Effect of Mixed Spraying of SA and ABA on the Growth and Development of Winter Oilseed Rape (Brassica napus L.) During the Post-Waterlogging Podding Period
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Subjects and Experimental Location
2.2. Experimental Design
2.3. Feature Evaluation
2.3.1. Net Photosynthetic Rate and Chlorophyll
2.3.2. Assays of POD, SOD, and CAT Enzyme Activities
2.4. Statistical Analysis
3. Results
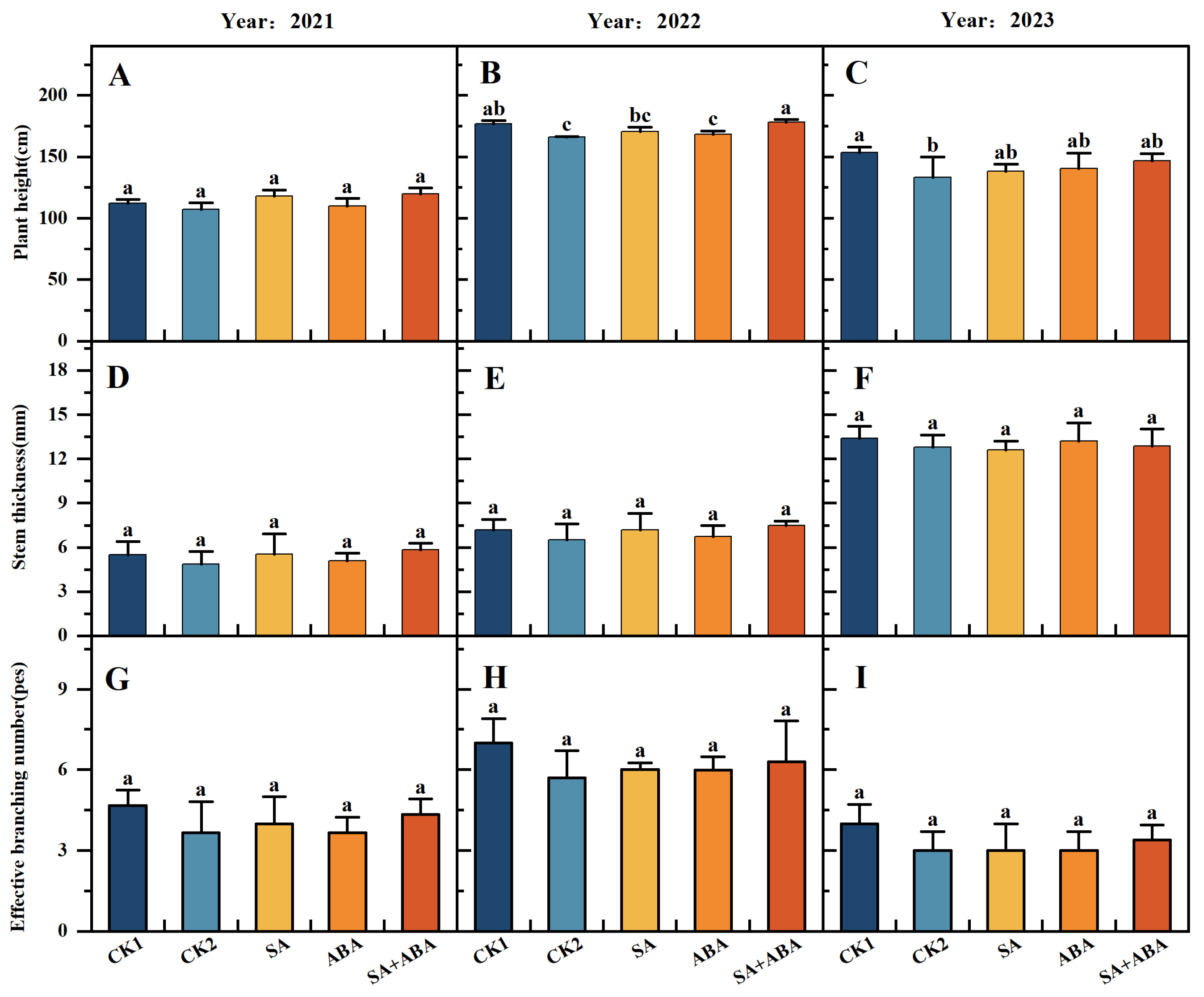
3.1. Exogenous SA + ABA Mixed Plant Growth Regulator Restores Damage to the Agronomic Trait of Oilseed Rape After Waterlogging
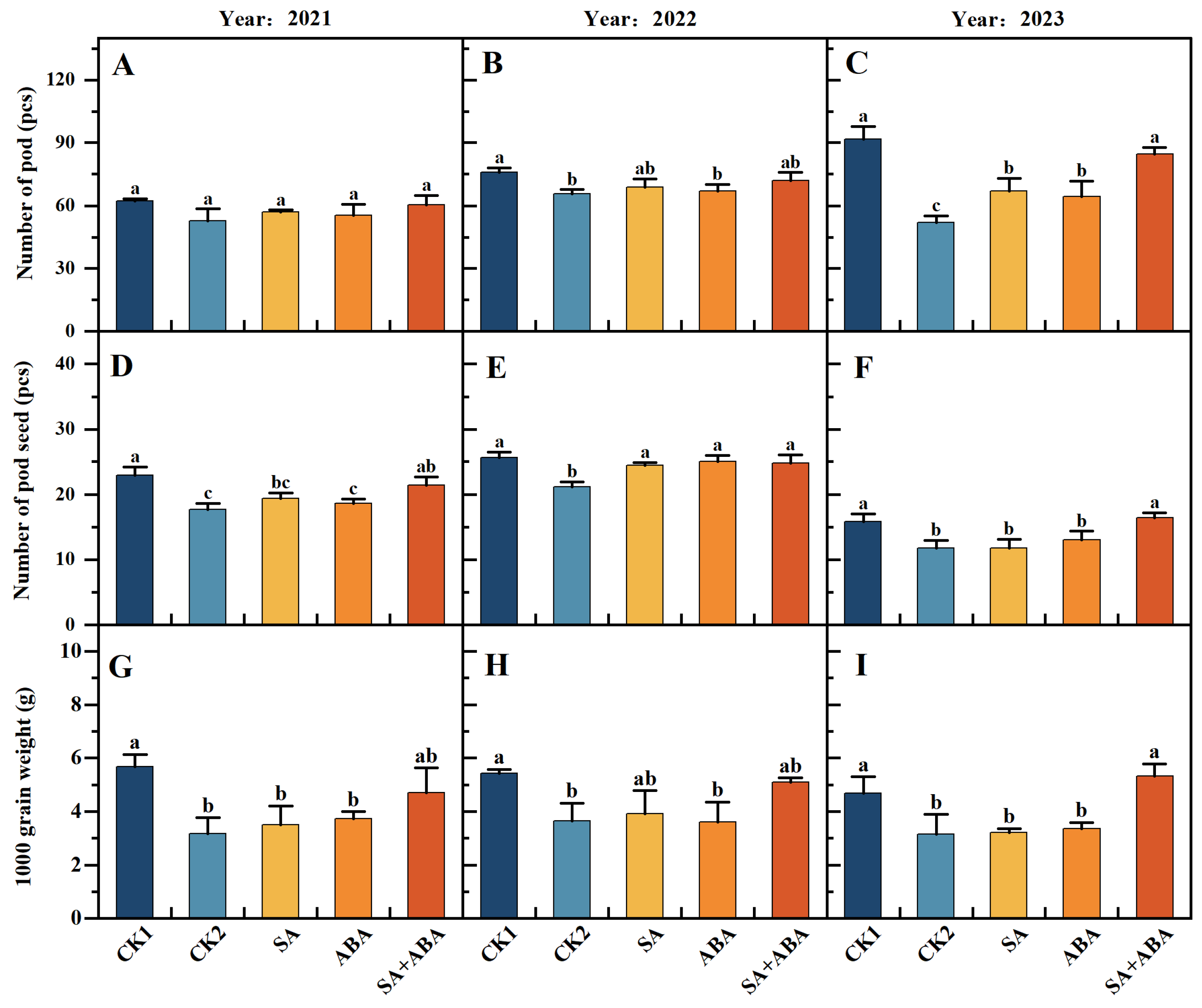
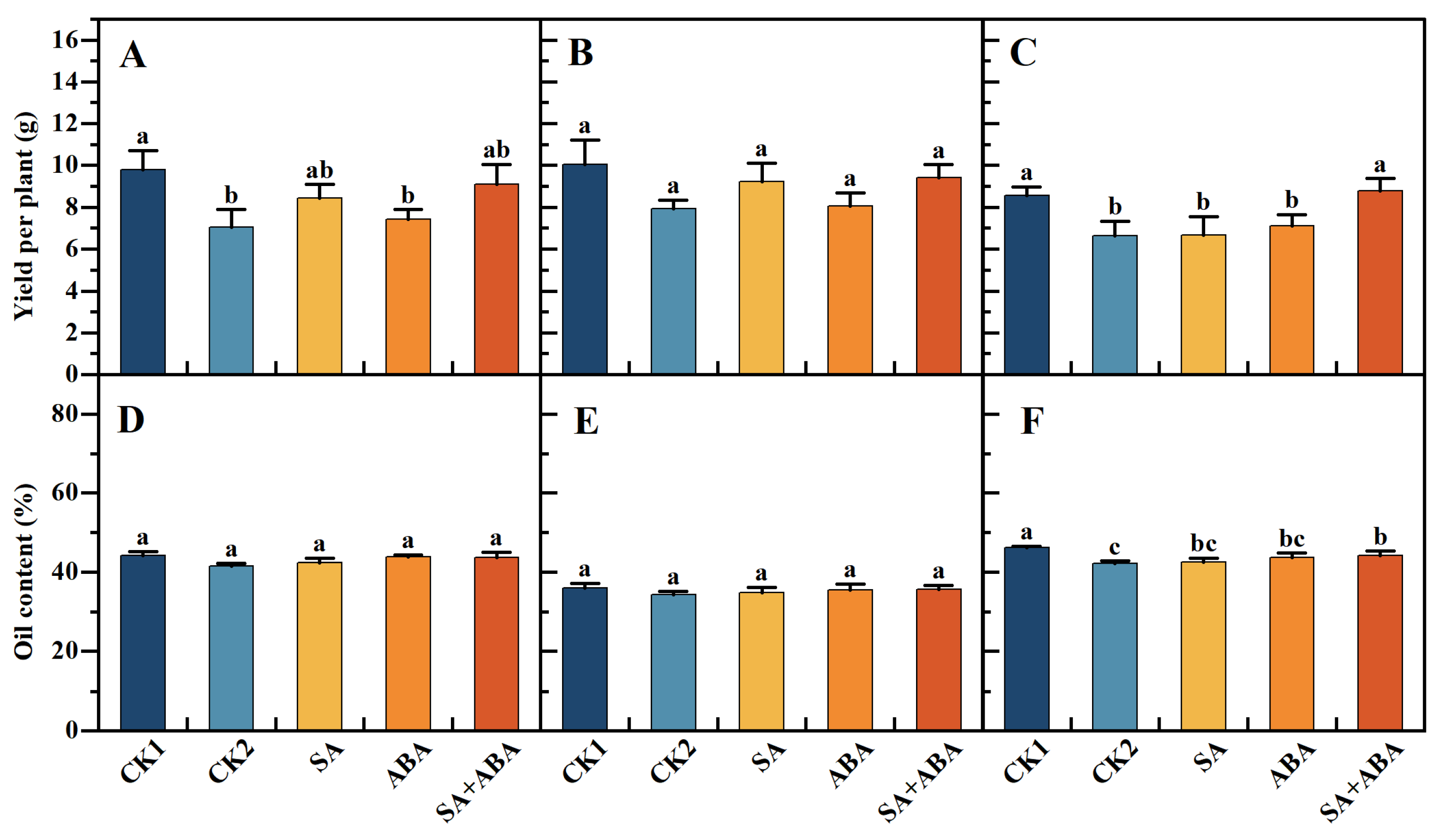
3.2. Exogenous SA + ABA Mixed Plant Growth Regulator Restores Damage to the Yield and Oil Content of Oilseed Rape After Waterlogging
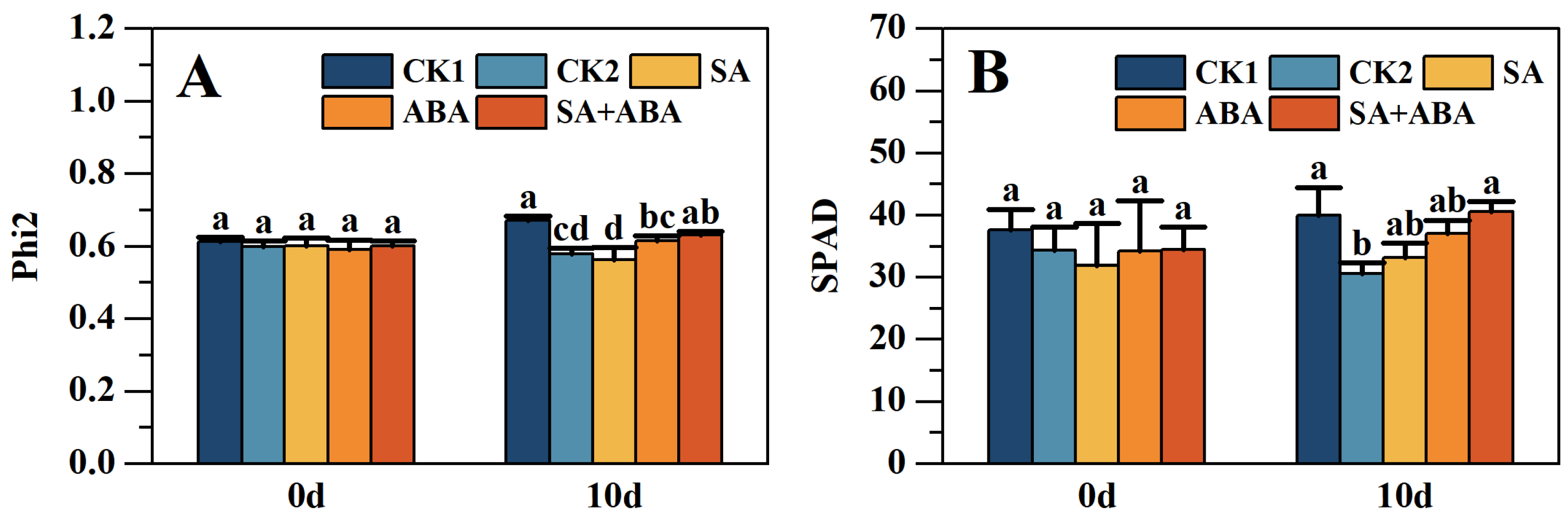
3.3. Exogenous SA + ABA Plant Growth Regulators Recovery phi2 and SPAD Reduction in Oilseed Rape After Waterlogging Stress
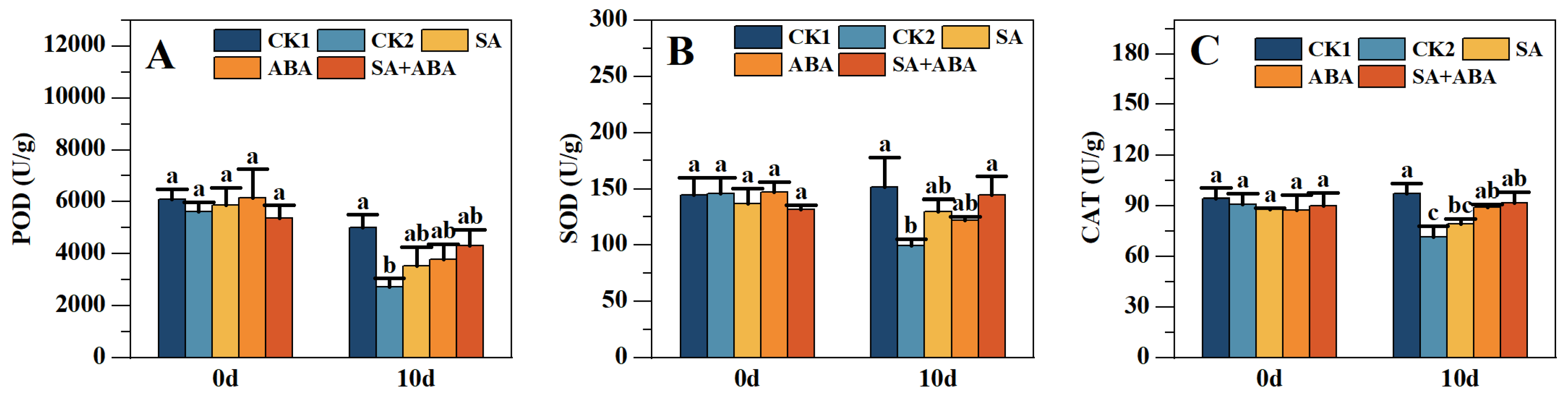
3.4. Effects of Plant Growth Regulators on POD, SOD, and CAT in Oilseed Rape After Waterlogging Stress
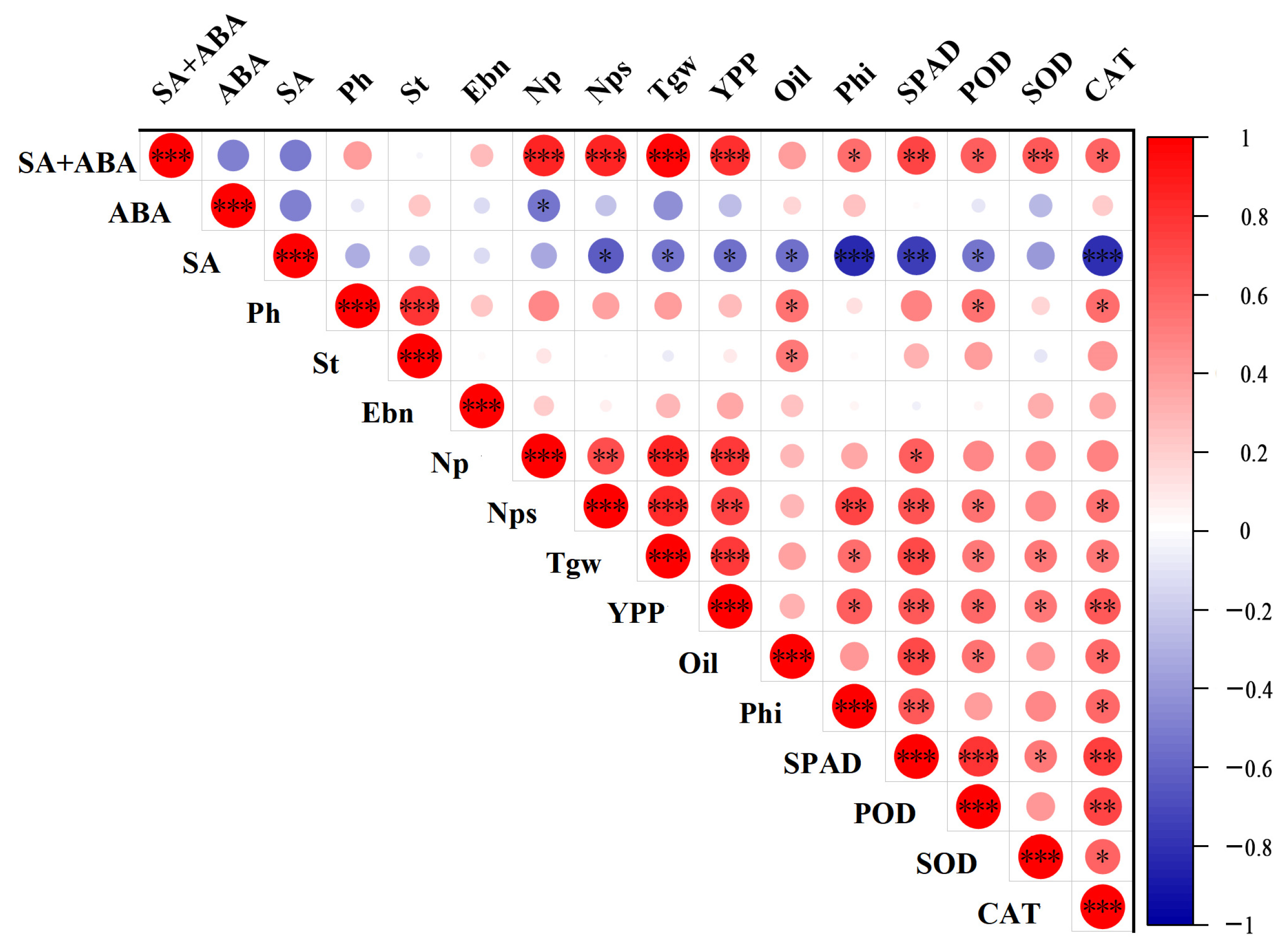
3.5. Correlation Between Individual and Combined Spraying of Plant Growth Regulators on Recovery of Oilseed Rape After Waterlogging
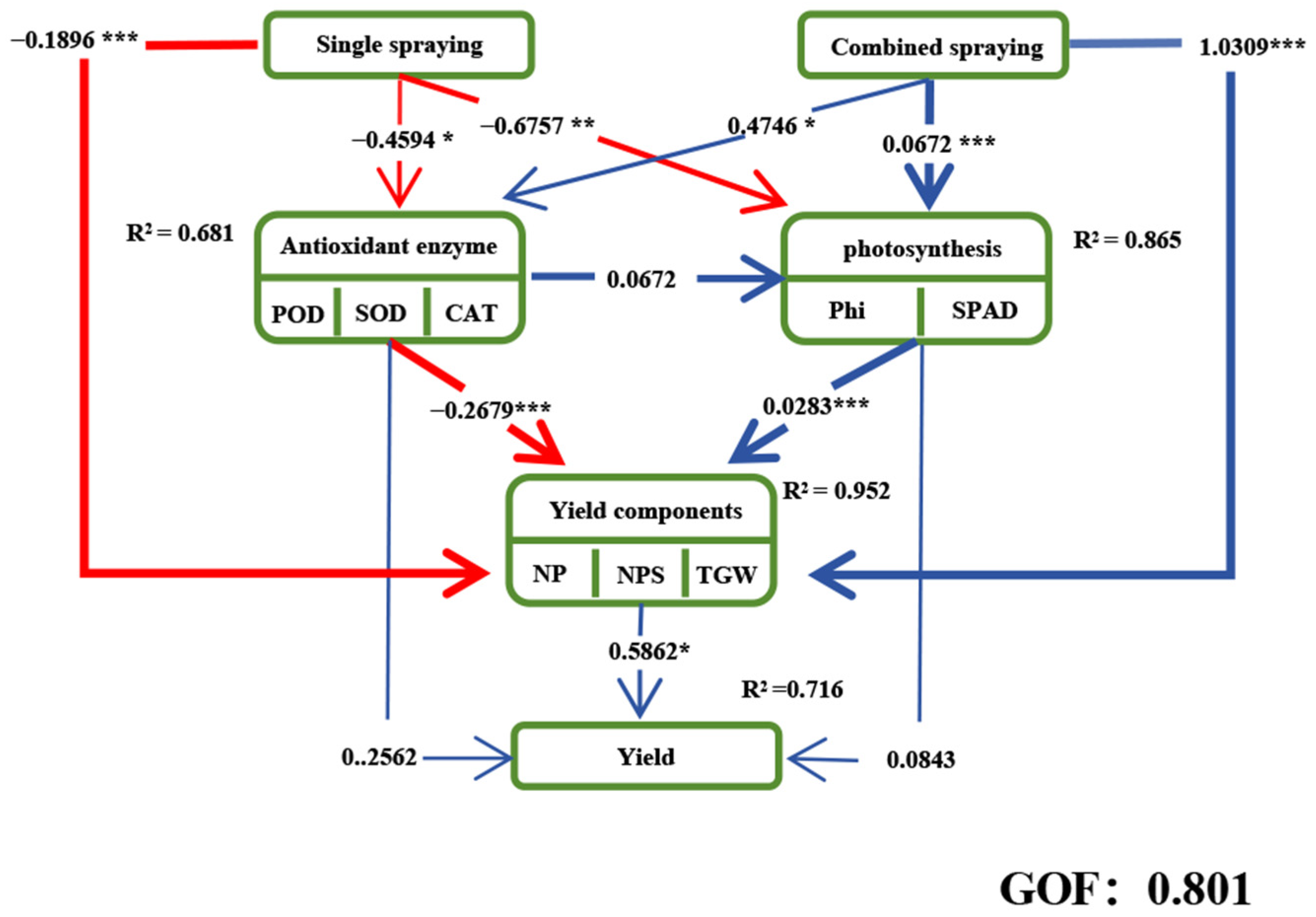
3.6. Structural Equations for Yield Recovery in Oilseed Rape Sprayed with Plant Growth Regulators After Waterlogging
4. Discussion
4.1. Effect of Exogenous SA +ABA on Yield per Plant and Oil Content of Oilseed Rape Plants at Podding Stage After Waterlogging
4.2. Effect of Exogenous SA +ABA on SPAD and Phi2 in Oilseed Rape at Podding Stage After Waterlogging
4.3. Effect of Exogenous SA +ABA on POD, SOD, and CAT Antioxidant Enzymes in Oilseed Rape at Podding Stage After Waterlogging
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SA | Salicylic acid |
| ABA | Abscisic acid |
| POD | Peroxidase |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| SPAD | Chlorophyll dynamics |
| Ph | Plant height |
| Sd | Stem thickness |
| Ebn | Effective branching number |
| Nps | Number of pod seeds |
| Tgw | 1000-grain weight |
| Phi2 | Net photosynthetic rate |
| ANOVA | Analysis of variance |
| ROS | Reactive oxygen species |
Appendix A
| Treat | Concentration of Drug | Number of Pod (pcs) | Number of Pod Seed (pcs) | Oil Content (%) | 1000 Grain Weight (g) | Yield per Plant (g) | RIR (%) | Treatment Options in 2021–2023 |
|---|---|---|---|---|---|---|---|---|
| CK1 | 0 | 60.07 ± 1.33 bc | 22.58 ± 1.15 a | 42.32 ± 0.98 bcd | 5.97 ± 0.61 a | 9.36 ± 0.84 a | 79.31 | |
| CK2 | 0 | 50.67 ± 4.2 6f | 17.40 ± 0.74 b | 41.63 ± 1.02 d | 3.41 ± 0.28 c | 5.22 ± 1.09 b | 0.00 | |
| SA | 1 mmol/L | 57 ± 0.63 cd | 17.54 ± 0.96 b | 43.76 ± 0.58 abcd | 3.5 ± 0.46 bc | 9.12 ± 0.16 a | 74.71 | |
| 2 mmol/L | 63.60 ± 1.08 b | 17.73 ± 0.91 b | 44.46 ± 0.42 abc | 4.178 ± 0.59 abc | 9.77 ± 0.96 a | 87.16 | √ | |
| 4 mmol/L | 56.80 ± 0.29 cde | 18.55 ± 0.64 b | 44.92 ± 1.03 ab | 3.37 ± 0.37 c | 8.62 ± 1.02 a | 65.13 | ||
| ABA | 0.08 g/L | 35.40 ± 1.18 g | 18.36 ± 0.90 b | 42.32 ± 1.13 bcd | 3.74 ± 1.13 bc | 7.03 ± 0.88 ab | 34.67 | |
| 0.16 g/L | 38.60 ± 0.39 g | 18.64 ± 0.63 b | 42.46 ± 0.36 bcd | 4.61 ± 0.76 abc | 9.12 ± 1.13 a | 74.71 | √ | |
| 0.32 g/L | 52.20 ± 1.44 ef | 19.18 ± 1.35 b | 43.77 ± 0.63 abcd | 3.43 ± 0.19 c | 7.04 ± 0.85 ab | 34.87 | ||
| SA + ABA | 0.08 g/L + 2 mmol/L | 61.80 ± 0.26 b | 13.27 ± 1.15 c | 44.79 ± 0.88 abc | 3.44 ± 0.42 bc | 7.56 ± 2.41 ab | 44.83 | |
| 0.16 g/L + 2 mmol/L | 68.80 ± 1.29 a | 16.45 ± 0.79 b | 45.29 ± 1.29 a | 5.24 ± 0.77 ab | 8.63 ± 1.06 a | 65.33 | √ | |
| 0.32 g/L + 2 mmol/L | 54.80 ± 0.96 def | 12.91 ± 0.69 c | 42.15 ± 0.69 cd | 4.31 ± 0.62 abc | 6.91 ± 0.98 ab | 32.38 |
References
- Kaur, G.; Singh, G.; Motavalli, P.P.; Nelson, K.A.; Orlowski, J.M.; Golden, B.R. Impacts and management strategies for crop production in waterlogged or flooded soils: A review. Agron. J. 2020, 112, 1475–1501. [Google Scholar] [CrossRef]
- Jianjun, Z.; Mingzhi, S. Advances in plant flooding physiology. J. Liaocheng Univ. (Nat. Sci. Ed.) 2004, 03, 54–56. [Google Scholar]
- Jingchen, W.; Xiaoying, X.; Kun-yin, L.; Chan, H.; Ying, Z.; Mingdong, Z. Agro-meteorological Disaster Situation and Disaster Prevention and Mitigation Suggestions in Hunan, 2019–2023. Hunan Agric. Sci. 2024, 06, 85–90. [Google Scholar] [CrossRef]
- Ullah, N.; Qian, F.; Geng, R.D.; Xue, Y.J.; Guan, W.J.; Ji, G.X.; Li, H.; Huang, Q.; Cai, G.Q.; Yan, G.X.; et al. Root system architecture change in response to waterlogging stress in a 448 global collection of rapeseeds (Brassica napus L.). Planta 2024, 259, 1–13. [Google Scholar] [CrossRef]
- Ding, L.N.; Liu, R.; Li, T.; Li, M.; Liu, X.Y.; Wang, W.J.; Yu, Y.K.; Cao, J.; Tan, X.L. Physiological and comparative transcriptome analyses reveal the mechanisms underlying waterlogging tolerance in a rapeseed anthocyanin more mutant. Biotechnol. Biof Biop 2022, 15, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Han, Y.L.; Luo, S.; Rong, X.M.; Song, H.X.; Jiang, N.; Li, C.W.; Yang, L. Calcium peroxide alleviates the waterlogging stress of rapeseed by improving root growth status in a rice-rape rotation field. Front. Plant Sci. 2022, 13, 1048227. [Google Scholar] [CrossRef] [PubMed]
- Li, M. Status quo and development prospect of oilseed rape industry in Hunan. Hunan Agric. 2022, 10, 9–10. [Google Scholar]
- Xiong, F.Y.; Infante, D.M.; Olden, J.D.; Gao, W.Q.; Wang, L.Z.; Chen, Y.S. River-lake connectivity, wetland, and human stress factors shape fish diversity (alpha and beta) patterns in the middle and lower Yangtze River, China. Landsc. Ecol. 2023, 38, 3809–3824. [Google Scholar] [CrossRef]
- Zhu, B.; Yu, J.; Shi, H.M.; Yue, K.X.; Lu, J.X.; Zhang, T. Effects of waterlogging stress on rapeseed yield, oil content, fatty acid composition, and transcriptome differences. Plant Growth Regul. 2023, 101, 769–779. [Google Scholar] [CrossRef]
- Xu, M.Y.; Ma, H.Q.; Zeng, L.; Cheng, Y.; Lu, G.Y.; Xu, J.S.; Zhang, X.K.; Zou, X.L. The effect of waterlogging on yield and seed quality at the early flowering stage in Brassica napus L. Field Crop Res. 2015, 180, 238–245. [Google Scholar] [CrossRef]
- Wollmer, A.C.; Pitann, B.; Mühling, K.H. Waterlogging events during stem elongation or flowering affect yield of oilseed rape (Brassica napus L.) but not seed quality. J. Agron. Crop Sci. 2018, 204, 165–174. [Google Scholar] [CrossRef]
- Manik, S.M.N.; Pengilley, G.; Dean, G.; Field, B.; Shabala, S.; Zhou, M.X. Soil and Crop Management Practices to Minimize the Impact of Waterlogging on Crop Productivity. Front. Plant Sci. 2019, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.E.; Mustafa, A.R.A.; Bedawy, I.M.A.; Ahmed, A.S.; Abdelsamie, E.A.; Mohamed, E.S.; Rebouh, N.Y.; Shokr, M.S. Utilizing Infrared Thermometry to Assess the Crop Water Stress Index of Wheat Genotypes in Arid Regions under Varying Irrigation Regimes. Agron. 2024, 14, 1814. [Google Scholar] [CrossRef]
- Gill, J.S.; Sale, P.W.G.; Peries, R.R.; Tang, C. Changes in soil physical properties and crop root growth in dense sodic subsoil following incorporation of organic amendments (vol 114, pg 137, 2009). Field Crop Res. 2010, 116, 205. [Google Scholar] [CrossRef]
- Rochester, I.J.; MB Peoples; Hulugalle, N.R.; Gault, R.; Constable, G.A. Using legumes to enhance nitrogen fertility and improve soil condition in cotton cropping systems. Field Crops Res. 2001, 70, 27–41. [Google Scholar] [CrossRef]
- Leul, M.; Zhou, W.J.F.C.R. Alleviation of waterlogging damage in winter rape by application of uniconazole: Effects on morphological characteristics, hormones and photosynthesis. Field Crop Res. 1998, 59, 121–127. [Google Scholar] [CrossRef]
- MA, G. Effects of kinetin on growth, grain yield and some mineral elements in wheat plants growing under excess salinity and oxygen deficiency. Plant Growth Regul. 1999, 27, 63–74. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.; Shim, I.S. Exogenous salicylic acid alleviates salt-stress damage in cucumber under moderate nitrogen conditions by controlling endogenous salicylic acid levels. Hortic. Environ. Biote 2017, 58, 247–253. [Google Scholar] [CrossRef]
- Li, Y.T.; Han, X.T.; Ren, H.; Zhao, B.; Zhang, J.W.; Ren, B.Z.; Gao, H.Y.; Liu, P. Exogenous SA or 6-BA maintains photosynthetic activity in maize leaves under high temperature stress. Crop J. 2023, 11, 605–617. [Google Scholar] [CrossRef]
- Liu, Y.M.; Chen, S.H.; Wei, P.P.; Guo, S.X.; Wu, J. A briefly overview of the research progress for the abscisic acid analogues. Front. Chem. 2022, 10, 967404. [Google Scholar] [CrossRef]
- Cisse, E.M.; Zhang, J.; Li, D.D.; Miao, L.F.; Yin, L.Y.; Yang, F. Exogenous ABA and IAA modulate physiological and hormonal adaptation strategies in under long-term waterlogging conditions. BMC Plant Biol. 2022, 22, 523. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Jin, Y.; Palta, J.A.; Liu, H.Y.; Chen, Z.; Li, F.M. Exogenous ABA Induces Osmotic Adjustment, Improves Leaf Water Relations and Water Use Efficiency, But Not Yield in Soybean under Water Stress. Agronomy 2019, 9, 395. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Exogenous application of salicylic acid for regulation of sunflower growth under abiotic stress: A systematic review. Biologia 2022, 77, 1685–1697. [Google Scholar] [CrossRef]
- Xin, L.; Wang, J.; Yang, Q. Exogenous salicylic acid alleviates water deficit stress by protecting photosynthetic system in maize seedlings. Agronomy 2023, 13, 2443. [Google Scholar] [CrossRef]
- Agehara, S.; Leskovar, D.I. Characterizing concentration effects of exogenous abscisic acid on gas exchange, water relations, and growth of muskmelon seedlings during water stress and rehydration. J. Am. Soc. Hortic. Sci. 2012, 137, 400–410. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.Q.; Liu, Q. Photosynthesis product allocation and yield in sweet potato with spraying exogenous hormones under drought stress. J. Plant Physiol. 2020, 253, 153265. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Bali, S.; Arora, S.; Sharma, A.; Kaur, R.; Bhardwaj, R. Modulation of antioxidative defense expression and osmolyte content by co-application of 24-epibrassinolide and salicylic acid in Pb exposed Indian mustard plants. Ecotox Environ. Safe 2018, 147, 382–393. [Google Scholar] [CrossRef]
- Darvizheh, H.; Zahedi, M.; Abbaszadeh, B.; Razmjoo, J. Changes in some antioxidant enzymes and physiological indices of purple coneflower (Echinacea purpurea L.) in response to water deficit and foliar application of salicylic acid and spermine under field condition. Sci. Hortic-Amst. 2019, 247, 390–399. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Ervin, E.H.; Wu, W.L.; Sharma, N.; Hamill, A. Auxin and Trinexapac-Ethyl Impact on Root Viability and Hormone Metabolism in Creeping Bentgrass under Water Deficit. Crop Sci. 2017, 57, S130–S137. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, J.; Soni, V.; Kalaji, H.M.; Elsheery, N.I.J.J.o.W.; Development, L. Waterlogging tolerance: A review on regulative morpho-physiological homeostasis of crop plants. J. Water Land Dev. 2021, 49, 16–28. [Google Scholar] [CrossRef]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Zhang, X.Y.; Liu, X.W.; Shao, L.W.; Sun, H.Y.; Chen, S.Y. Improving Winter Wheat Performance by Foliar Spray of ABA and FA Under Water Deficit Conditions. J. Plant Growth Regul. 2016, 35, 83–96. [Google Scholar] [CrossRef]
- Xia, W. Effect of exogenous salicylic acid on growth and reactive oxygen species metabolism in chilli seedlings under flooding stress. Chin. Squash 2022, 35, 73–78. [Google Scholar] [CrossRef]
- Wang, X.; Di, H.; Zhang, C.; Cao, Q. Effect of exogenous hormones on root vigour of cyclamen under high temperature stress. Zhejiang Agric. Sci. 2018, 59, 472–474. [Google Scholar] [CrossRef]
- Yuan, P.; Shen, W.Q.; Yang, L.Y.; Tang, J.L.; Xu, H.; Bu, F.W. Physiological and transcriptional analyses reveal the resistance mechanisms of kiwifruit mutant with enhanced heat tolerance. Plant Physiol. Biochem. 2024, 207, 108331. [Google Scholar] [CrossRef]
- Tian, L.X.; Zhang, Y.C.; Chen, P.L.; Zhang, F.F.; Li, J.; Yan, F.; Dong, Y.; Feng, B.L. How Does the Waterlogging Regime Affect Crop Yield? A Global Meta-Analysis. Front. Plant Sci. 2021, 12, 634898. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Zhang, L.; Tang, J.W.; Xing, H.C.; Zhao, L.; Jie, H.D.; Jie, Y.C. Waterlogging increases greenhouse gas release and decreases yield in winter rapeseed (Brassica napus L.) seedlings. Sci. Rep. 2023, 13, 1–9. [Google Scholar] [CrossRef]
- Zhou, W.; Lin, X.J.F.C.R. Effects of waterlogging at different growth stages on physiological characteristics and seed yield of winter rape (Brassica napus L.). Field Crop Res. 1995, 44, 103–110. [Google Scholar] [CrossRef]
- Song, W.Y.; Shao, H.B.; Zheng, A.Z.; Zhao, L.F.; Xu, Y.J. Advances in Roles of Salicylic Acid in Plant Tolerance Responses to Biotic and Abiotic Stresses. Plants-Basel 2023, 12, 3475. [Google Scholar] [CrossRef]
- Ma, Y.L.; Cao, J.; He, J.H.; Chen, Q.Q.; Li, X.F.; Yang, Y. Molecular Mechanism for the Regulation of ABA Homeostasis During Plant Development and Stress Responses. Int. J. Mol. Sci. 2018, 19, 3643. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, R.; Gheith, E.S.M.S.; Hussain, H.A.; Cholidah, L.; Zhang, K.P.; Zhang, S.; Wang, L.C. Role of exogenous-applied salicylic acid, zinc and glycine betaine to improve drought-tolerance in wheat during reproductive growth stages. BMC Plant Biol. 2021, 21, 574. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.T.; Li, W.; Wang, X.Y.; He, N.; Cao, D.; Wang, M.L.; Liu, J. Exogenous ABA foliar spray treatment alleviates flooding stress in adzuki bean (Vigna angularis) during the seedling stage. Not. Bot. Horti Agrobo 2021, 49, 12210. [Google Scholar] [CrossRef]
- Nguyen, D.; Rieu, I.; Mariani, C.; van Dam, N.M. How plants handle multiple stresses: Hormonal interactions underlying responses to abiotic stress and insect herbivory. Plant Mol. Biol. 2016, 91, 727–740. [Google Scholar] [CrossRef]
- Bideshki, A.; Arvin, M.J.; Darini, M. Interactive effects of Indole-3-butyric acid (IBA) and salicylic acid (SA) on growth parameters, bulb yield and allicin contents of garlic (Allium sativum) under drought stress in field. Int. J. Agron. Plant Productio 2013, 4, 271–279. [Google Scholar]
- Feng, Q.; Yang, S.; Wang, Y.; Lu, L.; Sun, M.; He, C.; Wang, J.; Li, Y.; Yu, X.; Li, Q. Physiological and molecular mechanisms of ABA and CaCl2 regulating chilling tolerance of cucumber seedlings. Plants 2021, 10, 2746. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Q.; Li, Z.; Liu, Y.H.; Li, W. Role of tillage measures in mitigating waterlogging damage in rapeseed. BMC Plant Biol. 2023, 23, 231. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Change Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef] [PubMed]
- Serrago, R.A.; Carretero, R.; Bancal, M.O.; Miralles, D.J.J.F.C.R. Grain weight response to foliar diseases control in wheat (Triticum aestivum L.). Field Crop Res. 2011, 120, 352–359. [Google Scholar] [CrossRef]
- Hossain, M.A.; Uddin, S.N.J.A.J.O.C.S. Mechanisms of waterlogging tolerance in wheat: Morphological and metabolic adaptations under hypoxia or anoxia. Aust. J. Crop Sci. 2011, 5, 1094–1101. [Google Scholar]
- Wu, X.; Tang, Y.; Li, C.; Wu, C.; Huang, G.J.P.P.S. Chlorophyll fluorescence and yield responses of winter wheat to waterlogging at different growth stages. Plant Prod. Sci. 2015, 18, 284–294. [Google Scholar] [CrossRef]
- Ren, B.Z.; Zhu, Y.L.; Zhang, J.W.; Dong, S.T.; Liu, P.; Zhao, B. Effects of spraying exogenous hormone 6-benzyladenine (6-BA) after waterlogging on grain yield and growth of summer maize. Field Crop Res. 2016, 188, 96–104. [Google Scholar] [CrossRef]
- Han, Y.J.; Song, P.S.; Kim, J.I. Phytochrome-mediated photomorphogenesis in plants. J. Plant Biol. 2007, 50, 230–240. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Bai, M.Y.; Oh, E.; Zhu, J.Y. Brassinosteroid Signaling Network and Regulation of Photomorphogenesis. Annu. Rev. Genet. 2012, 46, 701–724. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.X.; Wang, K.B.; Shangguan, Z.P. Cytokinin-induced photosynthetic adaptability of Zea mays L. to drought stress associated with nitric oxide signal: Probed by ESR spectroscopy and fast OJIP fluorescence rise. J. Plant Physiol. 2010, 167, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Haisel, D.; Pospíšilová, J.; Synková, H.; Schnablová, R.; Baťková, P. Effects of abscisic acid or benzyladenine on pigment contents, chlorophyll fluorescence, and chloroplast ultrastructure during water stress and after rehydration. Photosynthetica 2006, 44, 606–614. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Xia, X.J.; Li, X.; Shi, K.; Yu, J.Q.; Zhou, Y.H. Role of brassinosteroid in plant adaptation to abiotic stresses and its interplay with other hormones. Curr. Protein Pept. Sci. 2015, 16, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Lau, O.S.; Deng, X.W. Plant hormone signaling lightens up: Integrators of light and hormones. Curr. Opin. Plant Biol. 2010, 13, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.; Visser, R.G.; Bovy, A. Anthocyanin biosynthesis and degradation mechanisms in Solanaceous vegetables: A review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Jaksomsak, P.; Konseang, S.; Dell, B.; Rouached, H.; Prom-u-thai, C. Grain and Leaf Anthocyanin Concentration Varies among Purple Rice Varieties and Growing Condition in Aerated and Flooded Soil. Molecules 2022, 27, 8355. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.Q.; Wang, H.J.; Wang, Q.; Gao, Y.B.; Xu, K.; Sun, X.P. Exogenous treatment with melatonin enhances waterlogging tolerance of kiwifruit plants. Front. Plant Sci. 2022, 13, 1081787. [Google Scholar] [CrossRef]
- Xia, X.J.; Zhou, Y.H.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J.Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 2015, 66, 2839–2856. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Laloi, C.; Havaux, M. Key players of singlet oxygen-induced cell death in plants. Front. Plant Sci. 2015, 6, 39. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Collin, A.; Sitko, K.; Janiak, A.; Kalaji, H.M.; Szarejko, I. Genetic and physiological dissection of photosynthesis in barley exposed to drought stress. Int. J. Mol. Sci. 2019, 20, 6341. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Mpovo, C.L.; Khan, M.A.; Shaffique, S.; Ninson, D.; Bilal, S.; Khan, M.; Kwon, E.H.; Kang, S.M.; Yun, B.W.; et al. Synergistic Effect of Melatonin and Lysinibacillus fusiformis L. (PLT16) to Mitigate Drought Stress via Regulation of Hormonal, Antioxidants System, and Physio-Molecular Responses in Soybean Plants. Int. J. Mol. Sci. 2023, 24, 8489. [Google Scholar] [CrossRef]
- Qarehkhani, M. Effects of exogenous application of abscisic acid and melatonin on biochemical and molecular traits of Salicornia europaea under salinity stress. Res. Artic. 2023; PREPRINT (Version 1) available at Research Square. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Ahmed, N.; Saha, T.; Rahman, M.; Rahman, K.; Alam, M.M.; Rohman, M.M.; Nahar, K. Exogenous salicylic acid and kinetin modulate reactive oxygen species metabolism and glyoxalase system to confer waterlogging stress tolerance in soybean (Glycine max L.). Plant Stress. 2022, 3, 100057. [Google Scholar] [CrossRef]
- Mohi-Ud-Din, M.; Talukder, D.; Rohman, M.; Ahmed, J.U.; Jagadish, S.V.K.; Islam, T.; Hasanuzzaman, M. Exogenous Application of Methyl Jasmonate and Salicylic Acid Mitigates Drought-Induced Oxidative Damages in French Bean (L.). Plants 2021, 10, 2066. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, M.; He, Y.; Han, X.; Qu, H.; Zhang, X.; Chen, C.; Zhang, J.; Song, Q.; Zhou, J.; Jie, Y.; et al. Effect of Mixed Spraying of SA and ABA on the Growth and Development of Winter Oilseed Rape (Brassica napus L.) During the Post-Waterlogging Podding Period. Agronomy 2025, 15, 348. https://doi.org/10.3390/agronomy15020348
Shao M, He Y, Han X, Qu H, Zhang X, Chen C, Zhang J, Song Q, Zhou J, Jie Y, et al. Effect of Mixed Spraying of SA and ABA on the Growth and Development of Winter Oilseed Rape (Brassica napus L.) During the Post-Waterlogging Podding Period. Agronomy. 2025; 15(2):348. https://doi.org/10.3390/agronomy15020348
Chicago/Turabian StyleShao, Mingyu, Yejun He, Xinran Han, Hongyue Qu, Xiaoyang Zhang, Changqiang Chen, Jiamin Zhang, Qinxu Song, Jinghua Zhou, Yucheng Jie, and et al. 2025. "Effect of Mixed Spraying of SA and ABA on the Growth and Development of Winter Oilseed Rape (Brassica napus L.) During the Post-Waterlogging Podding Period" Agronomy 15, no. 2: 348. https://doi.org/10.3390/agronomy15020348
APA StyleShao, M., He, Y., Han, X., Qu, H., Zhang, X., Chen, C., Zhang, J., Song, Q., Zhou, J., Jie, Y., & Xing, H. (2025). Effect of Mixed Spraying of SA and ABA on the Growth and Development of Winter Oilseed Rape (Brassica napus L.) During the Post-Waterlogging Podding Period. Agronomy, 15(2), 348. https://doi.org/10.3390/agronomy15020348






