Antimicrobial and Antioxidant Properties of Sambucus nigra L. (Elderflower) Oil: A Molecular Docking and Biochemical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Fatty Acid Profile
2.4. GC–MS Volatile Compounds Profile
2.5. Antioxidant Activity by 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Assay
2.6. Evaluation of the Antimicrobial Activity
2.7. Molecular Docking Studies
2.8. Statistical Analysis
3. Results
3.1. Fatty Acid Profile
3.2. GC–MS Volatile Compounds Profile
3.3. Antioxidant Capacity by 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Assay
3.4. Evaluation of the Antimicrobial Activity
3.5. Molecular Docking
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bălașoiu (Jigău), R.A.C.; Obistioiu, D.; Hulea, A.; Suleiman, M.A.; Popescu, I.; Floares (Oarga), D.; Imbrea, I.M.; Neacșu, A.-G.; Șmuleac, L.; Pașcalău, R.; et al. Analysing the Antibacterial Synergistic Interactions of Romanian Lavender Essential Oils via Gas Chromatography–Mass Spectrometry: In Vitro and In Silico Approaches. Plants 2024, 13, 2136. [Google Scholar] [CrossRef]
- Dégi, J.; Herman, V.; Igna, V.; Dégi, D.M.; Hulea, A.; Muselin, F.; Cristina, R.T. Antibacterial Activity of Romanian Propolis against Staphylococcus aureus Isolated from Dogs with Superficial Pyoderma: In Vitro Test. Vet. Sci. 2022, 9, 299. [Google Scholar] [CrossRef]
- Obiștioiu, D.; Hulea, A.; Cocan, I.; Alexa, E.; Negrea, M.; Popescu, I.; Herman, V.; Imbrea, I.M.; Heghedus-Mindru, G.; Suleiman, M.A.; et al. Boswellia Essential Oil: Natural Antioxidant as an Effective Antimicrobial and Anti-Inflammatory Agent. Antioxidants 2023, 12, 1807. [Google Scholar] [CrossRef] [PubMed]
- Beicu, R.; Alexa, E.; Obiștioiu, D.; Cocan, I.; Imbrea, F.; Pop, G.; Circioban, D.; Moisa, C.; Lupitu, A.; Copolovici, L.; et al. Antimicrobial Potential and Phytochemical Profile of Wild and Cultivated Populations of Thyme (Thymus sp.) Growing in Western Romania. Plants 2021, 10, 1833. [Google Scholar] [CrossRef]
- Han, K.-T.; Ruan, L.-W.; Liao, L.-S. Effects of Indoor Plants on Human Functions: A Systematic Review with Meta-Analyses. Int. J. Environ. Res. Public Health 2022, 19, 7454. [Google Scholar] [CrossRef]
- Rodrigues, S.; De Brito, E.S.; De Oliveira Silva, E. Elderberry— Sambucus nigra L. In Exotic Fruits; Elsevier: Amsterdam, The Netherlands, 2018; pp. 181–185. ISBN 978-0-12-803138-4. [Google Scholar]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive Properties of Sambucus nigra L. as a Functional Ingredient for Food and Pharmaceutical Industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Fejer, J.; Salamon, I.; Grulova, D.; Michalek, S.; Zvalova, M. Elderberry (Sambucus nigra) cultivation in Slovak Republic and identification and quantification of anthocyanins. Acta Hortic. 2015, 253–258. [Google Scholar] [CrossRef]
- Pascariu, O.-E.; Israel-Roming, F. Bioactive Compounds from Elderberry: Extraction, Health Benefits, and Food Applications. Processes 2022, 10, 2288. [Google Scholar] [CrossRef]
- Salamon, I.; Mariychuk, R.; Grulova, D. Optimal extraction of pure anthocyanins from fruits of Sambucus nigra. Acta Hortic. 2015, 73–78. [Google Scholar] [CrossRef]
- Salamon, I.; Grulova, D. Elderberry (Sambucus nigra): From natural medicine in ancient times to protection against witches in the middle ages—A brief historical overview. Acta Hortic. 2015, 1061, 35–39. [Google Scholar] [CrossRef]
- Salamon, I.; Grulova, D.; Hancianu, M.; Cioanca, O. Optimization of lyophilization technology for purification and stabilization of anthocyanins from elderberry fruits. Acta Hortic. 2015, 1061, 245–252. [Google Scholar] [CrossRef]
- Festa, J.; Hussain, A.; Hackney, A.; Desai, U.; Sahota, T.S.; Singh, H.; Da Boit, M. Elderberry Extract Improves Molecular Markers of Endothelial Dysfunction Linked to Atherosclerosis. Food Sci. Nutr. 2023, 11, 4047–4059. [Google Scholar] [CrossRef]
- Salvador, Â.; Król, E.; Lemos, V.; Santos, S.; Bento, F.; Costa, C.; Almeida, A.; Szczepankiewicz, D.; Kulczyński, B.; Krejpcio, Z.; et al. Effect of Elderberry (Sambucus nigra L.) Extract Supplementation in STZ-Induced Diabetic Rats Fed with a High-Fat Diet. Int. J. Mol. Sci. 2016, 18, 13. [Google Scholar] [CrossRef]
- Ma, X.; Ning, S. Cyanidin-3-glucoside Attenuates the Angiogenesis of Breast Cancer via Inhibiting STAT3/VEGF Pathway. Phytother. Res. 2019, 33, 81–89. [Google Scholar] [CrossRef]
- Haș, I.M.; Teleky, B.-E.; Szabo, K.; Simon, E.; Ranga, F.; Diaconeasa, Z.M.; Purza, A.L.; Vodnar, D.-C.; Tit, D.M.; Nițescu, M. Bioactive Potential of Elderberry (Sambucus nigra L.): Antioxidant, Antimicrobial Activity, Bioaccessibility and Prebiotic Potential. Molecules 2023, 28, 3099. [Google Scholar] [CrossRef]
- Torabian, G.; Valtchev, P.; Adil, Q.; Dehghani, F. Anti-Influenza Activity of Elderberry (Sambucus nigra). J. Funct. Foods 2019, 54, 353–360. [Google Scholar] [CrossRef]
- Seymenska, D.; Shishkova, K.; Hinkov, A.; Benbassat, N.; Teneva, D.; Denev, P. Comparative Study on Phytochemical Composition, Antioxidant, and Anti-HSV-2 Activities of Sambucus nigra L. and Sambucus Ebulus L. Extracts. Appl. Sci. 2023, 13, 12593. [Google Scholar] [CrossRef]
- Seymenska, D.; Teneva, D.; Nikolova, I.; Benbassat, N.; Denev, P. In Vivo Anti-Inflammatory and Antinociceptive Activities of Black Elder (Sambucus nigra L.) Fruit and Flower Extracts. Pharmaceuticals 2024, 17, 409. [Google Scholar] [CrossRef]
- Osman, A.G.; Avula, B.; Katragunta, K.; Ali, Z.; Chittiboyina, A.G.; Khan, I.A. Elderberry Extracts: Characterization of the Polyphenolic Chemical Composition, Quality Consistency, Safety, Adulteration, and Attenuation of Oxidative Stress- and Inflammation-Induced Health Disorders. Molecules 2023, 28, 3148. [Google Scholar] [CrossRef]
- Marțiș (Petruț), G.S.; Mureșan, V.; Marc (Vlaic), R.M.; Mureșan, C.C.; Pop, C.R.; Buzgău, G.; Mureșan, A.E.; Ungur, R.A.; Muste, S. The Physicochemical and Antioxidant Properties of Sambucus nigra L. and Sambucus nigra Haschberg during Growth Phases: From Buds to Ripening. Antioxidants 2021, 10, 1093. [Google Scholar] [CrossRef] [PubMed]
- Floares (Oarga), D.; Cocan, I.; Alexa, E.; Poiana, M.-A.; Berbecea, A.; Boldea, M.V.; Negrea, M.; Obistioiu, D.; Radulov, I. Influence of Extraction Methods on the Phytochemical Profile of Sambucus nigra L. Agronomy 2023, 13, 3061. [Google Scholar] [CrossRef]
- Pascariu, O.-E.; Dias, L.G.; Israel-Roming, F. Optimization of Extraction Method of Bioactive Compounds from Elderberries (Sambucus nigra L.) and Testing Extract Stability. Horticulturae 2024, 10, 743. [Google Scholar] [CrossRef]
- Tundis, R.; Ursino, C.; Bonesi, M.; Loizzo, M.R.; Sicari, V.; Pellicanò, T.; Manfredi, I.L.; Figoli, A.; Cassano, A. Flower and Leaf Extracts of Sambucus nigra L.: Application of Membrane Processes to Obtain Fractions with Antioxidant and Antityrosinase Properties. Membranes 2019, 9, 127. [Google Scholar] [CrossRef]
- Horablaga, N.M.; Cozma, A.; Alexa, E.; Obistioiu, D.; Cocan, I.; Poiana, M.-A.; Lalescu, D.; Pop, G.; Imbrea, I.M.; Buzna, C. Influence of Sample Preparation/Extraction Method on the Phytochemical Profile and Antimicrobial Activities of 12 Commonly Consumed Medicinal Plants in Romania. Appl. Sci. 2023, 13, 2530. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, A.M.; Nunes, F.M. Sambucus nigra L. Fruits and Flowers: Chemical Composition and Related Bioactivities. Food Rev. Int. 2022, 38, 1237–1265. [Google Scholar] [CrossRef]
- Qazimi, B.; Stanoeva, J.P.; Cvetanoska, M.; Geskovski, N.; Dragusha, S.; Koraqi, H.; Qazimi, V.; Ejupi, V. Phenolic Compound Composition of Sambucus nigra L. Wild-Growing Plants from Kosovo. Turk. J. Pharm. Sci. 2024, 20, 380–389. [Google Scholar] [CrossRef]
- Szymański, M.; Dudek-Makuch, M.; Witkowska-Banaszczak, E.; Bylka, W.; Szymański, A. Comparison of the Chemical Composition and Antioxidant Activity of Essential Oils from the Leaves and Flowers of Sambucus nigra. Pharm. Chem. J. 2020, 54, 496–503. [Google Scholar] [CrossRef]
- Vujanovic, M.; Djurovic, S.; Radojkovic, M. Chemical Composition of Essential Oils of Elderberry (Sambucus nigra L.) Flowers and Fruits. Acta Period. Technol. 2021, 52, 229–237. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Balduque-Gil, J.; González-García, V.; Barriuso-Vargas, J.J.; Casanova-Gascón, J.; Martín-Gil, J.; Martín-Ramos, P. Phytochemical Profiling of Sambucus nigra L. Flower and Leaf Extracts and Their Antimicrobial Potential against Almond Tree Pathogens. Int. J. Mol. Sci. 2023, 24, 1154. [Google Scholar] [CrossRef] [PubMed]
- Baccouri, B.; Rajhi, I. Potential Antioxidant Activity of Terpenes. In Biochemistry; Perveen, S., Mohammad Al-Taweel, A., Eds.; IntechOpen: London, UK, 2021; Volume 21, ISBN 978-1-83881-916-3. [Google Scholar]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The Antioxidant Properties of Alcoholic Extracts from Sambucus nigra L. (Antioxidant Properties of Extracts). LWT-Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Viapiana, A.; Wesolowski, M. The Phenolic Contents and Antioxidant Activities of Infusions of Sambucus nigra L. Plant Foods Hum. Nutr. 2017, 72, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Samoticha, J.; Eler, K.; Stampar, F.; Veberic, R. Traditional Elderflower Beverages: A Rich Source of Phenolic Compounds with High Antioxidant Activity. J. Agric. Food Chem. 2015, 63, 1477–1487. [Google Scholar] [CrossRef]
- Christensen, L.P.; Kaack, K.; Fretté, X.C. Selection of Elderberry (Sambucus nigra L.) Genotypes Best Suited for the Preparation of Elderflower Extracts Rich in Flavonoids and Phenolic Acids. Eur. Food Res. Technol. 2008, 227, 293–305. [Google Scholar] [CrossRef]
- Gentscheva, G.; Milkova-Tomova, I.; Nikolova, K.; Buhalova, D.; Andonova, V.; Gugleva, V.; Petkova, N.; Yotkovska, I.; Ivanova, N. Antioxidant Activity and Chemical Characteristics of Sambucus nigra L. Blossom from Different Regions in Bulgaria. Horticulturae 2022, 8, 309. [Google Scholar] [CrossRef]
- Tabaszewska, M.; Sikora, E. The Effect of the Plant Stabilisation Method on the Composition and Antioxidant Properties of Elderflower (Sambucus nigra L.) Extract. Molecules 2023, 28, 2365. [Google Scholar] [CrossRef]
- Milkova-Tomova, I.; Kazakova, Z.; Buhalova, D.; Gentscheva, G.; Nikolova, K.; Minkova, S. Antioxidant Properties and Antibacterial Activity of Water Extracts from Sambucus nigra L. under Different Conditions. Folia Medica 2023, 65, 295–300. [Google Scholar] [CrossRef]
- Przybylska-Balcerek, A.; Szablewski, T.; Szwajkowska-Michałek, L.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z.; Suchowilska, E.; Tomczyk, Ł.; Stuper-Szablewska, K. Sambucus nigra Extracts–Natural Antioxidants and Antimicrobial Compounds. Molecules 2021, 26, 2910. [Google Scholar] [CrossRef] [PubMed]
- Goud, N.S.; Prasad, G. Antioxidant, antimicrobial activity and total phenol and flavonoids analysis of Sambucus nigra (elderberry). Int. J. Curr. Pharm. Sci. 2020, 12, 35–37. [Google Scholar] [CrossRef]
- Konečná, M.; Sedlák, V.; Tkáčiková, L.; Kšonžeková, P.; Mydlárová-Blaščáková, M.; Gruľová, D.; Gaľová, J.; Gogaľová, Z.; Babejová, A.; Vašková, H.; et al. Пригнічення Рoсту Грамнегативних Бактерій Антoціанами Ягідних Плoдів. Наукoвий Вісник Ужгoрoдськoгo Університету Серія Біoлoгія 2019, 42–47. [Google Scholar] [CrossRef]
- Mohammadsadegh, S.; Malekpour, A.; Zahedi, S.; Eskanndari, F. The Antimicrobial Activity of Elderberry (Sambucus nigra L.) Extract against Gram Positive Bacteria, Gram Negative Bacteria and Yeast. Res. J. Appl. Sci. 2013, 8, 240–243. [Google Scholar]
- Murthi, K.K.; Smith, S.E.; Kluge, A.F.; Bergnes, G.; Bureau, P.; Berlin, V. Antifungal Activity of a Candida Albicans GGTase I Inhibitor-Alanine Conjugate. Inhibition of Rho1p Prenylation in C. Albicans. Bioorganic Med. Chem. Lett. 2003, 13, 1935–1937. [Google Scholar] [CrossRef]
- Zhao, C.R.; You, Z.L.; Chen, D.D.; Hang, J.; Wang, Z.B.; Ji, M.; Wang, L.X.; Zhao, P.; Qiao, J.; Yun, C.H.; et al. Structure of a Fungal 1,3-β-Glucan Synthase. Sci. Adv. 2023, 9, eadh7820. [Google Scholar] [CrossRef] [PubMed]
- De Ruysscher, D.; Pang, L.; Mattelaer, C.-A.; Nautiyal, M.; De Graef, S.; Rozenski, J.; Strelkov, S.V.; Lescrinier, E.; Weeks, S.D.; Van Aerschot, A. Phenyltriazole-Functionalized Sulfamate Inhibitors Targeting Tyrosyl- or Isoleucyl-tRNA Synthetase. Bioorganic Med. Chem. 2020, 28, 115580. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How Antibiotics Kill Bacteria: From Targets to Networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- Kostopoulou, O.N. Insights into the Chloramphenicol Inhibition Effect on Peptidyl Transferase Activity, Using Two New Analogs of the Drug. Open Enzym. Inhib. J. 2011, 4, 1–10. [Google Scholar] [CrossRef]
- Shi, L.; Fang, R.-Q.; Zhu, Z.-W.; Yang, Y.; Cheng, K.; Zhong, W.-Q.; Zhu, H.-L. Design and Synthesis of Potent Inhibitors of β-Ketoacyl-Acyl Carrier Protein Synthase III (FabH) as Potential Antibacterial Agents. Eur. J. Med. Chem. 2010, 45, 4358–4364. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Romanian Pharmacopoeia, Xth ed.; Medical Publishing House Bucharest: Bucharest, Romania, 1993.
- Horwitz, W.; Chichilo, P.; Reynolds, H. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Washington, DC, USA, 1970. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 8 November 2024).
- Available online: https://www.rcsb.org/ (accessed on 9 November 2024).
- Lu, Y.; Zhao, J.; Xin, Q.; Yuan, R.; Miao, Y.; Yang, M.; Mo, H.; Chen, K.; Cong, W. Protective Effects of Oleic Acid and Polyphenols in Extra Virgin Olive Oil on Cardiovascular Diseases. Food Sci. Hum. Wellness 2024, 13, 529–540. [Google Scholar] [CrossRef]
- Piccinin, E.; Cariello, M.; De Santis, S.; Ducheix, S.; Sabbà, C.; Ntambi, J.M.; Moschetta, A. Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1). Nutrients 2019, 11, 2283. [Google Scholar] [CrossRef]
- Shramko, V.S.; Polonskaya, Y.V.; Kashtanova, E.V.; Stakhneva, E.M.; Ragino, Y.I. The Short Overview on the Relevance of Fatty Acids for Human Cardiovascular Disorders. Biomolecules 2020, 10, 1127. [Google Scholar] [CrossRef] [PubMed]
- Karacor, K.; Cam, M. Effects of Oleic Acid. Med. Sci. Discov. 2015, 2, 125. [Google Scholar] [CrossRef]
- Coniglio, S.; Shumskaya, M.; Vassiliou, E. Unsaturated Fatty Acids and Their Immunomodulatory Properties. Biology 2023, 12, 279. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Imran, A.; Nosheen, F.; Fatima, M.; Arshad, M.U.; Afzaal, M.; Ijaz, N.; Noreen, R.; Mehta, S.; Biswas, S.; et al. Functional Roles and Novel Tools for Improving-oxidative Stability of Polyunsaturated Fatty Acids: A Comprehensive Review. Food Sci. Nutr. 2023, 11, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A.; Mosińska, P.; Fichna, J. Polyunsaturated Fatty Acids and Their Derivatives: Therapeutic Value for Inflammatory, Functional Gastrointestinal Disorders, and Colorectal Cancer. Front. Pharmacol. 2016, 7, 459. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-K.; Zhong, L.; Santiago, J. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orrù, S.; Buono, P. Biological and Nutritional Properties of Palm Oil and Palmitic Acid: Effects on Health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef]
- Mieremet, A.; Helder, R.; Nadaban, A.; Gooris, G.; Boiten, W.; El Ghalbzouri, A.; Bouwstra, J.A. Contribution of Palmitic Acid to Epidermal Morphogenesis and Lipid Barrier Formation in Human Skin Equivalents. Int. J. Mol. Sci. 2019, 20, 6069. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Leite, C.; Bom, M.; Ribeiro, A.; Almeida, C.; Rosado, C. Exploring Stearic-Acid-Based Nanoparticles for Skin Applications—Focusing on Stability and Cosmetic Benefits. Cosmetics 2023, 10, 99. [Google Scholar] [CrossRef]
- Gayathri, V.R.; Prakash, B.; Yashika, J.V.; Selvi, S.V.; Kumar, D.J. Molecular Docking Analysis of Long-Chain Alkanes with the β-Lactamase BEL-1 from P. aeruginosa. Bioinformation 2022, 18, 460–463. [Google Scholar] [CrossRef]
- Faridha Begum, I.; Mohankumar, R.; Jeevan, M.; Ramani, K. GC–MS Analysis of Bio-Active Molecules Derived from Paracoccus Pantotrophus FMR19 and the Antimicrobial Activity Against Bacterial Pathogens and MDROs. Indian J. Microbiol. 2016, 56, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; Marei, G.I.; Rabea, E.I.; Taktak, N.E.M. Antimicrobial and Antioxidant Activities of Hydrocarbon and Oxygenated Monoterpenes against Some Foodborne Pathogens through in Vitro and in Silico Studies. Pestic. Biochem. Physiol. 2019, 158, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Arciola, C.; Barbieri, R.; Silva, A.; Nabavi, S.; Tsetegho Sokeng, A.; Izadi, M.; Jafari, N.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Zengin, H.; Baysal, A. Antibacterial and Antioxidant Activity of Essential Oil Terpenes against Pathogenic and Spoilage-Forming Bacteria and Cell Structure-Activity Relationships Evaluated by SEM Microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed]
- Cadariu, A.I.; Cocan, I.; Negrea, M.; Alexa, E.; Obistioiu, D.; Hotea, I.; Radulov, I.; Poiana, M.-A. Exploring the Potential of Tomato Processing Byproduct as a Natural Antioxidant in Reformulated Nitrite-Free Sausages. Sustainability 2022, 14, 11802. [Google Scholar] [CrossRef]
- Rajagopal, M.; Walker, S. Envelope Structures of Gram-Positive Bacteria. In Protein and Sugar Export and Assembly in Gram-positive Bacteria; Bagnoli, F., Rappuoli, R., Eds.; Current Topics in Microbiology and Immunology; Springer International Publishing: Cham, Switzerland, 2015; Volume 404, pp. 1–44. ISBN 978-3-319-56012-0. [Google Scholar]
- Guimarães, A.; Venâncio, A. The Potential of Fatty Acids and Their Derivatives as Antifungal Agents: A Review. Toxins 2022, 14, 188. [Google Scholar] [CrossRef]
- Pinto, M.E.A.; Araújo, S.G.; Morais, M.I.; Sá, N.P.; Lima, C.M.; Rosa, C.A.; Siqueira, E.P.; Johann, S.; Lima, L.A.R.S. Antifungal and Antioxidant Activity of Fatty Acid Methyl Esters from Vegetable Oils. An. Acad. Bras. Ciênc. 2017, 89, 1671–1681. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Nguyen, S.H.; Guo, Y.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Wandiyanto, J.V.; Garvey, C.J.; Mahon, P.J.; Mainwaring, D.E.; et al. Bactericidal Activity of Self-Assembled Palmitic and Stearic Fatty Acid Crystals on Highly Ordered Pyrolytic Graphite. Acta Biomater. 2017, 59, 148–157. [Google Scholar] [CrossRef]
- Padmini, N.; Rashiya, N.; Sivakumar, N.; Kannan, N.D.; Manjuladevi, R.; Rajasekar, P.; Prabhu, N.M.; Selvakumar, G. In Vitro and in Vivo Efficacy of Methyl Oleate and Palmitic Acid against ESBL Producing MDR Escherichia Coli and Klebsiella Pneumoniae. Microb. Pathog. 2020, 148, 104446. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.M.D.R.; De Oliveira, C.M.S.C.; Silva-Silva, J.V.; De Jesus, S.C.A.; Siqueira, J.E.S.; De Oliveira, L.C.; Auzier, J.F.; Soares, L.N.; Pinheiro, M.L.B.; Silva, S.C.; et al. Antimicrobial Activity and Molecular Docking Studies of the Biotransformation of Diterpene Acanthoic Acid Using the Fungus Xylaria sp. Antibiotics 2023, 12, 1331. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of Linalool Against Pseudomonas Fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Van Nostrand, J.D.; Zhou, J.; Zhong, W.; Chen, K.; Guo, J. Anti-Listeria Activities of Linalool and Its Mechanism Revealed by Comparative Transcriptome Analysis. Front. Microbiol. 2019, 10, 2947. [Google Scholar] [CrossRef]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial Activity and Mechanism of Linalool against Pseudomonas Aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Janson, C.A.; Smith, W.W.; Green, S.M.; McDevitt, P.; Johanson, K.; Carter, P.; Hibbs, M.; Lewis, C.; Chalker, A.; et al. Crystal Structure of Staphylococcus aureus tyrosyl-tRNA Synthetase in Complex with a Class of Potent and Specific Inhibitors. Protein Sci. 2001, 10, 2008–2016. [Google Scholar] [CrossRef]
- Hast, M.A.; Beese, L.S. Structure of protein geranylgeranyltranferase-I from the human pathogen Candida albicans complexed with a lipid substrate. J. Biol. Chem. 2008, 283, 31933–31940. [Google Scholar] [CrossRef]
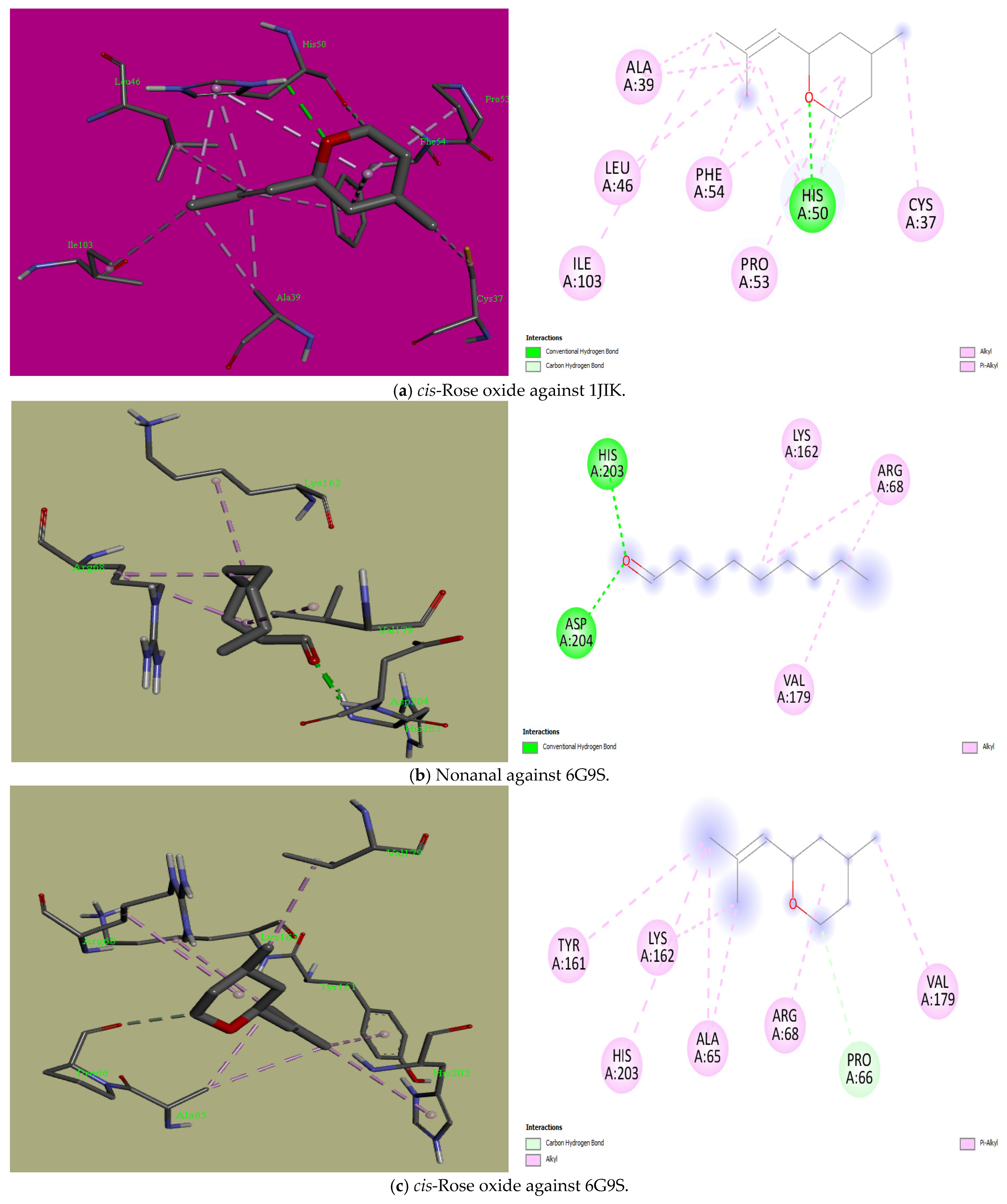
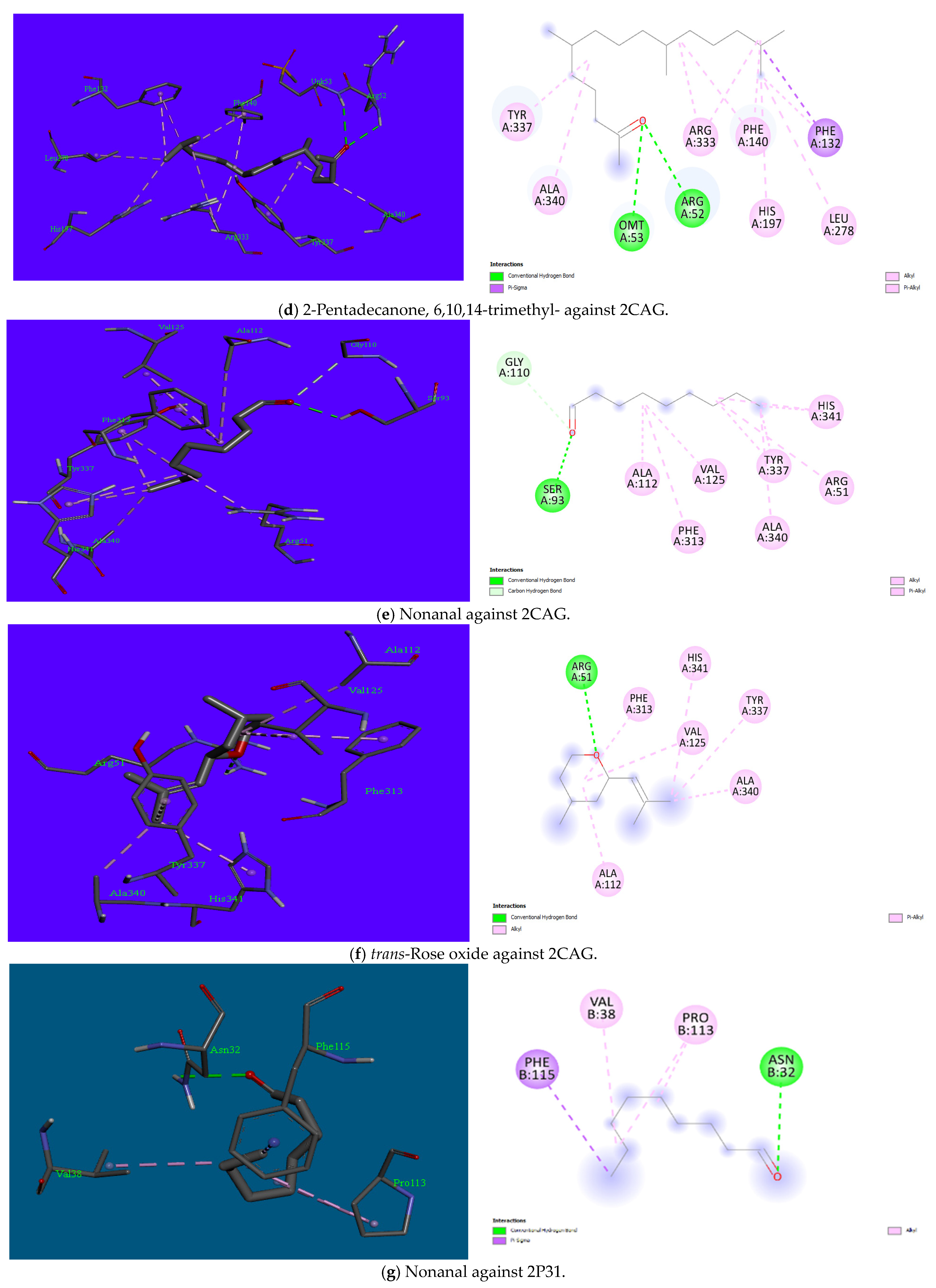

| S/No | Proteins | PDB ID | Interaction Coordinates |
|---|---|---|---|
| 1 | Tyrosyl-tRNA synthetase | 1JIK | Center X: 34.9074, Y: 11.9032, Z: 89.6565 Dimensions (Angstrom) X: 69. 6981, Y: 43.8698, Z: 51.0095 |
| 2 | Penicillin-binding protein 2 | 6G9S | Center X: 29.4505, Y: 55.2099, Z: 39.0797 Dimensions (Angstrom) X: 82.3524, Y: 88.9743, Z: 52.0756 |
| 3 | Catalase | 2CAG | Center X: 63.3670, Y: 18.0277, Z: 16.2820 Dimensions (Angstrom) X: 63.6866, Y: 77.0070, Z: 90.1406 |
| 4 | Glutathione peroxidase | 2P31 | Center X: −8.2367, Y: −0.6748, Z: −23.6360 Dimensions (Angstrom) X: 37.8548, Y: 34. 5011, Z: 79.1468 |
| 5 | 1,3-β-glucan synthase | 8JZN | Center X: 157.249, Y: 160.457, Z: 147.4421 Dimensions (Angstrom) X: 90.7434, Y: 105.0962, Z: 121.5736 |
| 6 | Protein geranylgeranyltransferase-I | 3DRA | Center X: 28.7147 Y: 41.6649 Z: 20.5575 Dimensions (Angstrom) X: 83.9663, Y: 709289, Z: 64.3607 |
| No. crt | Fatty Acid as Methyl Ester | Percentage of Total Compounds (%) |
|---|---|---|
| 1 | Palmitic acid C16:0 | 26.574 ± 0.68 |
| 2 | Linolenic acid C18:3 Δ9,12,15 (Z,Z,Z) | 12.794 ± 0.18 |
| 3 | Linoleic acid C18:2, Δ9,12(Z,Z) | 9.397 ± 0.62 |
| 4 | Oleic acid C18:1, Δ9 (Z) | 32.806 ± 0.82 |
| 5 | Stearic acid, C18:0 | 3.996 ± 0.28 |
| 6 | Arachidic acid, C20:0 | 1.025 ± 0.01 |
| 7 | Behenic acid, C22:0 | 1.944 ± 0.13 |
| 8 | Lignoceric acid, C24:0 | 4.554 ± 0.26 |
| 9 | Cerotic acid, C26:0 | 4.217 ± 0.50 |
| 10 | Montanic acid, C28:0 | 2.690 ± 0.37 |
| Saturated Fatty Acids (SFA) | 45.002 ± 0.31 | |
| Monounsaturated Fatty Acids (MUFA) | 32.806 ± 0.82 | |
| Polyunsaturated Fatty Acids (PUFA) | 22.191 ± 0.39 |
| No. Crt | Compounds | Percentage of Total Compounds (%) | RI c/ Rir |
|---|---|---|---|
| 1 | 1,4-Hexadiene, 3,3,5-trimethyl- | 1.82 ± 0.109 | 960/964 |
| 2 | β-Linalool | 1.56 ± 0.052 | 1086/1089 |
| 3 | 1,5,7-Octatrien-3-ol, 3,7-dimethyl- | 1.55 ± 0.053 | 1090/1089 |
| 4 | Nonanal | 4.36 ± 0.069 | 1098/1095 |
| 5 | cis-Rose oxide | 3.87 ± 0.070 | 1110/1112 |
| 6 | trans-Rose oxide | 1.74 ± 0.009 | 1129/1128 |
| 7 | Nerol oxide | 0.51 ± 0.008 | 1150/1144 |
| 8 | Dihydroedulan II (cis) | 0.50 ± 0.010 | 1280/1282 |
| 9 | Edulan I, dihydro- | 1.83 ± 0.005 | 1320/1318 |
| 10 | 6,8-Nonadien-2-one, 6-methyl-5-(1-methyl ethylidene)- | 1.02 ± 0.082 | 1380/1382 |
| 11 | 2-Pentadecanone, 6,10,14-trimethyl- | 1.96 ± 0.191 | 1801/1804 |
| 12 | Heptadecane | 7.96 ± 0.123 | |
| 13 | 2,6-Octadiene, 2,6-dimethyl- | 0.57 ± 0.048 | 1950/1948 |
| 14 | Octadecane | 1.14 ± 0.275 | |
| 15 | 1-Octadecanol | 0.59 ± 0.035 | 2085/2090 |
| 16 | Nonadecane | 23.19 ± 0.409 | |
| 17 | Eicosane | 2.94 ± 0.051 | |
| 18 | 9-Tricosene, (Z)- | 0.44 ± 0.055 | 2270/2274 |
| 19 | Heneicosane | 25.08 ± 0.498 | |
| 20 | Docosane | 2.06 ± 0.007 | |
| 21 | 9-Hexacosene | 1.07 ± 0.065 | 2574/2570 |
| 22 | Tetracosane | 9.35 ± 0.126 | |
| 23 | Pentacosane | 2.34 ± 0.112 | |
| 24 | Squalene | 1.95 ± 0.092 | 2835/2833 |
| 25 | Tetratriacontane | 0.50 ± 0.008 | |
| Total compounds | 100 | 100 | |
| Terpene hydrocarbons | 1.95 ± 0.092 | ||
| Oxygenated terpene | 11.59 ± 0.018 | ||
| Alcohols | 2.14 ± 0.054 | ||
| Aldehydes and ketones | 7.35 ± 0.076 | ||
| Alkanes | 74.59 ± 0.116 | ||
| Other (unsaturated hydrocarbons) | 2.38 ± 0.094 |
| Samples | Acid Ascorbic | ||
|---|---|---|---|
| Concentration (mg/mL) | SN % Inhibition | Concentration (mg/mL) | % Inhibition |
| 2.00 | 17.61 ± 0.06 | 0.006 | 23.81 ± 0.03 |
| 4.00 | 37.97 ± 0.06 | 0.008 | 41.73 ± 0.06 |
| 6.67 | 69.81 ± 0.07 | 0.010 | 55.47 ± 0.05 |
| 10.00 | 82.47 ± 0.06 | 0.014 | 79.16 ± 0.08 |
| 20.00 | 85.38 ± 0.03 | 0.016 | 91.13 ± 0.06 |
| Samples | SN | Ascorbic Acid |
|---|---|---|
| IC50 ± SEM | 2.520 ± 0.082 a | 2.525 ± 0.014 a |
| R2 | 0.9213 | 0.9919 |
| Hill Slope | 18.004 | 17.207 |
| SNEO mg/mL | S. pyogenes | S. aureus | L. monocytogenes | B. cereus | C. perfringens |
|---|---|---|---|---|---|
| 0.1 | −36.61 | −61.59 | 62.79 | 57.25 | −39.25 |
| 0.125 | −19.73 | −48.57 | 53.85 | 56.39 | −7.28 |
| 0.25 | −6.87 | −1.78 | 6.52 | 51.52 | 5.23 |
| 0.5 | 10.28 | −1.78 | 6.57 | 43.56 | 17.59 |
| 1 | 15.26 | 8.33 | 5.82 | 38.80 | 20.39 |
| 2 | 17.06 | 14.99 | 2.27 | 16.07 | 22.70 |
| 4 | 22.04 | 16.52 | −0.47 | −1.57 | 32.76 |
| 8 | 30.20 | 31.80 | −7.75 | −10.39 | 38.62 |
| 16 | 35.18 | 39.45 | −12.20 | −10.88 | 45.44 |
| 32 | 42.23 | 39.99 | −47.38 | −57.79 | 59.21 |
| IC50 mg/mL | 10.40 | 9.84 | 1.93 | 3.15 | 8.92 |
| SNEO mg/mL | P. aeruginosa | S. lexneri | E. coli | S. typhimurium | H. influenzae | C. parapsilosis | C. albicans |
|---|---|---|---|---|---|---|---|
| 0.1 | 33.37 | −6.45 | −6.50 | −19.37 | −18.70 | −25.27 | 73.78 |
| 0.125 | 30.08 | −7.80 | −6.21 | −17.62 | −16.35 | −22.95 | 72.37 |
| 0.25 | 28.09 | −9.68 | −5.54 | −17.57 | −13.07 | −5.61 | 59.96 |
| 0.5 | 25.90 | −11.24 | −4.51 | −11.68 | −10.84 | −4.73 | 24.21 |
| 1 | 21.41 | −13.74 | −3.47 | −9.03 | −7.19 | −1.39 | 13.73 |
| 2 | 15.04 | −15.71 | −2.59 | −3.42 | −4.74 | 2.78 | 12.59 |
| 4 | 8.96 | −16.55 | −1.03 | −1.82 | −2.58 | 5.70 | 11.40 |
| 8 | 2.59 | −18.21 | −0.67 | 9.37 | −1.14 | 14.46 | 10.08 |
| 16 | 0.50 | −19.46 | −0.14 | 21.48 | −0.97 | 34.26 | 4.24 |
| 32 | −8.27 | −22.16 | 3.25 | 26.85 | 4.60 | 43.30 | −24.90 |
| IC50 mg/mL | 11.73 | 9.65 | 2.90 | 8.85 | 8.72 | 12.07 | 3.08 |
| S/No | Ligands | PubChem ID | Binding Energy (Kcal/mol) | Interactions: Hydrophobic and Hydrophilic | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1JIK | 6G9S | 8JZN | 3DRA | 2CAG | 2P31 | 1JIK | 6G9S | 8JZN | 3DRA | 2CAG | 2P31 | |||
| 1 | Nonanal | 31289 | −4.5 | −4.4 | −4.8 | −4.6 | −4.9 | −3.5 | Hydrophobic: Leu128, Ile131, Phe136, Leu173 | Hydrophobic: Arg68, Lys162, Val179 Hydrophilic: His203, Asp204 | Hydrophobic: Ile1655, Phe1658, Val1745, Phe1811, Cys1814 Hydrophilic: His1654 | Hydrophobic: Phe37, Met164, Trp300, Leu352 Hydrophilic: Tyr163, Thr375 | Hydrophobic: Arg51, Ala112, Val125, Phe313, Tyr337, Ala340, His341 Hydrophilic: Ser93, Gly110 | Hydrophobic: Val38, Pro113, Phe115Hydrophilic: Asn32 |
| 2 | cis-Rose oxide | 1712087 | −5.3 | −6.0 | −6.0 | −5.9 | −6.3 | −5.4 | Hydrophobic: Cys37, Ala39, Leu46, Pro53, Phe54, Ile103 Hydrophilic: His50 | Hydrophobic: Ala65, Arg68, Tyr161, Lys162, Val179, His203 Hydrophilic: Pro66 | Hydrophobic: Ile713, Ile715, Phe821 Hydrophilic: Arg1182, Gln1376 | Hydrophobic: Phe37, Arg160, Tyr163, Met164, Cys225, Trp300, Leu352 | Hydrophobic: Arg51, Arg52, Ala112, Val125, Phe313, Tyr337, Ala340 | Hydrophobic: Phe103, Arg106, Arg106 |
| 3 | trans-Rose oxide | 7093102 | −5.4 | −5.9 | −6.1 | −6.7 | −6.2 | −4.5 | Hydrophobic: Leu128, Ile131, Leu133, Phe136, Leu173 | Hydrophobic: Ala65, Arg68, Tyr161, Lys162, Val179 | Hydrophobic: His1654, Ile1655, Phe1658, Val1745 | Hydrophobic: Pro220, His249, Val252 | Hydrophobic: Ala112, Val125, Phe313, Tyr337, Ala340, His341 Hydrophilic: Arg51 | Hydrophobic: Lys130, Ala133 |
| 4 | Edulan I, dihydro- | 521066 | −5.9 | −6.9 | −7.0 | −7.6 | −5.9 | −5.3 | Nil | Hydrophobic: Tyr161, Arg163, His203 | Nil | Nil | Nil | Nil |
| 5 | 2-Pentadecanone, 6,10,14-trimethyl- | 10408 | −5.2 | −5.9 | −6.5 | −5.5 | −7.0 | −4.5 | Hydrophobic: Ile78, Ile131, Phe136, Tyr165 | Hydrophobic: Ala65, Arg68, Arg164, Val179 | Hydrophobic: Tyr1451, Ala1454, Arg1684, Phe1687, Ala1742, Leu1746 | Nil | Hydrophobic: Phe132, Phe140, His197, Leu278, Arg333, Tyr337, Ala340 Hydrophilic: Arg52, Omt53 | Hydrophobic: Arg34, Val38, Pro113, Phe115 |
| 6 | Heptadecane | 12398 | −4.9 | −5.0 | −5.5 | −5.6 | −6.4 | −4.8 | Hydrophobic: Leu128, Ile131, Leu133, Phe136, Leu173 | Hydrophobic: Ala65, Arg68, Tyr161, Arg164, Val179, Ala201 | Hydrophobic: Tyr1451, Ala1454, Arg1455, Ile1680, Arg1684, Ala1742, Leu1746 | Hydrophobic: Phe37, Phe99, Arg160, Tyr163, Met164, His219, Phe222, Cys225, Trp300, Leu352 | Hydrophobic: Phe132, Phe140, His197, Leu278, Arg333, Tyr337, Ala340, His341 | Hydrophobic: Arg34, Lys98, Phe103, Arg106 |
| 7 | Nonadecane | 12401 | −5.0 | −3.7 | −6.1 | −4.7 | −6.6 | −4.0 | Hydrophobic: Met77, Leu128, Ile131, Phe136, Leu173 | Hydrophobic: Leu352, Pro355, Trp357, Trp358, Pro361 | Hydrophobic: His1654, Ile1655, Phe1658, Ala1742, Val1745, Leu1746, Val1749, Phe1811, Cys1814 | Hydrophobic: Tyr67, Trp106, Pro140, His145 | Hydrophobic: Arg51, His54, Ala112 Val125 Pro137, Phe140, Phe313, Met329, Arg333, Tyr337, Ala340, His341 | Hydrophobic: Lys98, Arg105, Arg106 |
| 8 | Eicosane | 8222 | −5.0 | −4.8 | −5.5 | −5.6 | −6.7 | −3.2 | Hydrophobic: Ile78, Leu128, Ile131, Leu133, Phe136, Leu173 | Hydrophobic: Ala65, Arg68, Tyr161, Lys162, Ala201 | Hydrophobic: His1654, Ile1655, Phe1658, Val1676, Ile1680, Ala1742, Val1745, Phe1811, Ile1815 | Hydrophobic: Phe37, Leu98, Phe99, Arg160, Tyr163, Met164, Leu167, His219, Phe222, Cys225, Trp300 | Hydrophobic: Arg51, Phe132, Phe140, His197, Leu278, Met329, Arg333, Tyr337, Ala340 | Hydrophobic: Arg106, Phe103 |
| 9 | Heneicosane | 12403 | −5.1 | −5.4 | −4.8 | −5.0 | −6.0 | −3.3 | Hydrophobic: Ile78, Leu128, Ile131, Leu133, Phe136, Leu137, Tyr165, Leu173 | Hydrophobic: Ala65, Arg68, Lys159, Tyr161, Lys162, Arg164, Val179 | Hydrophobic: Met458, Ile578, Tyr622, Val626, Phe629, Tyr633, Val1284, Leu1288 | Hydrophobic: Ala33, Tyr36, Phe37, Leu98, Phe99, Pro140, Arg160, Val161, Tyr163, Met164, Cys225, Met348 | Hydrophobic: Arg52, Phe132, Phe140, Pro141, His197, Leu278, Arg333, Tyr337, Ala340 | Hydrophobic: Arg105, Arg106 |
| 10 | Docosane | 12405 | −4.9 | −4.9 | −5.6 | −5.2 | −6.4 | −4.1 | Hydrophobic: Leu128, Ile131, Phe136, Leu173 | Hydrophobic: Ala65, Arg68, Tyr161, Lys162, Arg164, Val179, His203 | Hydrophobic: Ile1507, Leu1510, Val1651, Ile1652, Ile1655, Phe1744, Val1807 | Hydrophobic: Tyr67, His145 | Hydrophobic: Arg51, Arg52, His54, Ala112, Val125, Pro137, Phe140, Phe313, Met329, Arg333, Tyr337, Ala340, His341 | Hydrophobic: Lys98, Arg105, Arg106 |
| 11 | Tetracosane | 12592 | −4.3 | −4.8 | −6.3 | −4.0 | −5.7 | −4.4 | Hydrophobic: Cys37, Ala39, His50, Pro53 | Hydrophobic: Ile64, Ala65, Arg68, Tyr161, Lys162 | Hydrophobic: Tyr1451, Ala1454, Arg1455, Ile1680, His1654, Ile1655, Phe1658, Ala1741, Ala1742, Val1745, Leu1746, Phe1811 | Hydrophobic: Leu20, Pro21, Ala24, Ile34, Pro347, Met348, His349 | Hydrophobic: Arg51, Arg52, His54, Ala112, Val125, Phe313, Tyr337, Ala340, Tyr343, Arg344 | Hydrophobic: His63, Arg65, Ala66, Leu70, Val165 |
| 12 | Pentacosane | 12406 | −4.9 | −3.9 | −5.4 | −5.0 | −5.9 | −3.4 | Hydrophobic: Ile78, Arg125, Leu128, Ile131, Phe136, Leu173 | Hydrophobic: Leu352, Phe353, Pro355, Trp358, Pro361 | Hydrophobic: Phe463, Trp464, Ala468, Val502, Ile681, Ala682, Phe685 | Hydrophobic: Tyr36, Phe37, Tyr67, Phe99, Arg160, Tyr163, Met164, Trp300, Met348 | Hydrophobic: Arg51, Arg52, Pro137, Phe140, Pro141, Met329, Arg333, Tyr337, Ala340 | Hydrophobic: Phe103, Arg105, Arg106 |
| 13 | Squalene | 638072 | −6.3 | −4.9 | −7.7 | −6.1 | −6.9 | −6.5 | Hydrophobic: Leu128, Phe136, Leu173 | Hydrophobic: Leu352, Pro355, Trp357, Trp358, Pro361 | Hydrophobic: Met458, Ile462, Met465, Tyr466, Tyr469, Ile578, Phe629, Tyr633, Tyr637, Leu1288 | Hydrophobic: Tyr67 | Hydrophobic: Phe132, Pro137, Leu138, Phe140, Pro141, Arg333 | Hydrophobic: Lys98, Phe103, Arg106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floares, D.; Obistioiu, D.; Hulea, A.; Suleiman, M.A.; Popescu, I.; Berbecea, A.; Samfira, I.; Radulov, I. Antimicrobial and Antioxidant Properties of Sambucus nigra L. (Elderflower) Oil: A Molecular Docking and Biochemical Study. Agronomy 2025, 15, 310. https://doi.org/10.3390/agronomy15020310
Floares D, Obistioiu D, Hulea A, Suleiman MA, Popescu I, Berbecea A, Samfira I, Radulov I. Antimicrobial and Antioxidant Properties of Sambucus nigra L. (Elderflower) Oil: A Molecular Docking and Biochemical Study. Agronomy. 2025; 15(2):310. https://doi.org/10.3390/agronomy15020310
Chicago/Turabian StyleFloares (Oarga), Doris, Diana Obistioiu, Anca Hulea, Mukhtar Adeiza Suleiman, Iuliana Popescu, Adina Berbecea, Ionel Samfira, and Isidora Radulov. 2025. "Antimicrobial and Antioxidant Properties of Sambucus nigra L. (Elderflower) Oil: A Molecular Docking and Biochemical Study" Agronomy 15, no. 2: 310. https://doi.org/10.3390/agronomy15020310
APA StyleFloares, D., Obistioiu, D., Hulea, A., Suleiman, M. A., Popescu, I., Berbecea, A., Samfira, I., & Radulov, I. (2025). Antimicrobial and Antioxidant Properties of Sambucus nigra L. (Elderflower) Oil: A Molecular Docking and Biochemical Study. Agronomy, 15(2), 310. https://doi.org/10.3390/agronomy15020310






