DAS) | (100 DAS) | (100 DAS) | |||
| Treatment | Original | Control Pots | MP 2-Pots | LP 3-Pots | Olive Pots | |
| pH | Control | 5.0 ± 0.0 | 5.4 ± 0.0 f | 5.5 ± 0.0 e | 5.5 ± 0.0 eb | 5.6 ± 0.0 h |
| OB_10 | 7.3 ± 0.1 | 6.2 ± 0.0 cd | 6.3 ± 0.0 a | 6.6 ± 0.0 ab | 6.0 ± 0.0 d | |
| WB_10 | 6.6 ± 0.0 | 6.6 ± 0.3 b | 5.6 ± 0.3 d | 6.2 ± 0.3 db | 5.9 ± 0.0 e | |
| GC_10 | 6.2 ± 0.0 | 6.1 ± 0.1 de | 5.7 ± 0.1 d | 6.0 ± 0.1 db | 5.5 ± 0.0 h | |
| OB_5 + GC_5 | 6.2 ± 0.0 | 5.9 ± 0.1 e | 5.8 ± 0.1 c | 6.0 ± 0.1 cb | 5.8 ± 0.0 fg | |
| WB_5 + GC_5 | 6.2 ± 0.0 | 5.6 ± 0.0 f | 5.7 ± 0.0 d | 5.8 ± 0.0 db | 5.8 ± 0.0 f | |
| OB_20 | 6.0 ± 0.0 | 7.1 ± 0.1 a | 5.7 ± 0.1 d | 6.6 ± 0.1 db | 7.0 ± 0.1 a | |
| WB_20 | 5.7 ± 0.0 | 6.2 ± 0.1 cd | 5.8 ± 0.1 c | 6.3 ± 0.1 cb | 6.2 ± 0.0 c | |
| GC_20 | 5.8 ± 0.1 | 6.0 ± 0.0 de | 5.8 ± 0.0 c | 6.2 ± 0.0 cb | 6.2 ± 0.0 c | |
| OB_10 + GC_10 | 6.0 ± 0.0 | 6.4 ± 0.0 c | 6.3 ± 0.0 a | 6.5 ± 0.0 ab | 6.5 ± 0.0 b | |
| WB_10 + GC_10 | 5.3 ± 0.0 | 5.5 ± 0.1 f | 5.9 ± 0.1 b | 6.0 ± 0.1 cb | 5.7 ± 0.0 g | |
| TC 4 (g kg−1) | Control | 385 ± 22 e | 446 ± 0 c | 429 ± 2 de | 418 ± 2 f | 444 ± 2 d |
| OB_10 | 432 ± 11 c | 479 ± 3 b | 460 ± 7 c | 453 ± 1 d | 431 ± 6 e | |
| WB_10 | 438 ± 11 c | 485 ± 6 b | 586 ± 6 a | 513 ± 1 b | 532 ± 4 a | |
| GC_10 | 363 ± 33 f | 380 ± 5 e | 441 ± 4 d | 389 ± 3 h | 428 ± 5 e | |
| OB_5 + GC_5 | 412 ± 22 d | 421 ± 0 d | 417 ± 2 e | 407 ± 2 g | 450 ± 1 cd | |
| WB_5 + GC_5 | 440 ± 0 c | 428 ± 1 d | 490 ± 6 b | 430 ± 0 e | 457 ± 1 c | |
| OB_20 | 485 ± 22 b | 447 ± 3 c | 497 ± 2 b | 480 ± 2 c | 501 ± 0 b | |
| WB_20 | 506 ± 0 a | 554 ± 4 a | 578 ± 5 a | 582 ± 0 a | 537 ± 4 a | |
| GC_20 | 343 ± 55 g | 375 ± 11 e | 382 ± 7 f | 375 ± 5 i | 380 ± 0 g | |
| OB_10 + GC_10 | 384 ± 44 e | 436 ± 1 cd | 423 ± 1 e | 451 ± 4 d | 416 ± 5 f | |
| WB_10 + GC_10 | 451 ± 33 c | 429 ± 2 d | 489 ± 0 b | 483 ± 3 c | 461 ± 2 c | |
| TN 5 (g kg−1) | Control | 7.9 ± 3.1 | 13.8 ± 1.0 | 14.0 ± 0.3 | 9.1 ± 1.6 | 18.6 ± 1.1 |
| OB_10 | 13.4 ± 3.7 | 15.9 ± 0.4 | 14.7 ± 4.1 | 15.0 ± 0.8 | 18.1 ± 0.5 | |
| WB_10 | 7.8 ± 1.6 | 12.0 ± 0.1 | 11.8 ± 0.4 | 13.2 ± 0.3 | 14.8 ± 3.6 | |
| GC_10 | 8.0 ± 2.6 | 10.7 ± 2.6 | 16.9 ± 1.8 | 12.8 ± 1.4 | 21.3 ± 3.9 | |
| OB_5 + GC_5 | 6.9 ± 2.6 | 13.1 ± 1.5 | 12.9 ± 1.3 | 13.8 ± 0.2 | 18.7 ± 1.8 | |
| WB_5 + GC_5 | 9.2 ± 2.2 | 12.3 ± 0.5 | 16.3 ± 0.1 | 12.8 ± 0.6 | 15.6 ± 3.5 | |
| OB_20 | 4.2 ± 2.0 | 13.8 ± 0.1 | 18.7 ± 0.1 | 12.0 ± 0.3 | 18.4 ± 0.2 | |
| WB_20 | 7.9 ± 0.1 | 10.5 ± 2.2 | 13.2 ± 1.3 | 10.4 ± 0.9 | 13.7 ± 2.4 | |
| GC_20 | 8.4 ± 0.9 | 12.3 ± 0.1 | 14.7 ± 4.4 | 5.7 ± 1.9 | 14.4 ± 0.0 | |
| OB_10 + GC_10 | 11.5 ± 2.1 | 11.5 ± 1.4 | 18.5 ± 4.0 | 9.9 ± 3.4 | 15.9 ± 2.2 | |
| WB_10 + GC_10 | 8.4 ± 3.3 | 11.7 ± 2.7 | 17.1 ± 2.0 | 8.4 ± 1.4 | 17.4 ± 1.7 | |
| C/Nat | Control | 57 | 38 | 36 | 54 | 28 |
| OB_10 | 38 | 35 | 37 | 35 | 34 | |
| WB_10 | 65 | 47 | 58 | 45 | 34 | |
| GC_10 | 53 | 42 | 30 | 35 | 25 | |
| OB_5 + GC_5 | 70 | 38 | 38 | 34 | 28 | |
| WB_5 + GC_5 | 56 | 41 | 35 | 39 | 34 | |
| OB_20 | 134 | 38 | 31 | 47 | 32 | |
| WB_20 | 75 | 62 | 51 | 65 | 46 | |
| GC_20 | 48 | 35 | 30 | 77 | 31 | |
| OB_10 + GC_10 | 39 | 44 | 27 | 53 | 31 | |
| WB_10 + GC_10 | 63 | 43 | 33 | 67 | 31 | |
| Vegetation Development | Elemental Composition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M. polymorpha | L. perenne | M. polymorpha | L. perenne | |||||||
|
Fresh
Weight |
Dry
Weight |
Water
Content |
Fresh
Weight |
Dry
Weight |
Water
Content | TC | TN | TC | TN | |
| (g per Pot) | (g per Pot) | (%) | (g per Pot) | (g per Pot) | (%) | (g kg−1) | (g kg−1) | (g kg−1) | (g kg−1) | |
| Control | 5.5 ± 1.6 | 2.7 ± 0.6 | 50.3 ± 4.3 ab | 16.0 ± 2.5 | 0.6 ± 0.7 | 96.7 ± 3.8 | 428 ± 7 bc | 30.4 ± 3.8 a | 390 ± 4 b | 46.3 ± 2.2 abc |
| OB_10 | 8.8 ± 3.2 | 3.1 ± 2.0 | 67.6 ± 13.8 a | 13.0 ± 3.1 | 0.7 ± 0.4 | 95.2 ± 1.8 | 419 ± 5 c | 19.7 ± 5.2 c | 396 ± 2 b | 49.6 ± 2.4 a |
| WB_10 | 6.0 ± 0.8 | 2.0 ± 0.5 | 65.2 ± 13.2 a | 13.6 ± 0.5 | 0.7 ± 0.1 | 94.7 ± 0.5 | 426 ± 4 bc | 20.7 ± 4.7 bc | 408 ± 2 a | 45.6 ± 0.1 abc |
| GC_10 | 7.5 ± 1.5 | 2.9 ± 0.5 | 61.2 ± 7.1 ab | 12.0 ± 2.2 | 0.5 ± 0.3 | 95.7 ± 1.4 | 431 ± 7 ab | 26.8 ± 3.9 ab | 403 ± 1 a | 47.6 ± 1.0 ab |
| OB_5 + GC_5 | 9.1 ± 3.5 | 2.5 ± 1.2 | 73.0 ± 3.8 a | 14.7 ± 1.3 | 0.7 ± 0.5 | 95.6 ± 2.9 | 422 ± 1 bc | 20.5 ± 0.4 bc | 395 ± 1 b | 49.8 ± 1.8 a |
| WB_5 + GC_5 | 6.5 ± 3.1 | 2.7 ± 0.8 | 56.0 ± 10.6 ab | 13.0 ± 2.7 | 0.4 ± 0.4 | 97.1 ± 2.3 | 428 ± 0 bc | 20.9 ± 2.2 bc | 409 ± 2 a | 42.3 ± 2.5 bcd |
| OB_20 | - | - | - | - | - | - | - | - | - | - |
| WB_20 | 6.7 ± 0.3 | 2.7 ± 0.2 | 58.8 ± 2.3 ab | 13.3 ± 1.3 | 0.7 ± 0.2 | 95.1 ± 0.8 | 440 ± 5 a | 24.0 ± 0.1 abc | 406 ± 4 a | 46.6 ± 1.1 abc |
| GC_20 | 5.3 ± 2.1 | 1.7 ± 0.6 | 68.2 ± 2.1 a | 13.0 ± 1.9 | 0.7 ± 0.3 | 94.8 ± 1.5 | 424 ± 6 bc | 25.3 ± 0.7 abc | 403 ± 3 a | 44.9 ± 0.9 abc |
| OB_10 + GC_10 | 8.2 ± 5.4 | 3.0 ± 2.3 | 69.2 ± 12.7 a | 14.1 ± 3.2 | 0.6 ± 0.7 | 96.5 ± 4.0 | 429 ± 3 abc | 21.7 ± 1.1 bc | 403 ± 3 a | 40.3 ± 2.3 cd |
| WB_10 + GC_10 | 5.5 ± 0.2 | 3.1 ± 0.1 | 43.4 ± 2.0 b | 14.1 ± 1.7 | 0.9 ± 0.3 | 94.0 ± 1.3 | 431 ± 4 ab | 21.9 ± 0.9 bc | 406 ± 4 a | 37.8 ± 0.4 d |
| Plant Height | Fresh Weight of Leaves | Dry Weight of Leaves | Water Content in Leaves | Fresh Weight of Stems | Dry Weight of Stems |
SPAD Index
[Ms] |
Quantum Yield (F′ m F′ v−1)
[Ms] | |||
|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | (g) | (g) | (%) | (g) | (g) |
Before
Irrigation |
After
Irrigation |
Before
Irrigation |
After
Irrigation | |
| Control | 59.4 ± 8.8 ab | 14.6 ± 4.6 a | 6.7 ± 2.1 ab | 54.3 ± 2.1 b | 7.2 ± 1.4 a | 3.8 ± 0.7 a | 79 ± 4 | 83 ± 3 | 0.73 ± 0.02 | 0.73 ± 0.03 a |
| OB_10 | 10.7 ± 2.7 c | 0.5 ± 0.4 b | 0.2 ± 0.1 d | 62.1 ± 0.1 a | 0.5 ± 0.3 d | 0.3 ± 0.1 c | 68 ± 24 | 74 ± 19 | 0.69 ± 0.06 | 0.67 ± 0.07 ab |
| WB_10 | 58.8 ± 7.6 ab | 14.6 ± 1.6 a | 6.4 ± 0.8 ab | 56.5 ± 0.8 ab | 6.7 ± 2.3 ab | 3.3 ± 1.2 a | 75 ± 5 | 77 ± 6 | 0.68 ± 0.09 | 0.68 ± 0.07 ab |
| GC_10 | 61.2 ± 4.1 a | 14.7 ± 1.5 a | 6.5 ± 0.8 ab | 55.8 ± 0.8 ab | 6.9 ± 0.9 ab | 3.5 ± 0.5 a | 80 ± 4 | 82 ± 5 | 0.73 ± 0.05 | 0.70 ± 0.03 ab |
| OB_5 + GC_5 | 41.9 ± 25.0 ab | 7.4 ± 5.1 ab | 3.0 ± 1.9 c | 56.5 ± 2.0 ab | 4.3 ± 3.1 c | 1.9 ± 1.4 b | 67 ± 19 | 73 ± 17 | 0.62 ± 0.09 | 0.59 ± 0.11 b |
| WB_5 + GC_5 | 54.9 ± 10.9 ab | 15.2 ± 3.5 a | 6.8 ± 1.5 a | 55.5 ± 1.5 ab | 6.2 ± 1.2 ab | 3.1 ± 0.8 a | 79 ± 5 | 76 ± 7 | 0.68 ± 0.08 | 0.62 ± 0.07 ab |
| OB_20 | 7.6 ± 0.5 d | 0.0 ± 0.0 b | 0.0 ± 0.0 d | - | 0.2 ± 0.0 d | 0.1 ± 0.0 c | - | - | - | - |
| WB_20 | 55.5 ± 4.6 b | 10.3 ± 3.3 a | 4.7 ± 1.0 abc | 51.5 ± 1.0 ab | 5.5 ± 0.9 bc | 2.7 ± 0.4 ab | 71 ± 4 | 73 ± 5 | 0.73 ± 0.01 | 0.73 ± 0.02 a |
| GC_20 | 56.2 ± 5.6 ab | 10.3 ± 5.0 a | 4.6 ± 2.3 bc | 54.9 ± 2.3 ab | 5.8 ± 1.4 abc | 2.9 ± 0.8 ab | 80 ± 6 | 81 ± 3 | 0.71 ± 0.08 | 0.68 ± 0.09 ab |
| OB_10 + GC_10 | 8.8 ± 2.7 cd | 0.2 ± 0.2 b | 0.1 ± 0.1 d | 44.5 ± 0.1 b | 0.4 ± 0.2 d | 0.2 ± 0.1 c | 89 ± 3 * | 89 ± 4 * | 0.74 ± 0.02 * | - |
| WB_10 + GC_10 | 54.0 ± 4.1 b | 14.0 ± 3.3 a | 6.3 ± 1.5 ab | 55.2 ± 1.5 ab | 6.4 ± 1.5 ab | 3.3 ± 0.9 a | 79 ± 2 | 79 ± 4 | 0.64 ± 0.07 | 0.63 ± 0.10 ab |
| Nutrients | Elemental Composition | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | K | Mg | Na | P | S | B | Cu | Fe | Mn | Zn | TC | TN | |
| (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | (mg g−1 DW) | (µg g−1 DW) | (µg g−1 DW) | (µg g−1 DW) | (µg g−1 DW) | (µg g−1 DW) | (g kg−1) | (g kg−1) | |
| Control | 3.68 ± 0.10 ab | 12.8 ± 0.62 b | 0.94 ± 0.11 a | 0.06 ± 0.04 | 1.52 ± 0.26 | 1.80 ± 0.22 ab | 17.20 ± 1.79 | 0.82 ± 0.18 | 43.42 ± 8.30 | 21.26 ± 3.43 ab | 6.51 ± 3.4 ab | 480 ± 10 | 19.0 ± 1.9 ab |
| OB_10 * | 7.84 | 18.23 | 0.81 | 0.41 | 2.19 | 2.24 | 16.68 | 3.04 | 48.10 | 22.84 | 8.48 | 442 | 23.8 |
| WB_10 | 3.95 ± 0.26 ab | 14.0 ± 1.60 ab | 0.65 ± 0.04 b | 0.12 ± 0.07 | 1.22 ± 0.19 | 1.60 ± 0.22 ab | 16.90 ± 2.32 | 0.85 ± 0.17 | 36.86 ± 4.31 | 15.47 ± 0.56 b | 5.46 ± 0.5 ab | 467 ± 47 | 15.9 ± 3.2 ab |
| GC_10 | 3.69 ± 0.24 ab | 12.6 ± 0.39 b | 0.55 ± 0.03 b | 0.07 ± 0.05 | 1.34 ± 0.15 | 1.69 ± 0.33 ab | 17.40 ± 2.28 | 1.58 ± 1.24 | 35.50 ± 4.62 | 13.31 ± 1.52 b | 5.70 ± 1.5 ab | 474 ± 3 | 17.8 ± 1.8 ab |
| OB_5 + GC_5 | 3.13 ± 0.13 b | 19.2 ± 2.53 a | 0.52 ± 0.06 b | 0.38 ± 0.19 | 1.57 ± 0.27 | 1.95 ± 0.26 a | 19.15 ± 2.49 | 0.87 ± 0.07 | 44.86 ± 6.90 | 21.03 ± 1.30 a | 7.09 ± 1.3 a | 472 ± 15 | 23.1 ± 3.5 a |
| WB_5 + GC_5 | 4.33 ± 0.59 a | 14.4 ± 1.68 ab | 0.70 ± 0.09 b | 0.17 ± 0.14 | 1.51 ± 0.14 | 1.61 ± 0.17 ab | 21.88 ± 3.50 | 0.76 ± 0.10 | 37.33 ± 6.22 | 19.45 ± 9.51 ab | 6.06 ± 9.5 ab | 475 ± 26 | 16.7 ± 1.5 ab |
| OB_20 ** | - | - | - | - | - | - | - | - | - | - | - | - | - |
| WB_20 | 3.81 ± 0.47 ab | 16.3 ± 0.93 ab | 0.64 ± 0.09 b | 0.10 ± 0.09 | 1.31 ± 0.17 | 1.41 ± 0.20 b | 19.52 ± 1.80 | 1.88 ± 1.91 | 33.81 ± 3.74 | 17.30 ± 1.51 ab | 9.51 ± 1.5 ab | 470 ± 8 | 13.9 ± 2.5 b |
| GC_20 | 3.83 ± 0.22 ab | 16.0 ± 0.46 ab | 0.56 ± 0.10 b | 0.11 ± 0.07 | 1.44 ± 0.09 | 2.01 ± 0.19 a | 19.72 ± 0.75 | 2.75 ± 1.18 | 39.02 ± 2.87 | 16.39 ± 0.52 b | 9.80 ± 0.5 ab | 469 ± 19 | 20.0 ± 3.3 ab |
| OB_10 + GC_10 ** | - | - | - | - | - | - | - | - | - | - | - | - | - |
| WB_10 + GC_10 | 3.99 ± 0.10 ab | 14.1 ± 1.08 ab | 0.55 ± 0.06 b | 0.08 ± 0.09 | 1.42 ± 0.27 | 1.70 ± 0.35 ab | 19.76 ± 2.67 | 0.75 ± 0.18 | 36.47 ± 5.20 | 15.14 ± 4.03 b | 5.36 ± 4.0 b | 458 ± 15 | 18.0 ± 1.6 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, P.; Sánchez-Martín, Á.M.; Lucas, M.; Santa-Olalla, A.; Rosales, M.A.; de la Rosa, J.M. Biochar and Compost as Sustainable Alternatives to Peat. Agronomy 2025, 15, 2455. https://doi.org/10.3390/agronomy15112455
Campos P, Sánchez-Martín ÁM, Lucas M, Santa-Olalla A, Rosales MA, de la Rosa JM. Biochar and Compost as Sustainable Alternatives to Peat. Agronomy. 2025; 15(11):2455. https://doi.org/10.3390/agronomy15112455
Chicago/Turabian StyleCampos, Paloma, Águeda M. Sánchez-Martín, Marta Lucas, Arturo Santa-Olalla, Miguel A. Rosales, and José María de la Rosa. 2025. "Biochar and Compost as Sustainable Alternatives to Peat" Agronomy 15, no. 11: 2455. https://doi.org/10.3390/agronomy15112455
APA StyleCampos, P., Sánchez-Martín, Á. M., Lucas, M., Santa-Olalla, A., Rosales, M. A., & de la Rosa, J. M. (2025). Biochar and Compost as Sustainable Alternatives to Peat. Agronomy, 15(11), 2455. https://doi.org/10.3390/agronomy15112455








