The Impact of Abiotic Environmental Stressors on Fluorescence and Chlorophyll Content in Glycine max (L.) Merrill
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
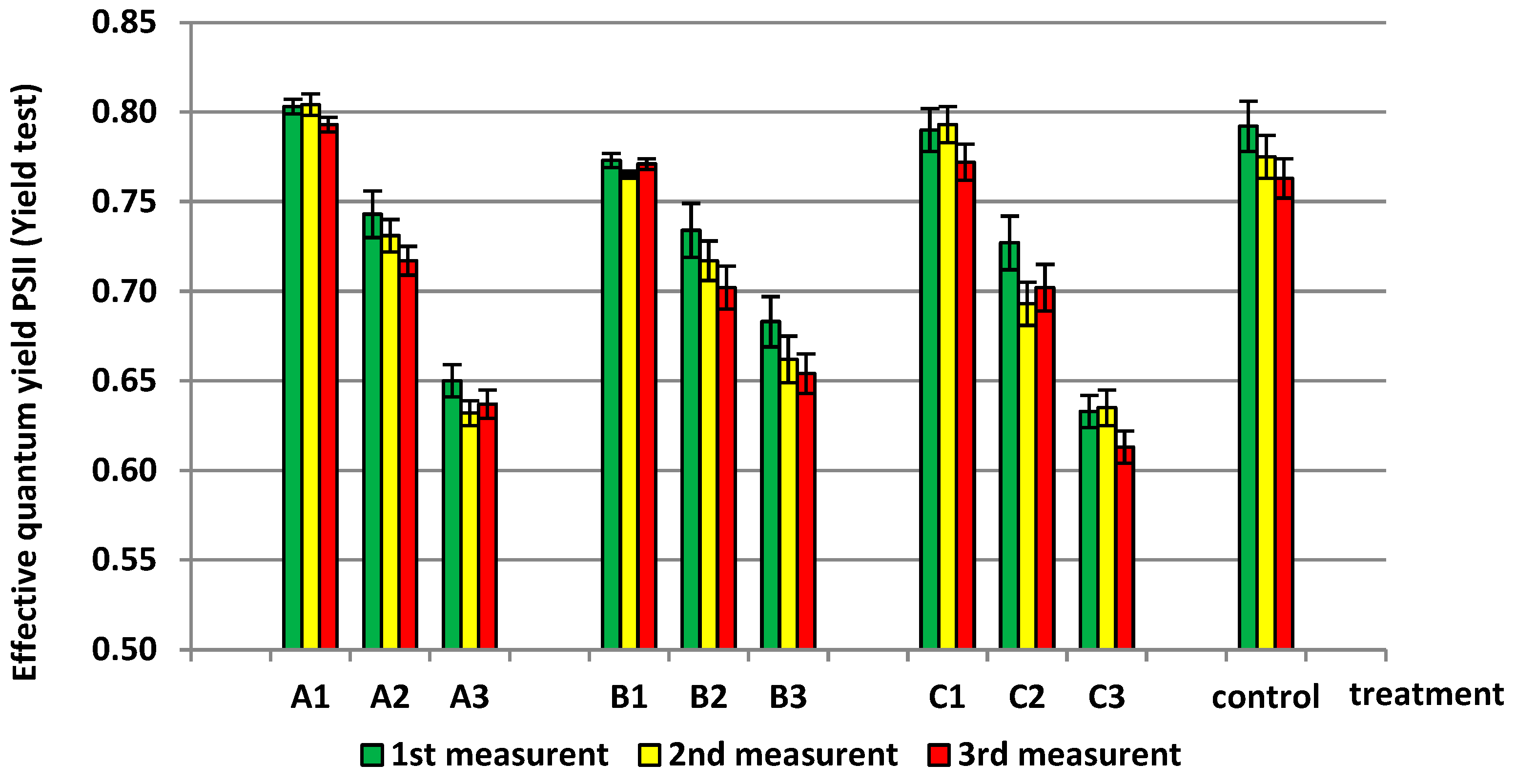
3.1. The Effect of Soil Salinity on Chlorophyll Fluorescence and Content in Soybean
3.2. The Effect of Soil pH on Chlorophyll Fluorescence and Content in Soybean
3.3. The Effect of Red Mud (Al) on Chlorophyll Fluorescence and Content in Soybean
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Angon, P.B.; Islam, M.S.; Shreejana, K.C.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef] [PubMed]
- Midula, P.; Andráš, P.; Ševciková, J.; Wiche, O. Phytoaccumulation of Mercury into Vascular Plants at Area of Abandoned Hg-Ore Deposit Malachov (Central Slovakia). Carpathian J. Earth Environ. Sci. 2023, 18, 225–229. [Google Scholar] [CrossRef]
- Ševčíková, J.; Midula, P.; Olšavský, M.; Mikušová, J.; Kupcová, E.; Benická, B. After-remediation monitoring of PAH concentration in groundwater of airport Sliač—Southern region (Slovakia). Carpathian J. Earth Environ. Sci. 2021, 16, 483–491. [Google Scholar] [CrossRef]
- Plesa, I.M.; González-Orenga, S.; Al Hassan, M.; Sestras, A.F.; Vicente, O.; Prohens, J.; Sestras, R.E.; Boscaiu, M. Effects of drought and salinity on European larch (Larix decidua Mill.) seedlings. Forests 2018, 9, 320. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Porcel, R.; Azcón, C.; Aroca, R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: New challenges in physiological and molecular studies. J. Exp. Bot. 2012, 63, 4033–4044. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bai, X.G.; Jiang, Z.D. The generic technology identification of saline–alkali land management and improvement based on social network analysis. Clust. Comput. 2019, 22, 13167–13176. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Pan, D.; Zhang, Y.; Luo, B.; Ji, J. Effects of drought stress on photosynthesis and chlorophyll fluorescence images of soybean (Glycine max) seedlings. Int. J. Agric. Biol. Eng. 2018, 11, 196–201. [Google Scholar] [CrossRef]
- Yu, Y.; Hou, W.S.; Yael, H.; Sun, S.; Wu, C.X.; Ifat, M.; Song, S.; Amir, R.; Han, T. Constitutive expression of feedback-insensitive cystathionine Y-synthase increases methionine levels in soybean leaves and seeds. J. Integr. Agric. 2018, 17, 54–62. [Google Scholar] [CrossRef]
- Wang, S.; Liu, S.; Wang, J.; Yokosho, K.; Zhou, B.; Yu, Y.C.; Liu, Z.; Frommer, W.B.; Ma, J.F.; Chen, L.Q. Simultaneous changes in seed size, oil content and protein content driven by selection of SWEET homologues during soybean domestication. Natl. Sci. Rev. 2020, 7, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- De Vargas, R.L.; Schuch, L.O.B.; Barros, W.S.; Rigo, G.A.; Szareski, V.J.; Carvalho, I.R.; Pimentel, J.R.; Troyjack, C.; Jaques, L.B.; De Souza, V.Q.; et al. Macronutrients and micronutrients variability in soybean seeds. J. Agric. Sci. 2018, 10, 209–222. [Google Scholar] [CrossRef]
- Guan, R.; Qu, Y.; Guo, Y.; Yu, L.; Liu, Y.; Jiang, J.; Chen, J.; Ren, Y.; Liu, G.; Tian, L. Salinity tolerance in soybean is modulated by natural variation in G m SALT 3. Plant J. 2014, 80, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.A.; Chowdhury, J.A.; Razzaque, M.; Ali, M.; Paul, S.; Aziz, M. Dry matter production and seed yield of soybean as affected by post-flowering salinity and water stress. Bangladesh Agron. J. 2016, 19, 21–27. [Google Scholar] [CrossRef]
- Phang, T.H.; Shao, G.; Lam, H.M. Salt tolerance in soybean. J. Integr. Plant Biol. 2008, 50, 1196–1212. [Google Scholar] [CrossRef]
- Soybean Maturity 00—ES MENTOR. ES MENTOR—Sója fazuľová—SAATBAU Slovensko. Available online: https://www.saatbau.com/sk/osiva/soja-fazulova/es-mentor/ (accessed on 22 October 2024). (In Slovak).
- Butcher, K.; Wick, A.F.; Desutter, T.; Chatterjee, A.; Harmon, J. Soil salinity: A threat to global food security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Soliman, M.; Elkelish, A.; Souad, T.; Alhaithloul, H.; Farooq, M. Brassinosteroid seed priming with nitrogen supplementation improves salt tolerance in soybean. Physiol. Mol. Biol. Plants 2020, 26, 501–511. [Google Scholar] [CrossRef]
- Chang, X.; Gao, Z.; Wang, S.; Chen, H. Modelling long-term soil salinity dynamics using SaltMod in Hetao Irrigation District, China. Comput. Electron. Agric. 2019, 156, 447–458. [Google Scholar] [CrossRef]
- Oral, E.; Altuner, F.; Tunçtürk, R.; Baran, İ. The impact of salt (NaCl) stress on germination characteristics of gibberellic acid (GA3) pretreated quinoa (Chenopodiumquinoa Willd.) seed. KSU J. Agric. Nat. 2020, 23, 349–356. [Google Scholar] [CrossRef]
- Miladinov, Z.; Maksimovic, I.; Balesević-Tubic, S.; Djukic, V.; Canak, P.; Miladinovic, J.; Djordjevic, V. Priming seed mitigates the effects of saline stress in soybean seedlings. Legume Res. 2020, 43, 263–267. [Google Scholar] [CrossRef]
- Oral, E.; Altuner, F.; Tunçtürk, R.; Tunçtürk, M. The impact of salt (NaCl) stress on germination characteristics of gibberellic acid pretreated wheat (Triticum durum Desf) seeds. Appl. Ecol. Environ. Res. 2019, 17, 12057–12071. [Google Scholar] [CrossRef]
- Çirka, M.; Kaya, A.R.; Eryigit, T. Influence of temperature and salinity stress on seed germination and seedling growth of soybean (Glycine max L.). Legume Res. 2021, 44, 1053–1059. [Google Scholar] [CrossRef]
- Oyebowale, M.A.; Egbichi, I.M.; Ludidi, N. Comparative analysis of responses to field salinity stress in contrasting soybean accessions highlights NaCl exclusion in leaves as a key mechanism for salinity stress tolerance. J. Oasis Agric. Sustain. Dev. 2021, 3, 20–24. [Google Scholar] [CrossRef]
- Roy, P.R.; Tahjib-Ul-Arif, M.; Polash, M.A.S.; Hossen, M.Z.; Hossain, M.A. Physiological mechanisms of exogenous calcium on alleviating salinity-induced stress in rice (Oryza sativa L.). Physiol. Mol. Biol. Plants 2019, 25, 611–624. [Google Scholar] [CrossRef]
- Luo, D.; Shi, Y.J.; Song, F.H.; Li, J.C. Effects of salt stress on growth, photosynthetic and fluorescence characteristics, and root architecture of Corylus heterophylla× C. avellan seedlings. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2019, 30, 3376–3384. [Google Scholar] [CrossRef]
- Grewal, H.S. Water uptake, water use efficiency, plant growth and ionic balance of wheat, barley, canola and chickpea plants on a sodic vertosol with variable subsoil NaCl salinity. Agric. Water Manag. 2010, 97, 148–156. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front. Plant Sci. 2016, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.S.; Abdelhamid, M.T. Influence of amino acids mixture application on some biochemical aspects, antioxidant enzymes and endogenous polyamines of Vicia faba plant grown under seawater salinity stress. Gesunde Pflanz. 2015, 67, 119–129. [Google Scholar] [CrossRef]
- Soliman, M.H.; Abdulmajeed, A.M.; Alhaithloul, H.; Alharbi, B.M.; El-Esawi, M.A.; Hasanuzzaman, M.; Elkelish, A. Saponin biopriming positively stimulates antioxidants defense, osmolytes metabolism and ionic status to confer salt stress tolerance in soybean. Acta Physiol. Plant. 2020, 42, 114. [Google Scholar] [CrossRef]
- Peña Calzada, K.; Olivera Viciedo, D.; Habermann, E.; Calero Hurtado, A.; Lupino Gratão, P.; De Mello Prado, R.; Lata-Tenesaca, L.F.; Martinez, C.A.; Ajila Celi, G.E.; Rodríguez, J.C. Exogenous Application of Amino Acids Mitigates the Deleterious Effects of Salt Stress on Soybean Plants. Agronomy 2022, 12, 2014. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Hossain, A.; Barutçular, C.; Iqbal, M.A.; Islam, M.S.; Fahad, S.; Sytar, O.; Çiğ, F.; Meena, R.S.; Erman, M. Consequences of Salinity Stress on the Quality of Crops and Its Mitigation Strategies for Sustainable Crop Production: An Outlook of Arid and Semi-arid Regions. In Environment, Climate, Plant and Vegetation Growth; Fahad, S., Hasanuzzaman, M., Alam, M., Ullah, H., Saeed, M., Khan, I.A., Adnan, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 503–533. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Nath, J.; Singh, A.K.; Pareek, A.; Joshi, R. Ion transporters and their regulatory signal transduction mechanisms for salinity tolerance in plants. Physiol. Plant. 2022, 174, e13702. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef]
- Kafle, A.; Garcia, K.; Peta, V.; Yakha, J.; Soupir, A.; Bücking, H. Beneficial plant microbe interactions and their effect on nutrient uptake, yield, and stress resistance of soybeans. In Soybean-Biomass, Yield and Productivity; Kassai, M., Ed.; IntechOpen: London, UK, 2018; pp. 1–20. [Google Scholar] [CrossRef]
- Hou, Q.Z.; Sun, K.; Zhang, H.; Su, X.; Fan, B.Q.; Feng, H.Q. The responses of photosystem II and intracellular ATP production of Arabidopsis leaves to salt stress are affected by extracellular ATP. J. Plant Res. 2018, 131, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.A. Salinity tolerance in plants: Trends and perspectives. Int. J. Mol. Sci. 2019, 20, 2408. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Ding, C.Q.; Li, W.H.; Wang, D.C.; Cui, D. Applications of metabolomics in the research of soybean plant under abiotic stress. Food Chem. 2020, 310, 125914. [Google Scholar] [CrossRef]
- Luo, B.; Wang, C.; Wang, X.; Zhang, H.; Zhou, Y.; Wang, W.; Song, P. Changes in photosynthesis and chlorophyll fluorescence in two soybean (Glycine max) varieties under NaCl stress. Int. J. Agric. Biol. Eng. 2021, 14, 76–82. [Google Scholar] [CrossRef]
- Liu, W.; Li, R.J.; Han, T.T.; Cai, W.; Fu, Z.W.; Lu, Y.T. Salt stress reduces root meristem size by nitric oxide-mediated modulation of auxin accumulation and signaling in Arabidopsis. Plant Physiol. 2015, 168, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Vratarić, M.; Sudarić, A. Soja Glycine max (L.) Merr; Poljoprivredni institut Osijek: Osijek, Croatia, 2008; pp. 1–460. [Google Scholar]
- Shea, Z.; Singer, W.M.; Zhang, B. Soybean production, versatility, and improvement. In Legume Crops-Prospects, Production and Uses; Hasanuzzaman, M., Ed.; IntechOpen: London, UK, 2020; pp. 29–50. [Google Scholar] [CrossRef]
- Toomer, O.T.; Oviedo, E.O.; Ali, M.; Patino, D.; Joseph, M.; Frinsko, M.; Vu, T.; Maharjan, P.; Fallen, B.; Mian, R. Current Agronomic Practices, Harvest & Post-Harvest Processing of Soybeans (Glycine max)—A Review. Agronomy 2023, 13, 427. [Google Scholar] [CrossRef]
- Shamsi, I.H.; Wei, K.; Jilani, G.; Zhang, G.P. Interactions of cadmium and aluminum toxicity in their effect on growth and physiological parameters in soybean. J. Zhejiang Univ. Sci. B 2007, 8, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yang, Y.S.; Xu, G.; Guo, S.; Zheng, X.; Wang, M. Physiological response of four southern herbaceous plants to aluminium stress in South China. Front. Biol. China 2006, 1, 295–302. [Google Scholar] [CrossRef]
- Ying, X.F.; Liu, P.; Xu, G.D. Effect of aluminum on the isozymes of the seedlings of two soybeans Glycine max (L.) Merrill varieties. Plant Soil Environ. 2006, 52, 262–270. [Google Scholar] [CrossRef]
- Qin, R.; Chen, F. The aluminum toxicity of some crop seedlings in red soil of southern Hunan. J. Plant Nutr. Fertil. 1999, 5, 50–55. [Google Scholar] [CrossRef]
- Mmayi, M.P.; Musyimi, D.M.; Netondo, G.W.; Sikuku, P.A. Chlorophyll fluorescence parameters and photosynthetic pigments of four Glycine max varieties under Aluminium chloride stress. Sci. Agric. 2015, 10, 84–94. [Google Scholar] [CrossRef]
- Dhara, A.; Satish, C.B. Melatonin and nitric oxide relulate sunflower deedling growth under salt stress accompanying differential expression of Cu/Zn SOD and Mn SOD. Free Radic. Biol. Med. 2017, 106, 315–328. [Google Scholar] [CrossRef]
- Gorbunov, M.Y.; Falkowski, P.G. Using chlorophyll fluorescence kinetics to determine photosynthesis in aquatic ecosystems. Limnol. Oceanogr. 2021, 66, 1–13. [Google Scholar] [CrossRef]
- Zurek, G.; Rybka, K.; Pogrzeba, M.; Krzyzak, J.; Prokopiuk, K. Chlorophyll a Fluorescence in Evaluation of the Effect of Heavy Metal Soil Contamination on Perennial Grasses. PLoS ONE 2014, 9, e91475. [Google Scholar] [CrossRef]
- Hric, P.; Jancovic, J.; Kovar, P.; Vozar, L. The dynamics of the assimilation pigments content of turf fertilized by various forms of fertilizers. Agrochemistry 2016, 20, 3–7. (In Slovak) [Google Scholar]
- Hric, P.; Vozar, L.; Kovar, P. The changes of the assimilation pigments content of turf Festuca spp. leaves after application of different nutrition forms. Acta Fytotech. Zootech. 2018, 21, 6–10. [Google Scholar] [CrossRef][Green Version]
- Kovar, P.; Vozar, L.; Hric, P. Establishment and Care of Lawns, 1st ed.; Slovak Agricultural University: Nitra, Slovakia, 2022; pp. 1–180. [Google Scholar]
- Schwarz, M.; Lalik, V.; Vanek, M. Possibility of Exploitation of Mud from Alumina Production. Chem. Listy 2011, 105, 114–121. [Google Scholar]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef]
- Osório, J.; Osório, M.L.; Correia, P.J.; de Varennes, A.; Pestana, M. Chlorophyll fluo-rescence imaging as a tool to understand the impact of iron deficiency and resupply on photosynthetic performance of strawberry plants. Sci. Hortic. 2014, 165, 148–155. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Kromdijk, J.; Govindjee. Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Nath, K.; O’Donnell, J.P.; Lu, Y. Chlorophyll Fluorescence for High-Throughput Screening of Plants During Abiotic Stress, Aging, and Genetic Perturbation. In Photosynthesis: Structures, Mechanisms, and Applications; Hou, H., Najafpour, M., Moore, G., Allakhverdiev, S., Eds.; Springer: Cham, Switzerland, 2017; pp. 261–273. [Google Scholar] [CrossRef]
- Linda, R.; Zádrapová, D.; Křížová, K.; Kuneš, I. Měření obsahu a fluorescence chlorofylu v listech sadebního materiálu vybraných dřevin pomocí přenosných přístrojů (certifikovaná metodika) = Measurement of Chlorophyll Content and Fluorescence in the Leaves of Planting Material of Selected Tree Species Using Portable Devices (Certified Methodology); Linda, R., Ed.; Výzkumný ústav lesního hospodářství a myslivosti: Strnady, Czech Republic, 2019; pp. 1–46. (In Czech) [Google Scholar]
- Malinská, H.; Pidlisnyuk, V.; Nebeská, D.; Erol, A.; Medžová, A.; Trögl, J. Physiological Response of Miscanthus x giganteus to Plant Growth Regulators in Nutritionally Poor Soil. Plants 2020, 9, 194. [Google Scholar] [CrossRef]
- Martinez-Ferri, E.; Zumaquero, A.; Ariza, M.T.; Barceló, A.; Pliego, M.C. Nondestructive detection of white root rot disease in avocado rootstocks by leaf chlorophyll fluorescence. Plant Dis. 2016, 100, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, D.; Nikolić, B.; Đurović, S.; Waisi, H.; Anđelković, A.; Marisavljević, D. Chlorophyll as a measure of plant health: Agroecological aspects. Pestic. Fitomedicina 2014, 29, 21–34. [Google Scholar] [CrossRef]
- Parry, C.; Blonquist, J.M., Jr.; Bugbee, B. In situ measurement of leaf chlorophyll concentration: Analysis of the optical/absolute relationship. Plant Cell Environ. 2014, 37, 2508–2520. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Kalanaki, M.; Ritzema, H.; Bamshad, R.; Jones, E.; Fazilatnia, M. Application of bio-desalinization for reclamation of salt-affected soil under composted cow manure and deficit irrigation with saline water. Paddy Water Environ. 2020, 18, 469–479. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Daly, P.; Sharma, A.; Shaghaleh, H.; Hamoud, Y.A.; El-Esawi, M.A.; Pan, R.; Wan, Q.; et al. Seed priming and foliar application with jasmonic acid enhance salinity stress tolerance of soybean (Glycine max L.) seedlings. J. Sci. Food Agric. 2021, 101, 2027–2041. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.M.; Jan, S.; Abd Allah, E.F.; Rashid, B.; John, R.; Ahmad, P. Soybean under abiotic stress:proteomic approach. In Plant-Environment Interaction: Responses and Approaches to Mitigate Stress; Azooz, M.M., Ahmad, P., Eds.; John Wiley: Chichester, UK, 2015; pp. 28–42. [Google Scholar] [CrossRef]
- Hu, H.; Wang, L.; Wang, Q.; Jiao, L.; Hua, W.; Zhou, Q.; Huang, X. Photosynthesis, chlorophyll fluorescence characteristics, and chlorophyll content of soybean seedlings under combined stress of bisphenol A and cadmium. Environ. Toxicol. Chem. 2014, 33, 2455–2462. [Google Scholar] [CrossRef]
- Prieto, P.; Pecluelas, J.; Llusia, J.; Asensio, D.; Marc, E. Effects of long-term experimental night-time warming and drought on photosynthesis, Fv/Fm and stomatal conductance in the dominant species of a Mediterranean shrubland. Acta Physiol. Plant. 2009, 31, 729–739. [Google Scholar] [CrossRef]
- Alharbi, B.M.; Elhakem, A.H.; Alnusairi, G.S.H.; Mona, H.; Soliman, M.H.; Hakeem, K.R.; Hasan, M.M.; Abdelhamid, M.T. Exogenous application of melatonin alleviates salt stress-induced decline in growth and photosynthesis in Glycine max (L.) seedlings by improving mineral uptake, antioxidant and glyoxalase system. Plant Soil Environ. 2021, 67, 208–220. [Google Scholar] [CrossRef]
- Buczek, J.; Bobrecka-Jamro, D.; Jańczak-Pieniążek, M. Photosynthesis, yield and quality of soybean (Glycine max (L.) Merr.) under different soil-tillage systems. Sustainability 2022, 14, 4903. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Gresshoff, P.M. Physiological Implications of Legume Nodules Associated with Soil Acidity. In Legume Nitrogen Fixation in a Changing Environment; Sulieman, S., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2015; pp. 113–125. [Google Scholar] [CrossRef]
- Miransari, M. Soybean and Acidity Stress. In Environmental Stresses in Soybean Production; Miransari, M., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2016; Volume 2, pp. 229–250. [Google Scholar] [CrossRef]
- Evans, L.S.; Lewin, K.F.; Santucci, K.A.; Owen, E.M. Yields of field-grown soybeans exposed to simulated acidic deposition. Environ. Pollut. 1989, 61, 47–57. [Google Scholar] [CrossRef]
- Pham, H.T.T.; Nguyen, A.T.; Do, A.T.N.; Hens, L. Impacts of Simulated Acid Rain on the Growth and the Yield of Soybean (Glycine max (L.) Merr.) in the Mountains of Northern Vietnam. Sustainability 2021, 13, 4980. [Google Scholar] [CrossRef]
- Hu, H.; Wang, L.; Liao, C.; Fan, C.; Zhou, Q.; Huang, X. Combined effects of lead and acid rain on photosynthesis in soybean seedlings. Biol. Trace Elem. Res. 2014, 161, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, I.H.; Wei, K.; Zhang, G.P.; Jilani, G.H.; Hassan, M.J. Interactive effects of cadmium and aluminum on growth and antioxidative enzymes in soybean. Biologia Plantarum 2008, 52, 165–169. [Google Scholar] [CrossRef]
- Javor, Ľ.; Surovčík, J.; Škrobáková, E.; Beluský, J.; Šanta, M.; Frančáková, H. Legume Cultivation Technology (Technológia pestovania strukovín); VÚ rastlinnej výroby: Piešťany, Slovakia, 2001; pp. 1–60. ISBN 809685531X. (In Slovak) [Google Scholar]
- Davarpanah, M.N.; Poozesh, V.; Rezaei, A. Biochemical Responses of Two Soybean (Glycine max) Varieties to Aluminum Stress in Nutrient Solution. J. Chem. Health Risks 2016, 6, 237–247. [Google Scholar]
- Ying, X.; Liu, P. Effects of aluminum stress on photosynthetic characters of soybean. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2005, 16, 166–170. [Google Scholar] [PubMed]
- Karnwal, A.; Shrivastava, S.; Al-Tawaha, A.R.M.S.; Kumar, G.; Kumar, A.; Kumar, A. PGPR-Mediated Breakthroughs in Plant Stress Tolerance for Sustainable Farming. J. Plant Growth Regul. 2024, 43, 2955–2971. [Google Scholar] [CrossRef]
- Du, B.; Haensch, R.; Alfarraj, S.; Rennenberg, H. Strategies of plants to overcome abiotic and biotic stresses. Biol. Rev. 2024, 99, 1524–1536. [Google Scholar] [CrossRef]





| Treatment | Abiotic Chemical Stressor | Dose of Chemical Stressor | Volume of Gardening Substrate (dm3) |
|---|---|---|---|
| A1 | 20 g NaCl/2 dm3 H2O = 10‰ | 4.0 | |
| A2 | Salinity | 30 g NaCl/2 dm3 H2O = 15‰ | 4.0 |
| A3 | 60 g NaCl/2 dm3 H2O = 30‰ | 4.0 | |
| B1 | pH 6 | 4.0 | |
| B2 | Acidity | pH 5 | 4.0 |
| B3 | pH 4 | 4.0 | |
| C1 | 200 g | 3.8 | |
| C2 | Red mud | 400 g | 3.6 |
| C3 | 600 g | 3.4 | |
| Control | - | - | 4.0 |
| Factor | Quantum Yields PSII | Chlorophyll Content (CCI) | ||
|---|---|---|---|---|
| Y(II) | Fv/Fm | |||
| Treatment | A1 | 0.800 ± 0.006 g | 0.805 ± 0.022 f | 37.467 ± 1.206 e |
| A2 | 0.730 ± 0.013 d | 0.768 ± 0.013 d | 30.800 ± 1.044 d | |
| A3 | 0.640 ± 0.009 a | 0.687 ± 0.006 b | 26.033 ± 0.666 c | |
| B1 | 0.770 ± 0.004 e | 0.752 ± 0.008 c | 35.633 ± 2.684 e | |
| B2 | 0.718 ± 0.016 cd | 0.695 ± 0.012 b | 23.233 ± 2.458 b | |
| B3 | 0.666 ± 0.015 b | 0.668 ± 0.008 a | 22.833 ± 2.040 b | |
| C1 | 0.785 ± 0.011 f | 0.797 ± 0.009 ef | 24.533 ± 1.429 bc | |
| C2 | 0.707 ± 0.018 c | 0.782 ± 0.008 de | 18.133 ± 1.343 a | |
| C3 | 0.627 ± 0.012 a | 0.687 ± 0.009 b | 15.533 ± 0.751 a | |
| Control | 0.777 ± 0.015 ef | 0.798 ± 0.004 f | 35.767 ± 1.607 e | |
| LSD α0.05 | 0.01326 | 0.01467 | 2.63611 | |
| Measurement | 1 | 0.733 ± 0.060 c | 0.752 ± 0.057 b | 27.840 ± 7.525 b |
| 2 | 0.721 ± 0.064 b | 0.741 ± 0.055 a | 26.860 ± 7.369 ab | |
| 3 | 0.712 ± 0.063 a | 0.739 ± 0.051 a | 26.290 ± 8.275 a | |
| LSD α0.05 | 0.00727 | 0.00803 | 1.44386 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaškinová, J.; Tomaškin, J.; Drimal, M.; Bellido, J. The Impact of Abiotic Environmental Stressors on Fluorescence and Chlorophyll Content in Glycine max (L.) Merrill. Agronomy 2025, 15, 263. https://doi.org/10.3390/agronomy15020263
Tomaškinová J, Tomaškin J, Drimal M, Bellido J. The Impact of Abiotic Environmental Stressors on Fluorescence and Chlorophyll Content in Glycine max (L.) Merrill. Agronomy. 2025; 15(2):263. https://doi.org/10.3390/agronomy15020263
Chicago/Turabian StyleTomaškinová, Judita, Ján Tomaškin, Marek Drimal, and Jesus Bellido. 2025. "The Impact of Abiotic Environmental Stressors on Fluorescence and Chlorophyll Content in Glycine max (L.) Merrill" Agronomy 15, no. 2: 263. https://doi.org/10.3390/agronomy15020263
APA StyleTomaškinová, J., Tomaškin, J., Drimal, M., & Bellido, J. (2025). The Impact of Abiotic Environmental Stressors on Fluorescence and Chlorophyll Content in Glycine max (L.) Merrill. Agronomy, 15(2), 263. https://doi.org/10.3390/agronomy15020263





