Sly-miR398 Participates in Heat Stress Tolerance in Tomato by Modulating ROS Accumulation and HSP Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Condition
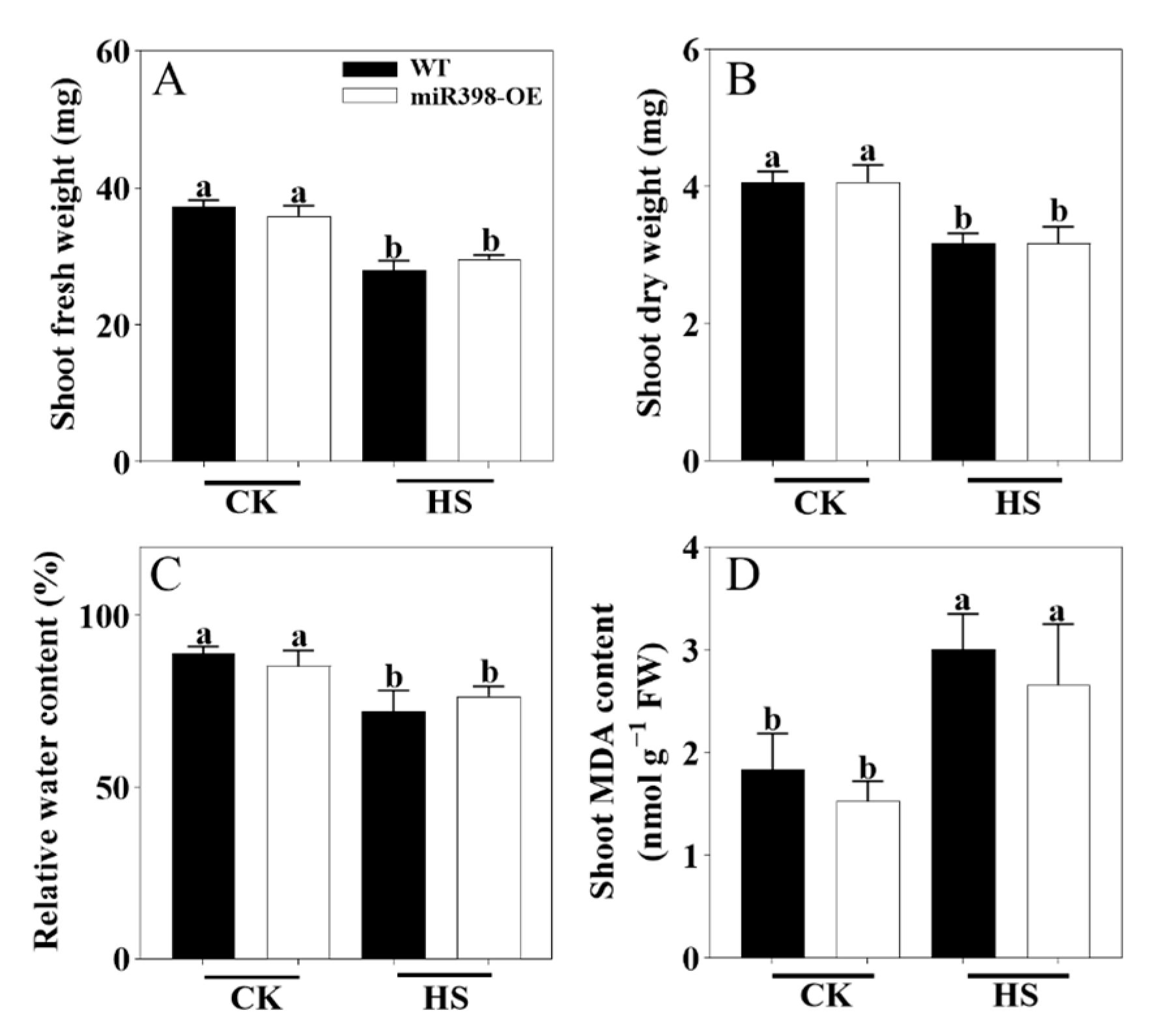
2.2. Biomass, Relative Water Content, and MDA Content
2.3. ROS Accumulation
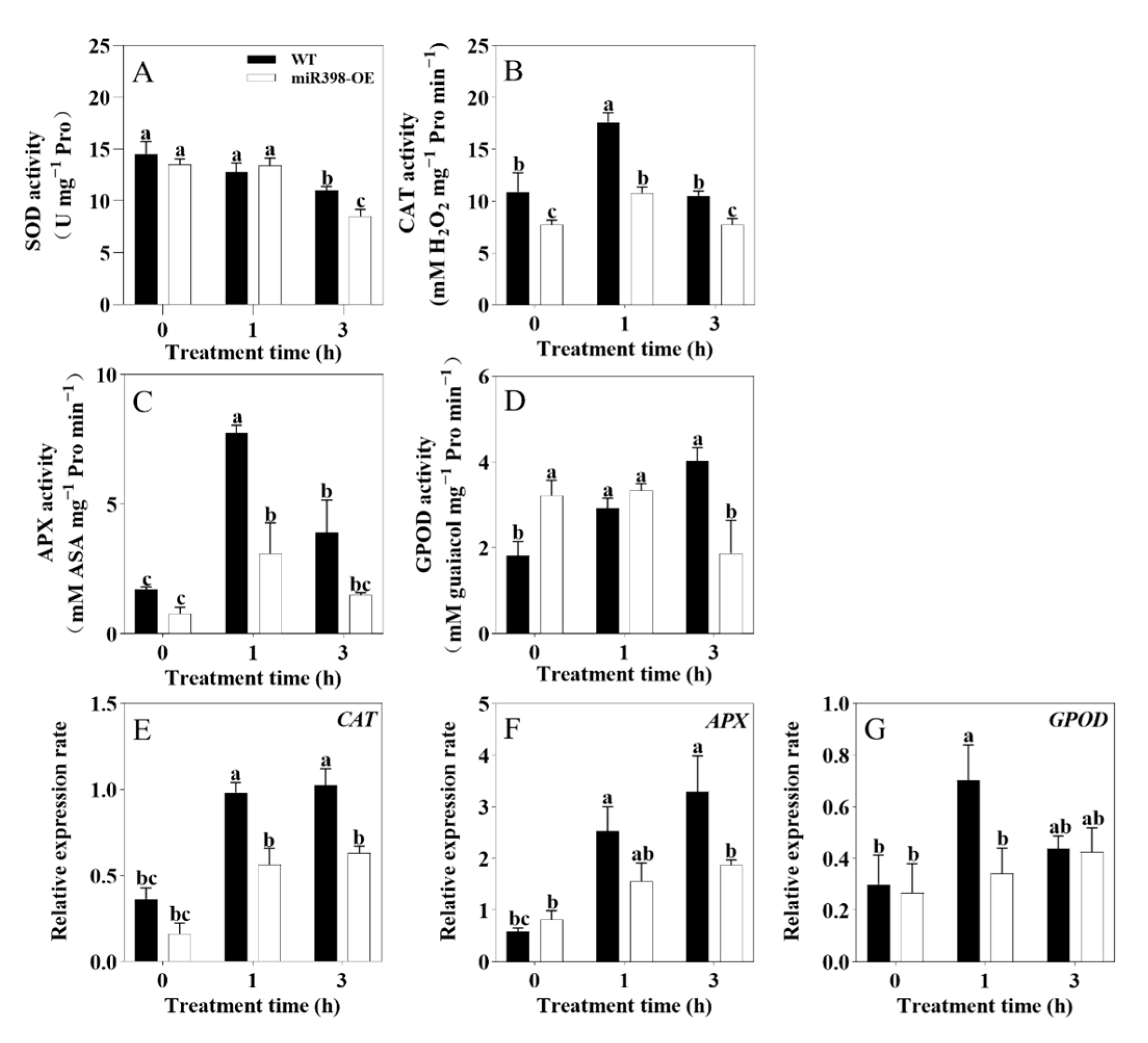
2.4. Antioxidant Enzyme Activity
2.5. H2O2 Scavenger Treatment
2.6. Gene Expression
2.7. Statistical Analysis
3. Results
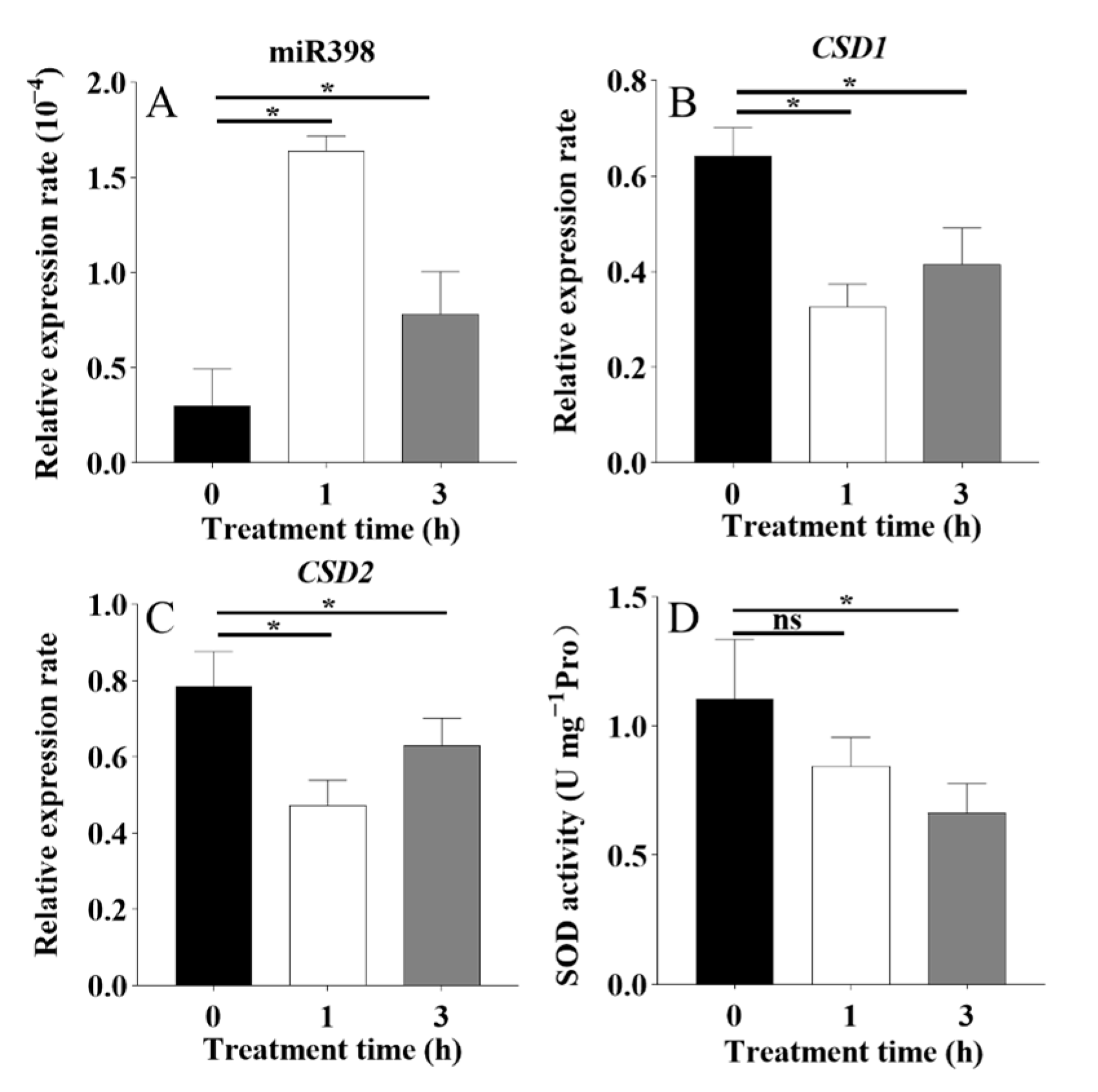
3.1. Response of miR398 to Heat Stress in Tomato
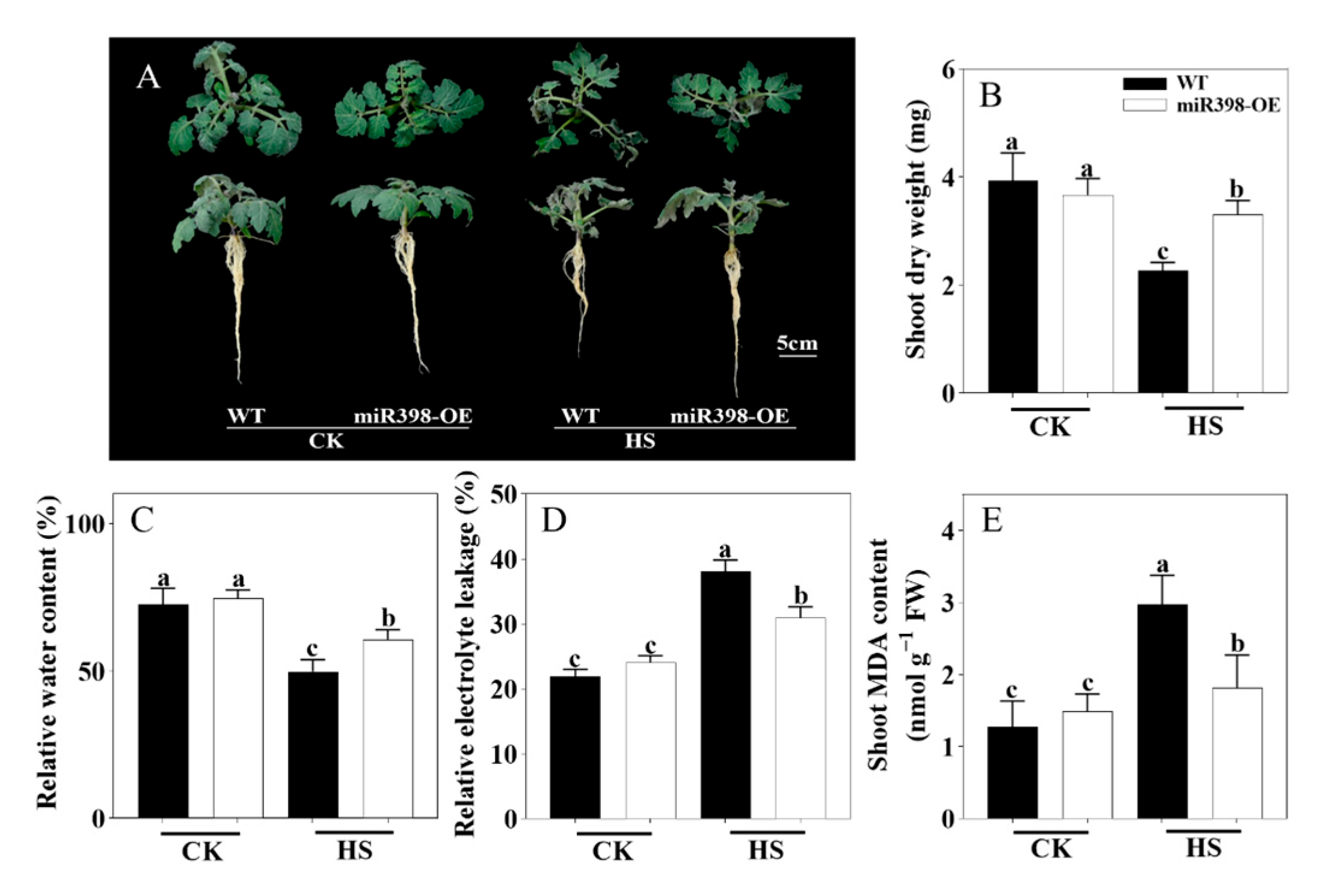
3.2. miR398-OE Was More Tolerant to Heat Stress than WT
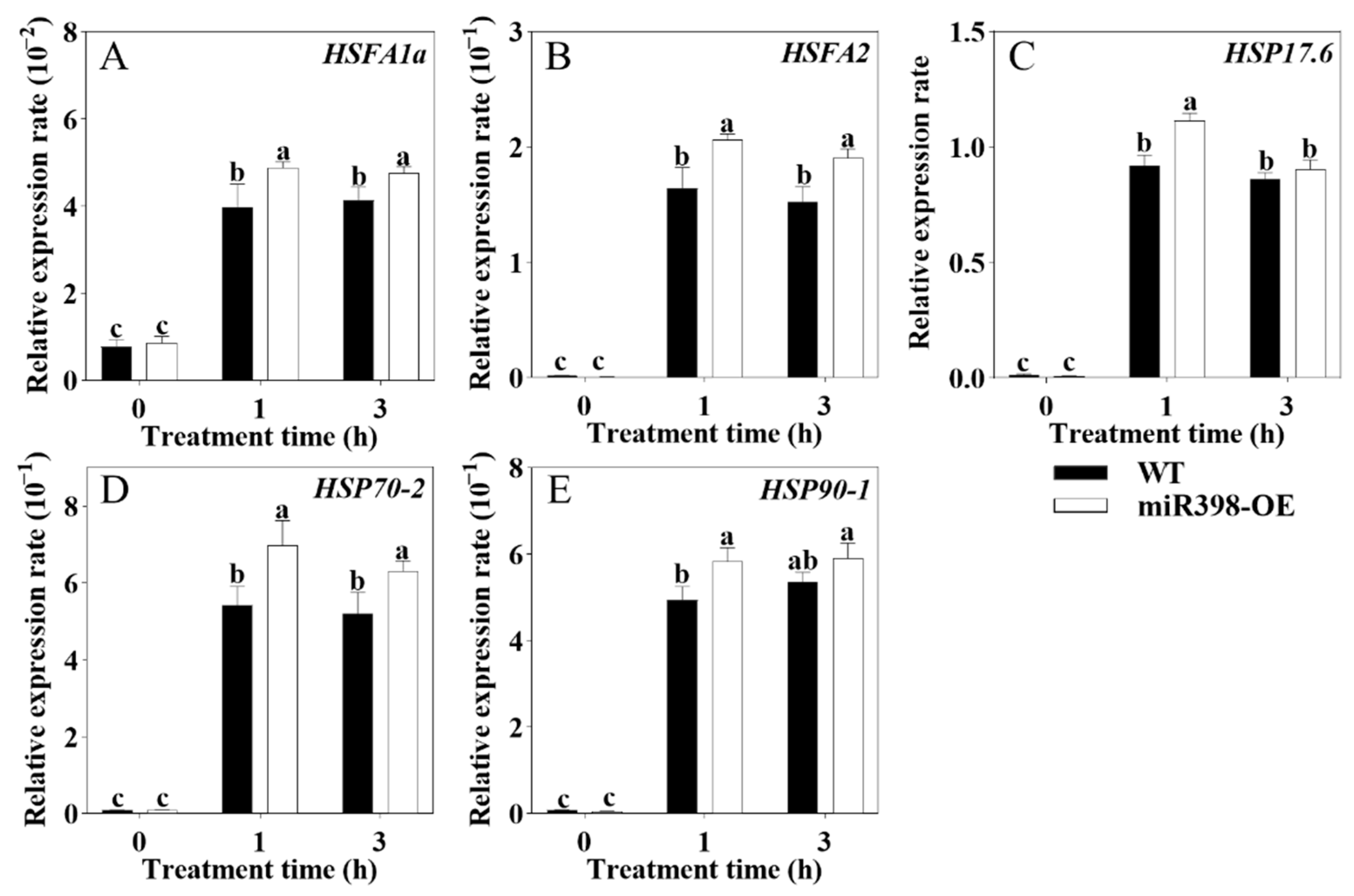
3.3. The Expression of HSFs/HSPs Was Enhanced in miR398-OE
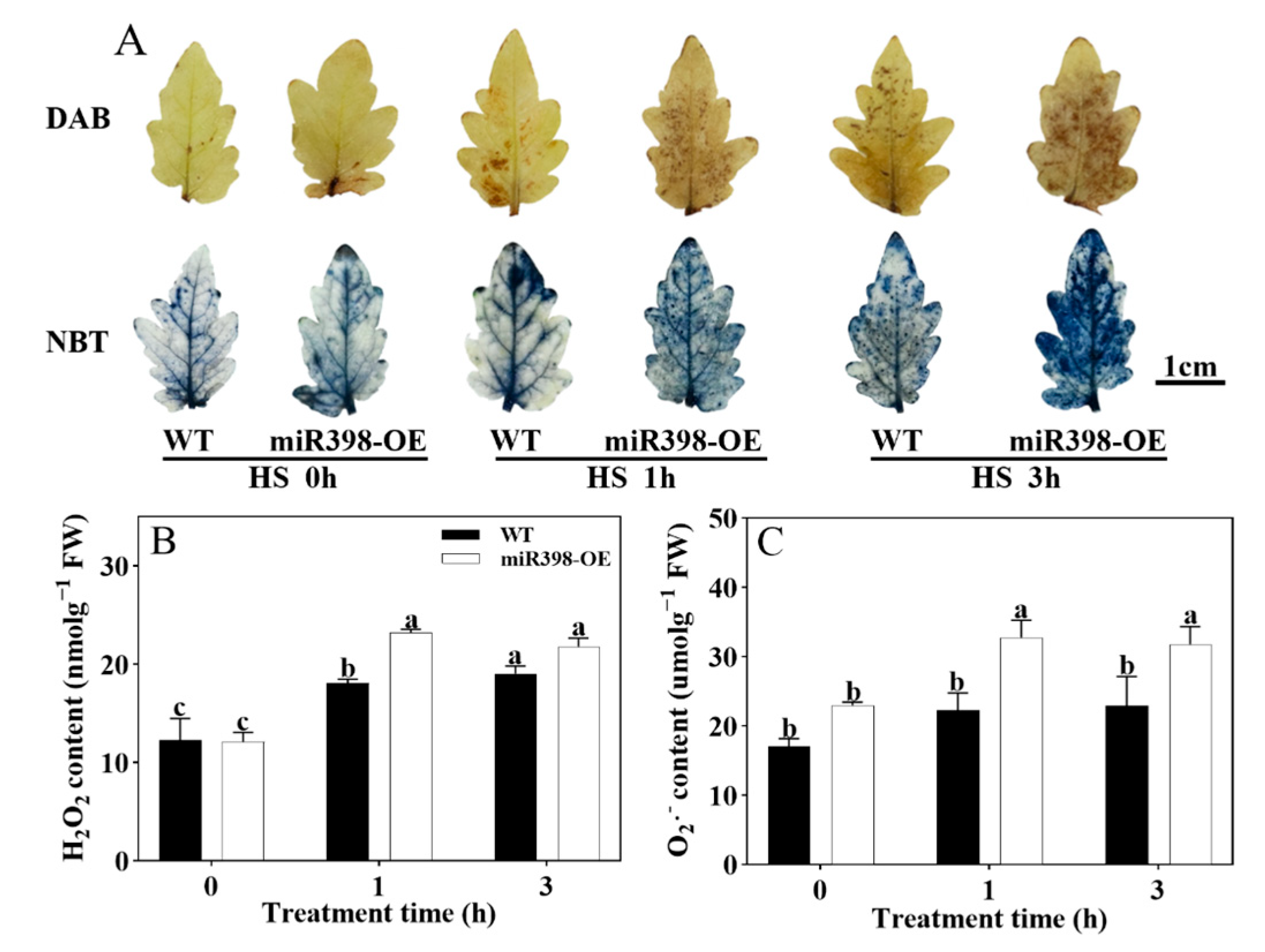
3.4. miR398-OE Accumulated More ROS Under Heat Stress than WT
3.5. Overexpression of miR398 Decreased Antioxidant Enzyme Activity in Tomato Under Heat Stress
3.6. H2O2 Scavenger Treatment Eliminated the Difference of Growth and HSFs/HSPs Expression Between WT and miR398-OE Under Heat Stress
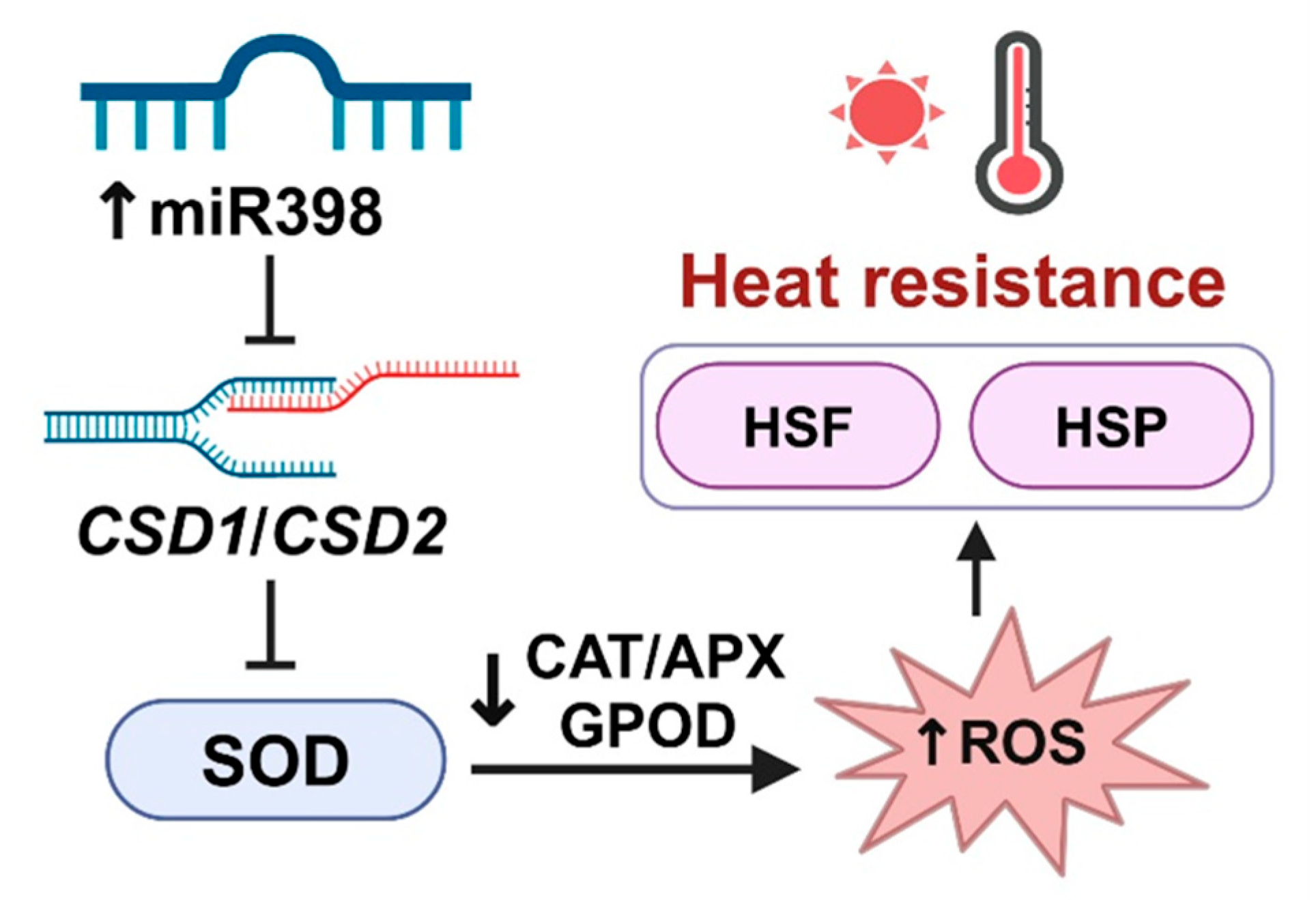
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing climate-resilient crops: Improving plant tolerance to stress combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Mu, X.; Gao, J.; Lin, H.; Lin, Y. The molecular basis of heat stress responses in plants. Mol. Plant 2023, 16, 1612–1634. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.A.; Hameed, A.; Nawaz, M.A.; Wei, M.A.; Noor, M.A.; Hussain, M.; Mehboob-ur-Rahman. Mechanisms and molecular approaches for heat tolerance in rice (Oryza sativa L.) under climate change scenario. J. Interg. Agric. 2018, 17, 726–738. [Google Scholar] [CrossRef]
- Goraya, G.K.; Kaur, B.; Asthir, B.; Bala, S.; Kaur, G.; Farooq, M. Rapid injuries of high temperature in plants. J. Plant Biol. 2017, 60, 298–305. [Google Scholar] [CrossRef]
- Lesk, C.; Anderson, W.; Rigden, A.; Coast, O.; Jaegermeyr, J.; McDermid, S.; Davis, K.F.; Konar, M. Compound heat and moisture extreme impacts on global crop yields under climate change. Nat. Rev. Earth Environ. 2022, 3, 872–889. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, S. Surviving and thriving: How plants perceive and respond to temperature stress. Dev. Cell 2022, 57, 947–958. [Google Scholar] [CrossRef]
- Pfleiderer, P.; Schleussner, C.F.; Kornhuber, K.; Coumou, D. Summer weather becomes more persistent in a 2 °C world. Nat. Clim. Change 2019, 9, 666–671. [Google Scholar] [CrossRef]
- Asthir, B. Mechanisms of heat tolerance in crop plants. Biol. Plant. 2015, 59, 620–628. [Google Scholar] [CrossRef]
- Gallardo, P.; Salas-Pino, S.; Daga, R.R. Reversible protein aggregation as cytoprotective mechanism against heat stress. Curr. Genet. 2021, 67, 849–855. [Google Scholar] [CrossRef]
- Zahra, N.; Hafeez, M.B.; Ghaffar, A.; Kausar, A.; Al Zeidi, M.; Siddique, K.H.M.; Farooq, M. Plant photosynthesis under heat stress: Effects and management. Environ. Exp. Bot. 2023, 206, 105178. [Google Scholar] [CrossRef]
- Andrasi, N.; Pettko-Szandtner, A.; Szabados, L. Diversity of plant heat shock factors: Regulation, interactions, and functions. J. Exp. Bot. 2021, 72, 1558–1575. [Google Scholar] [CrossRef]
- Fragkostefanakis, S.; Roeth, S.; Schleiff, E.; Scharf, K.-D. Prospects of engineering thermotolerance in crops through modulation of heat stress transcription factor and heat shock protein networks. Plant Cell Environ. 2015, 38, 1881–1895. [Google Scholar] [CrossRef] [PubMed]
- Bourgine, B.; Guihur, A. Heat shock signaling in land plants: From plasma membrane sensing to the transcription of small heat shock proteins. Front. Plant Sci. 2021, 12, 710801. [Google Scholar] [CrossRef] [PubMed]
- Driedonks, N.; Xu, J.; Peters, J.L.; Park, S.; Rieu, I. Multi-level interactions between heat shock factors, heat shock proteins, and the redox system regulate acclimation to heat. Front. Plant Sci. 2015, 6, 999. [Google Scholar] [CrossRef] [PubMed]
- Jones-Rhoades, M.W.; Bartel, D.P.; Bartel, B. MicroRNAs and their regulatory roles in plants. Annu. Rev. Plant Biol. 2006, 57, 19–53. [Google Scholar] [CrossRef]
- Tang, J.Y.; Chu, C.C. MicroRNAs in crop improvement: Fine-tuners for complex traits. Nat. Plants 2017, 3, 17077. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Cao, X.; Qi, Y. MicroRNAs and their regulatory roles in plant-environment interactions. Annu. Rev. Plant Biol. 2019, 70, 489–525. [Google Scholar] [CrossRef]
- Sunkar, R.; Zhu, J.K. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 2004, 16, 2001–2019. [Google Scholar] [CrossRef]
- Sunkar, R.; Kapoor, A.; Zhu, J.K. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 2006, 18, 2051–2065. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Kliebenstein, D.J.; Monde, R.A.; Last, R.L. Superoxide dismutase in Arabidopsis: An eclectic enzyme family with disparate regulation and protein localization. Plant Physiol. 1998, 118, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, Q.; Zuo, Z.F.; Liu, L. MicroRNA398: A master regulator of plant development and stress responses. Int. J. Mol. Sci. 2022, 23, 10803. [Google Scholar] [CrossRef]
- Lin, K.Y.; Wu, S.Y.; Hsu, Y.H.; Lin, N.S. MiR398-regulated antioxidants contribute to Bamboo mosaic virus accumulation and symptom manifestation. Plant Physiol. 2022, 188, 593–607. [Google Scholar] [CrossRef]
- Li, Y.J.; Li, X.R.; Yang, J.; He, Y.K. Natural antisense transcripts of MIR398 genes suppress microR398 processing and attenuate plant thermotolerance. Nat. Commun. 2020, 11, 5351. [Google Scholar] [CrossRef]
- Guan, Q.M.; Lu, X.Y.; Zeng, H.T.; Zhang, Y.Y.; Zhu, J.H. Heat stress induction of miR398 triggers a regulatory loop that is critical for thermotolerance in Arabidopsis. Plant J. 2013, 74, 840–851. [Google Scholar] [CrossRef]
- Yu, X.; Wang, H.; Lu, Y.Z.; de Ruiter, M.; Cariaso, M.; Prins, M.; van Tunen, A.; He, Y.K. Identification of conserved and novel microRNAs that are responsive to heat stress in Brassica rapa. J. Exp. Bot. 2012, 63, 1025–1038. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox signaling in plants. Antioxid. Redox Signal. 2013, 18, 2087–2090. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Alsamir, M.; Mahmood, T.; Trethowan, R.; Ahmad, N. An overview of heat stress in tomato (Solanum lycopersicum L.). Saudi J. Biol. Sci. 2021, 28, 1654–1663. [Google Scholar] [CrossRef]
- Liu, B.; Song, L.; Deng, X.; Lu, Y.; Lieberman-Lazarovich, M.; Shabala, S.; Ouyang, B. Tomato heat tolerance: Progress and prospects. Sci. Hortic. 2023, 322, 112435. [Google Scholar] [CrossRef]
- He, Y.; Zhou, J.; Hu, Y.; Fang, C.; Yu, Y.; Yang, J.; Zhu, B.; Ruan, Y.; Zhu, Z. Overexpression of sly-miR398b increased salt sensitivity likely via regulating antioxidant system and photosynthesis in tomato. Environ. Exp. Bot. 2021, 181, 104273. [Google Scholar] [CrossRef]
- Weatherley, P.E. Studies in the water relations of the cotton plant. 1. The field measurement of water deficits in leaves. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Yan, G.C.; Fan, X.P.; Zheng, W.N.; Gao, Z.X.; Yin, C.; Li, T.Q.; Liang, Y.C. Silicon alleviates salt stress-induced potassium deficiency by promoting potassium uptake and translocation in rice (Oryza sativa L.). J. Plant Physiol. 2021, 258–259, 153379. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kkinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Jabs, T.; Dietrich, R.A.; Dangl, J.L. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 1996, 273, 1853–1856. [Google Scholar] [CrossRef]
- Vanacker, H.; Carver, T.L.; Foyer, C.H. Early H2O2 accumulation in mesophyll cells leads to induction of glutathione during the hyper-sensitive response in the barley-powdery mildew interaction. Plant Physiol. 2000, 123, 1289–1300. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, Z.; Jia, B.; Shi, Y.; Dong, J.; Jiang, S.; Li, Z. DNA origami as a nanomedicine for targeted rheumatoid arthritis therapy through reactive oxygen species and nitric oxide scavenging. ACS Nano 2022, 16, 12520–12531. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Yan, G.C.; Fan, X.P.; Peng, M.; Yin, C.; Xiao, Z.X.; Liang, Y.C. Silicon improves rice salinity resistance by alleviating ionic toxicity and osmotic constraint in an organ-specific pattern. Front. Plant Sci. 2020, 11, 260. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases. 1. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Egley, G.H.; Paul, R.N.; Vaughn, K.C.; Duke, S.O. Role of peroxidase in the development of water-impermeable seed coats in Sida spinosa L. Planta 1983, 157, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, X.L.; Zhu, Y.; Yang, X.M.; Zhang, K.N.; Xiao, Z.Y.; Wang, H.; Zhao, J.H.; Zhang, L.L.; Li, G.B.; et al. Osa-miR398b boosts H2O2 production and rice blast disease-resistance via multiple superoxide dismutases. New Phytol. 2019, 222, 1507–1522. [Google Scholar] [CrossRef]
- De la Rosa, C.; Covarrubias, A.A.; Reyes, J.L. A dicistronic precursor encoding miR398 and the legume-specific miR2119 coregulates CSD1 and ADH1 mRNAs in response to water deficit. Plant Cell Environ. 2019, 42, 133–144. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, J.F.; Song, A.P.; Chen, S.M.; Shan, H.; Luo, H.L.; Gu, C.S.; Sun, J.; Zhu, L.; Fang, W.M.; et al. Ambient temperature enhanced freezing tolerance of Chrysanthemum dichrum CdICE1 Arabidopsis via miR398. BMC Biol. 2013, 11, 121. [Google Scholar] [CrossRef]
- Yan, G.C.; Hua, Y.C.; Jin, H.; Huang, Q.Y.; Zhou, G.F.; Xu, Y.M.; He, Y.; Zhu, Z.J. Sly-miR398 participates in cadmium stress acclimation by regulating antioxidant system and cadmium transport in tomato (Solanum lycopersicum). Int. J. Mol. Sci. 2023, 24, 1953. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, K.; Hoshikawa, K.; Kang, M.; Jang, S. Molecular bases of heat stress responses in vegetable crops with focusing on heat shock factors and heat shock proteins. Front. Plant Sci. 2022, 13, 837152. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Wang, P.; Zhao, S.; Dong, J.; Mao, S.; Zhu, X.; Yuan, T.; Qiu, H.; Cao, L.; Xu, Y.; et al. Sly-miR398 Participates in Heat Stress Tolerance in Tomato by Modulating ROS Accumulation and HSP Response. Agronomy 2025, 15, 294. https://doi.org/10.3390/agronomy15020294
Li B, Wang P, Zhao S, Dong J, Mao S, Zhu X, Yuan T, Qiu H, Cao L, Xu Y, et al. Sly-miR398 Participates in Heat Stress Tolerance in Tomato by Modulating ROS Accumulation and HSP Response. Agronomy. 2025; 15(2):294. https://doi.org/10.3390/agronomy15020294
Chicago/Turabian StyleLi, Baoyu, Peiwen Wang, Shuaijing Zhao, Jiaqi Dong, Shengming Mao, Xuyongjie Zhu, Tiantian Yuan, Haiying Qiu, Long Cao, Yunmin Xu, and et al. 2025. "Sly-miR398 Participates in Heat Stress Tolerance in Tomato by Modulating ROS Accumulation and HSP Response" Agronomy 15, no. 2: 294. https://doi.org/10.3390/agronomy15020294
APA StyleLi, B., Wang, P., Zhao, S., Dong, J., Mao, S., Zhu, X., Yuan, T., Qiu, H., Cao, L., Xu, Y., He, Y., Zhu, Z., & Yan, G. (2025). Sly-miR398 Participates in Heat Stress Tolerance in Tomato by Modulating ROS Accumulation and HSP Response. Agronomy, 15(2), 294. https://doi.org/10.3390/agronomy15020294





